Abstract
Amplified fragment length polymorphism (AFLP) was used for typing avian mycoplasma species. Forty-four avian mycoplasma strains were successfully typed into eight distinct groups, with each representing a different species. Homology of AFLP patterns of 35% or less was used as a cutoff value to differentiate avian mycoplasma strains into different species.
Some species of avian mycoplasma are important disease factors which adversely affect the commercial poultry industry in the United States. Avian mycoplasmas are very small prokaryotes devoid of cell walls. They tend to grow very slowly on a protein-rich medium containing 10 to 15% added animal serum and are rather resistant to certain antibiotics which are frequently employed in medium to retard growth of contaminant bacteria and fungi (4). Mycoplasma gallisepticum is the most pathogenic avian mycoplasma species, causing chronic respiratory diseases in chickens and infectious sinusitis in turkeys (11). When the infection becomes systemic, M. synoviae can also cause infectious synovitis in chickens and turkeys (5). M. iowae infection leads to reduced hatchability and embryo mortality in turkeys.
Condemnation of the infected flocks and reduction in feed conversion and egg production are the major factors related with economic losses. However, nonpathogenic species such as M. gallinarum and M. gallinaceum are often isolated and must be differentiated from pathogenic species. Amplified fragment length polymorphism (AFLP) has been extensively tested by Kokotovic et al. for typing mycoplasma species isolated from food animals such as cattle (9), swine (8), and goats (6) and from humans (7). The major advantage of this technique compared to other molecular typing methods is that it requires a relatively small amount of DNA and has great discriminatory power and reproducibility (15). So far, the system has not been evaluated for typing avian mycoplasma species. The objective of this study was to apply AFLP for differentiating avian mycoplasma species.
A total of 44 strains of avian mycoplasma representing eight different species were used in this study (Table 1). They were obtained from the depository at the Poultry Diagnostic and Research Center in Athens, Ga. Mycoplasma strains were cultured in Frey's medium with 12% swine serum (2). Unlike Kokotovic's procedure (7), the DNA template in this study was prepared by a simple boiling method described by Fan et al. (2). Mycoplasma culture (1.5 ml) harvested at late log phase was used for DNA extraction. DNA was stored at −20°C until used.
TABLE 1.
Mycoplasma strains used in this study
| Organism | Strain | Isolated from: | Source or history |
|---|---|---|---|
| M. gallisepticum | K703 | Chicken | Atypical field isolate |
| K730 | Chicken | Atypical field isolate | |
| K4110A | Turkey trachea | Field isolate | |
| K4110B | Turkey trachea | Field isolate | |
| K4492A-1 | Chicken trachea | Field isolate | |
| Ts-11 | Chicken | Vaccine (Merial) | |
| 6/85 | Unknown | Vaccine (Intervet) | |
| F | Chicken trachea | Vaccine (Schering-Plough) | |
| R | Chicken trachea | Pathogenic strain | |
| M. synoviae | K1968 | Turkey joint | Pathogenic field isolate |
| K4822B | Turkey trachea | Field isolate | |
| K4822C | Turkey trachea | Field isolate | |
| K4822D | Turkey trachea | Field isolate | |
| K5001-2 | Chicken trachea | Field isolate | |
| K5001-3 | Chicken trachea | Field isolate | |
| K5001-5 | Chicken trachea | Field isolate | |
| WVU-1853 | Chicken joint | Type strain | |
| M. iowae | 695 | Turkey air sac | Type strain |
| 693 | Turkey joint | Reference strain | |
| DK-CPA | Turkey embryo | Reference strain | |
| DNA-O | Turkey air sac | Reference strain | |
| L3-10B | Turkey yolk sac | Reference strain | |
| DRA-O | Turkey air sac | Reference strain | |
| K5378-10 | Turkey embryo | Field isolate | |
| K5413-2 | Turkey embryo | Field isolate | |
| K5521-5 | Turkey embryo | Field isolate | |
| M. meleagridis | K4766-1 | Turkey trachea | Field isolate |
| K4766-2 | Turkey trachea | Field isolate | |
| K5428A-11 | Turkey trachea | Field isolate | |
| RY39A | Turkey | Pathogenic strain | |
| CA-6 | Turkey | Reference strain | |
| E-2 | Turkey embryo | Hemagglutinating strain | |
| M. gallinarum | LPG-16 | Chicken trachea | Reference strain |
| K5446A-15 | Chicken trachea | Field isolate | |
| SB.B | Chicken trachea | Field isolate | |
| 1504 | Chicken trachea | Reference strain | |
| M. gallinaceum | SA | Chicken | Reference strain |
| R85A | Chicken | Reference strain | |
| Tully DD | Chicken | Reference strain | |
| S-594TT | Chicken | Reference strain | |
| M. pullorum | K285-496A | Chicken | Field isolate |
| K285D-2403 | Chicken | Field isolate | |
| y96 | Chicken | Reference strain | |
| M. imitans | 4229 | Duck | ATCC type strain |
Genomic DNA digestion, adapter ligation, and selective amplification were performed following the procedure described by Kokotovic et al. (7). Restriction endonucleases BglII and MfeI were used in the digestion of genomic DNA. The MFE1 primer used in this study, as in Kokotovic's study, was modified by adding a selective nucleotide A at its 3′ end to increase the selectivity of the amplification reaction and to obtain better banding pattern resolution.
Fragment detection was carried on an ABI 310 automatic sequencer (ABI Applied Biosystems, Foster City, Calif.). A mixture consisting of 2.0 μl of PCR products, 12.0 μl of 100% deionized formamide, and 0.5 μl of GeneScan 500 ROX size standard (ABI Applied Biosystems, Foster City, Calif.) was heated to 95°C for 5 min and quickly chilled on ice before electrophoresis on the machine. Fragment size determination and pattern analysis were performed by using GeneScan 3.1 fragment analysis software (ABI Applied Biosystems). ABI chromatograms were converted into schematic gel images with GelCompar II 3.5 (Applied Math Inc., Austin, Texas). Background subtraction and data normalization were subsequently conducted. Cluster analysis was performed using the Pearson correlation and unweighted pair group methods with average linkages.
AFLP analysis in this study provided an optimal separation and a uniform sizing of the amplified fragments. Fragments of between 75 and 500 bp were used in numerical and cluster analysis for species differentiation. We found that M. gallisepticum strains had the highest banding pattern complexity, consisting of about 90 AFLP fragments, while M. meleagridis profiles had the lowest complexity, having approximately only 20 amplified fragments. Both M. gallinarum and M. iowae strains contained about 50 fragments. About 60, 40, and 30 fragments were generated for M. gallinaceum, M. pullorum, and M. synoviae, respectively. M. imitans had 70 fragments in its AFLP banding pattern. Reproducibility of AFLP analysis has been extensively tested by Kokotovic et al. (7). In this study, AFLP procedure was repeated three times on three randomly chosen strains from each species and highly reproducible results were obtained. Minor changes in band intensities existed but were insignificant for determining identity of strains (data not shown).
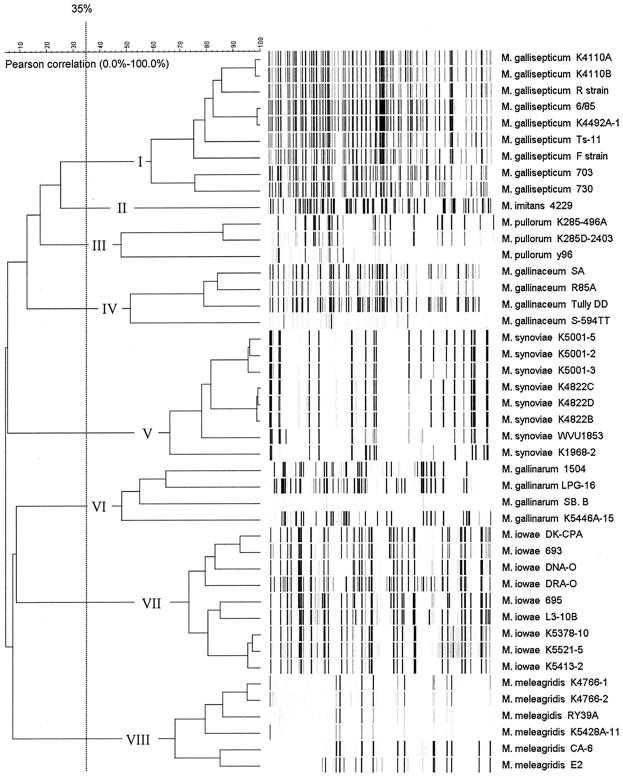
On the basis of cluster analysis, we chose the 35.0% linkage level (percent homology) as a cutoff value for discriminating mycoplasma strains at the species level (Fig. 1). AFLP data revealed eight distinct groups (I to VIII), each consisting of strains belonging to a single species. All strains within each group had different AFLP patterns, some of which were nearly identical. To validate this clustering method, we calculated error flags (not shown in the dendrogram) representing the value of the mean linkage level plus standard deviation for those groups of strains as indicated in the following paragraphs. The 35.0% cutoff line did not cross any of those error flags.
FIG. 1.
AFLP fingerprints of avian mycoplasma species. The dendrogram was constructed using Pearson correlation and the unweighted pair group method with average linkages. The eight avian mycoplasma species groups generated at a 35.0% linkage level cutoff point are indicated.
M. pullorum, M. gallinarum, and M. gallinaceum strains showed high AFLP pattern heterogeneity, with linkage levels of 47.8% ± 4.5% (mean plus standard deviation) (group III), 48.0% ± 9.0% (group VI), and 51.4% ± 6.8% (group IV), respectively. Our study is the first to report the genetic heterogeneity among those saprophytic avian mycoplasma species, though a larger number of strains, as well as other restriction enzymes, needs to be tested to make final conclusions.
On the other hand, M. iowae and M. meleagridis strains showed high homogeneity in their AFLP profiles and clustered at linkage levels of 73.2% ± 6.2% (group VII) and 68.0% ± 7.8% (group VIII), respectively. Previous studies revealed that although heterogeneity in serological responses was observed for M. iowae (10), less-variable protein profiles and random amplified polymorphic DNA (RAPD) patterns were obtained for different strains (3, 14). Among pathogenic avian mycoplasmas, M. gallisepticum strains revealed the widest intraspecies heterogeneity by AFLP analysis, with a linkage level of 59.2% ± 2.2% (group I). The genetic variation of this species has been documented by several other molecular typing techniques, such as pulse field gel electrophoresis (12), random amplified polymorphic DNA (2, 12), restriction fragment length polymorphism (13), and Southern blotting (17). M. synoviae strains, with a linkage level of 66.1% ± 3.0% (group V), exhibited more genetic homogeneity than M. gallisepticum, and the same conclusion was made using an rRNA gene hybridization test conducted by Yogev et al. (16).
M. imitans shared many phenotypic properties with M. gallisepticum but had low genetic homology with M. gallisepticum in a DNA-DNA hybridization study (1). In this study, the two species were typed into related groups and linked at the 25.3% homology level.
As determined on the basis of these results, AFLP can be used as an additional confirmatory tool for identification of avian mycoplasma species. This can be achieved by setting up a database for reference strains; such a database was partially created in this study and can be easily expanded. Our follow-up studies will focus on using AFLP for typing avian mycoplasma strains within each species.
Acknowledgments
This work was supported by grant IS-3126-99 from BARD (United States-Israel Binational Agricultural Research and Development) and by project no. 464 from the U.S. Poultry and Egg Association.
REFERENCES
- 1.Bradbury, J. M., O. M. Abdul-Wahab, C. A. Yavari, J. P. Dupiellet, and J. M. Bove. 1993. Mycoplasma imitans sp. nov. is related to Mycoplasma gallisepticum and found in birds. Int. J. Syst. Bacteriol. 43:721-728. [DOI] [PubMed] [Google Scholar]
- 2.Fan, H. H., S. H. Kleven, and M. W. Jackwood. 1995. Application of polymerase chain reaction with arbitrary primers to strain identification of Mycoplasma gallisepticum. Avian Dis. 39:729-735. [PubMed] [Google Scholar]
- 3.Fan, H. H., S. H. Kleven, and M. W. Jackwood. 1995. Studies of intraspecies heterogeneity of Mycoplasma synoviae, M. meleagridis, and M. iowae with arbitrarily primed polymerase chain reaction. Avian Dis. 39:766-777. [PubMed] [Google Scholar]
- 4.Kleven, S. H. 2003. Mycoplasmosis-introduction, p. 719-721. In Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadley, L. R. McDougald, and D. E. Swayne (ed.), Diseases of poultry. Iowa State Press, Ames, Iowa.
- 5.Kleven, S. H. 2003. Mycoplasma synoviae infection, p. 756-766. In Y. M. Saif, H. J. Barnes, J. R. Glisson, A. M. Fadley, L. R. McDougald, and D. E. Swayne (ed.), Diseases of poultry. Iowa State Press, Ames, Iowa.
- 6.Kokotovic, B., G. Bolske, P. Ahrens, and K. Johansson. 2000. Genomic variations of Mycoplasma capricolum subsp. capripneumoniae detected by amplified fragment length polymorphism (AFLP) analysis. FEMS Microbiol. Lett. 184:63-68. [DOI] [PubMed] [Google Scholar]
- 7.Kokotovic, B., N. F. Friis, J. S. Jensen, and P. Ahrens. 1999. Amplified-fragment length polymorphism fingerprinting of Mycoplasma species. J. Clin. Microbiol. 37:3300-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokotovic, B., N. F. Friis, E. O. Nielsen, and P. Ahrens. 2002. Genomic diversity among Danish field strains of Mycoplasma hyosynoviae assessed by amplified fragment length polymorphism analysis. Vet. Microbiol. 85:221-231. [DOI] [PubMed] [Google Scholar]
- 9.Kusiluka, L. J., B. Kokotovic, B. Ojeniyi, N. F. Friis, and P. Ahrens. 2000. Genetic variations among Mycoplasma bovis strains isolated from Danish cattle. FEMS Microbiol. Lett. 192:113-118. [DOI] [PubMed] [Google Scholar]
- 10.Leiting, V. A., and S. H. Kleven. 2000. Preparation of a heterogeneous conjugate to detect Mycoplasma iowae by immunofluorescence. Avian Dis. 44:697-700. [PubMed] [Google Scholar]
- 11.Ley, D. H., H. W. Yoder (ed.). 1997. Mycoplasma gallisepticum infection, 10th ed. Iowa State University Press, Ames, Iowa.
- 12.Marois, C., F. Dufour-Gesbert, and I. Kempf. 2001. Molecular differentiation of Mycoplasma gallisepticum and Mycoplasma imitans strains by pulsed-field gel electrophoresis and random amplified polymorphic DNA. J. Vet. Med. B Infect. Dis. Vet. Public Health 48:695-703. [DOI] [PubMed] [Google Scholar]
- 13.Pillai, S. R., H. L. Mays, Jr., D. H. Ley, P. Luttrell, V. S. Panangala, K. L. Farmer, and S. R. Roberts. 2003. Molecular variability of house finch Mycoplasma gallisepticum isolates as revealed by sequencing and restriction fragment length polymorphism analysis of the pvpA gene. Avian Dis. 47:640-648. [DOI] [PubMed] [Google Scholar]
- 14.Rhoades, K. R. 1984. Comparison of strains of Mycoplasma iowae. Avian Dis. 28:710-717. [PubMed] [Google Scholar]
- 15.Savelkoul, P. H., H. J. Aarts, J. de Haas, L. Dijkshoorn, B. Duim, M. Otsen, J. L. Rademaker, L. Schouls, and J. A. Lenstra. 1999. Amplification-fragment length polymorphism analysis: the state of an art. J. Clin. Microbiol. 37:3083-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yogev, D., S. Levisohn, S. H. Kleven, D. Halachmi, and S. Razin. 1988. Ribosomal RNA gene probes to detect intraspecies heterogeneity in Mycoplasma gallisepticum and M. synoviae. Avian Dis. 32:220-231. [PubMed] [Google Scholar]
- 17.Yogev, D., S. Levisohn, and S. Razin. 1989. Genetic and antigenic relatedness between Mycoplasma gallisepticum and Mycoplasma synoviae. Vet. Microbiol. 19:75-84. [DOI] [PubMed] [Google Scholar]