Abstract
Background
Breastfeeding is a widely encouraged practice due to its benefits for mother and the infant. Little is known about the impact of disease states, such as kidney dysfunction and childbirth complications, on the composition of breast milk.
Methods
We describe a case of a 35-year-old woman who suffered a postpartum hemorrhage, was administered a contrast dye prior to computer tomography, and developed an acute kidney injury. Using nuclear magnetic resonance spectrometry, we measured composition of milk in acute kidney injury. The amount of dye secreted into milk was determined using a spectroscopic assay.
Results
Here we show that acute kidney injury results in changes in milk composition, but it does not significantly affect major macronutrients. We also determine that iodinated computer tomography contrast dye does not accumulate in milk in appreciable amounts.
Conclusion
Acute kidney injury has impact on breast milk. Intravenous administration of computer tomography contrast dye does not result in significantly elevated levels in milk.
Keywords: Maternal–fetal medicine, nephrology, complications
Introduction
Breast milk is widely regarded as the preferred form of newborn nutrition. It is an excellent source of nutrients for infants, and provides an abundance of proteins, sugars, lipids, and minerals.1 In addition to the nutritional benefits, breastfeeding is demonstrated to be protective against a number of ailments, such as upper respiratory tract infections, asthma, necrotizing enterocolitis, obesity, inflammatory bowel disease, diabetes, and sudden infant death syndrome.2 While the benefits are well demonstrated for healthy mothers and babies, little is known about how breast-milk composition is affected by disease states, such as kidney disease and complications of childbirth.
To our knowledge, there are no definite guidelines about breastfeeding in either chronic kidney disease (CKD) or acute kidney injury (AKI). Studies of breast milk composition in the setting of CKD or AKI and of the short- or long-term health outcomes of nursing children whose mothers had CKD/AKI are lacking. AKI/CKD are characterized by systemic inflammation, accumulation of nitrogenous and other metabolic wastes, and alterations in pH, and electrolyte balance.3 It is therefore possible that breast milk composition is altered in these states.
The work-up of a variety of different complications peri- or post-partum might require the intravenous administration of radiocontrast dyes such as iodixanol (Vispaque) or iohexol (Omnipaque). The radiocontrast dyes allow for better visualization of vasculature and organs on computer tomography (CT) imaging. These dyes are freely filtered at the glomerulus and therefore rapidly eliminated from the systemic circulation in the setting of normal kidney function.4 They are also secreted in milk, but concentrations in breast milk of healthy mothers levels are sufficiently low that radio-contrast administration is not considered a firm contraindication to breast feeding.5,6 Given that contrast is renally excreted, it is possible in the setting of AKI that increased serum contrast levels could translate into elevated concentrations in breast milk.4,7 To our knowledge, there are no studies examining contrast accumulation in breast milk in the setting of AKI and the available breast feeding guidelines do not address this issue.7–9
Here we describe a case of a 35-year-old woman who experienced a severe peri-partum hemorrhage, received iodixanol, and who developed AKI. She was highly motivated to breast feed and inquired about the impact of her illness and treatment on her breast milk. In this report, we look at how (a) AKI affects milk composition in the immediate postpartum period, and (b) the extent to which iodinated dyes such as iodinaxol accumulate in milk in AKI.
Case report
The patient was a healthy 35-year-old woman with no history of kidney disease, hypertension, diabetes, nephrolithiasis, recurrent urinary tract infections, or vasculitis. She had a healthy pregnancy and presented to a regional health centre in Canada, with premature rupture of membranes at 36 weeks and 2 days. During spontaneous vaginal delivery, she developed severe pelvic pain, hemodynamic instability, and oliguria (133 mL/12 h). A contrast-enhanced CT scan (100 mL of iodixanol 320 mg I/mL) showed a 13.7 × 1.8 × 15 cm3 hematoma in the right lateral lower pelvis, extending into the perivesicular space and into the extra-peritoneal space beside the labia majora. There was no hydronephrosis. She was transferred to a tertiary hospital via air ambulance. On arrival, the hemoglobin (Hb) was 85 g/L (133 g/L immediately post-delivery), creatinine (Cr) was 222 µmol/L (only previous available Cr was 64 µmol/L 43 months prior), and urea was 6.7 mmol/L. Sodium was 130 mmol/L, potassium was 4.5 mmol/L, and bicarbonate was 23 mmol/L.
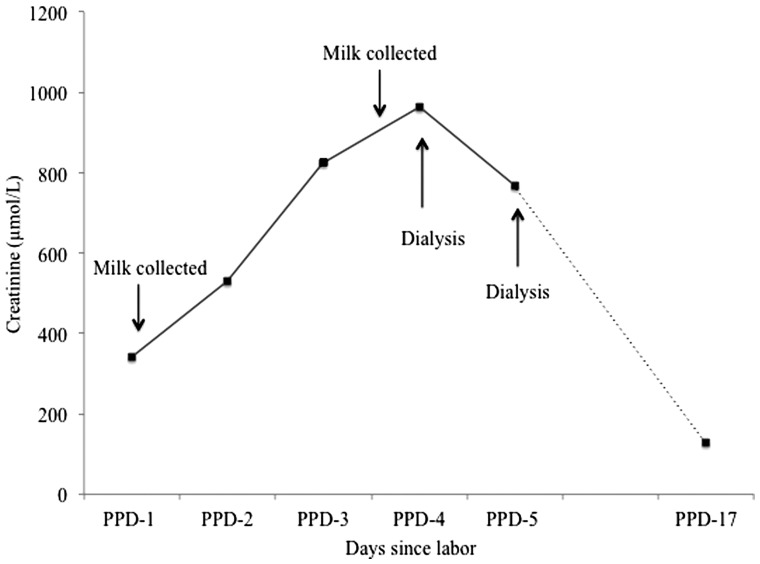
She remained oliguric and was volume resuscitated using colloid, crystalloid, and blood products. Nephrology was consulted. Urine microscopy revealed many muddy brown casts and she was diagnosed with ischemic and toxic acute tubular necrosis (ATN). She stabilized clinically with no further drop in Hb and did not require vasopressor support. Her subsequent biochemistry and clinical course is illustrated in Figure 1. Hemodialysis (HD) was initiated on postpartum day (PPD)-4 with second treatment on PPD-5. Breast milk was collected prior to HD on PPD-4. She was transferred back to her regional health care center on PPD-6 where dialysis was eventually discontinued due to renal recovery.
Figure 1.
Event timeline; the rise in creatinine during the post-partum period is shown, and the instances of milk collection and dialysis are indicated on the graph.
Methods
Milk samples were collected as part of regular expression from the patient with an AKI on PPD-1 and PPD-4 (pre-HD) after informed consent. A midwife identified a suitable healthy control and, after informed consent was obtained, a control milk sample was collected on PPD-4. Milk samples were frozen at −80℃ until analysis.
The composition of breast milk from the woman with AKI was compared with the healthy control PPD-4 milk were determined using nuclear magnetic resonance (NMR) spectroscopy. A single set of NMR spectroscopy measurements was performed at a commercial center—The Metabolomics Innovation Centre using established methods.10 Lipids from samples were extracted and measured according to protocol described by Bligh and Dyer.11
The levels of ioxidanol in PPD-1 and PPD-4 breast milk from the woman with AKI were measured using a spectroscopic assay. The assay takes advantage of the extremely high water and low lipid solubility of ioxidanol.4 Briefly, 100 µL aliquots of milk were mixed with 50 µL of trichloroacetic acid (TCA) and precipitated overnight at 4℃. The supernatant was separated by centrifugation at 16,000 g for 30 min at 4℃. In all, 50 µL of the supernatant was removed, carefully avoiding the lipid layer; 50 µL of Tris base was then added prior to measurement with Nanodrop at UV–vis 244 nm and 260 nm. The measurements were compared to a standard curve, obtained by adding fixed dye concentration to the control milk sample.
Results
Table 1 shows the metabolite concentration of 33 different compounds in PPD-4 breast milk from both the AKI case and healthy volunteer control. The PPD-4 AKI milk contained lower levels of detectable amino acids than the healthy milk: alanine, glutamine, glutamate, glycine, and valine. The PPD-4 AKI milk contained similar levels of lactose as the healthy control, less glucose, and more maltose. There was no difference in the fatty acid content between the two samples. The PPD-4 AKI milk had a higher pH than the control sample. The PPD-4 AKI milk contained higher levels of creatinine, hippurate, creatine, creatine phosphate as compared to control PPD-4 milk. Concentrations of acetone, choline, and pyruvate were elevated in the AKI PPD-4 milk as compared to the PPD-4 control. On the other hand, citrate, glycerol, phosphocholine, and valerate were lower in the setting of AKI.
Table 1.
Metabolite concentrations in milk.
| Metabolite | PPD-4 healthy control milk | PPD-4 AKI milk | Compound ratio in AKI/normal |
|---|---|---|---|
| Amino acids (µmol/L) | |||
| Alanine | 144 | 44 | 0.3 |
| Glutamate | 566 | 63 | 0.1 |
| Glutamine | 248 | 232 | 0.9 |
| Glycine | 4736 | 4194 | 0.9 |
| S-Sulfocysteine | 71 | 65 | 0.9 |
| Valine | 41 | 24 | 0.6 |
| Sugars (µmol/L) | |||
| 1,6-Anhydro-β-D-glucose | 516 | 316 | 0.6 |
| Cellobiose | 2413 | 2118 | 0.9 |
| Glucose | 2699 | 641 | 0.2 |
| Lactose | 309,038 | 318,398 | 1.0 |
| Maltose | 133 | 603 | 4.5 |
| Sucrose | 614 | 850 | 1.4 |
| Lipids (g/L) | |||
| Total lipids | 1 | 1 | 1 |
| Catabolites (µmol/L) | |||
| Hippurate | 45 | 1724 | 38.3 |
| Lactate | 261 | 426 | 1.6 |
| Citrate | 12,469 | 5942 | 0.5 |
| Creatine | 170 | 266 | 1.6 |
| Creatine phosphate | 100 | 520 | 5.2 |
| Creatinine | 97 | 734 | 7.6 |
| O-Phosphocholine | 1220 | 281 | 0.2 |
| Other (µmol/L) | |||
| Acetate | 44 | 37 | 0.8 |
| Acetone | 9 | 62 | 7.1 |
| Betaine | 1144 | 479 | 0.4 |
| Butyrate | 32 | 23 | 0.7 |
| Choline | 115 | 302 | 2.6 |
| Ethanol | 142 | 135 | 1.0 |
| Formate | 178 | 110 | 0.6 |
| Fumarate | 11 | 11 | 0.9 |
| Glycerol | 11,937 | 7941 | 0.7 |
| Methanol | 398 | 352 | 0.9 |
| Pyruvate | 31 | 71 | 2.3 |
| Succinate | 22 | 13 | 0.6 |
| Valerate | 34 | 21 | 0.6 |
| sn-Glycero-3-phosphocholine | 474 | 136 | 0.3 |
Using the spectroscopy-based assay, we estimated that the PPD-1 AKI breast milk contained 55 µg I/mL of iodixanol and that decreased to 28 µg I/mL of iodixanol in PPD-4 milk.
Discussion
Composition of milk in AKI
This is the first report of breast milk composition in AKI. Our study of a single patient suggests that AKI may have an acute effect on the composition of breast milk with lower amino acid concentrations, higher catabolites, but similar levels of the major nutrients of lactose and lipids. The AKI milk had a pH similar to that described in the literature.12
Breast milk is produced in the mammary gland and its major constituents are proteins, sugars, lipids and micronutrients.1 Nutrient levels vary significantly throughout lactation.1 Lactose is the major carbohydrate in human milk.13 Lactose concentrations were similar between the AKI and control mother’s milk. Levels in both were higher than that reported in the literature at PPD-4 (161,000, SE 8760 µmol/L) but methodologies of measurement were very dissimilar.14 Lipids are another major energetic constituent of milk.13 The lipid concentration was also similar between AKI milk and healthy control milk. Our reported values however were lower than those in literature but again the methodologies used were different.15
Proteins represent about 10% of milk by weight.13 We were unable to measure the total amount of proteins in our sample. However, concentrations of individual amino acids differ between the milk in AKI and in the healthy control with higher levels in the control healthy milk. The control milk had levels similar to those reported in healthy women measured using similar technology albeit at PPD-90 (alanine 231 ± 67.2 µmol/L, glutamine 616 ± 314 µmol/L, valine 48.7 ± 11.2 µmol/L).16 Concentrations of amino acids early post-partum are not available for comparative purposes. It is possible that the decreased AKI breast milk amino acid concentrations could result from increased metabolic derangements and protein catabolism that characterizes early AKI.17,18
The concentrations of creatine, creatinine, and hippuric acid in our AKI sample were from 2.5 t 38 times higher than in the healthy control. This is not surprising given their well-documented accumulation in the serum in AKI.3 An analysis of breast milk from a mother with HD dependent CKD found that concentrations of creatinine and urea were higher pre-HD than post-HD and higher than in control milk.19 The significance of increase in these catabolites on the infant is not known and no studies have examined their serum levels in breast fed infants of mothers with CKD/AKI.
Breast milk pH in AKI has never been examined. The pH of the AKI milk is similar to the pH of PPD 4 breast milk from healthy controls reported in the literature (pH approximately 7.45 PPD1–3 and 7.15 PPD 4–7)14 despite the apparent maternal metabolic acidosis (serum HCO3 15 mmol/L PPD-4 prior to HD). This is important, as physiological pH is necessary for the antimicrobial and enzymatic properties and leukocyte function of milk to be retained.12 The “normal pH” of AKI milk suggests that breast milk pH in AKI is not significantly influenced by systemic pH. We speculate that the comparatively lower pH of the control milk could be due to person-to-person variation or sample handling. Comparatively, higher citrate levels in the control milk support the former.
Serum citrate levels are not affected by AKI in the absence of liver disease.20,21 Breast milk citrate has been reported to be inversely related to breast milk pH with lower citrate concentrations and higher pH early in lactogenesis and higher citrate levels and lower pH later on.14 In healthy post-partum women, citrate are low in the very early post period PPD-2 to 3 (2181 ± 307 µmol/l) climbing by PPD-4 to 7 (6074 ± 297 µmol/l) and then progressively dropping from PPD-21 onward.22 It has been hypothesized that this trend reflects citrate utilization for de-novo fatty acid synthesis. The breast milk citrate levels of our PPD-4 AKI mother is in keeping with those of healthy women.22
Impact of AKI on breast milk iodinated dye concentrations
To our knowledge, there are currently no studies looking at the amount of iodixanol secreted into breast milk. One study in an animal model (goat) suggests that another iodinated dye—iobitridol is minimally secreted in milk with only 0.7% of the iobitrol administered to the mother secreted into milk after 30 h.23 In another study in three human mothers at different stages of breast feeding (1 week to 4 months) with presumed normal kidney function, breast milk secretion of iohexol, another iodinated dye, was estimated by AUC analysis to be 0.5% of the total maternal dose at 24 h.5
The concentration of iodixanol in PPD-1 breast milk was 55 µg I/mL, decreasing to 28 µg I/mL by PPD-4. Assuming approximately 100 mL of milk ingestion on PPD1 and 600 mL on PPD4,1 the newborn would consume a total of 5500 µg I on PPD-1 and 16,800 µg I on PPD-4 of iodixanol with presumably doses between those values on PPD-2 and 3. This represents 0.017% of the total administered maternal dose on PPD-1 and 0.05% on PPD-4. Even for an older infant consuming 800 mL/breast milk per day,24 0.14% and 0.07% of the maternal dose would be ingested day 1 and 4 post contrast. It should also be noted that although safety in children has not been established for iodixanol,4 similar agents are used intravenously in radiographic studies in the pediatric setting at significantly higher doses. A recent review of patterns of intravenous contrast use in children found that iodinated dyes were commonly used at doses of 600 mg I/kg.24 The dose ingested orally in the current case on PPD-1 and 4 combined represents 0.9% of the standard intravenous dose for one IVP study for an infant with a weight of 4 kg.
Our results suggest that newborn iodinated dye exposure was minimal despite the maternal AKI and impaired renal contrast excretion. Further, iodinated contrast agents are poorly absorbed orally,25 and therefore the amount of systemic exposure to breast fed children would presumably be even lower. No study has examined serum contrast concentrations in the breastfed children of mothers who received intravenous contrast.
Manufacturer’s guidelines suggest temporary cessation of breastfeeding in mothers administered iodixanol.4 The American College of Radiology (ACR) guidelines state that because of a very limited amount of radiocontrast dye secreted in milk, it is safe to proceed with breastfeeding after contrast administration.7 The Canadian Family Physician Mother Risk Update also questions the need to cease breastfeeding, and highlights the risks associated with the use of formula.6 This approach is echoed by the guidelines from the European Society of Urogenital Radiology and the Italian Society of Radiology, which state that cessation of breastfeeding should be an informed decision made by the mother, as the small amounts secreted only pose a minimal and theoretical risk.8,26
This study adds to this small literature by inclusion of one case subject with AKI and one healthy control. We have limited ability to generalize our findings as we studied only one subject and one control. We were also unable to detect some important metabolites like urea, uric acid, or phosphate and to measure concentrations of minerals due to the technical limitations of our methodology. Because of a limited amount of milk available, we were only able to perform a single set of NMR measurements. Last, we did not validate the iodixanol assay in an independent laboratory, as we could not identify one despite extensive searching and contacting the manufacturer (GE healthcare).
This study reveals lower concentrations of some amino acids, higher concentrations of certain metabolic waste products, and similar concentrations of lactose and fatty acids in one individual with AKI as compared to a healthy control at the same post partum day. Whether these changes have any impact on the nutritional value and safety of breast milk remains to be shown. The results should be considered preliminary and further study in larger groups of nursing women with kidney dysfunction and their children are required. Similarly, the finding of low iodixanol concentrations in the milk of lactating mothers with AKI is reassuring regarding the safety of breast feeding but requires confirmation.
Acknowledgements
We would like to acknowledge Dr. Nancy N Fang for her invaluable help with the completion of this work. We would also like to thank the Metabolomics Innovation Centre (TMIC) for their help with the sample analysis.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
The local medical ethics research board approved this study (SMED 162-15). Written consent was obtained from the patient for publication.
Guarantor
CW.
Contributorship
Research idea and study design: AC and CW; data acquisition: AC; analysis/interpretation: AC and CW; supervision and mentorship: CW, RM, and AA.
References
- 1.Neville M, Allen J, Archer P, et al. Studies in human lactation: Milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr 1991; 54: 81–92. [DOI] [PubMed] [Google Scholar]
- 2.Breastfeeding and the Use of Human Milk. Section on Breastfeeding. Pediatrics 2012; 129: e827–e841. DOI: 10.1542/peds.2011-3552. [DOI] [PubMed]
- 3.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 2012; 380: 756–766. [DOI] [PubMed] [Google Scholar]
- 4.GE Healthcare. Visipaque, http://www3.gehealthcare.com/en/products/categories/contrast_media/visipaque.
- 5.Nielsen ST, Matheson I, Rasmussen JN, et al. Excretion of iohexol and metizoate in human breast milk. Acta Radiol 1987; 28: 523–526. [PubMed] [Google Scholar]
- 6.Newman J. Breastfeeding and radiologic procedures. Can Fam Physician 2007; 53: 630–631. [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis J, Davenport M, Dillman J, et al. (eds) ACR manual on contrast media. 10.1 ed. ACR Committee on Drugs, published online 2015.
- 8.Webb J, Thomsen H, Morcos S. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol 2005; 15: 1234–1240. [DOI] [PubMed] [Google Scholar]
- 9.Piccoli GB, Cabiddu G, Attini R, et al. Pregnancy in chronic kidney disease: Questions and answers in a changing panorama. Best Pract Res Clin Obstet Gynaecol 2015; 29: 625–642. [DOI] [PubMed] [Google Scholar]
- 10.Psychogios N, Hau DD, Peng J, et al. The human serum metabolome. PLoS One 2011; 6: e16957–e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bligh E, Dyer W. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 12.Harrison VC, Peat G. Significance of milk pH in newborn infants. Br Med J 1972; 4: 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballard O, Morrow A. Human milk composition: Nutrients and bioactive factors. Pediatr Clin North Am 2014; 60: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morriss FH, Brewer ED, Spedale SB, et al. Relationship of lactation fatty acids of human milk to concentrations pH during course of citrate and fatty acids. Pediatrics 2015; 78: 458–464. [PubMed] [Google Scholar]
- 15.Kelishadi R, Hadi B, Iranpour R. A study on lipid content and fatty acid of breast milk and its association with mother’s diet composition. J Res Med Sci 2012; 17: 824–827. [PMC free article] [PubMed] [Google Scholar]
- 16.Smiltowitz J, O’Sullivan A, Barile D, et al. The human milk metabolome reveals diverse oligosaccharide profiles 1–3. J Nutr 2013; 143: 1709–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiaccadori E, Regolisti G, Cabassi A. Specific nutritional problems in acute kidney injury, treated with non-dialysis and dialytic modalities. NDT Plus 2010; 3: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Druml W. Protein metabolism in acute renal failure. Min Electrolyte Metab 1998; 27: 45–54. [DOI] [PubMed] [Google Scholar]
- 19.Balzer M, Gross M, Lichtinghagen R, et al. Got milk? Breastfeeding and milk analysis of a mother on chronic hemodialysis. PLoS One 2015; 10: e0143340–e0143340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y, Xu Z, Zhu Q, et al. Citrate pharmacokinetics in critically ill patients with acute kidney injury. PLoS One 2013; 8: e65992–e65992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer E, Derfler K, Joukhadar C, et al. Citrate kinetics in patients receiving long-term hemodialysis therapy. Am J Kidney Dis 2005; 46: 903–907. [DOI] [PubMed] [Google Scholar]
- 22.Allen J, Keller R, Archer P, et al. Studies in human lactation: Milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr 1991; 54: 69–80. [DOI] [PubMed] [Google Scholar]
- 23.Bourrinet P, Dencausse A, Havard P, et al. Transplacental passage and milk excretion of iobitridol. Invest Radiol 1995; 30: 156–158. [DOI] [PubMed] [Google Scholar]
- 24.Trout A, Dillman J, Ellis J, et al. Patterns of intravenous contrast material use and corticosteroid premedication in children–A survey of Society of Chairs of Radiology in Children’s Hospitals (SCORCH) Member Institutions. Pediatr Radiol 2011; 41: 1272–1283. [DOI] [PubMed] [Google Scholar]
- 25.Johanses JG. Assessment of a non-ionic contrast medium (amipaque) in the gastrointestinal tract. Invest Radiol 1978; 13: 523–527. [DOI] [PubMed] [Google Scholar]
- 26.Cova MA, Stacul F, Quaranta R, et al. Radiological contrast media in the breastfeeding woman: A Position Paper of the Italian Society of Radiology (SIRM), the Italian Society of Paediatrics (SIP), the Italian Society of Neonatology (SIN) and the Task Force on Breastfeeding, Ministry of Health. Eur Radiol 2014; 24: 2012–2022. [DOI] [PubMed] [Google Scholar]