Abstract
BACKGROUND
The vein of Labbé (VOL) is a superficial cortical vein which drains the lateral surface of the temporal lobe. Thrombosis of the VOL can occur in the neonatal period. The developmental outcomes of infants who had VOL thrombosis are unknown as few studies of outcomes exist.
METHODS
Retrospective chart review of infants born ≥34 weeks gestation, diagnosed with VOL thrombosis and/or infarction on neuroimaging during the first 30 days of life. Size of each temporal lobe infarction was estimated based on number of temporal lobe’s segments involved. Primary outcomes were presence of major neurodevelopmental impairments in childhood and Bayley scores at 2 years.
RESULTS
Our cohort of 19 infants had a median gestational age of 38 weeks [IQR 36–39] and mean birthweight 2892±920 grams. The most common presenting symptoms of VOL thrombosis and infarction of surrounding tissue were seizures, apnea, lethargy and either hyper- or hypotonia. At the latest clinical follow-up appointment documented in the electronic medical record (mean 4.4±3.08 years), 44% had major neurodevelopmental impairment. Patients with large VOL infarctions had significantly worse average Bayley scores than those with small to moderate lesions, differences in language composite were statistically significant (72.7 vs 107.8, p = 0.017).
CONCLUSIONS
Neonates with large VOL infarctions are more likely to have poor language outcomes. This suggests a need for targeted surveillance to ensure early identification of deficits and referral for intervention.
Keywords: cerebral sinovenous thrombosis, vein of Labbé, outcome, neonate, neurodevelopment
INTRODUCTION
Cerebral sinovenous thrombosis (CSVT) is a rare but important cause of morbidity and mortality in neonates. Nearly half of pediatric CSVT cases occur in neonates1,2. This age group is at risk of thrombosis secondary to immature hemostatic systems, and maternal and perinatal risk factors.3 The former include maternal diabetes, pre-eclampsia and chorioamnionitis. The latter include perinatal fetal distress, hypoxic ischemic injury, neonatal infection and pro-thrombotic disorders.1–3 Neurologic manifestations and presenting signs and symptoms of CSVT in children are similar to those seen in adult stroke. In neonates, however, the symptoms are non-specific. Such ambiguity hampers a timely and accurate diagnosis.1
Advances in CNS imaging techniques allow accurate localization of thrombosis or infarction in children and adults. Difficulties visualizing the drainage territories of the cortical veins in neonates, has resulted in a paucity of information for this population.4–6 The vein of Labbé (VOL), however, part of the superficial venous system draining the lateral surface of the temporal lobe, is one of the most consistently identified cortical veins. The VOL arises from the Sylvian fissure and travels posteriorly and inferiorly into the transverse sinus.6,7 Thrombosis in the VOL results in a pattern of hemorrhagic infarction in the lateral aspect of the underlying temporal lobe, with medial temporal sparing and surrounding ischemic changes.6,7 Outcomes of other types of CSVT include epilepsy and significant neurologic disability.2 However, the outcomes of VOL thrombosis and ensuing infarction of the surrounding tissue are unknown, presenting a challenge for clinicians who counsel parents in the neonatal period.
To aid clinicians caring for infants with VOL thrombosis and ensuing infarction of the surrounding tissue, we studied the hypothesis that increasing size of VOL infarctions would be associated with worse neurodevelopmental outcomes in childhood. We also investigated whether perinatal factors could assist in early identification of VOL thrombosis and ensuing infarction of the surrounding tissue. To accomplish our aims, we searched a Pediatric Neuroradiology database of CSVT and the corresponding electronic medical record of the patients diagnosed with VOL thrombosis and ensuing infarction of the surrounding tissue to characterize the patients’ development.
MATERIALS AND METHODS
We conducted a retrospective chart review of all patients with a diagnosis of VOL thrombosis and ensuing infarction of the surrounding tissue in a pediatric neuroradiology database since its creation in 2002. All subjects in the database were admitted the Neonatal Intensive Care Unit (NICU) between 2002 and 2014. This level IV NICU cares for patients referred from level-II and level-III nurseries in a 200 mile surrounding area when a higher level of neonatal care or sub-specialty services are needed. Included in the review were 19 infants born at ≥34 weeks gestation, diagnosed with VOL thrombosis and ensuing infarction of the surrounding tissue on neuroimaging in the first 30 days after birth. Exclusion criteria were major congenital central nervous system anomalies, culture confirmed meningitis, major congenital anomalies, additional large non-VOL superficial or deep venous thrombosis and ensuing infarction of the surrounding tissue or thrombosis of the cerebral sinuses. This study was approved by our hospital’s Institutional Review Board. Neurodevelopmental data included neurologic exams, physical, occupational and speech therapy assessments, follow-up imaging and the Bayley-III Scales of Infant and Toddler Development (Bayley) cognitive, language and motor scales.8 Data from all available standardized neurodevelopmental testing were collected (see supplementary material for specific tests). In the Neonatal Follow-up Clinic, trained examiners administered the Bayley at 6, 12, 18 and 24 months corrected age. Cerebral palsy (CP) diagnosis was confirmed through review of neurology and NICU follow-up clinic exams and notes, and classified according to the Gross Motor Function Classification System on the basis of the neurologic exam at the last visit.
Primary outcomes were presence of major neurodevelopmental impairment (NDI) at the latest clinical follow-up appointment documented in the electronic medical record and Bayley scores in the first two years of life. Major neurodevelopmental impairment was defined as a Bayley score <80 in any domain, major vision impairment (cortical blindness, bilateral visual field loss, hemianopsia), major hearing impairment (bilateral hearing loss requiring amplification), major neurobehavioral impairment or a diagnosis of CP.
Two pediatric radiologists independently reviewed all imaging and consensus findings were used for data analysis. Location of thrombus in the VOL and presence of infarction was recorded. The vein of Labbé drains the entire lateral temporal lobe from the anterior tip to the overlap zone of the posterior temporal lobe and the lateral occipital lobe. It drains into the tentorial leafs about a centimeter from the junction of the transverse sinus and the sigmoid sinus. Therefore, the segmental temporal lobe landmarks were the anterior tip of the temporal lobe and the insertion point of the VOL into the tentorium. This distance was divided into 3 equal segments and the superior to inferior distance of the lateral temporal lobe was also divided into 3 equal segments making 9 segments. A 10th segment was also added composed of the anterior lateral occipital lobe within the drainage area of the VOL. Size and severity of each temporal lobe infarction was estimated based on number of temporal lobe’s segments involved: small (1–3 segments), moderate (4–6 segments), or large (7–10 segments). The following were also recorded: A) presence of intraventricular or choroid plexus hemorrhage B) vascular anomalies and other small venous thromboses C) deep white matter abnormalities. Figure 1 illustrates the range of VOL thrombosis and ensuing infarction of the surrounding tissue. Study data were managed using REDCap electronic data capture tools hosted at our institution.9
Figure 1.
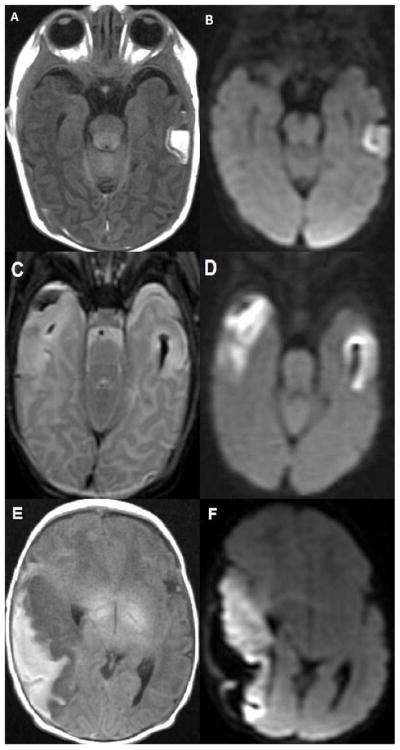
A. and B. 8 day old infant with small left VOL hemorrhagic venous infarction (HVI). A. Axial T1 weighted image demonstrates the typical appearance of a small mid left temporal lobe infarct with peripheral hemorrhage. B. Axial diffusion weighted image demonstrates a medial band of bright signal consistent with restricted diffusion (infarction) within the cortex of the VOL HVI.
C. and D. 3 day old infant with moderate right VOL hemorrhagic venous infarction (HVI) and small left VOL HVI. C. Axial T2 weighted image demonstrates peripheral hemorrhage in the moderate right VOL HVI as well as T2 bright edema in the involved right temporal lobe cortex and underlying white matter. The small left VOL HVI shows focal cortical and white matter edema with deeper T2 dark hemorrhage. D. Axial diffusion weighted image demonstrates bright signal consistent with restricted diffusion (infarction) in both moderate and small VOL HVIs.
E. and F. 1 day old infant with large right VOL hemorrhagic venous infarction (HVI) who presented with apneic events at 8 hours of life, confirmed as electrographic seizures. He was discharged on anti-epilepsy medication and was ultimately diagnosed with Lennox Gastaut Syndrome at age 3 years. He has marked language delays. E. Axial T1 weighted image demonstrates peripheral T1 bright hemorrhage along the large right temporal VOL HVI with low T1 signal edema in the underlying infarcting cortex and subcortical white matter. F. Axial diffusion weighted image demonstrates bright signal consistent with restricted diffusion (infarction) within the cortex and subcortical white matter of the entire right temporal lobe. The peripheral hemorrhage lateral to the infarcting right temporal lobe has dark signal.
Analysis
Continuous variables were summarized using mean ± standard deviation or median [interquartile range]. Categorical variables were summarized using percentiles. Unpaired two-tailed t-tests and chi-squared tests were used to respectively compare continuous and categorical variables. Significance was assigned at p<0.05.
RESULTS
Our cohort consisted of 19 patients with VOL thrombosis and ensuing infarction of the surrounding tissue. The demographic and clinical data are detailed in Table 1. Of 19 infants, 13 (68%) were male. Most infants (84%) were admitted to the NICU within the first week after birth. The majority of the cohort presented with common clinical signs and symptoms of perinatal stroke prompting imaging evaluation. However, 5 of 19 patients had a lesion discovered on imaging performed for unrelated reasons (e.g., research). More than half of infants presented in the first day of life, with seizures, apnea, lethargy and either hyper- or hypotonia.
Table 1.
| Number of patients (%) | |
|---|---|
| Male sex | 13 (68) |
| Admitted within the 1st week of life | 16 (84) |
| Median Gestational Age | 38 weeks [IQR 36–39] |
| Birth weight (mean±SD) | 2892±920 grams |
| Vaginal Delivery | 15 (79) |
| APGAR score 1 minute (median) | 7 [IQR 5–8] |
| APGAR score 5 minute (median) | 9 [IQR 9–9] |
| Race | 15 (79) White |
| 2 (11) African American | |
| 1 (5) Hispanic | |
| 1 (5) Other | |
| Clinical signs/symptoms of lesion | 14 (74) |
| Clinical symptoms in the first day of life | 10 (53) |
| Presenting symptom | 8 (42) Apnea |
| 8 (42) Seizure | |
| 7 (37) Lethargy | |
| 6 (32) Hyper- or Hypo-tonia | |
| 5 (26) Asymptomatic | |
| 5 (26) Encephalopathy | |
| 1 (5) Signs of increased ICP |
ICP, intracranial pressure
Infants with VOL thrombosis or infarction of the surrounding tissue often had identified maternal risk factors (Table 2), with the most common being pre-eclampsia (21%), hypertension (32%) and maternal chorioamnionitis (26%). Nearly half of the infants demonstrated signs of fetal distress (decelerations or non-reassuring biophysical profile) throughout the labor and delivery process. While, half of the infants exhibited coagulopathy at presentation, only 1/19 patients had a demonstrated pro-thrombotic abnormality.
Table 2.
Maternal, Perinatal and Neonatal Conditions in VOL Subjects N= 19
| Number of patients (%) | |
|---|---|
| Maternal | |
| Pre-eclampsia or Eclampsia | 4 (21) |
| Chronic or pregnancy induced hypertension | 6 (32) |
| Diabetes (pre-existing or pregnancy induced) | 1 (5) |
| Maternal infection or chorioamnionitis | 5 (26) |
| Maternal pro-thrombotic abnormalities | 2 (11) |
| Perinatal | |
| Fetal distress (decelerations or non-reassuring BPP) | 9 (47) |
| Instrument assisted delivery | 1 (5) vacuum |
| Birth asphyxia/HIE | 3 (16) |
| Neonatal | |
| Sepsis/infection | 1 (5) |
| Complex congenital heart disease | 2 (11) |
| Polycythemia (Hematocrit >65) | 1 (5) |
| Pro-thrombotic disorder | 1 (5) |
BPP, biophysical profile; HIE, hypoxic-ischemic encephalopathy
The first neuroimaging study was usually performed within 1 day of presenting symptoms, with the first neuroimaging study suggestive of VOL thrombosis and ensuing infarction of the surrounding tissue performed on day 4.5±4.5 (Table 3). On average, MRI confirming the VOL thrombosis and ensuing infarction of the surrounding tissue occurred 15.4±29.3 days after initial imaging. Left-side lesions were more common (11/19 infants) and 64% were small to moderate. In the group with large VOL lesions, 6/7 (86%) infants were male with median gestational age 38 weeks [IQR 36–40]. In the group with large VOL lesions, 2/7 (29%) infants were diagnosed with CP, 2/7 (29%) infants were diagnosed with epilepsy, 1 infant had a major vision impairment and none of the seven infants had a major hearing impairment. Ventriculomegaly, midline shift and deep gray lesions were seen almost exclusively in large VOL thrombosis and ensuing infarction of the surrounding tissue (Table 3). Seven infants were discharged on an anti-epilepsy medication and two of these infants were later diagnosed with epilepsy. One patient was diagnosed with Lennox Gastaut syndrome at three years of age based on EEG patterns; the other did not have any known seizure events in the neonatal period and was diagnosed at 9 months as having a lowered threshold for focal seizures on EEG, with frequent epileptiform activity in the left and right occipital regions.
Table 3.
Neuroimaging
| Number of patients/Total (%) | |
|---|---|
| Day of life of 1st neuroimaging study (mean±SD) | 3±3.8 |
| Day of life of initial neuroimaging study suggestive of VOLI (mean±SD) | 4.5±4.5 |
| 1st neuroimaging study done within 1 day of presenting symptoms | 12/14 (86) |
| Type of 1st neuroimaging study | 9/19 (47) HUS |
| 9/19 (47) CT | |
| 1/19 (5) MRI | |
| Patients who needed 2nd neuroimaging study to confirm diagnosis | 18/19 (95) |
| 1 week or less between initial imaging and confirmatory | 13/19 (68) |
| Location of lesions | 11/19 (58) Left |
| 2/19 (11) Right | |
| 6/19 (32) Bilateral | |
| Size of lesion | 6/19 (32) Small |
| 6/19 (32) Moderate | |
| 7/19 (37) Large | |
| Other neuroimaging findings N=19 | |
| Number of patients (%) | |
| Ventriculomegaly | 5 (26) |
| Diffuse edema | 2 (11) |
| Corpus callosum infarct | 2 (11) |
| Periventricular white matter edema | 11 (58) |
| White matter lesions | 6 (32) |
| Deep gray matter lesions | 2 (11) |
| Prominent deep medullary veins | 10 (53) |
| Other small superficial venous thrombosis | 4 (21) |
| Small deep venous thrombosis | 2 (11) |
| Intraventricular hemorrhage | 7 (37) |
| Choroid plexus hemorrhage | 12 (63) |
| Extra-axial fluid collections | 7 (37) |
| Midline shift | 3 (16) |
| Posterior fossa abnormality (cerebellar hemorrhage, posterior fossa dependent SDH) | 3 (16) |
| Scalp abnormality (hematoma, edema) | 3 (16) |
SDH, subdural hematoma
The entire cohort survived to hospital discharge and to the latest follow-up time point at a mean of 4.4±3.08 years (Table 4). Follow-up data were available for 95% of patients and Bayley scores for 53%. At the latest clinical follow-up appointment documented in the electronic medical record, 44% had major neurodevelopmental impairment, with no difference between those with unilateral versus bilateral lesions or between those with left versus right side lesions. Of those patients with major NDI, 5/8 (63%) had large VOL lesions (Table 5). The two patients who were later diagnosed with epilepsy, both had large VOL lesions and major NDI. There were no statistically significant differences in sex or gestational age between the groups with and without major NDI. Patients with large VOL infarctions appeared to have worse average Bayley scores than those with small to moderate lesions, although only differences in language composite scores were statistically significant (Table 5. 72.7 vs 107.8, p = 0.01).
Table 4.
Cohort Outcomes
| Number of patients/total (%) | |
|---|---|
| Survived | 19/19 (100) |
| Follow-up data available | 18/19 (95) |
| Major neurodevelopmental impairment (at latest follow-up) | 8/18 (44) |
| Diagnosed with epilepsy at follow-up | 2/18 (11) |
| Bayley scores available | 10/19 (53) |
| Child has one Bayley subset score <80* | 3/10 (30) |
refers to composite scores in either cognition, language or motor subsets
Table 5.
Bayley Scores by VOL Infarction Size
| Small-mod | Large | p-value | |
|---|---|---|---|
| Bayley cognitive (mean±SD) | 106±17.5 | 88.75±16.5 | 0.09 |
| Bayley communication (mean±SD) | 107.8±17.1 | 72.7±23.3 | 0.017 |
| Bayley motor (mean±SD) | 104.2±17.7 | 88.8±19.8 | 0.14 |
| Major NDI | 3 | 5 | 0.07 |
DISCUSSION
In this large series we show that infants with large VOL infarctions were more likely to have significant deficits in language development than infants with small or moderate sized VOL infarctions. Risk factors and presentation of VOL thrombosis and ensuing infarction of the surrounding tissue were comparable to those of CSVT and could be utilized to identify these infants prompting neuroimaging evaluation.
Maternal risk factors for CSVT include chronic or pregnancy-induced hypertension, pre-eclampsia, diabetes, autoimmune disease, cocaine use, maternal pro-thrombotic abnormalities, placental abruption and chorioamnionitis.3,10 The majority of our cohort had at least 1 of these risk factors with the most common being pre-eclampsia, hypertension and chorioamnionitis. Neonatal or perinatal complications identified as CSVT risk factors were present in 58% of our cohort consistent with previous reports that risk factors were present in 61–84% of infants at the time of diagnosis of CSVT. These include both delivery (fetal distress, birth asphyxia) and neonatal (dehydration, cardiac defects, pro-thrombotic disorders, ECMO) complications.3,10–11 Signs of fetal distress throughout the labor and delivery process were common in our group, and concurred with 21% of infants requiring intubation in the delivery room. This is far higher than the 1% expected for routine term or near-term deliveries. Although pro-thrombotic disorders have been identified as an important CSVT risk factor,1,3 in our group, only 1 infant was diagnosed with a pro-thrombotic abnormality, both infant and mother were positive for beta-2-glycoprotein I antibodies.
Half of neonates (53%) with VOL thrombosis and ensuing infarction of the surrounding tissue presented on the first day of life, with seizures, apnea, lethargy, and either hyper- or hypotonia.3,12 These are non-specific signs and symptoms and would require clinicians to carefully consider risk factors and promptly use neuroimaging to assist with diagnosis. In most infants, the first neuroimaging (CT or ultrasound) study was performed within 1 day of the presenting symptoms. Although MRI and MRV are methods for establishing the diagnosis of CSVT in neonates,1 ultrasound and CT are acceptable screening exams. However, these modalities have inherent false positive and negative findings and MRI should be used for confirmation.3 One third of infants did not have a confirmatory MRI within 1 week of initial abnormal imaging, suggesting opportunities for improvement in timing of imaging, as this information can impact management decisions, family counseling regarding patient prognosis, and risk of recurrence as well as planned follow-up. Neonatologists could strive to order this important confirmatory test as soon as an infant is clinically stable.
Outcomes of neonates with isolated VOL thrombosis and ensuing infarction of the surrounding tissue are available in only a few case reports and a small case series.6–7,13 In a report of 7 newborns with unilateral temporal lobe infarcts secondary to superficial cortical venous thrombosis, all but one had normal development and neurologic exams at latest follow-up (ranging from 10 months to 5 years). In contrast, half of the infants and children with CSVT had evidence of neurodevelopmental impairment at latest follow-up (mean interval from thrombosis to last follow-up visit 1.6 years) with 80% of neurologic deficits being motor.1 Nearly half of our patients had evidence of major neurodevelopmental impairment at their latest clinical follow-up (mean 4.4±3.08 years), with speech or language impairments being the most common. A mixed receptive-expressive language disorder was diagnosed in 5 patients. Due to the mixed nature of language disorders in these subjects, it is possible that the VOL lesions affected a variety of areas involved in production and understanding of language, from auditory processing to complex association areas. We found an association with worse language composite scores on the Bayley and larger VOL thrombosis and ensuing infarction of the surrounding tissue, consistent with demonstrated impairments in auditory processing in patients with other temporal lobe lesions.14–15 Because of the synergy between cortical auditory and language systems, targeted early hearing, speech and language testing may ensure early identification of impairments and timely referrals for hearing amplification or speech/language therapy.
Although our series represents the largest of its kind to date, it is limited by small size. Nearly all patients had some type of follow-up data available, but with a wide range of developmental instruments. Furthermore, some follow-up exams or data were not completed by a neurologist or neonatal neurodevelopmental specialist. This limitation highlights the need for continued specialized follow-up through school age of any infant with CSVT. We were also limited in our ability to show major differences in outcomes between children with either unilateral vs. bilateral lesions or in those with left vs. right-sided lesions. Lastly, maternal education level was not available for our patients, an important socio-economic factor usually accounted for in neurodevelopmental analyses.
CONCLUSIONS
Vein of Labbé superficial venous thrombosis and ensuing infarction of the surrounding tissue is associated with maternal and perinatal risk factors and early imaging can help confirm this diagnosis. Survival after VOL thrombosis and ensuing infarction of the surrounding tissue is the norm, however infants often have significant long-term neurodevelopmental morbidities. Neonates with large VOL infarctions are more likely to have poor language outcomes, suggesting the need for targeted surveillance with audiology, speech and language testing, to ensure early identification and referral for interventions.
Supplementary Material
Acknowledgments
Funding: This work was supported by 1R01HD081120-01A1 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to N.L.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.deVeber G, Andrew M, Adams C, et al. Canadian Pediatric Ischemic Stroke Study Group. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–423. doi: 10.1056/NEJM200108093450604. [DOI] [PubMed] [Google Scholar]
- 2.Moharir MD, Shroff M, Pontigon AM, Askalan R, Yau I, Macgregor D, et al. A prospective outcome study of neonatal cerebral sinovenous thrombosis. J Child Neurol. 2011;26(9):1137–44. doi: 10.1177/0883073811408094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JY, Chan AK, Callen DJ, Paes BA. Neonatal cerebral sinovenous thrombosis: sifting the evidence for a diagnostic plan and treatment strategy. Pediatrics. 2010;126:e693700. doi: 10.1542/peds.2010-1035. [DOI] [PubMed] [Google Scholar]
- 4.Coutinho JM, Gerritsma JJ, Zuurbier SM, Stam J. Isolated cortical vein thrombosis: systematic review of case reports and case series. Stroke. 2014;45:1836–1838. doi: 10.1161/strokeaha.113.004414. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Cope WP, Zhou Z, De Witt ME, Boockvar JA, Tsiouris AJ. Isolated cortical vein thrombosis: case series. J Neurosurg. 2015;123:427–433. doi: 10.3171/2014.9.jns141813. [DOI] [PubMed] [Google Scholar]
- 6.Kalpatthi R, Coley B, Rusin J, Blanchong C. Neonatal temporal lobar hemorrhage secondary to thrombosis of the vein of Labbé. J Perinatol. 2005;25:605–607. doi: 10.1038/sj.jp.7211298. [DOI] [PubMed] [Google Scholar]
- 7.Jones B. Case 62: lobar hemorrhage from thrombosis of the vein of Labbe. Radiology. 2003;228:693–696. doi: 10.1148/radiol.2283011829. [DOI] [PubMed] [Google Scholar]
- 8.Bayley N. Bayley scales of infant and toddler development. 3. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berfelo FJ, Kersbergen KJ, van Ommen CH, et al. Neonatal cerebral sinovenous thrombosis from symptom to outcome. Stroke. 2010;41:1382–1388. doi: 10.1161/strokeaha.110.583542. [DOI] [PubMed] [Google Scholar]
- 11.Wu YW, Miller SP, Chin K, et al. Multiple risk factors in neonatal sinovenous thrombosis. Neurology. 2002;59(3):438–440. doi: 10.1212/wnl.59.3.438. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald KC, Williams LS, Garg BP, Carvalho KS, Golomb MR. Cerebral sinovenous thrombosis in the neonate. Arch Neurol. 2006;63(3):405–409. doi: 10.1001/archneur.63.3.405. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter L, Egelhoff J, Balmakund T. Neurologic outcome in neonatal temporal lobe hemorrhagic venous infarcts. J Child Neurol. 2009;24(10):1236–1242. doi: 10.1177/0883073809333529. [DOI] [PubMed] [Google Scholar]
- 14.Boatman DF, Lesser RP, Crone NE, Krauss G, Lenz FA, Miglioretti DL. Speech recognition impairments in patients with intractable right temporal lobe epilepsy. Epilepsia. 2006;47:1397–1401. doi: 10.1111/j.1528-1167.2006.00562.x. [DOI] [PubMed] [Google Scholar]
- 15.Han MW, Ahn JH, Kang JK, Lee EM, Lee JH, Bae JH, et al. Central auditory processing impairment in patients with temporal lobe epilepsy. Epilepsy Behav. 2011;20:370–374. doi: 10.1016/j.yebeh.2010.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


