Abstract
Neural plasticity is considered the neurophysiological correlate of learning and memory, although several studies have also noted that it plays crucial roles in a number of neurological and psychiatric diseases. Indeed, impaired brain plasticity may be one of the pathophysiological mechanisms that underlies both cognitive decline and major depression. Moreover, a degree of cognitive impairment is frequently observed throughout the clinical spectrum of mood disorders, and the relationship between depression and cognition is often bidirectional. However, most evidence for dysfunctional neural plasticity in depression has been indirect. Transcranial magnetic stimulation has emerged as a noninvasive tool for investigating several parameters of cortical excitability with the aim of exploring the functions of different neurotransmission pathways and for probing in vivo plasticity in both healthy humans and those with pathological conditions. In particular, depressed patients exhibit a significant interhemispheric difference in motor cortex excitability, an imbalanced inhibitory or excitatory intracortical neurochemical circuitry, reduced postexercise facilitation, and an impaired long-term potentiation-like response to paired-associative transcranial magnetic stimulation, and these symptoms may indicate disrupted plasticity. Research aimed at disentangling the mechanism by which neuroplasticity plays a role in the pathological processes that lead to depression and evaluating the effects of modulating neuroplasticity are needed for the field to facilitate more powerful translational research studies and identify novel therapeutic targets.
Keywords: cortical excitability, major depression, mood disorders, non-invasive brain stimulation, synaptic plasticity
Introduction
The cerebral cortex possesses the intrinsic ability to compensate, adapt, and reorganize itself in response to environmental stimuli or pathological conditions. The term neural plasticity refers to the dynamic and persistent reorganization of cortical properties, including synaptic connection strength, representation patterns, and functional or structural neuronal activity. Several mechanisms are involved in the origin and modulation of neural plasticity, including long-term potentiation (LTP) and long-term depression (LTD), second messenger pathway activation, gene transcription, and morphological changes in neuronal membranes, axons, and postsynaptic cells. Studies aimed at determining when neural plasticity plays a compensatory versus a maladaptive role would be of substantial interest (Cohen et al., 1995).
Plastic cortical rearrangement is also considered one of the substrates for learning and memory and is known to be involved in major depressive disorder (MDD). Despite a considerable literature, the neurobiology of depression and related cognitive-behavioral changes remains poorly understood, and the evidence supporting the role of impaired cortical plasticity has generally been indirect. Neurotrophic changes, including the loss of pyramidal neurons (Rajkowska, 2000) and glial cells in the dorsolateral prefrontal cortex (dlPFC; Rajkowska and Stockmeier, 2013) in addition to a reduction in gamma-aminobutyric acid (GABA)-ergic connections in the hippocampus (Stockmeier et al., 2004), have been observed in postmortem studies.
Other evidence is provided by studies using animal models of depression and chronic stress (Liu and Aghajanian, 2008). The abnormal chronic activation of the hypothalamic-pituitary-adrenal axis can cause atrophy at the level of the PFC and hippocampus, and these data provide support for the hypothesis that exposure to chronic stress leads to negative effects, including structural modifications of the central nervous system (CNS; McEwen et al., 2012). In this context, stress-related cortical atrophy is frequently observed in patients with MMD and is particularly prevalent at the level of the frontal, medial temporal, and limbic areas (Treadway et al., 2015), even in subjects with subclinical depressive symptoms (Webb et al., 2014). A reduction in the number of synapses within the dlPFC (Kang et al., 2012) in combination with indirect signs of disrupted synaptic signaling processes (Feyissa et al., 2009; Duric et al., 2013) indicates that abnormal synaptic functioning is involved in depressive disorders. In particular, impaired cortical activity (He et al., 2016) and the dysregulation of the connectivity between the PFC and limbic regions have been observed (Price and Drevets, 2010), suggesting the loss of physiological phenomena including cortical excitability and plasticity.
The pathophysiological picture of depression becomes more complex when considering the late-onset form of depression. In patients in whom depression appears later in life (Bella et al., 2010), the typical clinical presentations include psychomotor retardation, difficulty at work, apathy, lack of insight, and executive dysfunction. These clinical symptoms, in combination with neuroimaging evidence indicating vascular white matter lesions, support the “vascular depression” (VD) hypothesis. It has been hypothesized that cognitive-behavioral and mood abnormalities reflect ischemic disruption at the level of the dlPFC or the dorsal portion of the head of the caudate nucleus, which are structures that have been implicated in mood-affect regulation and cognition (Cummings, 1993; Bella et al., 2010).
Finally, synaptic plasticity-related dysfunction is also viewed as an early event during the development and course of neurological disorders, and depression and other neuropsychiatric symptoms are frequently found to be comorbid manifestations or early symptoms of a more disabling condition, such as Alzheimer’s disease (Pennisi et al., 2011a; Briggs et al., 2017), vascular dementia (Pennisi et al., 2011b; Pennisi et al., 2015), and atypical Parkinsonism (Cantone et al., 2014). These symptoms may even present in the preclinical or early stages of these diseases (Bella et al., 2011a, 2011b, 2013; Lanza et al., 2013; Pennisi et al., 2016; Lanza et al., 2017).
Transcranial Magnetic Stimulation: Basic Principles and Applications for Exploring the Neurochemical Correlates of Neural Plasticity
Among neurophysiological techniques, transcranial magnetic stimulation (TMS) was originally introduced as a valuable noninvasive tool that was specifically useful for evaluating excitability in the primary motor cortex (M1) and conductivity along the cortical-spinal tract. Nevertheless, today, the applications involving TMS go well beyond the simple assessment of the pyramidal tract (Pennisi et al., 2015). Indeed, TMS can be used to provide novel insights into the pathophysiology of the circuitries underlying neurological and psychiatric diseases, to probe the in vivo excitability and plasticity of the human brain, and to assess the functional integrity of intracortical neuronal and callosal fibers (Kobayashi and Pascual-Leone, 2003; Chen et al., 2008; Pennisi et al., 2011a, 2011b; Lanza et al., 2013, 2015). TMS is well suited for studies aimed at exploring and monitoring motor system impairment during the preclinical phase of several neurological disorders (Cantone et al., 2014) or systemic diseases involving the CNS (Pennisi et al., 2014; Bella et al., 2015). Moreover, when integrated with other neurophysiological techniques (e.g., electroencephalography—EEG) or structural and functional imaging, TMS also allows the exploration of connectivities across motor and nonmotor areas (Groppa, 2016; Kimiskidis, 2016). Finally, because it can be used to evaluate the effects of drugs that are agonists or antagonists for specific neurotransmitters, TMS can selectively test the activity of glutamatergic, GABAergic, monoaminergic, and cholinergic central circuits (e.g., so called pharmaco-TMS; Ziemann et al., 2015).
In this article, we aim to critically and systematically review the literature regarding the use of TMS to probe cortical excitability and neural plasticity in depressive disorders.
Technical principles and brief description of a standard TMS exam
TMS is based on Faraday’s law of electromagnetic induction to activate cortical neurons (Barker et al., 1985). A transducing coil attached to a high-voltage, high-current discharge system produces a strong time-varying and short-lasting magnetic field at right angles to the stimulation coil (Jalinous, 1991). When the stimulation coil is placed tangential to the head, the magnetic field penetrates the scalp and skull with minimal attenuation and induces a secondary eddy current in conductive intracranial tissue. The electrical field in the tissue is oriented perpendicular to the magnetic field and opposite the direction of the electrical current in the stimulation coil (Groppa et al., 2012; Rossini et al., 2015). The two following coil shapes are most commonly used: a figure-eight-shaped coil and a circular coil. The former provides a more focal stimulation, which allows for fairly detailed mapping of cortical representations, while the latter induces a more widely distributed electric field, which is desirable when evaluating central motor conduction times.
A standard examination involves bilateral recordings from distal limb muscles while the patient is seated or lying on a bed or armchair. Motor evoked potentials (MEPs) are produced by stimulating M1 at the optimum scalp position to elicit motor responses in the contralateral target muscles. MEPs are usually recorded using bipolar surface electrodes (e.g., Ag/AgCl cup electrodes) and a belly tendon montage (Groppa et al., 2012). During TMS, the operator can control the intensity of the current flowing through the coil and thereby change the magnitude of both the induced magnetic field and the secondarily induced electrical field. In addition to controlling its intensity and focus, the operator can also manipulate the frequency and interstimuli interval of the delivered stimuli, which together critically determine the effects of TMS on the targeted brain region. Indeed, TMS can be delivered as a single pulse, as a pair of stimuli applied to the same or different brain areas, as paired cortical and peripheral stimuli, or as trains of repetitive stimuli. Anatomically precise localization of the stimulus can be achieved by using a stereotactic neuro-navigational system (Kobayashi and Pascual-Leone, 2003).
Single-pulse TMS
A single TMS pulse applied an adequate stimulator intensity to M1 elicits a MEP in the contralateral target muscles (Di Lazzaro et al., 2001). The MEP latency and central motor conduction time are considered indexes of the integrity of cortical-spinal pathways, whereas the MEP amplitude is used to measure the excitation state of the neurons connecting the motor cortex to the muscles (Rossini et al., 2015). The resting motor threshold (rMT), when defined according to the recommendations of the International Federation of Clinical Neurophysiology Committee (Rossini et al., 2015), is considered a global parameter of brain excitability because it is a compound measure of the membrane excitability of cortical-spinal neurons, of neural inputs into pyramidal cells within the cortex, and of spinal motor neurons, neuromuscular junctions, and muscles (Ziemann et al., 1996; Rossini and Rossi, 2007). The stimulus–response curve between TMS stimulus intensity and MEP amplitude provides another useful measurement of excitation (Ridding and Rothwell, 1997).
Applying TMS after a brief period of exercise provides valuable information regarding the cortical excitability and intracortical synaptic reorganization that underlie motor learning (Caramia et al., 2000). In particular, in normal subjects, postexercise facilitation is defined as a period of increased motor excitability that occurs after transient muscle activation and decays to baseline over 2 to 4 min. This phenomenon is thought to originate in the cortex because transcranial electric stimulation fails to elicit exercise facilitation when cortical-spinal cells are stimulated at their proximal axons (Samii et al., 1996).
The ability to perform focal stimulation using butterfly coils has allowed mapping studies to determine muscle representation. The results can then be used to determine the center of gravity of a motor representation (Wassermann et al., 1992) that underlies a type of cortical plasticity (Cohen et al., 1995). Experiments have repeatedly shown that neurons can assume the properties of nearby neurons in other, usually adjacent, areas. Cortical map plasticity has been demonstrated in both animal models and human cortex, as have changes in motor maps, such as those resulting from exercise or practicing movement tasks (Classen et al., 1998).
Applying a suprathreshold TMS pulse to M1 during a tonic voluntary contraction of contralateral muscles suppresses electromyographic activity in those muscles for a few hundred milliseconds (Chen et al., 1999). This phenomenon, called the contralateral silent period (CSP), can be exploited to functionally measure intracortical inhibitory circuits (Cantello et al., 1992), which are mediated mainly by GABA-B transmission (Siebner et al., 1998). If the pulse is delivered to the ipsilateral M1, an electromyographic silence that lasts approximately 30 ms and is called the ipsilateral silent period (iSP) can be recorded. The iSP is thought to reflect the ability of the motor cortex to excite the inhibitory interneurons in its contralateral counterpart, and it seems to be induced via transcallosal pathways (Ferbert et al., 1992).
Paired-pulse TMS
Inhibitory and excitatory interneuronal activity within the human cortex can be explored noninvasively using a paired-pulse TMS paradigm (Kujirai et al., 1993; Ziemann, 2004). The conventional protocol uses a “conditioning stimulus” (subthreshold) followed by a “test stimulus” (suprathreshold). By varying the intensity of the conditioning stimulus and the interval between the pair of TMS pulses (the interstimulus interval—ISI), a number of measures of intracortical interneuronal function and interaction can be obtained. At an ISI of 1–4 ms, the conditioning stimulus suppressed the MEP amplitude, a phenomenon that has been called short-latency intracortical inhibition (SICI). At a longer ISI (7–20 ms), the stimulus results in intracortical facilitation of motor responses (Kujirai et al., 1993). The mechanisms underlying these phenomena are thought to reflect the activity of distinctive neurochemical circuits. Hence, while SICI is likely mediated by GABA-A interneuron activity (Di Lazzaro et al., 2000), facilitation is more complex (Di Lazzaro et al., 2006), although it does appear to be produced mainly by the activation of glutamatergic cells (Kujirai et al., 1993; Paulus et al., 2008).
Sensory-motor modulation
TMS can be used to test the functional connectivity of different cortical areas and monitor how connectivity changes over time. For instance, the short-latency afferent inhibition (SAI) of MEP responses reflects the sensory stimuli-mediated inhibitory modulation of M1 (Tokimura et al., 2000). This effect depends on the time that elapses between the peripheral nerve electrical stimulus and the TMS pulse, and it typically occurs at an ISI of 20 ms (Sailer et al., 2003). SAI may represent a neurophysiological correlate of central cholinergic activity in vivo because it is reduced or abolished by the muscarinic receptor antagonist scopolamine (Di Lazzaro et al., 2000) and positively modulated by acetylcholine (Di Lazzaro et al., 2005).
TMS for measuring neural plasticity
Changes in cortical plasticity can be induced by and studied using a repetitive TMS (rTMS) paradigm. Repetitive TMS can be used to modify the excitability of the human cortex in a predictable manner, with low frequencies (stimulus rates <1 Hz) usually used to inhibit and high frequencies (5–20 Hz) used to induce excitation in the stimulated cortical area (Chen et al., 1997). The changes in excitability that are produced by rTMS share several characteristics with the induction of LTP and LTD by tetanic stimulation in cortical slices (Wang et al., 1996). Indeed, most, but not all, rTMS paradigms are N-Methyl-D-aspartate (NMDA)-receptor activity dependent (Cheeran et al., 2010) and modulated by prior synaptic activation (Jung and Ziemann, 2009), and the induced change in MEP amplitude is dependent on the frequency of stimulation (Di Lazzaro et al., 2010). Plasticity involves the rapid down-regulation of GABA-related inhibitory circuits and short-term changes in synaptic efficacy, which are dependent on sodium and calcium channels (Ziemann et al., 1998). The rTMS-induced long-lasting reduction in SICI appears to involve the activation of NMDA receptors and is probably associated with a LTP-like mechanism. However, rTMS is also able to avoid saturation by inducing LTD-like responses that decrease the efficacy of connections between synapses. LTD refers to a prolonged use-dependent decrease in the strength of excitatory synapses and is predominantly mediated by the activation of synaptic NMDA receptors or metabotropic glutamate receptors at the level of hippocampal CA3:CA1 synapses (Gladding et al., 2009). However, because rTMS affects a wide range of cell types that possess a variety of properties, obtaining a comprehensive understanding of the induction of LTP or LTD is not enough to determine the actual impact of rTMS on the human brain. The powerful role of TMS in modulating human neuroplasticity is of crucial clinical relevance because it could be used to support plasticity where it is needed or, conversely, to down-regulate neuronal excitability where it such excitation is found to be maladaptive. However, TMS can also be used to induce long-lasting effects in cerebral regions outside M1, such as the prefrontal areas or the cerebellum (Kobayashi and Pascual-Leone, 2003).
These findings reflect an interest in identifying therapeutic applications in neurological or psychiatric disorders. This interest is also demonstrated by the development of paired associative stimulation (PAS), which is considered a repetitive application of SAI (Stefan et al., 2002). The PAS protocol includes applying an electrical stimulus to a peripheral nerve (usually the median nerve) and then a single TMS pulse over the hand area of M1. PAS induces long-lasting LTP-/LTD-like changes in sensory-motor pathways, which are considered a marker of motor cortex plasticity.
Finally, new rTMS paradigms have recently been developed to facilitate further investigations into cortical excitability and synaptic plasticity. For example, theta burst stimulation (TBS) and quadripulse stimulation (QPS) can be used to produce neuroplastic effects that range broadly from MEP suppression to MEP facilitation, depending on the interval of the pulses within a burst. Moreover, they can be used to measure so called metaplasticity, which refers to the ability of a synapse to undergo a second plastic change subsequent to a recent induction of plasticity (Yger and Gilson, 2015).
Data Source and Selection
We performed a PubMed-based literature review to identify all studies that have been published on neural plasticity and MDD. The following keywords were used: “transcranial magnetic stimulation,” “plasticity,” and “depression.” The following data were extracted: (a) study design; (b) patient characteristics, such as sample size and the age, gender, laterality, diagnostic criteria, and drug exposure of participants; (c) TMS features; and (d) results and main translational findings. In an initial search performed using the above-mentioned keywords, a total of 212 articles were screened. We then excluded articles on TMS and plasticity that were performed in healthy subjects or studies conducted using animals because their content did not fit the aim of this review. Moreover, we excluded preliminary or low-quality data to avoid drawing misleading conclusions. We also excluded articles that were not research studies (e.g., reviews, letters to the Editor, and commentaries) and papers published in language other than English. Publications that addressed only the treatment of psychiatric disorders were also excluded. Finally, we reviewed the articles listed in the reference sections of the selected papers to locate any further relevant data. We identified 25 studies as a result of this process.
Tables 1 and 2 provide a summary of the main findings of the identified TMS studies related to cortical excitability and synaptic plasticity, respectively, in depressive disorders. Figure 1 provides a summary overview of the main findings regarding the use of TMS techniques and the implications of these findings for therapeutic strategies.
Table 1.
TMS Studies of Cortical Excitability in Depressed Subjects.
| Study | Number of participants (M/F) | Age (years) mean ± SD (or range) | Diagnosis/ diagnostic criteria used | Drug exposure | Measures of plasticity investigated | Results in depressed subjects | Main translational findings |
|---|---|---|---|---|---|---|---|
| Pennisi et al., 2016 | 16 VD (NR) vs 11 VCI-ND (NR) vs 15 controls (NR) | 68.1 ± 8.6 vs 70.0 ± 7.0 vs 63.8 ± 7.2 | DSM-IV-TR | N | rMT CSP Paired-pulse curve | ▾ rMT ▾ CSP ▪ SICI ▪ ICF | The mechanisms that enhance the risk of dementia in VD might be related to either subcortical vascular lesions or a lack of compensatory functional cortical changes. |
| Veronezi et al., 2016 | 60 patients (19/41) vs 21 controls (11/10) | 37 vs 28 | MDD subtypes/ DSM-V | N | rMT CSP Paired-pulse curve | ▴ rMT ▾ CSP ▾ SICI ▴ ICF | Cortical excitability, glutamatergic and GABA-ergic balance are neurophysiological markers of different subtypes of depression. |
| Concerto et al., 2013 | 11 VD (6/5) vs 11 MDD (5/6) vs 11 controls (6/5) | 67.72 ± 3.29 vs 57.18 ± 7.12 vs 67.36 ± 3.75 | DSM-IV-TR | Y | rMT CSP Paired-pulse curve | ▴ rMT (L) ▴ CSP (R) ▪ SICI ▪ ICF | Distinctive TMS patterns between late-onset depression with subcortical vascular disease and early-onset drug-resistant MDD. Possible drug effects of inhibitory measures. |
| Croarkin et al., 2013 | 24 patients (10/14) vs 22 controls (11/11) | 13.9 ± 2.1 vs 13.8 ± 2.2 | MDD/K-SADS-PL and CDRS-R | N | rMT CSP Paired-pulse curve | ▪ rMT ▾CSP (L) ▪ SICI ▴ICF | Neurophysiological abnormalities are also present in children and adolescents. |
| Bella et al., 2011 | 15 VD (7/8) vs 10 VCI-ND (6/4) vs 10 controls (5/5) | 70.5 ± 6.6 vs 70.8 ± 6.3 vs 67.7 ± 3.9 | DSM-IV-TR | N | rMT CSP Paired-pulse curve | ▪ rMT ▪ CSP ▾ SICI ▪ CSP | The neurophysiological mechanisms underlying VD differ from those reported in MDD and seem to be similar to those in patients with subcortical ischemic vascular disease. |
| Levinson et al., 2010 | 60 patients (22/38) vs 25 controls (12/13) | 47.2 ± 11.2 vs 43.8 ± 8.9 | MDD/DSM-IV | Y | rMT CSP Paired-pulse curve | ▴ rMT (L) ▾ CSP ▾ SICI ▪ ICF | Cortical excitability and glutamatergic and GABAergic balance are neurophysiological markers of different subtypes of depression. |
| Navarro et al., 2009 | 91 patients (39/52) No control group | 46.1 ± 10.5 | MDD/DSM-IV | Y | rMT | ▪ rMT | A trend toward a lower rMT on the left hemisphere. Probable influence of long-term benzodiazepine intake. |
| Lefaucheur et al., 2008 | 35 patients (14/21) vs 35 controls (17/18) | 56 ± 2.8 vs 43 | MDD/DSM-IV | Y | rMT CSP Paired-pulse curve | ▴ rMT (L) ▾ CSP (L) ▾ SICI (L) ▾ ICF (L) | Significant reduction of excitability of both inhibitory and facilitatory inputs in the left hemisphere that correlates with depression severity. |
| Levinson et al., 2007 | 15 patients (9/6) vs 15 controls (11/4) | 36.8 ± 9.1 vs 35.1 ± 9.2 | Bipolar disorder | Y | CSP Paired-pulse curve transcallosal inhibition | ▾ CSP ▾ SICI ▪ ICF ▾ iSP | Bipolar patients exhibited impairment of inhibitory pathways |
| Bajbouj et al., 2006 | 20 patients (14/6) vs 20 controls (14/6) | 42.9 ± 11.9 vs 44 ± 14.6 | MDD/DSM-IV | N | rMT CSP Paired-pulse curve | ▾ rMT (R) ▾ CSP ▾ ICI (B) | Reduction of GABAergic TMS parameters, suggesting a central inhibitory deficit in medication-free MDD patients. |
| Fitzgerald et al., 2004 | 60 patients (NR) No control group | - | MDD (unipolar or bipolar)/DSM- IV | Y | rMT CSP Paired-pulse curve rTMS | ▴ rMT (R) ▾ CSP ▾ ICI (L) | Relationship between excitability of the right hemisphere and the severity of psychopathology, and between inhibition in the left hemisphere and poor response to rTMS. |
| Maeda et al., 2000 | 8 patients (5/3) vs 8 controls (6/2) | 46.8 vs 44.9 | MDD/DSM-IV | N | rMT Paired-pulse curve | ▴ rMT (L) ▴ SICI (L) ▾ ICF (L) | Significant interhemispheric difference that correlates with depression severity. |
| Steele et al., 2000 | 16 patients (4/12) vs 19 controls (6/13) | 43.1 ± 8.9 vs 40.2 ± 11.5 | MDD/DSM-IV | Y | rMT CSP | ▴ CSP | Data do not support motor retardation due to a dopaminergic parkinsonian-like mechanism. |
| Abarbanel et al., 1996 | 10 patients (7/3) vs 10 controls (6/4) | 39.4 ± 11.3 vs 39.7 ± 6.7 | MDD | Y | rMT CMCT | Neither asymmetry nor variation | Motor cortex as an intriguing window into neuropsychiatric disorders. |
Note. TMS = transcranial magnetic stimulation; rTMS = repetitive TMS; M = male; F = female; SD = standard deviation; R = right hemisphere; L = left hemisphere; Y = yes; N = no; MDD = Major Depressive Disorder; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition; DSM-IV-TR = DSM – Fourth Edition – Text Revision; DSM-V = DSM – Fifth Edition; K-SADS-PL = Kiddie-Sads-Present and Lifetime Version; CDRS-R = Children’s Depression Rating Scale–Revised; rMT = resting motor threshold; CSP = cortical silent period; iSP = ipsilateral silent period; SICI = short-latency afferent inhibition; ICI = intracortical inhibition; ICF = intracortical facilitation; CMCT = central motor conduction time; GABA = gamma-aminobutyric acid; VD = patients with vascular depression; VCI-ND = patients with vascular cognitive impairment – no dementia; NR = not reported; ▴ = increase/enhancement; ▾ = decrease/reduction; ▪ = no significant change.
Table 2.
Investigations of Neural Plasticity That Used TMS-Related Protocols in Patients With Depression.
| Study | Number of participants (M/F) | Age (years) mean ± SD (or range) | Diagnosis/ diagnostic criteria used | Drug exposure | Measures of p lasticity investigated | Results in depressed subjects | Main translational findings |
|---|---|---|---|---|---|---|---|
| Kuhn et al., 2016 | 27 patients (15/12) vs 27 controls (14/13) | 19-58 vs 18-55 | MDD/ICD-10 | Y | PAS | ▾ LTP-like plasticity restored after remission | State-dependent synaptic plasticity as a treatment target. |
| Player et al., 2013 | 23 patients (10/13) vs 23 controls (NR) | 38.0 ± 12.8 vs 38.5 ± 13.1 | MDD (20) and bipolar disorder (3)/DSM-IV | Y | PAS | ▾ LTP-like plasticity | Altered plasticity in depression might influence learning or response to treatment. |
| Spampinato et al., 2013 | 12 drug-resistant MDD (8/4) vs 10 drug-free patients (6/4) | 50.5 ± 8.7 vs 55.6 ± 8.8 | MDD/DSM-IV-TR | Y | rMT, paired-pulse curve before and after 10-Hz rTMS to the dlPFC | Restored rMT asymmetry and paired-pulse curve imbalance | Brain stimulation techniques are clinically effective for modulating altered excitability and plasticity in drug-resistant MDD. |
| Croarkin et al., 2013 | 8 patients (1/7) No control group | 16.1 ± 1.1 | MDD/ K-SADS-PL and CDRS-R | Y | rMT after 10-Hz rTMS to the left M1 | ▾ rMT | rTMS is safe for studying and treating affective disorders in children. |
| Bajwa et al., 2008 | 13 patients (1/12) vs 14 controls (2/12) | 36.3 ± 12.9 vs 33.7 ± 7.1 | MDD/DSM-IV | Y | rMT before and after 1-Hz rTMS to the left M1 | ▪ rMT baseline ▴ rMT bilateral after rTMS | Brain stimulation techniques are valid tools for restoring plasticity |
| Reid et al., 2002 | 10 patients (4/6) vs 13 controls (4/9) | 48.3 ± 12.8 vs 35.1 ± 7.9 | MDD (unipolar or bipolar)/DSM-IV | Y | Postexercise facilitation | ▾ facilitation | Fatigue as a phenomenon of cortical origin in depression. |
| Shajahan et al., 1999a | 10 patients (2/8) vs 10 controls (3/7) | 41.1 ± 10.9 vs 39.3 ± 10.8 | MDD (unipolar or bipolar)/DSM-IV | Y | Postexercise facilitation | ▾ facilitation | Fatigue as a phenomenon of cortical origin in depression. |
| Triggs et al., 1999 | 10 patients (5/5) No control group | 52 | MDD/DSM-IV | N | rMT after 20-Hz rTMS to the left dlPFC | ▾ rMT | Brain stimulation techniques are valid tools for restoring plasticity. |
| Samii et al., 1996 | 10 patients (NR) vs 18 controls (NR) | 42 vs 42 | MDD (unipolar or bipolar)/ DSM-III-TR | N | Postexercise facilitation | ▾ facilitation | Neurophysiological correlates of fatigue and motor inertia in depression. |
Note. TMS = transcranial magnetic stimulation; rTMS = repetitive TMS; PAS = paired-associative stimulation; M = male; F = female; SD = standard deviation; Y = yes; N = no; MDD = Major Depressive Disorder; DSM-III-TR = Diagnostic and Statistical Manual of Mental Disorders – Third Edition – Text Revision; DSM-IV = DSM – Fourth Edition; DSM-IV-TR = DSM – Fourth Edition – Text Revision; K-SADS-PL = Kiddie-Sads-Present and Lifetime Version; CDRS-R = Children’s Depression Rating Scale–Revised; ICD-10 = International Statistical Classification of Diseases and Related Health Problems – Tenth revision; LTP = long-term potentiation; dlPFC = dorsolateral prefrontal cortex; M1 = primary motor cortex; rMT = resting motor threshold; NR = not reported; ▴ = increase/enhancement; ▾ = decrease/reduction; ▪ = no significant change.
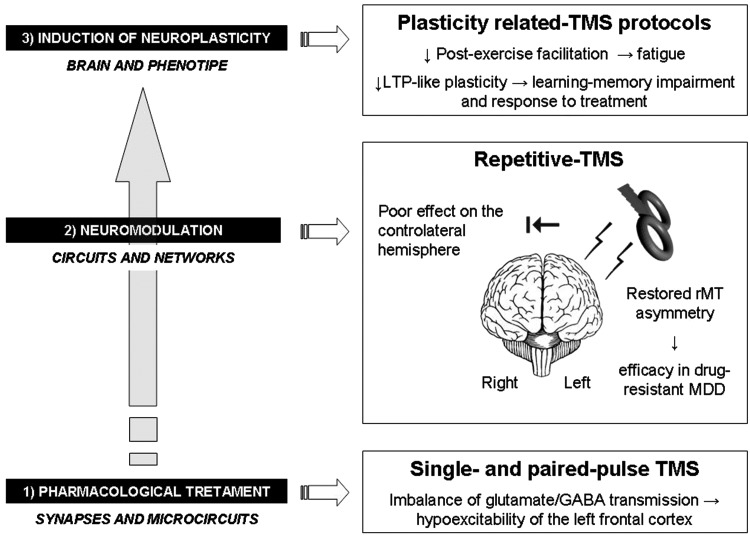
Figure 1.
Imbalance in the “depressed brain”: a summary overview of the main findings related to TMS techniques and their implications for therapeutic strategies. TMS = transcranial magnetic stimulation; GABA = gamma-amino-butyric acid; MDD = major depressive disorder; rMT = resting motor threshold; LTP = long-term potentiation.
TMS in Depression
Global Cortical Excitability and Interhemispheric Asymmetry
Studies that used rMT in depression (Abarbanel et al., 1996; Lefaucheur et al., 2008; Bajwa et al., 2008; Navarro et al., 2009) revealed that in these individuals, there is a global reduction in cortical excitability in the frontal cortex (Maeda et al., 2000; Fitzgerald et al., 2004; Levinson et al., 2010; Spampinato et al., 2013; Concerto et al., 2013; Croarkin et al., 2013; Veronezi et al., 2016), an interhemispheric imbalance between the prefrontal and motor cortices that manifested as reduced excitability in the left hemisphere (Maeda et al., 2000; Fitzgerald et al., 2004; Levinson et al., 2010; Spampinato et al., 2013; Concerto et al., 2013) or increased excitability in the right hemisphere (Bajbouj et al., 2006). In one study that investigated interhemispheric asymmetry in the prefrontal and motor cortices, 1 Hz (inhibitory) rTMS was applied to the left M1, and the results showed that the rTMS reduced cortical excitability in the ipsilateral side but did not induce any change (i.e., an increase in excitability) in the right hemisphere (Bajwa et al., 2008). These data suggest that normal interhemispheric modulation had been lost in these individuals.
In other investigations, rMT was reevaluated after rTMS. In one study, high-frequency rTMS was applied to the left prefrontal area, and the results showed that treatment was associated with a significant increase in cortical excitability in the ipsilateral hemisphere (Triggs et al., 1999). In line with these results, another recent study showed that applying high-frequency rTMS to the left dlPFC for 1 month reduced the difference in excitability between the left and right hemispheres (Spampinato et al., 2013) and was associated with clinical improvements that persisted over time (Concerto et al., 2015).
Measures of Cortical Inhibition
Unlike measures of excitation, conflicting results have emerged regarding indexes of inhibition, namely CSP and SICI. In particular, in some studies, patients with either unipolar or bipolar depression have a prolonged CSP (Steele et al., 2000), while other studies, both a reduced CSP and SICI were observed (Fitzgerald et al., 2004; Bajbouj et al., 2006; Lefaucheur et al., 2008). Shortened CSP has also been reported in patients with a previous diagnosis of MDD and in those with treatment-resistant depression. Interestingly, only the latter group exhibited significantly reduced SICI (Levinson et al., 2010). These results are consistent with the “cortical disinhibition” hypothesis, in which GABAergic transmission is involved in the pathophysiology of depression, as was previously suggested by animal, neurochemical, and neuroimaging studies (Brambilla et al., 2003; Croarkin et al., 2011).
The influences of physiological, technical, and experimental factors should be taken into account because variability in these measures has been observed even in healthy individuals. For instance, because CSP duration is known to be dependent on stimulus intensity and type of stimulating coil that is used (Kimiskidis et al., 2005), these parameters need to be considered. Moreover, different TMS methods have been employed, and differences in the ages, clinical presentation, and severity of included patients as well as the effects of neuroactive drugs on measures of cortical excitation and inhibition may contribute to differences in conclusions between studies (for recent comprehensive reviews, see Ziemann et al., 2015; Bhandari et al., 2016).
Indexes of Neuroplasticity
The impaired postexercise facilitation of MEPs is characterized by an initial facilitation followed by an early return to the baseline MEP amplitude. This sequence has been demonstrated in both nonmedicated and medicated MDD subjects (Shajahan et al., 1999a; Reid et al., 2002). These studies support the view that the motor cortex is less dynamically excitable in depressed patients and that this phenomenon may be a neurophysiological sign of an imbalance between the excitatory and inhibitory mechanisms involved in plasticity. A subsequent study revealed that this facilitation was normalized in previously depressed patients who had clinically recovered (Shajahan et al., 1999b).
Using a PAS protocol in a sample of 23 medicated depressed patients, Player et al. (2013) observed that neuroplasticity was significantly lower in these patients than in age- and gender-matched healthy controls. More recently, an investigation using a PAS protocol in patients with MDD further supported this hypothesis by showing that cortical LTP-like plasticity was significantly decreased in a state-dependent manner in a group of 27 patients who suffered an acute episode of MDD. Interestingly, this study clearly showed that LTP-like plasticity was restored after remission (Kuhn et al., 2016). Finally, after facilitatory PAS, changes in cortical excitability were linked to associative LTP. This connection is believed to involve glutamate signaling and the postsynaptic depolarization of cells via the activation of NMDA and α-amino-3-hydroxy-5-methylisoxazole propionate (AMPA) receptors (Stefan et al., 2002).
Vascular Depression and TMS
Few data are available regarding cortical excitability in VD, and the relevant studies have not provided any evidence demonstrating overall changes in motor cortical excitability or interhemispheric asymmetry. Hence, in these patients, there is a pattern of cortical excitability that is clearly different from that in MDD, as described earlier. Moreover, the normality of CSP and SICI in VD patients indicates that the mechanisms regulating GABAergic intracortical inhibitory circuits are not involved in these individuals. These data provide a new and intriguing neurophysiological explanation for the differences in the neurobiological processes that underlie nonvascular early onset major depression and late-onset VD (Bella et al., 2011; Concerto et al., 2013; Lanza et al., 2017). Furthermore, a longitudinal study evaluated the electrophysiological changes and progression of cognitive decline in patients with subcortical cerebrovascular lesions, and the results showed that glutamate-related neuroplasticity was differentially enhanced between patients with and without VD. In particular, a high level of intracortical facilitation was observed in the nondepressed patients, and this seemed to provide a relative level of protection from cognitive and functional deterioration (Pennisi et al., 2016).
Discussion
Main Findings
The articles reviewed here provide neurophysiological evidence for altered cortical excitability (Maeda et al., 2000; Fitzgerald et al., 2004; Levinson et al., 2010; Spampinato et al., 2013; Concerto et al., 2013; Croarkin et al., 2013; Veronezi et al., 2016) and synaptic plasticity (Chroni et al., 2008; Bajwa et al., 2008; Spampinato et al., 2013; Croarkin et al., 2013; Player et al., 2013; Kuhn et al., 2016) in MDD. These data confirm the findings of previous investigations that have used different approaches (Debener et al., 2000; Normann et al., 2007; Teyler and Cavus, 2007; Salustri et al., 2007; Nissen et al., 2010).
Moreover, a number of studies have shown that there is a significant decrease in postexercise facilitation in these individuals (Samii et al., 1996; Shajahan et al., 1999a, 1999b; Reid et al., 2002; Chroni et al., 2008) that is probably linked to an imbalance between inhibitory and excitatory inputs at the level of cortical-spinal neurons. This observation, which has also been reported in schizophrenia (Reid et al., 2002; Chroni et al., 2002), might explain the symptoms reported by these patients, which can include fatigue, weakness, motor inertia, and apathy, and indicates the existence of a potentially strong relationship between psychiatric disorders and motor output.
Finally, it should be kept in mind that changes in cortical measures of inhibition may also provide important contributions to these conditions. Accordingly, applying electroconvulsive therapy in combination with 3 Hz or 10 Hz rTMS (i.e., facilitatory rTMS) to the left prefrontal cortex of subjects with MDD led not only to clinical improvement and enhanced cortical excitability in the left motor cortex (indicated by an increased MEP/M-wave ratio and decreased motor threshold but also to the modulation of measures of GABA-mediated intracortical inhibition (i.e., shortened CSP and reduced SICI; Chistyakov et al., 2005a, 2005b). Taken together, these data suggest that in depressed subjects, the motor cortex is more refractory to modulatory inputs from both adjacent areas and other nonmotor areas within the CNS. Moreover, several studies have demonstrated that structural and functional abnormalities in trans-callosal connections may play crucial roles in patients with depression and other mood disorders (Bajwa et al., 2008; Yasuno et al. 2016; Zalsman et al., 2016; Matsuoka et al., 2017).
In summary, when tested using TMS, both glutamatergic and GABAergic pathways seem to be impaired in MDD. Abnormal activity in the glutamatergic system that regulates synaptic plasticity is centrally important to the pathological mechanism underlying and treatment for MDD (Sanacora et al., 2008). The excessive glutamatergic activation of NMDA receptors induces LTD, which may be responsible for the disruptions observed in glutamatergic receptor plasticity-related processes, including reduced motor cortex excitability and postexercise facilitation and insensitivity to PAS protocols (Samii et al., 1996; Shajahan et al., 1999a; Maeda et al., 2000; Reid et al., 2002; Fitzgerald et al., 2004; Chroni et al., 2008; Levinson et al., 2010; Spampinato et al., 2013; Concerto et al., 2013; Player et al., 2013).
Neurotrophin and Metaplasticity: The Contribution of Repetitive TMS
Another possible mechanism by which noninvasive brain stimulation techniques may operate is the modulation of neurotrophin release, which is particularly relevant in depression. It is widely accepted that brain-derived neurotrophic factor (BDNF) is implicated in neuronal survivability and the functions involved in activity-dependent synaptic plasticity, which affects dendrite complexity and spine density (Li et al., 2010; Dumas, 2012). Exposure to stress decreases BDNF expression in the hippocampus and PFC, and different studies have shown that there is a deficit of this neurotrophin in depressed patients. These data further support the concept that dysfunctional neuroplasticity contributes to both mood and cognitive disorders (Pittenger and Duman, 2008; Krishnan and Nestler, 2008; Li et al., 2010; Dumas, 2012). In this regard, rTMS has been shown to increase serum BDNF concentrations in depressed patients (Zanardini et al., 2006) and to improve refractory depression by influencing the release of catecholamines and BDNF (Yukimasa et al., 2006). Finally, applying low-frequency rTMS in model rats with vascular dementia improved learning and memory, protected pyramidal cells from apoptosis, and promoted hippocampal plasticity by increasing Bcl-2 expression and reducing Bax expression (Yang et al., 2015).
Finally, relatively little is currently known regarding aberrant metaplasticity phenomena in mood disorders or other neuropsychiatric diseases. It is widely accepted that metaplasticity is necessary to modulate the excitability and functions of neuronal networks. Hence, the modulation of metaplasticity might increase or even restore the innate ability of neurons to exhibit synaptic plasticity under pathological conditions. Based on the recently introduced concept of “interhemispheric rivalry” in stroke, several studies have used a “priming” protocol to enhance recovery in stroke survivors (Di Lazzaro et al., 2013). The application of a protocol including TBS or QPS may be useful for modulating brain regions exhibiting disrupted plasticity in depressed subjects.
Limitations and Critical Aspects
Although TMS provides exciting insights into different aspects of neural excitability and plasticity, the following limitations and aspects critical to both the techniques and designs or methodologies used in different studies should be taken into account:
as previously shown, the spatial resolution of TMS is more limited than that of neuroimaging techniques, even when a figure-eight-shaped coil is used;
an absence of changes in TMS measures does not necessarily rule out the presence of subtle neuroplastic changes;
TMS is primarily useful for assessing the motor cortex, which is not always the most-involved area in patients with depression or other psychiatric disorders; generalizing findings from motor to nonmotor areas therefore requires caution, although TMS has been shown to reliably probe the excitability and connectivity of both motor and nonmotor cortical-subcortical networks (Reis et al., 2008);
there is a degree of variability in the results of the studies reviewed here that might be explained by the heterogeneous phenotypes of the included affective disorders and the diagnostic overlap among them;
the depressed patients included in the studies were often on antidepressant medications and other psychotropic drugs that may have affected TMS measures of excitability and plasticity. Furthermore, only a few studies have evaluated patients in a remission state, and it is therefore difficult to determine whether neuroplasticity may be an underlying pathophysiological mechanism of the disease or a state-dependent phenomenon.
finally, although the hypothesis that disrupted synaptic plasticity contributes to depression may open paths to novel TMS-driven drugs, very few such studies have so far been carried out.
Conclusions
Studying cortical excitability and synaptic plasticity using TMS may lead to new insights into the electrophysiology and neurochemistry underlying a wide spectrum of depressive disorders. The observed TMS patterns may indicate the different pathological substrates of neuropsychiatric diseases and explain some symptoms, such as fatigue and motor inertia, which are frequently reported in depressed patients. In the near future, interventions aimed at enhancing neuroplasticity should merit special attention. Particular consideration should be given to the role of noninvasive brain stimulation techniques, the genetic profiles of subjects, and the pharmacological effects of drugs that act on multiple neurotransmission pathways.
Summary statement
Transcranial magnetic stimulation sheds light on the in vivo neurochemical mechanisms underlying cortical plasticity in patients with major depression.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Abarbanel J. M., Lemberg T., Yaroslavski U., Grisaru N., Belmaker R. H. (1996) Electrophysiological responses to transcranial magnetic stimulation in depression and schizophrenia. Biol Psychiatry 40: 148–150. [DOI] [PubMed] [Google Scholar]
- Bhandari A., Radhu N., Farzan F., Mulsant B. H., Rajji T. K., Daskalakis Z. J., Blumberger D. M. (2016) A meta-analysis of the effects of aging on motor cortex neurophysiology assessed by transcranial magnetic stimulation. Clin Neurophysiol 127: 2834–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajbouj M., Lisanby S. H., Lang U. E., Danker-Hopfe H., Heuser I., Neu P. (2006) Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry 59: 395–400. [DOI] [PubMed] [Google Scholar]
- Bajwa S., Bermpohl F., Rigonatti S. P., Pascual-Leone A., Boggio P. S., Fregni F. (2008) Impaired interhemispheric interactions in patients with major depression. J Nerv Ment Dis 196: 671–677. [DOI] [PubMed] [Google Scholar]
- Barker A. T., Jalinous R., Freeston I. L. (1985) Non-invasive magnetic stimulation of human motor cortex. Lancet 1: 1106–1107. [DOI] [PubMed] [Google Scholar]
- Bella R., Pennisi G., Cantone M., Palermo F., Pennisi M., Lanza G., Zappia M., Paolucci S. (2010) Clinical presentation and outcome of geriatric depression in subcortical ischemic vascular disease. Gerontology 56: 298–302. [DOI] [PubMed] [Google Scholar]
- Bella R., Ferri R., Cantone M., Pennisi M., Lanza G., Malaguarnera G., Spampinato C., Giordano D., Raggi A., Pennisi G. (2011. a) Motor cortex excitability in vascular depression. Int J Psychophysiol 82: 248–253. [DOI] [PubMed] [Google Scholar]
- Bella R., Ferri R., Pennisi M., Cantone M., Lanza G., Malaguarnera G., Spampinato C., Giordano D., Alagona G., Pennisi G. (2011. b) Enhanced motor cortex facilitation in patients with vascular cognitive impairment-no dementia. Neurosci Lett 503: 171–175. [DOI] [PubMed] [Google Scholar]
- Bella R., Ferri R., Lanza G., Cantone M., Pennisi M., Puglisi V., Vinciguerra L., Spampinato C., Mazza T., Malaguarnera G., Pennisi G. (2013) TMS follow-up study in patients with vascular cognitive impairment-no dementia. Neurosci Lett 534: 155–159. [DOI] [PubMed] [Google Scholar]
- Bella R., Lanza G., Cantone M., Giuffrida S., Puglisi V., Vinciguerra L., Pennisi M., Ricceri R., D'Agate C. C., Malaguarnera G., Ferri R., Pennisi G. (2015) Effect of a gluten-free diet on cortical excitability in adults with celiac disease. PLoS One 10: e0129218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P., Perez J., Barale F., Schettini G., Soares J. C. (2003) GABAergic dysfunction in mood disorders. Mol Psychiatry 8: 715, 721–737. [DOI] [PubMed] [Google Scholar]
- Briggs C. A., Chakroborty S., Stutzmann G. E. (2017) Emerging pathways driving early synaptic pathology in Alzheimer's disease. Biochem Biophys Res Commun 483: 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantello R., Gianelli M., Civardi C., Mutani R. (1992) Magnetic brain stimulation: The silent period after the motor evoked potential. Neurology 42: 1951–1959. [DOI] [PubMed] [Google Scholar]
- Cantone M., Di Pino G., Capone F., Piombo M., Chiarello D., Cheeran B., Pennisi G., Di Lazzaro V. (2014) The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin Neurophysiol 125: 1509–1532. [DOI] [PubMed] [Google Scholar]
- Caramia M. D., Scalise A., Gordon R., Michalewski H. J., Starr A. (2000) Delayed facilitation of motor cortical excitability following repetitive finger movements. Clin Neurophysiol 111: 1654–1660. [DOI] [PubMed] [Google Scholar]
- Cheeran B., Koch G., Stagg C. J., Baig F., Teo J. (2010) Transcranial magnetic stimulation: From neurophysiology to pharmacology, molecular biology and genomics. Neuroscientist 16: 210–221. [DOI] [PubMed] [Google Scholar]
- Chen R., Gerloff C., Classen J., Wassermann E. M., Hallett M., Cohen L. G. (1997) Safety of different inter-train intervals for repetitive transcranial magnetic stimulation and recommendations for safe ranges of stimulation parameters. Electroencephalogr Clin Neurophysiol 105: 415–421. [DOI] [PubMed] [Google Scholar]
- Chen R., Lozano A. M., Ashby P. (1999) Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res 128: 539–542. [DOI] [PubMed] [Google Scholar]
- Chen R., Cros D., Curra A., Di Lazzaro V., Lefaucheur J. P., Magistris M. R., Mills K., Rösler K. M., Triggs W. J., Ugawa Y., Ziemann U. (2008) The clinical diagnostic utility of transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol 119: 504–532. [DOI] [PubMed] [Google Scholar]
- Chistyakov A. V., Kaplan B., Rubichek O., Kreinin I., Koren D., Hafner H., Feinsod M., Klein E. (2005. a) Effect of electroconvulsive therapy on cortical excitability in patients with major depression: A transcranial magnetic stimulation study. Clin Neurophysiol 116: 386–392. [DOI] [PubMed] [Google Scholar]
- Chistyakov A. V., Kaplan B., Rubichek O., Kreinin I., Koren D., Feinsod M., Klein E. (2005. b) Antidepressant effects of different schedules of repetitive transcranial magnetic stimulation vs. clomipramine in patients with major depression: relationship to changes in cortical excitability. Int J Neuropsychopharmacol 8: 223–233. [DOI] [PubMed] [Google Scholar]
- Chroni E., Lekka N. P., Tsoussis I., Nikolakopoulou A., Paschalis C., Beratis S. (2002) Effect of exercise on motor evoked potentials elicited by transcranial magnetic stimulation in psychiatric patients. J Clin Neurophysiol 19: 240–244. [DOI] [PubMed] [Google Scholar]
- Chroni E., Lekka N. P., Argyriou A. A., Polychronopoulos P., Beratis S. (2008) Persistent suppression of postexercise facilitation of motor evoked potentials during alternate phases of bipolar disorder. J Clin Neurophysiol 25: 115–118. [DOI] [PubMed] [Google Scholar]
- Classen J., Liepert J., Wise S. P., Hallett M., Cohen L. G. (1998) Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79: 1117–1123. [DOI] [PubMed] [Google Scholar]
- Cohen L. G., Gerloff C., Ikoma K., Hallett M. (1995) Plasticity of motor cortex elicited by training of synchronous movements of hand and shoulder. Abstracts in Neuroscience 21: 517. [Google Scholar]
- Concerto C., Lanza G., Cantone M., Pennisi M., Giordano D., Spampinato C., Ricceri R., Pennisi G., Aguglia E., Bella R. (2013) Different patterns of cortical excitability in major depression and vascular depression: A transcranial magnetic stimulation study. BMC Psychiatry 13: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concerto C., Lanza G., Cantone M., Ferri R., Pennisi G., Bella R., Aguglia E. (2015) Repetitive transcranial magnetic stimulation in patients with drug-resistant major depression: A six-month clinical follow-up study. Int J Psychiatry Clin Pract 19: 252–258. [DOI] [PubMed] [Google Scholar]
- Croarkin P. E., Levinson A. J., Daskalakis Z. J. (2011) Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev 35: 818–825. [DOI] [PubMed] [Google Scholar]
- Croarkin P. E., Nakonezny P. A., Husain M. M., Melton T., Buyukdura J. S., Kennard B. D., Emslie G. J., Kozel F. A., Daskalakis Z. J. (2013) Evidence for increased glutamatergic cortical facilitation in children and adolescents with major depressive disorder. JAMA Psychiatry 70: 291–299. [DOI] [PubMed] [Google Scholar]
- Cummings J. L. (1993) Frontal-subcortical circuits and human behavior. Arch Neurol 50: 873–880. [DOI] [PubMed] [Google Scholar]
- Debener S., Beauducel A., Nessler D., Brocke B., Heilemann H., Kayser J. (2000) Is resting anterior EEG alpha asymmetry a trait marker for depression? Findings for healthy adults and clinically depressed patients. Neuropsychobiology 41: 31–37. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Oliviero A., Profice P., Pennisi M. A., Di Giovanni S., Zito G., Tonali P., Rothwell J. C. (2000) Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res 135: 455–461. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Oliviero A., Mazzone P., Insola A., Pilato F., Saturno E., Accurso A., Tonali P., Rothwell J. C. (2001) Comparison of descending volleys evoked by monophasic and biphasic magnetic stimulation of the motor cortex in conscious humans. Exp Brain Res 141: 121–127. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Oliviero A., Pilato F., Saturno E., Dileone M., Marra C., Ghirlanda S., Ranieri F., Gainotti G., Tonali P. (2005) Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiatry 76: 1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V., Pilato F., Oliviero A., Dileone M., Saturno E., Mazzone P., Insola A., Profice P., Ranieri F., Capone F., Tonali P. A., Rothwell J. C. (2006) Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol 96: 1765–1771. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Profice P., Pilato F., Dileone M., Oliviero A., Ziemann U. (2010) The effects of motor cortex rTMS on corticospinal descending activity. Clin Neurophysiol 121: 464–473. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Rothwell J. C., Talelli P., Capone F., Ranieri F., Wallace A. C., Musumeci G., Dileone M. (2013) Inhibitory theta burst stimulation of affected hemisphere in chronic stroke: A proof of principle, sham-controlled study. Neurosci Lett 553: 148–152. [DOI] [PubMed] [Google Scholar]
- Dumas T. C. (2012) Postnatal alterations in induction threshold and expression magnitude of long-term potentiation and long-term depression at hippocampal synapses. Hippocampus 22: 188–199. [DOI] [PubMed] [Google Scholar]
- Duric V., Banasr M., Stockmeier C. A., Simen A. A., Newton S. S., Overholser J. C., Jurjus G. J., Dieter L., Duman R. S. (2013) Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol 16: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbert A., Priori A., Rothwell J. C., Day B. L., Colebatch J. G., Marsden C. D. (1992) Interhemispheric inhibition of the human motor cortex. J Physiol 453: 525–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa A. M., Chandran A., Stockmeier C. A., Karolewicz B. (2009) Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 33: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. B., Brown T. L., Marston N. A., Daskalakis Z. J., de Castella A., Bradshaw J. L., Kulkarni J. (2004) Motor cortical excitability and clinical response to rTMS in depression. J Affect Disord 82: 71–76. [DOI] [PubMed] [Google Scholar]
- Gladding C. M., Fitzjohn S. M., Molnár E. (2009) Metabotropic glutamate receptor-mediated long-term depression: Molecular mechanisms. Pharmacol Rev 61: 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L. G., Mall V., Kaelin-Lang A., Mima T., Rossi S., Thickbroom G. W., Rossini P. M., Ziemann U., Valls-Solé J., Siebner H. R. (2012) A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin Neurophysiol 123: 858–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S. (2016) Multifocal TMS for temporo-spatial description of cortico-cortical connectivity patterns. Clin Neurophysiol 127: 1005–1006. [DOI] [PubMed] [Google Scholar]
- He H., Yu Q., Du Y., Vergara V., Victor T. A., Drevets W. C., Savitz J. B., Jiang T., Sui J., Calhoun V. D. (2016) Resting-state functional network connectivity in prefrontal regions differs between unmedicated patients with bipolar and major depressive disorders. J Affect Disord 190: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalinous R. (1991) Technical and practical aspects of magnetic nerve stimulation. J Clin Neurophysiol 8: 10–25. [DOI] [PubMed] [Google Scholar]
- Jung P., Ziemann U. (2009) Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J Neurosci 29: 5597–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. J., Voleti B., Hajszan T., Rajkowska G., Stockmeier C. A., Licznerski P., Lepack A., Majik M. S., Jeong L. S., Banasr M., Son H., Duman R. S. (2012) Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18: 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimiskidis V. K., Papagiannopoulos S., Sotirakoglou K., Kazis D. A., Kazis A., Mills K. R. (2005) Silent period to transcranial magnetic stimulation: Construction and properties of stimulus-response curves in healthy volunteers. Exp Brain Res 163: 21–31. [DOI] [PubMed] [Google Scholar]
- Kimiskidis V. K. (2016) Transcranial magnetic stimulation (TMS) coupled with electroencephalography (EEG): Biomarker of the future. Rev Neurol (Paris) 172: 123–126. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Pascual-Leone A. (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2: 145–156. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Nestler E. J. (2008) The molecular neurobiology of depression. Nature 455: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Mainberger F., Feige B., Maier J. G., Mall V., Jung N. H., Reis J., Klöppel S., Normann C., Nissen C. (2016) State-dependent partial occlusion of cortical LTP-like plasticity in major depression. Neuropsychopharmacology 41: 1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T., Caramia M. D., Rothwell J. C., Day B. L., Thompson P. D., Ferbert A., Wroe S., Asselman P., Marsden C. D. (1993) Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza G., Bella R., Giuffrida S., Cantone M., Pennisi G., Spampinato C., Giordano D., Malaguarnera G., Raggi A., Pennisi M. (2013) Preserved transcallosal inhibition to transcranial magnetic stimulation in nondemented elderly patients with leukoaraiosis. Biomed Res Int 2013: 351680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza G., Cantone M., Lanuzza B., Pennisi M., Bella R., Pennisi G., Ferri R. (2015) Distinctive patterns of cortical excitability to transcranial magnetic stimulation in obstructive sleep apnea syndrome, restless legs syndrome, insomnia, and sleep deprivation. Sleep Med Rev 19: 39–50. [DOI] [PubMed] [Google Scholar]
- Lanza G., Bramanti P., Cantone M., Pennisi M., Pennisi G., Bella R. (2017) Vascular cognitive impairment through the looking glass of transcranial magnetic stimulation. Behav Neurol 2017: 1421326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J. P., Lucas B., Andraud F., Hogrel J. Y., Bellivier F., Del Cul A., Rousseva A., Leboyer M., Paillère-Martinot M. L. (2008) Inter-hemispheric asymmetry of motor corticospinal excitability in major depression studied by transcranial magnetic stimulation. J Psychiatr Res 42: 389–398. [DOI] [PubMed] [Google Scholar]
- Levinson A. J., Fitzgerald P. B., Favalli G., Blumberger D. M., Daigle M., Daskalakis Z. J. (2010) Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry 67: 458–464. [DOI] [PubMed] [Google Scholar]
- Levinson A. J., Young L. T., Fitzgerald P. B., Daskalakis Z. J. (2007) Cortical inhibitory dysfunction in bipolar disorder: a study using transcranial magnetic stimulation. J Clin Psychopharmacol 27: 493–497. [DOI] [PubMed] [Google Scholar]
- Li B., Arime Y., Hall F. S., Uhl G. R., Sora I. (2010) Impaired spatial working memory and decreased frontal cortex BDNF protein level in dopamine transporter knockout mice. Eur J Pharmacol 628: 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. J., Aghajanian G. K. (2008) Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A 105: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F., Keenan J. P., Pascual-Leone A. (2000) Interhemispheric asymmetry of motor cortical excitability in major depression as measured by transcranial magnetic stimulation. Br J Psychiatry 177: 169–173. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Yasuno F., Kishimoto T., Yamamoto A., Kiuchi K., Kosaka J., Nagatsuka K., Iida H., Kudo T. (2017) Microstructural differences in the corpus callosum in patients with bipolar disorder and major depressive disorder. J Clin Psychiatry 78: 99–104. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Eiland L., Hunter R. G., Miller M. M. (2012) Stress and anxiety: Structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 62: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro R., Zarkowski P., Sporn A., Avery D. (2009) Hemispheric asymmetry in resting motor threshold in major depression. J ECT 25: 39–43. [DOI] [PubMed] [Google Scholar]
- Nissen C., Holz J., Blechert J., Feige B., Riemann D., Voderholzer U., Normann C. (2010) Learning as a model for neural plasticity in major depression. Biol Psychiatry 68: 544–552. [DOI] [PubMed] [Google Scholar]
- Normann C., Schmitz D., Fürmaier A., Döing C., Bach M. (2007) Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry 62(5): 373–380. [DOI] [PubMed] [Google Scholar]
- Paulus, W., Classen, J., Cohen, L. G., Large, C. H., Di Lazzaro, V., Nitsche, M., Pascual-Leone, A., Rosenow, F., Rothwell, J. C., & Ziemann, U. (2008). State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul, 1, 151–163. [DOI] [PubMed]
- Pennisi G., Ferri R., Lanza G., Cantone M., Pennisi M., Puglisi V., Malaguarnera G., Bella R. (2011. a) Transcranial magnetic stimulation in Alzheimer’s disease: A neurophysiological marker of cortical hyperexcitability. J Neural Transm (Vienna) 118: 587–598. [DOI] [PubMed] [Google Scholar]
- Pennisi G., Ferri R., Cantone M., Lanza G., Pennisi M., Vinciguerra L., Malaguarnera G., Bella R. (2011. b) A review of transcranial magnetic stimulation in vascular dementia. Dement Geriatr Cogn Disord 31: 71–80. [DOI] [PubMed] [Google Scholar]
- Pennisi G., Lanza G., Giuffrida S., Vinciguerra L., Puglisi V., Cantone M., Pennisi M., D'Agate C. C., Naso P., Aprile G., Malaguarnera G., Ferri R., Bella R. (2014) Excitability of the motor cortex in de novo patients with celiac disease. PLoS One 9: e102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi G., Bella R., Lanza G. (2015) Motor cortex plasticity in subcortical ischemic vascular dementia: What can TMS say? Clin Neurophysiol 126: 851–852. [DOI] [PubMed] [Google Scholar]
- Pennisi M., Lanza G., Cantone M., Ricceri R., Spampinato C., Pennisi G., Di Lazzaro V., Bella R. (2016) Correlation between motor cortex excitability changes and cognitive impairment in vascular depression: Pathophysiological insights from a longitudinal TMS study. Neural Plast 2016: 8154969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C., Duman R. S. (2008) Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 33: 88–109. [DOI] [PubMed] [Google Scholar]
- Player M. J., Taylor J. L., Weickert C. S., Alonzo A., Sachdev P., Martin D., Mitchell P. B., Loo C. K. (2013) Neuroplasticity in depressed individuals compared with healthy controls. Neuropsychopharmacology 38: 2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. L., Drevets W. C. (2010) Neurocircuitry of mood disorders. Neuropsychopharmacology 35: 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. (2000) Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry 48: 766–777. [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Stockmeier C. A. (2013) Astrocyte pathology in major depressive disorder: Insights from human postmortem brain tissue. Curr Drug Targets 14: 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. D., Daniels B., Rybak M., Turnier-Shea Y., Pridmore S. (2002) Cortical excitability of psychiatric disorders: Reduced post-exercise facilitation in depression compared to schizophrenia and controls. Aust N Z J Psychiatry 36: 669–673. [DOI] [PubMed] [Google Scholar]
- Reis J., Swayne O. B., Vandermeeren Y., Camus M., Dimyan M. A., Harris-Love M., Perez M. A., Ragert P., Rothwell J. C., Cohen L. G. (2008) Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 586: 325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding M. C., Rothwell J. C. (1997) Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol 105: 340–344. [DOI] [PubMed] [Google Scholar]
- Rossini P. M., Rossi S. (2007) Transcranial magnetic stimulation: Diagnostic, therapeutic, and research potential. Neurology 68: 484–488. [DOI] [PubMed] [Google Scholar]
- Rossini P. M., et al. (2015) Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126: 1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A., Molnar G. F., Paradiso G., Gunraj C. A., Lang A. E., Chen R. (2003) Short and long latency afferent inhibition in Parkinson's disease. Brain 126: 1883–1894. [DOI] [PubMed] [Google Scholar]
- Salustri C., Tecchio F., Zappasodi F., Bevacqua G., Fontana M., Ercolani M., Milazzo D., Squitti R., Rossini P. M. (2007) Cortical excitability and rest activity properties in patients with depression. J Psychiatry Neurosci 32: 259–266. [PMC free article] [PubMed] [Google Scholar]
- Samii A., Wassermann E. M., Ikoma K., Mercuri B., George M. S., O'Fallon A., Dale J. K., Straus S. E., Hallett M. (1996) Decreased postexercise facilitation of motor evoked potentials in patients with chronic fatigue syndrome or depression. Neurology 47: 1410–1414. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Zarate C. A., Krystal J. H., Manji H. K. (2008) Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 7: 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajahan P. M., Glabus M. F., Gooding P. A., Shah P. J., Ebmeier K. P. (1999. a) Reduced cortical excitability in depression. Impaired post-exercise motor facilitation with transcranial magnetic stimulation. Br J Psychiatry 174: 449–454. [DOI] [PubMed] [Google Scholar]
- Shajahan P. M., Glabus M. F., Jenkins J. A., Ebmeier K. P. (1999. b) Postexercise motor evoked potentials in depressed patients, recovered depressed patients, and controls. Neurology 53: 644–646. [DOI] [PubMed] [Google Scholar]
- Siebner H. R., Dressnandt J., Auer C., Conrad B. (1998) Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve 21: 1209–1212. [DOI] [PubMed] [Google Scholar]
- Spampinato C., Aguglia E., Concerto C., Pennisi M., Lanza G., Bella R., Cantone M., Pennisi G., Kavasidis I., Giordano D. (2013) Transcranial magnetic stimulation in the assessment of motor cortex excitability and treatment of drug-resistant major depression. IEEE Trans Neural Syst Rehabil Eng 21: 391–403. [DOI] [PubMed] [Google Scholar]
- Steele J. D., Glabus M. F., Shajahan P. M., Ebmeier K. P. (2000) Increased cortical inhibition in depression: A prolonged silent period with transcranial magnetic stimulation (TMS). Psychol Med 30: 565–570. [DOI] [PubMed] [Google Scholar]
- Stefan K., Kunesch E., Benecke R., Cohen L. G., Classen J. (2002) Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol 543: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier C. A., Mahajan G. J., Konick L. C., Overholser J. C., Jurjus G. J., Meltzer H. Y., Uylings H. B., Friedman L., Rajkowska G. (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56: 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler T. J., Cavus I. (2007) Depressed neuroplasticity in major depressive disorder? Biol Psychiatry 62: 371–372. [DOI] [PubMed] [Google Scholar]
- Tokimura H., Di Lazzaro V., Tokimura Y., Oliviero A., Profice P., Insola A., Mazzone P., Tonali P., Rothwell J. C. (2000) Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 523: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M. T., Waskom M. L., Dillon D. G., Holmes A. J., Park M. T., Chakravarty M. M., Dutra S. J., Polli F. E., Iosifescu D. V., Fava M., Gabrieli J. D., Pizzagalli D. A. (2015) Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry 77: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs W. J., McCoy K. J., Greer R., Rossi F., Bowers D., Kortenkamp S., Nadeau S. E., Heilman K. M., Goodman W. K. (1999) Effects of left frontal transcranial magnetic stimulation on depressed mood, cognition, and corticomotor threshold. Biol Psychiatry 45: 1440–1446. [DOI] [PubMed] [Google Scholar]
- Veronezi B. P., Moffa A. H., Carvalho A. F., Galhardoni R., Simis M., Benseñor I. M., Lotufo P. A., Machado-Vieira R., Daskalakis Z. J., Brunoni A. R. (2016) Evidence for increased motor cortical facilitation and decreased inhibition in atypical depression. Acta Psychiatr Scand 134: 172–182. [DOI] [PubMed] [Google Scholar]
- Wang H., Wang X., Scheich H. (1996) LTD and LTP induced by transcranial magnetic stimulation in auditory cortex. Neuroreport 7: 521–525. [DOI] [PubMed] [Google Scholar]
- Wassermann E. M., McShane L. M., Hallett M., Cohen L. G. (1992) Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol 85: 1–8. [DOI] [PubMed] [Google Scholar]
- Webb C. A., Weber M., Mundy E. A., Killgore W. D. (2014) Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: A voxel-based morphometric analysis. Psychol Med 44: 2833–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Liu Y., Xie J. C., Liu N. N., Tian X. (2015) Effects of repetitive transcranial magnetic stimulation on synaptic plasticity and apoptosis in vascular dementia rats. Behav Brain Res 281: 149–155. [DOI] [PubMed] [Google Scholar]
- Yasuno F., Kudo T., Matsuoka K., Yamamoto A., Takahashi M., Nakagawara J., Nagatsuka K., Iida H., Kishimoto T. (2016) Interhemispheric functional disconnection because of abnormal corpus callosum integrity in bipolar disorder type II. BJPsych Open 2: 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yger, P., & Gilson, M. (2015). Models of metaplasticity: A review of concepts. Front Comput Neurosci, 9, 138. [DOI] [PMC free article] [PubMed]
- Yukimasa T., Yoshimura R., Tamagawa A., Uozumi T., Shinkai K., Ueda N., Tsuji S., Nakamura J. (2006) High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry 39: 52–59. [DOI] [PubMed] [Google Scholar]
- Zalsman G., Weller A., Shbiro L., Barzilay R., Gutman A., Weizman A., Mann J. J., Wasserman J., Wasserman D. (2016) Fibre tract analysis using diffusion tensor imaging reveals aberrant connectivity in a rat model of depression. World J Biol Psychiatry 7: 1–9. [DOI] [PubMed] [Google Scholar]
- Zanardini R., Gazzoli A., Ventriglia M., Perez J., Bignotti S., Rossini P. M., Gennarelli M., Bocchio-Chiavetto L. (2006) Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients. J Affect Disord 91: 83–86. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Rothwell J. C., Ridding M. C. (1996) Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 496: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U., Hallett M., Cohen L. G. (1998) Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci 18: 7000–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. (2004) TMS and drugs. Clin Neurophysiol 115: 1717–1729. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Reis J., Schwenkreis P., Rosanova M., Strafella A., Badawy R., Müller-Dahlhaus F. (2015) TMS and drugs revisited 2014. Clin Neurophysiol 126: 1847–1868. [DOI] [PubMed] [Google Scholar]