Abstract
Objective
To establish the efficacy of motivational-interviewing (MI)-based postoperative care navigation in improving functional status post-total knee arthroplasty (TKA) and to identify subgroups likely to benefit from the intervention.
Methods
We conducted a parallel randomized controlled trial in TKA recipients with two arms: postoperative care with frequent follow-up by a care navigator or usual care. The primary outcome was the difference between the arms in WOMAC function score change, over six months post-surgery. We performed a pre-planned subgroup analysis of differential efficacy by obesity and exploratory subgroup analyses on sex and pain catastrophizing.
Results
We enrolled 308 subjects undergoing TKA for osteoarthritis. Mean preoperative function score was 41 (SD 17) (0–100 scale, 100 worst). At six months, subjects in the navigation arm improved by 30 (SD 16) points on average compared to 27 (SD 18) points in the usual care arm (p=0.148). Participants with moderate to high levels of pain catastrophizing were unlikely to benefit from navigation compared to those with lower levels of pain catastrophizing (p-value for interaction 0.013).
Conclusion
Subjects assigned to the navigation intervention did not demonstrate greater functional improvement compared to those in the control group. The negative overall result could be explained by the large effect on functional improvement of TKA itself compared to the smaller, additional benefit from care navigation, as well as potential differential effects for subjects with moderate to high degrees of pain catastrophizing. Greater focus on developing programs for reducing pain catastrophizing could lead to better functional outcomes following TKA.
Keywords: total knee arthroplasty, care navigation, pain catastrophizing
Introduction
Total knee arthroplasty (TKA) is a common and effective surgical procedure for patients with end-stage knee osteoarthritis (OA). More than 600,000 TKA procedures are performed annually in the US, providing pain relief and functional benefit in over 80% of patients, with approximately 20% of patients experiencing suboptimal outcomes following surgery, including inadequate reduction in pain or unsatisfactory improvement in function.1–6
Recovery from TKA is a difficult process that requires patients to ambulate and complete physical therapy regimens despite significant pain.7,8 The transition from the hospital to home can be difficult for patients to manage, as they need to adjust to different medication regimens, new self-care responsibilities, and reliance on social support systems at home or in their communities.9,10
The success of rehabilitation that takes place within the first few months post-surgery can have lasting effects on long-term functional outcomes such as muscle strength and range of motion.7,11–14 While the importance of adherence to rehabilitation regimens following TKA has been established, little has been done to identify ways of helping TKA patients achieve their functional goals. Motivational Interviewing (MI) is a technique for promoting behavior change by guiding individuals through the stages of change to achieve their goals.15 We conducted the Adding Value in Knee Arthroplasty (AViKA) postoperative care navigation trial to establish the efficacy of MI-based enhanced postoperative care navigation in improving functional outcomes in persons undergoing TKA and to identify patient groups that could benefit the most from such interventions.
Methods
STUDY DESIGN AND OVERSIGHT
The AViKA trial (NCT01540851) was a parallel two-arm randomized controlled trial conducted at Brigham and Women’s Hospital (BWH), a tertiary academic medical center in Boston, MA. The details of the trial design and conduct have been previously published.16 The study was approved by the Partners Human Research Committee, the institutional review board of BWH (protocol 2010P002597).
ENROLLMENT AND RANDOMIZATION
We recruited patients over the age of 40 with knee osteoarthritis as the principal underlying diagnosis who were scheduled to undergo a primary TKA from the practices of one of five orthopedic surgeons at BWH. Eligible participants were mailed a recruitment letter and contacted by a research assistant if they did not opt out of the study. The research assistant met interested participants in person to obtain written informed consent and collect a baseline questionnaire. This baseline preoperative visit was usually completed within two weeks before the scheduled surgery date.
Following surgery, subjects were randomized in a 1:1 ratio to either the navigator arm or the usual care arm. Randomization was conducted using permutated blocks with variable block size. Patients were stratified according to sex, age (<65 vs. 65+), and surgeon surgery volume (low volume vs. high volume). Further details on the screening and enrollment process are available elsewhere.16
INTERVENTIONS
Enhanced postoperative management (navigator arm)
Patients randomized to the navigator arm received care navigation in addition to all usual practices for TKA recovery. Prior to speaking with patients, navigators studied the theory and technique behind MI.15 They also received training in MI techniques by an expert with doctoral training in behavioral science. To ensure that the navigators were well-equipped to carry out this intervention, they observed inpatient and outpatient care specific to post-TKA patients.
Patients assigned to the navigator arm were scheduled to receive ten calls from navigators trained in MI over the course of the six-month post-TKA recovery period. The calls were made weekly for the first month following surgery, biweekly for months two and three, and monthly for months four through six. During these calls, the navigator asked open-ended questions to elicit the patient’s objectives for post-surgical recovery and to help the patient identify barriers to achieving these goals. The navigator used MI-driven techniques to elicit statements of self-efficacy and aid the patient in developing specific strategies to achieve goals. The navigator also celebrated progress towards goal achievement with subjects and validated adherence to therapy. Navigators did not provide medical advice and encouraged the patient to contact his or her surgeon, physical therapist, or other healthcare provider when appropriate.
Usual care arm
Patients randomized to the usual care arm underwent the standard of care at BWH for TKA recipients. The standard rehabilitation protocol consisted of inpatient physical therapy for range of motion and gait therapy. Most patients were then discharged home with a prescribed physical therapy regimen and eventually transitioned to outpatient physical therapy. Although the duration and number of outpatient physical therapy visits varied among patients, most patients received some form of therapy for three to six months following surgery.
OUTCOME MEASURES
The primary outcome of the study was the change in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function scores from preoperative to six months postoperative. Secondary outcomes included the change in the WOMAC pain scores from preoperative to six months postoperative, the percent of participants who were very satisfied with the results of their TKA (satisfaction was assessed on a 5-item Likert scale), and the percent of participants who achieved satisfactory range of motion at six months post-surgery (able to straighten knee completely and able to bend knee to at least 120 degrees). An additional outcome was the number of participants who were able to achieve the minimal clinically important difference (MCID) in WOMAC function score by six months post-surgery. The MCID was calculated using the method described by Escobar and colleagues in which baseline WOMAC function score is accounted for in determining the MCID.17 Specifically, higher (worse) baseline scores require a larger gain in function score in order to meet the MCID.
ASSESSMENTS
Assessments were completed using self-reported questionnaires. Range of motion was measured using a standardized protocol at the time of the preoperative visit. They were also asked to complete questionnaires at three and six months post-surgery. Six-month range of motion was assessed using a validated self-reported tool.18 The principal investigator and the study team members responsible for enrolling and assessing patients were blinded to intervention group assignments. Patients were terminated from the study if they had a TKA on the contralateral knee within six months of their first procedure or if their surgeon requested early termination from the study. Adverse events relating to medical or surgical complications within six months of surgery were assessed for their relevance to the trial and reported to the Partners IRB according to IRB policies.
STATISTICAL ANALYSIS
Sample size and power
The sample size considerations were based on the detection of a ten-point difference in primary outcome between the two arms. This difference is approximately one-half of the standard deviation of change noted in observational data,19,20 and falls in the range of the MCID in the WOMAC function scale among OA patients estimated by Angst et al.21,22 We calculated the sample size assuming a Type I error rate of 5% and power of 80% and taking into account a projected 10% loss to follow-up. Our sample size estimation included one pre-planned subgroup analysis. On the basis of these considerations, we set the target sample size at 300 patients.
Analytic approach
We first compared study participants across arms to ensure that randomization led to a balanced allocation of measured characteristics. In the primary analysis we used a general linear model to evaluate the difference in primary outcome between the arms, using an “intention to treat” (ITT) approach, in which subjects were analyzed in the group to which they were randomly assigned. Secondary outcomes were assessed using general linear models for continuous outcomes and logistic regression for dichotomous outcomes. We conducted one pre-planned and several exploratory subgroup analyses. We examined whether the intervention had a differential effect for different obesity groups (pre-planned), for participants with high and low levels of pain catastrophizing (high levels were defined by values greater than 20 on the pain catastrophizing scale (PCS), the cutoff proposed by the developers of the scale),23 and for men and women.
In our primary analysis we used a complete case approach, only including data for patients completing both the preoperative and six month postoperative WOMAC function assessments. In sensitivity analysis we used a last observation carried forward approach to account for missing data. A large body of literature suggests that there are small differences in functional status between three and six months post-surgery, so we used three month data for those study participants for whom we did not have six month data.24–26 In addition to the ITT analysis, we conducted an ‘as treated’ analysis, analyzing the data of 17 study participants randomized to the care navigator arm but who did not receive any intervention as part of the ‘usual care’ arm.
Results
CHARACTERISTICS OF THE STUDY SAMPLE
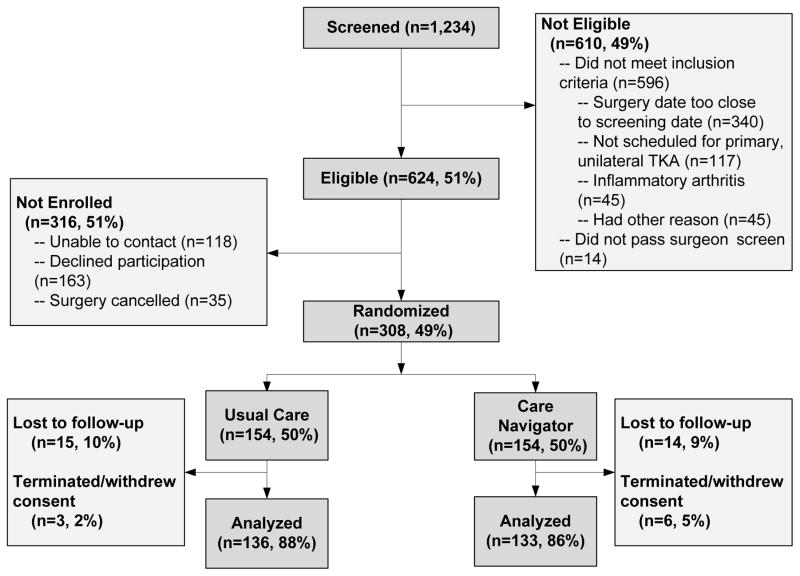
Between August 2011 and November 2013, we screened 1,234 patients scheduled to undergo TKA at BWH, and identified 624 (51%) patients eligible for enrollment. Of the eligible patients, 308 (49%) agreed to participate and were randomized into one of the two treatment groups, with 154 randomized to each arm (Figure 1). Participants in each group were similar with respect to age, sex, body mass index (BMI), Kellgren-Lawrence (KL) grade,27 and WOMAC pain and physical function scores. The mean age of the sample was 66 years (SD 8), and 60% were females. Half of the sample had BMI>30 kg/m2. About two-thirds of the knees in the sample had KL 4 OA, the highest severity. The average baseline function score was 41 (SD 17). The groups were balanced with respect to pain catastrophizing scores, with an average pain catastrophizing score of 11 (Table 1). Among those randomized to the usual care arm, 15 (10%) were lost to follow up and 3 (2%) were terminated or withdrew from the study. Among subjects randomized to the navigator arm, losses to follow up and terminations/withdrawals were similar at 9% and 5% respectively. The final analytical sample for the primary analysis consisted of 269 participants (Figure 1).
Figure 1. Study enrollment and follow-up.
1,234 persons were assessed for eligibility, of whom 624 were deemed eligible for enrollment. Of those eligble who agreed to participate, 154 subjects were randomized to each study arm. 136 and 133 subjects were analyzed in the usual care and care navigator study arms, respectively.
Table 1.
Baseline Characteristics of the Study Participants*
| Characteristic | Care Navigator (N=154) | Usual Care (N=154) |
|---|---|---|
| Mean age – years | 66 (SD 8) | 67 (SD 8) |
| Sex – n. (%) | ||
| Female | 93 (60%) | 93 (60%) |
| Male | 61 (40%) | 61 (40%) |
| BMI Range – n. (%)† | ||
| 1. <30 | 73 (49%) | 76 (50%) |
| 2. 30–35 | 46 (31%) | 40 (27%) |
| 3. >35 | 30 (20%) | 35 (23%) |
| Kellgren-Lawrence Grade – n. (%)‡ | ||
| 1–2 | 13 (9%) | 20 (13%) |
| 3 | 42 (28%) | 33 (22%) |
| 4 | 94 (63%) | 100 (65%) |
| WOMAC physical function score¶ | 42 (SD 16) | 41 (SD 19) |
| WOMAC pain score ** | 42 (SD 16) | 40 (SD 19) |
| Pain catastrophizing score*** | 11 (SD 9) | 11 (SD 10) |
| Pain catastrophizing level – n. (%) | ||
| Low | 130 (84%) | 129 (84%) |
| Moderate/High | 24 (16%) | 25 (16%) |
Values shown are for all patients who completed a baseline visit and were randomized to one of the study arms.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters
A Kellgren-Lawrence grade of 1 (no joint-space narrowing, questionable osteophite) indicates possible osteoarthritis; a grade of 2 (no joint-space narrowing, definite osteophite) indicates mild osteoarthritis; a grade of 3 (less than 50% joint-space narrowing) indicates moderate osteoarthritis; a grade of 4 (more than 50% joint-space narrowing) indicates severe osteoarthritis.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function score is scored from 0 to 100, with higher scores indicating more limited physical function
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score is a five-item scale scored from 0 to 100, with higher scores indicating higher pain levels
The Pain Catastrophizing Scale quantifies the extent to which an individual engages in catastrophic thoughts as a response to pain on a scale from 0 to 52, with 52 being the worst catastrophizing. Scores greater than 20 are indicative of moderate/high pain catastrophizing
FIDELITY TO THE INTERVENTION PROTOCOL
Four care navigators made a total of 2,504 phone calls to the 154 patients randomized to the navigator arm. Ninety seven percent of patients in the navigator arm had a consistent navigator. Out of all calls made, navigators reached study participants on 71% of the calls. Among completed calls, 55% were complete on a first attempt, with an additional 27% complete on the second attempt, and 18% required more than two attempts. The average call length among completed calls was 13 minutes, with the average length of the first call being 15 minutes. Among 421 unsuccessful contacts (27% of all planned contacts), the compliance with the protocol (four call attempts) was 32%, with 39% of unsuccessful contacts having at least two calls. Reaching study participants varied by the time from the surgery. During the first post-operative week, only 64% of calls were successful, in the interval between 2 and 10 weeks post-surgery, 76% of planned contacts were successful, and after week 10, 64% of all planned contacts were successful.
Out of the 154 subjects randomized to the navigation arm, 109 (71%) had 7 or more completed calls, 23 (15%) had 5 or 6 completed calls, and 22 (14%) had fewer than 5 completed calls. 137 patients (89%) had at least one completed call. The mean number of completed calls per patient was 7. Patients with a BMI over 35 showed slightly greater adherence to calls, with 77% of patients completing 7 or more calls, compared to 67% of patients with BMI between 30 and 35, and 70% of patients with BMI less than 30.
OUTCOMES
Primary outcome
The mean change in WOMAC function score from baseline to six months post-operatively for patients assigned to the navigator group was −30 points, compared to −27 points for the usual care group, with a difference of −3 points (95% confidence interval [CI] −7 to 1) (Table 2). In both arms improvement from the baseline (pre-TKA) was substantial, reaching 1.5 SD.
Table 2.
Primary and Secondary Outcomes of the AViKA Trial at 6 Months Post-TKA*
| Outcome | Care Navigator (N= 133) | Usual Care (N= 136) | Improvement from Baseline|| | Between-Group Difference in Improvement from Baseline† | |
|---|---|---|---|---|---|
| Care Navigator | Usual Care | ||||
| Primary Outcome | |||||
|
| |||||
| WOMAC physical-function score - mean (95% CI) | 12 (10 to 14) | 13 (11 to 16) | −30 (−33 to −27) | −27 (−30 to −23) | −3 (−7 to 1) |
|
| |||||
| Secondary Outcomes | |||||
|
| |||||
| WOMAC pain score - mean (95% CI) | 11 (9 to 14) | 11 (9 to 14) | −31 (−33 to −28) | −28 (−31 to −24) | −3 (−7 to 1) |
| n (%) | n (%) | p-value | |||
|
| |||||
| Failure to achieve MCID (Escobar 2014) in WOMAC function score‡ | 26 (20%) | 37 (27%) | 0.18 | ||
|
| |||||
| Very satisfied with the results of TKA¶ | 105 (80%) | 97 (72%) | 0.14 | ||
|
| |||||
| Able to straighten knee completely** | 98 (74%) | 92 (69%) | 0.36 | ||
|
| |||||
| Able to bend knee ≥120 degrees*** | 91 (70%) | 97 (72%) | 0.60 | ||
CI denotes 95% confidence interval
Improvement from baseline calculated as the baseline value subtracted from the six month value
Between-group differences in improvement were calculated by subtracting the improvement from baseline for the usual care group from the improvement from baseline for the navigator group
MCID is based on a method described by Escobar et al. (2014) in which baseline score is taken into account to determine MCID
Assessed using a 5-item Likert scale on the 6 month follow-up questionnaire
Patients who self-reported on their 6 month questionnaire that they were able to extend their leg to be completely straight
Patients who self-reported on their 6 month questionnaire that they were able to bend their index knee to greater than 120 degrees
Secondary outcomes
The improvements from baseline to six months in WOMAC pain score were similar to WOMAC function. The proportion of participants with improvements in functional status below accepted levels of MCID at six months postoperatively was 20% in the care navigation arm and 27% in the usual care arm (p=0.178) (Table 2). Satisfaction with surgery was 80% in the care navigator arm and 72% in the usual care arm (p=0.143). Seventy percent of subjects randomized to the care navigation arm and 72% of those randomized to the usual care arm reported being able to bend their operative knee to more than 120 degrees six months after surgery (Table 2).
Subgroup analyses
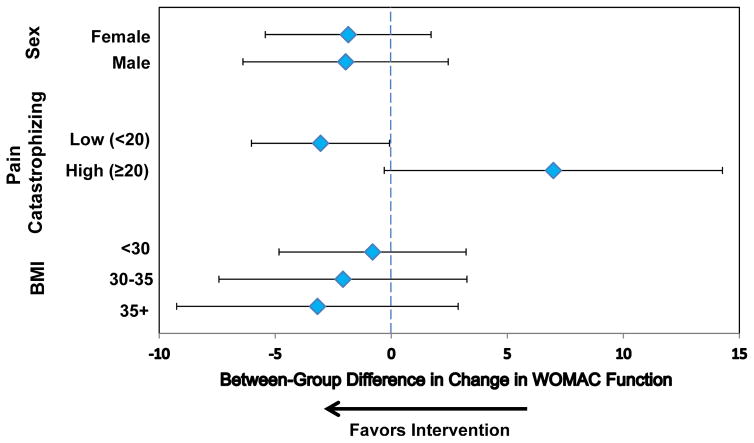
Figure 2 depicts results of one pre-planned and several exploratory subgroup analyses. The effect of the intervention was −1 point (95% CI: −5 to 3) for study participants with BMI<30 kg/m2, −2 points (95% CI: −7 to 3) for those with BMI between 30 and 35 kg/m2, and −3 points (95% CI: −9 to 3) for those with BMI>35 kg/m2 (p-value for interaction 0.802).
Figure 2. Pre-planned and exploratory subgroup analyses showing changes in WOMAC function scores from baseline to six months post-surgery.
The effectiveness of the care navigator intervention was analyzed for subgroups divided by sex, pain catastrophizing score (cutoff of 20), and BMI (cutoffs of 30 and 35). Diamonds represent the mean difference in the 6 month change in WOMAC function between the care navigator group and the usual care group, adjusted for baseline WOMAC function scores. The lines show 95% confidence intervals. Negative values indicate functional improvement.
For study participants with a moderate degree of pain catastrophizing (scores of 20 and higher) the intervention had a detrimental effect of 7 points (95% CI: 0 to 14) compared to small positive effect among those with a low degree of pain catastrophizing (−3 points, 95% CI: −6 to 0) (p-value for interaction 0.013).
The data did not suggest a difference in the effect of the care navigation intervention between men and women.
As treated analysis
When the 17 study participants randomized to the navigator arm who did not have any contacts with the study navigator were analyzed in the ‘usual care’ arm the results of the analyses remained virtually unchanged.
Last observation carried forward analysis
When we used three month data for those study participants for whom we did not have six month data, no appreciative differences compared to main analysis were observed in the results.
Discussion
This report summarizes results of a randomized controlled trial of a motivational-interviewing-based care navigation intervention for patients undergoing TKA. The six month intervention did not lead to clinically important improvements in functional status during the six months post-TKA. Satisfaction with the surgery was high, with 80% of subjects randomized to the navigator arm and 72% of subjects randomized to the usual care arm reporting being “very satisfied” with the results of surgery. Both arms reported similar range of motion at six months postoperatively.
The results of our study suggest that during the relatively short rehabilitation period most subjects may have been sufficiently supported and motivated by the medical team (surgeon, home care nurse and physical therapist) and that therefore the care navigator intervention was not necessary. That is, the high motivation to recover, the comprehensive support of the medical team, and the powerful effect of the surgical intervention appeared to be sufficient for the vast majority of patients to achieve satisfactory functional improvement.
To our knowledge, this is the first published randomized controlled trial to investigate the effectiveness of a motivational interviewing intervention on improvement in pain and function following TKA. Rosal and colleagues have published a study protocol for a telephone-delivered Patient Self-Management Support intervention partially based on the principles of MI to improve pain and functional outcomes following TKA, but the results have not yet been published.28
Previous studies have had success implementing motivational interviewing techniques for the management of chronic diseases ranging from rheumatoid arthritis and diabetes to obesity and substance abuse.29–32 For example, a study of teenagers with type I diabetes showed that patients in the motivational interviewing group had significantly lower A1C levels compared to baseline than the control group at 12 months, and this difference was maintained at 24 months.31 Motivational interviewing has also been shown to help diabetic patients lower blood pressure and improve their depression symptom scores.33 Most of these studies applied MI techniques to improve adherence to the management of chronic conditions. The major difference between management of chronic conditions and post-surgical recovery may lie in the stage of change of enrolled subjects. While many patients with chronic diseases may be in lower stages of behavioral change (‘precontemplation’ or ‘contemplation’), those undergoing surgery are likely to be in higher stages (‘planning’ or ‘action’).34
In the current study, short-term navigation was used to further improve outcomes of a surgical intervention that has been shown to substantially improve functional status in about 80% of the subjects. Since there are no clear algorithms that prospectively identify TKA recipients at high risk of suboptimal outcomes, offering the intervention to all TKA recipients rather than only to those at high risk for poor outcomes may have diluted the efficacy of the intervention. Since MI is based on techniques related to long-term behavioral change, it is possible that instilling behavioral changes over the short period and focusing on adherence to a short-term rehabilitation regimen is not as effective as helping people manage chronic conditions such as diabetes or obesity, where forming and maintaining healthy habits can have major impacts on disease symptoms.
The results of this study suggest that instead of implementing the MI-based program for all TKA recipients, it is conceivable that a better approach would be to identify particular subgroups more likely to benefit from an MI-based intervention. Tailored MI-based interventions could be useful for patients with high BMI. In fact, these patients showed somewhat greater adherence to calls, compared to non-obese study participants. Results of our subgroup analysis show a trend, though non-significant, toward somewhat greater effect in more obese subjects, suggesting that future studies might focus on the value of MI-based interventions in obese patients.
Exploratory results of the subgroup analyses suggest that TKA recipients with higher levels of pain catastrophizing likely need more individualized step-up interventions that first focus on coping skills development with subsequent shift toward adherence to the post-TKA rehabilitation regimen. Pain catastrophizing has shown to be a significant predictor of poor outcomes following TKA in several previous studies.35–38 It has also been associated with longer hospital stays following TKA.39 Additionally, studies have shown that women are more likely to engage in pain catastrophizing than men.40,41 As evidence regarding pain catastrophizing as a risk factor for suboptimal outcomes evolves, researchers have sought to identify interventions to alleviate the impact of pain catastrophizing. A study conducted by Riddle and colleagues tested the efficacy of pain coping skills training on patients undergoing TKA who had elevated levels of pain catastrophizing.42 Their intervention involved teaching adaptive coping strategies such as distraction and relaxation and steering patients away from harmful coping strategies. Patients who received pain coping skills training reported significantly greater reductions in pain severity and catastrophizing, and saw more improvement in function compared to the control group.42 While our motivational interviewing model focuses on behavioral change and overcoming obstacles, patients with high levels of pain catastrophizing may not benefit from the intervention until they receive psychological training and learn coping strategies. The results of our study provide preliminary support to this hypothesis, suggesting that patients with a high degree of pain catastrophizing may not be responsive to behavioral interventions focused on goal setting and adherence to rehabilitation regimens.
Results of this study should be viewed within several limitations. The study was conducted at a single academic medical center and the results may not be generalizable to geographically or demographically distinct populations. A majority of the study sample reported having good access to physical therapy, home health care, and adequate transportation to follow-up appointments. It is possible that a care navigation intervention may be more beneficial in a community with less access to health care or with lower socio-economic status. Although fewer than 15% of study participants dropped out, it is possible that the missing data may not be missing completely at random. Additionally, there were several navigators who were trained in motivational interviewing and delivered the care navigator intervention, and each one could have differed slightly in their approach to the intervention. Care was taken to audit the navigator calls and ensure consistency among the navigators by standardization of training and common discussion of the case-based studies, but it is possible that small differences remained. Using telephone calls to deliver the MI intervention may not have generated a strong enough relationship between the navigators and the patients. It is possible that other modes of delivery, such as in-person or using video calls may allow patients to form a stronger relationship with the navigator and benefit more from the intervention.
In summary, patients receiving a TKA at Brigham and Women’s Hospital in Boston were randomly assigned to receive either usual care or a navigator intervention following surgery. The navigator intervention incorporated motivational interviewing techniques to help patients make behavioral changes to aid in TKA recovery. The results of our study suggest that while it is unlikely that an MI-based rehabilitation support program would lead to better overall functional status 6 months post TKA, such a program could be of value to particular subgroups or if focused on global outcomes of TKA such as greater engagement in physical activity and reduction in sedentary behavior.43,44
This study suggests that pain catastrophizing may present a barrier to successful patient navigation interventions for functional improvements following TKA. The lack of the positive effect of the intervention on patients with pain catastrophizing may be due to the difficulty these patients experience coping with pain. Patients with high levels of pain catastrophizing might achieve better post-operative outcomes with interventions such as cognitive behavioral therapy and other methods that teach coping skills rather than MI alone. Patients with high pain catastrophizing levels should be identified early so that they can benefit from such supportive therapies.
The results of this study suggest that instead of implementing the MI-based program for all TKA recipients, a better approach may be to identify particular subgroups more likely to benefit from an MI-based intervention. Risk stratification may be critical prior to selecting persons who could benefit from behavioral interventions, and such interventions should be tailored to underlying factors such as degree of pain catastrophizing. In order for patient navigation to be helpful for patients following TKA, persons with high levels of pain catastrophizing may benefit from a pain coping skills intervention before participating in a MI-based intervention. Future studies should address the effect of MI on additional outcomes following TKA such as increasing physical activity or reducing sedentary behavior.
Significance and Innovations.
The data did not support the hypothesis that a postoperative care navigation program would lead to better functional status six months post-TKA compared to those receiving the standard post-TKA protocol. These results suggest that implementing postoperative care navigating programs without tailoring them to specific patient groups may not lead to overall improvements in functional status in persons undergoing total knee arthroplasty.
Exploratory subgroup analyses suggested that the intervention was more efficacious in subjects with low levels of pain catastrophizing.
Pain catastrophizing could be a barrier for successful patient navigation interventions aimed at improving postoperative functional status, and interventions addressing pain catastrophizing could be beneficial for improving outcomes following TKA.
Acknowledgments
Support: NIH/NIAMS K24AR057827
Footnotes
Potential Conflicts of Interest:
Losina: Deputy Editor, Journal of Bone and Joint Surgery
Wright: DePuy, a Johnson & Johnson Company
Katz: Deputy Editor, Journal of Bone and Joint Surgery; President-elect, Osteoarthritis Research Society International
References
- 1.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2:e000435. doi: 10.1136/bmjopen-2011-000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker PN, van der Meulen JH, Lewsey J, Gregg PJ. The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br. 2007;89:893–900. doi: 10.1302/0301-620X.89B7.19091. [DOI] [PubMed] [Google Scholar]
- 3.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152:566–572. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) Rockville, MD: Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 5.Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308:1227–1236. doi: 10.1001/2012.jama.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright RJ, Sledge CB, Poss R, Ewald FC, Walsh ME, Lingard EA. Patient-reported outcome and survivorship after Kinemax total knee arthroplasty. J Bone Joint Surg Am. 2004;86-A:2464–2470. doi: 10.2106/00004623-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Meier W, Mizner RL, Marcus RL, Dibble LE, Peters C, Lastayo PC. Total knee arthroplasty: muscle impairments, functional limitations, and recommended rehabilitation approaches. J Orthop Sports Phys Ther. 2008;38:246–256. doi: 10.2519/jospt.2008.2715. [DOI] [PubMed] [Google Scholar]
- 8.Petterson SC, Mizner RL, Stevens JE, Raisis L, Bodenstab A, Newcomb W, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61:174–183. doi: 10.1002/art.24167. [DOI] [PubMed] [Google Scholar]
- 9.Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists. J Hosp Med. 2007;2:314–323. doi: 10.1002/jhm.228. [DOI] [PubMed] [Google Scholar]
- 10.Cain CH, Neuwirth E, Bellows J, Zuber C, Green J. Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7:382–387. doi: 10.1002/jhm.1918. [DOI] [PubMed] [Google Scholar]
- 11.Moffet H, Collet JP, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: A single-blind randomized controlled trial. Arch Phys Med Rehabil. 2004;85:546–556. doi: 10.1016/j.apmr.2003.08.080. [DOI] [PubMed] [Google Scholar]
- 12.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005;35:424–436. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 13.Ebert JR, Munsie C, Joss B. Guidelines for the early restoration of active knee flexion after total knee arthroplasty: implications for rehabilitation and early intervention. Arch Phys Med Rehabil. 2014;95:1135–1140. doi: 10.1016/j.apmr.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Mizner RL, Stevens JE, Snyder-Mackler L. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83:359–365. [PubMed] [Google Scholar]
- 15.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2. New York: The Guilford Press; 2002. [Google Scholar]
- 16.Losina E, Collins JE, Daigle ME, Donnell-Fink LA, Prokopetz JJ, Strnad D, et al. The AViKA (Adding Value in Knee Arthroplasty) postoperative care navigation trial: rationale and design features. BMC Musculoskelet Disord. 2013;14:290. doi: 10.1186/1471-2474-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escobar A, Riddle DL. Concordance between important change and acceptable symptom state following knee arthroplasty: the role of baseline scores. Osteoarthritis Cartilage. 2014;22:1107–1110. doi: 10.1016/j.joca.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Collins JE, Rome BN, Daigle ME, Lerner V, Katz JN, Losina E. A comparison of patient-reported and measured range of motion in a cohort of total knee arthroplasty patients. J Arthroplasty. 2014;29:1378–1382. e1371. doi: 10.1016/j.arth.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escobar A, Quintana JM, Bilbao A, Azkarate J, Guenaga JI, Arenaza JC, et al. Effect of patient characteristics on reported outcomes after total knee replacement. Rheumatology (Oxford) 2007;46:112–119. doi: 10.1093/rheumatology/kel184. [DOI] [PubMed] [Google Scholar]
- 20.Nunez M, Lozano L, Nunez E, Segur JM, Sastre S, Macule F, et al. Total knee replacement and health-related quality of life: factors influencing long-term outcomes. Arthritis Rheum. 2009;61:1062–1069. doi: 10.1002/art.24644. [DOI] [PubMed] [Google Scholar]
- 21.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–138. [PubMed] [Google Scholar]
- 22.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–391. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan M, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 24.Davis AM, Perruccio AV, Ibrahim S, Hogg-Johnson S, Wong R, Streiner DL, et al. The trajectory of recovery and the inter-relationships of symptoms, activity and participation in the first year following total hip and knee replacement. Osteoarthritis Cartilage. 2011;19:1413–1421. doi: 10.1016/j.joca.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy DM, Stratford PW, Riddle DL, Hanna SE, Gollish JD. Assessing recovery and establishing prognosis following total knee arthroplasty. Phys Ther. 2008;88:22–32. doi: 10.2522/ptj.20070051. [DOI] [PubMed] [Google Scholar]
- 26.Naylor JM, Harmer AR, Heard RC. Severe other joint disease and obesity independently influence recovery after joint replacement surgery: an observational study. Aust J Physiother. 2008;54:57–64. doi: 10.1016/s0004-9514(08)70067-9. [DOI] [PubMed] [Google Scholar]
- 27.Kellgren JH, Lawrence JS. Radiographic assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosal MC, Ayers D, Li W, Oatis C, Borg A, Zheng H, et al. A randomized clinical trial of a peri-operative behavioral intervention to improve physical activity adherence and functional outcomes following total knee replacement. BMC Musculoskelet Disord. 2011;12:226. doi: 10.1186/1471-2474-12-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabbay RA, Anel-Tiangco RM, Dellasega C, Mauger DT, Adelman A, Van Horn DH. Diabetes nurse case management and motivational interviewing for change (DYNAMIC): results of a 2-year randomized controlled pragmatic trial. J Diabetes. 2013;5:349–357. doi: 10.1111/1753-0407.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knittle K, De Gucht V, Hurkmans E, Peeters A, Ronday K, Maes S, et al. Targeting motivation and self-regulation to increase physical activity among patients with rheumatoid arthritis: a randomised controlled trial. Clin Rheumatol. 2015;34:231–238. doi: 10.1007/s10067-013-2425-x. [DOI] [PubMed] [Google Scholar]
- 31.Channon SJ, Huws-Thomas MV, Rollnick S, Hood K, Cannings-John RL, Rogers C, et al. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care. 2007;30:1390–1395. doi: 10.2337/dc06-2260. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12:709–723. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 33.Gabbay RA, Anel-Tiangco RM, Dellasega C, Mauger DT, Adelman A, Horn DH. Diabetes Nurse Case Management and Motivational Interviewing for Change (DYNAMIC): Results of a 2-year Randomized Controlled Pragmatic Trial. J Diabetes. 2013 doi: 10.1111/1753-0407.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prochaska JO, Velicer WF. The Transtheoretical Model of Health Behavior Change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan M, Tanzer M, Reardon G, Amirault D, Dunbar M, Stanish W. The role of presurgical expectancies in predicting pain and function one year following total knee arthroplasty. Pain. 2011;152:2287–2293. doi: 10.1016/j.pain.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Riddle DL, Wade JB, Jiranek WA, Kong X. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res. 2010;468:798–806. doi: 10.1007/s11999-009-0963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vissers MM, Bussmann JB, Verhaar JA, Busschbach JJ, Bierma-Zeinstra SM, Reijman M. Psychological factors affecting the outcome of total hip and knee arthroplasty: a systematic review. Semin Arthritis Rheum. 2012;41:576–588. doi: 10.1016/j.semarthrit.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Forsythe ME, Dunbar MJ, Hennigar AW, Sullivan MJ, Gross M. Prospective relation between catastrophizing and residual pain following knee arthroplasty: two-year follow-up. Pain Res Manag. 2008;13:335–341. doi: 10.1155/2008/730951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witvrouw E, Pattyn E, Almqvist KF, Crombez G, Accoe C, Cambier D, et al. Catastrophic thinking about pain as a predictor of length of hospital stay after total knee arthroplasty: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2009;17:1189–1194. doi: 10.1007/s00167-009-0817-x. [DOI] [PubMed] [Google Scholar]
- 40.Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87:325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan M, Tripp D, Santor D. Gender differences in pain and pain behavior: The role of catastrophizing. Cognitive Therapy and Research. 2000;24:121–134. [Google Scholar]
- 42.Riddle DL, Keefe FJ, Nay WT, McKee D, Attarian DE, Jensen MP. Pain coping skills training for patients with elevated pain catastrophizing who are scheduled for knee arthroplasty: a quasi-experimental study. Arch Phys Med Rehabil. 2011;92:859–865. doi: 10.1016/j.apmr.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martins RK, McNeil DW. Review of Motivational Interviewing in promoting health behaviors. Clin Psychol Rev. 2009;29:283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Harland J, White M, Drinkwater C, Chinn D, Farr L, Howel D. The Newcastle exercise project: a randomised controlled trial of methods to promote physical activity in primary care. BMJ. 1999;319:828–832. doi: 10.1136/bmj.319.7213.828. [DOI] [PMC free article] [PubMed] [Google Scholar]