Abstract
Background
Pneumonia is a leading cause of morbidity and mortality in children worldwide; however, its diagnosis can be challenging, especially in settings where skilled clinicians or standard imaging are unavailable. We sought to determine the diagnostic accuracy of lung ultrasound when compared to radiographically-confirmed clinical pediatric pneumonia.
Methods
Between January 2012 and September 2013, we consecutively enrolled children aged 2–59 months with primary respiratory complaints at the outpatient clinics, emergency department, and inpatient wards of the Instituto Nacional de Salud del Niño in Lima, Peru. All participants underwent clinical evaluation by a pediatrician and lung ultrasonography by one of three general practitioners. We also consecutively enrolled children without respiratory symptoms. Children with respiratory symptoms had a chest radiograph. We obtained ancillary laboratory testing in a subset.
Results
Final clinical diagnoses included 453 children with pneumonia, 133 with asthma, 103 with bronchiolitis, and 143 with upper respiratory infections. In total, CXR confirmed the diagnosis in 191 (42%) of 453 children with clinical pneumonia. A consolidation on lung ultrasound, which is our primary endpoint for pneumonia, had a sensitivity of 88.5%, specificity of 100%, and an area under-the-curve of 0.94 (95% CI 0.92–0.97) when compared to radiographically-confirmed clinical pneumonia. When any abnormality on lung ultrasound was compared to radiographically-confirmed clinical pneumonia the sensitivity increased to 92.2% and the specificity decreased to 95.2%, with an area under-the-curve of 0.94 (95% CI 0.91–0.96).
Conclusions
Lung ultrasound had high diagnostic accuracy for the diagnosis of radiographically-confirmed pneumonia. Added benefits of lung ultrasound include rapid testing and high inter-rater agreement. Lung ultrasound may serve as an alternative tool for the diagnosis of pediatric pneumonia.
Keywords: Lung ultrasound, Pediatric pneumonia, Point-of-care diagnosis
Highlights
-
•
Lung ultrasound is emerging as a promising imaging alternative for the diagnosis of pneumonia in children.
-
•
Existing studies in children are limited by small sample size, heterogeneity of populations, variable reference standards, and selection bias.
-
•
We found that lung ultrasound can be implemented at a busy healthcare center with high diagnostic accuracy and high inter-rater agreement.
-
•
Lung ultrasound was conducted without major disruptions in workflow, and it took <10 minutes to perform in most instances.
-
•
This is the largest study demonstrating high diagnostic accuracy of lung ultrasound in children for the diagnosis of pneumonia.
1. Introduction
Pneumonia is a leading cause of death in children worldwide. It is responsible for 120 million episodes and 1 million deaths each year in children aged <5 years [1], [2]. Despite a decrease in child mortality in the past 20 years, the proportion of pneumonia deaths has remained constant at approximately 20% [3]. In low resource settings, where skilled providers are not widely available, the World Health Organization (WHO) developed a case management algorithm for the treatment of pneumonia. This involved training community health workers to identify respiratory signs and symptoms, centered on cough, difficulty breathing, respiratory rate and danger signs, for diagnosis, treatment, and referral [5]. While this algorithm achieved important mortality reductions after implementation [6], multiple studies thereafter have shown that it lacks specificity [7], [8], [9]. A high false positive rate has resulted in overuse of antibiotics and improper therapy for children with acute bronchospasm that may instead require bronchodilators.
Pediatric pneumonia is a heterogeneous disease with bacterial and viral causes. Additionally, there is a large overlap with bronchiolitis and reactive airways disease. Therefore, the diagnosis of pneumonia can be challenging and requires integration of a variety of diagnostic tools [9], [10]. In low resource settings, previous studies have tested additional, non-respiratory, diagnostic features, such as fever [10] and oxygen saturation [11], [12]. But when resources are available, imaging is an important adjunct [13]. In fact, the American Academy of Pediatrics endorsed chest X-ray (CXR) as the imaging modality of choice for complicated or ambiguous cases [10]. However, CXR has its own disadvantages including radiation exposure, high inter-observer variability [14], and lack of impact on clinical outcome [15].
Lung ultrasound (LUS) is an emerging diagnostic tool for pneumonia in both adults [16] and children [17]. It also has many advantages for pediatric respiratory disease in low resource settings, including portability, rapid and repeat testing, no ionizing radiation, and ease of use [18], [19], [20]. We sought to evaluate the diagnostic accuracy of LUS as a point-of-care diagnostic tool for pediatric pneumonia in a tertiary care setting in Lima, Peru.
2. Materials and methods
2.1. Study design
Between January 2012 and September 2013, we consecutively enrolled children with respiratory symptoms in the Emergency Department, General Pediatric Wards, and Outpatient Clinics at the Instituto Nacional de Salud del Niño; a large children's hospital treating more than 170,000 children annually, in Lima, Peru. Inclusion criteria were children aged 2–59 months and the presence of respiratory symptoms. Exclusion criteria were: chronic lung disease excluding asthma, significant cardiac disease, and need for mechanical ventilation. We also enrolled children of a similar age range without respiratory complaints and acute non-respiratory illnesses (fever, vomiting or diarrhea) at the same institution. These children were usually those presenting to the hospital for well-child visits or siblings of child participants recruited into the study. We described detailed methods about study procedures elsewhere [21]. Of note, we amended the original protocol to allow for an increase in sample size. We followed STARD guidelines for the reporting of diagnostic accuracy [22]. The study was approved by the Institutional Review Board committees of the Instituto Nacional de Salud del Niño (Lima, Peru), A.B. PRISMA (Lima, Peru), and the Johns Hopkins School of Medicine (Baltimore, USA).
2.2. Clinical diagnosis
Clinical assessment included a history and physical exam performed by a pediatrician. Pediatricians on service provided a diagnosis following standard of care with input from international clinical guidelines (Table 1 ) [23], [24], [25]. An anteroposterior CXR was obtained for all children with respiratory symptoms. Lateral view CXRs were not obtained because they were not consistent with clinical practice in our setting. CXR used a scanner with 4800 × 4800 dots-per-inch resolution for CXR image digitation. Films were digitized and sent to a third party reading group of three study radiologists. We did not obtain a CXR on children without respiratory symptoms.
Table 1.
Guidelines for clinical diagnosis and definitions and grading system for lung ultrasound and chest radiograph.
| Clinical diagnoses | |
| Pneumonia | Difficulty breathing as described by tachypnea, chest indrawing, nasal flaring, or grunting; history of fever, cough, chest pain or abdominal pain; adventitious lung sounds, including rales, rhonchi, crackles, or diminished sounds. Associated CXR findings of interstitial abnormalities or consolidation as reviewed by hospital clinician or on-site radiologist at time of diagnosis [3]. Severity was assessed by presence of chest indrawing, seizures, lethargy, or unconsciousness [4]. |
| Asthma | Presence of wheeze or difficulty breathing on physical exam, history of wheeze and responsive to bronchodilators [5]. Response to bronchodilators assessed clinically without formal pulmonary function testing. |
| Bronchiolitis | Less than 18 months of age; presence of wheeze or coarse breath sounds, difficulty breathing, and viral symptoms (cough, rhinorrhea); not responsive to bronchodilators if attempted [24]. |
| Upper respiratory infection | Associated with nasal secretions, nasal congestion, sore or red throat or hoarseness without evidence of lower airways involvement (tachypnea and abnormal lung sounds on exam). |
| Lung ultrasound findings | |
| Lung sliding | Rhythmic movement of pleural line with respiration. It represents sliding of the visceral pleura against the parietal pleura. |
| A-lines | Horizontal lines equally spaced from pleural line, representing artifact generated by subpleural air, a normal finding. |
| B-lines | Vertical lines arising from pleura and extending to base of the image. |
| Pleural abnormality | Disruption of pleural line. |
| Consolidation | Hypoechoic circumscribed disruption of pleural line extending inferiorly and may contain any of the following; Air bronchogram, Shred sign, Pleural effusion. |
| Air bronchogram | Punctate or branching hyperechoic images reflecting airways made visible by surrounding fluid/inflammation |
| Shred sign | Irregular border of consolidation |
| Pleural effusion | Anechoic space between visceral and parietal pleura. |
| Sonographic grading | |
| Lung consolidation | |
| Small | Consolidation contained within one intercostal space and extending just below pleural line. |
| Large | Consolidation extends to more than 1 intercostal space, extends beyond the pleural line, and may be associated with a pleural effusion. |
| Interstitial abnormality | |
| Focal | Multiple B lines (≥3) present in single view or unilaterally. |
| Diffuse | Multiple B lines (≥3) present bilaterally. |
| Radiographic grading | |
| Lung consolidation | Alveolar pneumonia: dense, fluffy consolidation of a portion of a lobe or entire lung. Often contains air bronchograms and may be associated with pleural effusion. |
| Interstitial abnormality | Linear and patchy densities in a lacy pattern involving both lungs, featuring peri-bronchial thickening and multiple areas of atelectasis. Lung inflation is normal to increased. |
2.3. Lung ultrasound imaging
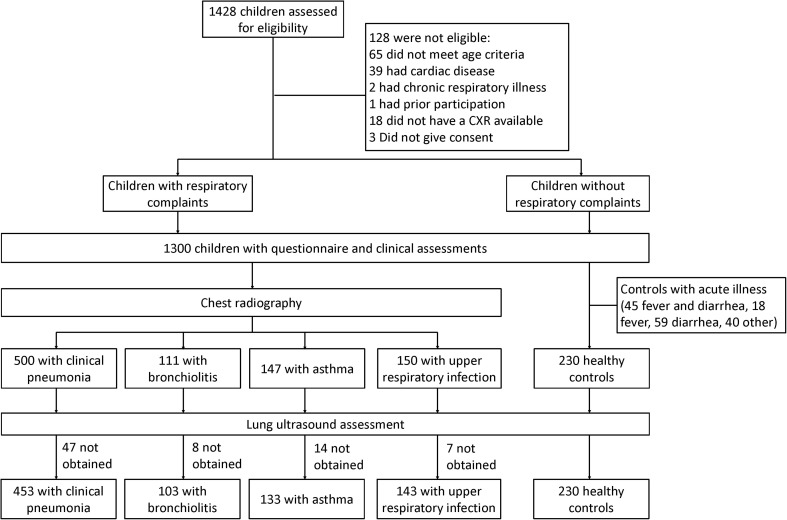
All participants underwent a complete LUS evaluation using a MicroMaxx® portable ultrasound machine (Sonosite, Bothwell, WA) with a HFL38/13-6 MHz linear transducer. This device is approximately the size of a 13” laptop computer and used at the bedside as a point-of-care tool. One of three general practitioners (LEE, MAC, and JMC) performed LUS after completing a 7-day standardized training protocol [21] based on international recommendations [26]. Both conduct and interpretation of LUS findings were performed independent of clinical evaluation or radiographic findings. LUS was conducted on almost all children who had CXR as well as on all children without respiratory symptoms (Fig. 1 ).
Fig. 1.
Flowchart of study participants.
2.4. Imaging interpretation
Interpretation CXR and LUS images was performed at a later date by three board-certified pediatric radiologists (PCC, EAM, and JB) and three general practitioners (LEE, MAC, and JMC), respectively. These groups were blinded to clinical information and results from the alternative imaging modality. We used standardized protocols for interpretation of both CXR [14] and LUS [26]. We defined radiographic pneumonia as the presence of a lobar consolidation with or without a pleural effusion (Table 1). Pneumonia on LUS was defined as the identification of artifacts consistent with lobar consolidation or patchy lobar consolidation, if it occupied more than one intercostal space in vertical view, or small consolidation with a pleural effusion (Table 1). We did not consider the isolated findings of interstitial changes on LUS or CXR as positive for pneumonia. Agreement by two out of three readers was required for final diagnosis.
2.5. Laboratory assessment
We conducted laboratory testing in a subset of participants to offer additional objective data. Complete blood counts and blood cultures were obtained in a subset of 361 and 137 children, respectively. Serum or plasma levels of procalcitonin (PCT) were measured in a convenience sample of 259 children. Batched samples were maintained at −20 °C until tested in a reference laboratory. PCT levels were measured using the Kryptor chemiluminescence immunoassay (BRAHMS, Hennigsdorf, Germany) according to manufacturer specifications. The inter-assay coefficient of variation was <10%, and the functional sensitivity of the assay was 0.06 ng/mL. Pharyngeal swabs were analyzed for viruses and bacteria in a convenience sample of 251 children. Nucleic acid extraction for viral testing was accomplished utilizing the Arrow Viral NA kit (Diasorin Inc., Stillwater, MN), while bacterial extraction utilized the MagNA Pure LC with DNA Isolation Kit I (Roche Diagnostics, Indianapolis, IN). For viral testing, respiratory virus controls were utilized in each run (NATrol Respiratory Validation Panel 3, Zeptometrix Corporation, Buffalo, NY). Reverse Transcription polymerase chain reaction/Electrospray ionization mass spectrometry (RT-PCR/ESI-MS, Abbott Molecular, Des Plaines, IL) was performed for viral and bacterial pathogens with Respiratory Virus Surveillance 2.5 kit and the Bacterial Antibiotic Candida kit. Positive detection were defined as reactions identified by RT-PCR/ESI-MS as having a Q score >0.9.
2.6. Biostatistical methods
The primary goal of our analysis was to establish the diagnostic accuracy of sonographically-confirmed pneumonia when compared to radiographically-confirmed clinical pneumonia as the reference standard. This comparison was conducted in child participants with respiratory findings that were diagnosed with clinical pneumonia by a study physician, and had both CXR and LUS conducted as part of the study. We focused on clinical pneumonia only because asthma, bronchiolitis and upper respiratory infection had low rates of consolidation on CXR. We defined positive sonographic findings as either finding a consolidation or any abnormality on LUS. Any abnormality on LUS was included to show that there was a difference in diagnostic accuracy between consolidation and any abnormality on LUS, when compared to radiographically-confirmed clinical pneumonia. Diagnostic accuracy was measured using positive predictive value (PPV), negative predictive value (NPV), sensitivity, specificity and the C-statistic as a measure of area under the Receiving Operator Characteristic curve. We analyzed continuous variables as means using analysis of variance, and categorical variables as proportions using chi-square or Fisher exact tests, as appropriate. Inter-rater agreement among readers was determined using the kappa coefficient. We conducted statistical analyses in R (www.r-project.org) and STATA version 13 (StatCorp, College Station, Texas).
3. Results
3.1. Participant characteristics
From January 2012 to September 2013, 1428 children were assessed for eligibility (Fig. 1). Of those, 1062 (74%) children underwent LUS. Upon initial assessment, 128 children were found to be ineligible due to age, and presence of cardiac or chronic respiratory disease, and 163 children without respiratory symptoms were found to have an acute non-respiratory illness upon further evaluation. Additionally, 76 ultrasounds were not obtained due to time constraints or parents refused. Median age was 19 months and 57% were male. The final clinical diagnoses were as follows: 453 had clinical pneumonia, 133 had asthma, 103 had bronchiolitis, 143 had an upper respiratory infection, and 230 were children without respiratory symptoms (Table 2 ). CXR identified abnormalities consistent with pneumonia in 360 (79%) children, and 191 (42%) were found to have consolidations and 169 (37%) had an interstitial abnormality.
Table 2.
Demographic information and clinical characteristics according to study group. Tachycardia was defined as a heart rate ≥190 beats/minute in children aged <12 months and heart rate ≥140 beats/minute in children aged ≥12 months. Tachypnea was defined as respiratory rate >50 breaths/minute for children aged 2–11 months and respiratory rate >40 breaths/minute for children aged ≥12 months.
| Characteristics | Children without respiratory symptoms (n = 230) | Pneumonia (n = 453) | Asthma (n = 133) | Bronchiolitis (n = 103) | Upper respiratory infection (n = 143) | P Value |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age in months, mean (SD) | 41.3 (15.2) | 20.0 (14.6) | 30.4 (18.2) | 10.1 (10.1) | 24.7 (16.9) | <0.001 |
| Number < 12 months (%) | 30 (13) | 168 (37) | 33 (25) | 77 (75) | 44 (31) | <0.001 |
| Number of boys (%) | 117 (51) | 258 (57) | 87 (65) | 46 (35) | 88 (62) | 0.058 |
| Clinical characteristics | ||||||
| Symptoms, n (%) | ||||||
| Cough | 0 (0) | 453 (100) | 130 (98) | 103 (100) | 138 (97) | <0.001 |
| Difficulty breathing | 0 (0) | 416 (92) | 118 (89) | 91 (88) | 71 (50) | <0.001 |
| Rhinorrhea | 0 (0) | 376 (83) | 107 (80) | 83 (81) | 123 (86) | <0.001 |
| Fever | 0 (0) | 348 (77) | 69 (52) | 57 (55) | 55 (38) | <0.001 |
| Diarrhea | 0 (0) | 96 (21) | 19 (14) | 23 (22) | 16 (11) | <0.001 |
| Chest indrawing, n (%) | 0 (0) | 203 (45) | 48 (36) | 48 (46) | 1 (1) | <0.001 |
| Temperature, mean (SD) | 36.4 (0.4) | 36.9 (0.7) | 36.9 (0.6) | 36.8 (0.6) | 36.7 (0.6) | <0.001 |
| Number with ≥38.0 °C (%) | 1 (0) | 48 (11) | 9 (7) | 8 (8) | 8 (6) | <0.001 |
| Heart Rate | ||||||
| Infants, mean (SD) | 122 (14) | 138 (17) | 127 (19) | 135 (17) | 133 (15) | |
| Children, mean (SD) | 107 (10) | 129 (18) | 127 (17) | 129 (18) | 114 (16) | |
| Number with tachycardia (%) | 3 (1) | 89 (20) | 22 (17) | 7 (7) | 8 (6) | <0.001 |
| Respiratory Rate | ||||||
| Infants, mean (SD) | 29 (4) | 45 (11) | 46 (13) | 44 (13) | 35 (11) | |
| Children, mean (SD) | 23 (3) | 39 (11) | 36 (11) | 42 (13) | 27 (6) | |
| Number with tachypnea (%) | 1 (0) | 203 (45) | 43 (32) | 42 (41) | 13 (9) | <0.001 |
| Oxyhemoglobin Saturation (SpO2) %, mean (SD) | 99 (1) | 96 (3) | 96 (3) | 97 (2) | 99 (2) | |
| Number with SpO2 <95% (%) | 1 (0) | 47 (10) | 18 (14) | 7 (7) | 1 (1) | |
| Number with SpO2 <92% (%) | 1 (0) | 175 (39) | 51 (38) | 23 (22) | 10 (7) | <0.001 |
| Auscultation findings, n (%) | ||||||
| Normal | 230 (100) | 2 (0) | 1 (1) | 0 (0) | 74 (52) | <0.001 |
| Wheezes | 0 (0) | 200 (44) | 100 (75) | 65 (63) | 8 (6) | <0.001 |
| Crackles | 0 (0) | 335 (74) | 53 (40) | 50 (49) | 7 (5) | <0.001 |
| Decreased breath sounds | 0 (0) | 80 (19) | 13 (10) | 4 (4) | 1 (1) | <0.001 |
| Rhonchi | 0 (0) | 157 (35) | 42 (32) | 46 (45) | 9 (6) | <0.001 |
3.2. Inter-reader agreement
For LUS, kappa coefficients for agreement among readers was 0.65 (95% CI 0.61–0.66) overall, 0.73 (0.70–0.74) for a normal lung ultrasound, 0.38 (0.27–0.41) for interstitial abnormalities, and 0.77 (0.75–0.78) for lung consolidation. Medium and large consolidation findings yielded a kappa coefficient of 0.87 (0.86–0.89) and small consolidation yielded a kappa coefficient of 0.77 (0.74–0.81). For CXR, kappa coefficients for agreement among readers was 0.37 (95% CI 0.34–0.40) overall, 0.40 (0.37–0.42) for normal chest radiograph, 0.20 (0.16–0.23) for interstitial abnormalities, and 0.51 (0.48–0.58) for lung consolidation.
3.3. Imaging characteristics
A total of 1062 ultrasounds were available for review. Among children without respiratory symptoms, none had lung consolidation, and 11 (1%) had an interstitial abnormality. Of the 832 participants with respiratory symptoms, 414 (50%) had a sonographic lung consolidation and 530 (64%) had any abnormality (Table 3 ). On average, LUS took less than 10 min to perform; 5.5 ± 2.1 min in controls compared to 8.2 ± 3.1 min in children with pneumonia (p < 0.001). We summarized characteristics of LUS findings in Table 2. A total of 832 CXRs were available for review. Of these, 221 (26%) had a lobar consolidation and 264 (32%) had interstitial abnormality.
Table 3.
Lung ultrasound diagnosis and characteristics.
| Final ultrasound diagnosis | Children without Respiratory Symptoms (n = 230) | Pneumonia (n = 453) | Asthma (n = 133) | Bronchiolitis (n = 103) | Upper respiratory infection (n = 143) | P Value |
|---|---|---|---|---|---|---|
| Procedure time in minutes, mean (SD) | 5.5 (2.1) | 8.2 (3.1) | 7.2 (2.5) | 7.5 (2.6) | 5.9 (4.2) | <0.001 |
| Normal ultrasound, n (%) | 219 (95) | 93 (21) | 67 (50) | 36 (35) | 107 (75) | <0.001 |
| Large Consolidation | 0 (0) | 127 (28) | 3 (2) | 8 (8) | 0 (0) | <0.001 |
| Shred sign | 0 (0) | 24 (19) | 0 (0) | 2 (25) | 0 (0) | 0.81 |
| Air Bronchogram | 0 (0) | |||||
| Pleural Abnormalities | 0 (0) | 23 (18) | 2 (67) | 3 (38) | 0 (0) | 0.03 |
| Pleural Effusion | 0 (0) | 22 (17) | 1 (33) | 1 (13) | 0 (0) | 0.64 |
| Interstitial Findings | 0 (0) | |||||
| Small Consolidation | 0 (0) | 175 (39) | 46 (35) | 41 (40) | 15 (10) | <0.001 |
| Shred sign | 0 (0) | 35 (20) | 0 (0) | 5 (12) | 7 (47) | <0.001 |
| Air Bronchogram | 0 (0) | |||||
| Pleural Abnormalities | 0 (0) | 59 (34) | 18 (19) | 14 (34) | 2 (13) | 0.34 |
| Pleural Effusion | 0 (0) | 1 (1) | 1 (2) | 0 (0) | 0 (0) | 0.60 |
| Interstitial Findings | 0 (0) | |||||
| Focal Interstitial syndrome | 9 (4) | 43 (9) | 8 (6) | 10 (10) | 13 (9) | 0.09 |
| Diffuse Interstitial syndrome | 1 (0) | 9 (2) | 5 (4) | 6 (6) | 4 (3) | 0.02 |
| Pleural abnormalities only | 1 (0) | 6 (1) | 4 (3) | 2 (2) | 4 (3) | 0.26 |
3.4. Diagnostic performance
Consolidation on lung ultrasound had a sensitivity of 88.5%, specificity of 100%, and AUC of 0.94 (95%CI 0.92–0.97) when compared with radiographically-confirmed clinical pneumonia (Table 4 ). Any abnormality on lung ultrasound had a sensitivity of 92.2%, specificity of 95.2%, and AUC of 0.94 (95% CI 0.91–0.96) with compared with radiographically-confirmed clinical pneumonia (Table 5 ).
Table 4.
Definitions and values for analysis of diagnostic Accuracy.
| True positive | True negative | Test | Total population | True positive | True negative | False positive | False negative |
|---|---|---|---|---|---|---|---|
| Radiographically-confirmed clinical pneumonia | Children without Respiratory Symptoms | Consolidation on lung ultrasound | 421 | 169 | 230 | 0 | 22 |
| Radiographically-confirmed clinical pneumonia | Children without Respiratory Symptoms | Any abnormality on lung ultrasound | 421 | 176 | 219 | 11 | 15 |
Table 5.
Analysis of diagnostic Accuracy.
| Analysis | PPV | NPV | Sensitivity | Specificity | AUC (95% CI) | |
|---|---|---|---|---|---|---|
| Radiographically-Confirmed Clinical Pneumonia vs. finding a consolidation on lung ultrasound | 100 | 91.3 | 88.5 | 100 | 0.94 | (0.92–0.97) |
| Radiographically-Confirmed Clinical Pneumonia vs. finding any abnormality on lung ultrasound | 94.1 | 93.6 | 92.2 | 95.2 | 0.94 | (0.91–0.96) |
3.5. Laboratory findings
We summarized differences in ancillary laboratory data by final diagnosis status (Table 6 ). Procalcitonin levels supported our clinical definitions of pneumonia and bronchiolitis, with highest levels in children with pneumonia. Children with bronchiolitis were more likely to have respiratory syncytial virus isolated on a pharyngeal swab when compared to other groups. Streptococcus pneumoniae was present in over half of all participants, with no difference found between children with or without respiratory symptoms. There were no differences in demographic variables like age and sex between those with and without ancillary laboratory data (data not shown).
Table 6.
Laboratory evaluation in subset of participants.
| Children without respiratory symptoms | Pneumonia | Asthma | Bronchiolitis | Upper respiratory infection | P value | |
|---|---|---|---|---|---|---|
| Blood cell count | ||||||
| Sample size | 54 | 138 | 54 | 49 | 66 | |
| Hemoglobin (mg/dl), mean (SD) | 10.9 (1.3) | 10.6 (1.3) | 10.8 (1.7) | 10.8 (1.4) | 10.7 (1.4) | 0.79 |
| Number with <11 mg/dL (%) | 26 (49) | 56 (41) | 26 (48) | 19 (39) | 29 (44) | 0.74 |
| Platelets x 103/L, mean (SD) | 420 (136) | 414 (146) | 405 (124) | 460 (130) | 421 (136) | 0.28 |
| White blood cells x 103/L, mean (SD) | 11.9 (5.5) | 11.7 (5.1) | 13.8 (6.7) | 13.0 (6.0) | 13.1 (5.0) | 0.08 |
| Number with >17 × 103/L (%) | 8 (15) | 20 (15) | 13 (24) | 7 (15) | 16 (25) | 0.26 |
| Throat swabs result | ||||||
| Sample size | 32 | 32 | 41 | 46 | 50 | |
| Respiratory virus, n (%) | ||||||
| Any positive virus | 4 (13) | 29 (35) | 18 (44) | 23 (50) | 11 (22) | 0.002 |
| Viral etiology | ||||||
| Respiratory Synctitial Virus | 0 (0) | 12 (18) | 8 (26) | 14 (38) | 4 (9) | <0.001 |
| Adenovirus | 3 (10) | 6 (10) | 6 (21) | 1 (4) | 4 (9) | 0.45 |
| Parainfluenza 1-3 | 0 (0) | 5 (9) | 2 (8) | 5 (18) | 1 (3) | 0.07 |
| Metapneumovirus | 1 (3) | 5 (9) | 1 (4) | 2 (8) | 1 (3) | 0.77 |
| Influenza virus | 0 (0) | 2 (4) | 1 (4) | 0 (0) | 2 (5) | 0.79 |
| Coronavirus | 0 (0) | 0 (0) | 0 (0) | 2 (8) | 0 (0) | 0.04 |
| Bacteria and Candida | ||||||
| Any positive | 32 (100) | 79 (96) | 36 (88) | 42 (91) | 50 (100) | 0.03 |
| Microorganism etiology | ||||||
| Streptococcus pneumoniae | 18 (56) | 52 (63) | 27 (66) | 30 (65) | 33 (66) | 0.91 |
| Candida albicans | 2 (6) | 6 (7) | 2 (5) | 9 (20) | 7 (14) | 0.11 |
| Haemophilus influenzae | 1 (3) | 6 (7) | 0 (0) | 2 (4) | 2 (4) | 0.45 |
| Moraxella catarrhalis | 1 (3) | 2 (2) | 0 (0) | 1 (2) | 1 (2) | 0.89 |
| Neisseria meningitidis | 1 (3) | 1 (1) | 1 (2) | 1 (2) | 0 (0) | 0.80 |
| Procalcitonin, ng/ml | ||||||
| Sample size | 28 | 113 | 50 | 27 | 41 | |
| Median (IQR) | 0.01 (0.00–0.03) | 0.14 (0.06–0.55) | 0.06 (0.04–0.08) | 0.06 (0.04–0.09) | 0.06 (0.04–0.11) | <0.001 |
| Geometric mean (95% CI) | 0.03 (0.03–0.04) | 0.19 (0.15–0.25) | 0.07 (0.06–0.09) | 0.07 (0.05–0.09) | 0.08 (0.06–0.10) | <0.001 |
4. Discussion
Our study supports the use of point-of-care lung ultrasound as a reliable and accurate method of diagnosing radiographically-confirmed clinical pediatric pneumonia. LUS averaged less than 10 min to perform with high inter-reader agreement. Moreover, there is a high diagnostic accuracy between identification of consolidation on LUS and radiographically-confirmed clinical pneumonia in children.
Two recent meta-analyses by our group also demonstrated high accuracy of LUS for the diagnosis of pneumonia in adults [16] and children [17], however, these analyses were limited by small sample sizes and heterogeneity among studies, decreasing the overall power. Moreover, five of the eight studies focused on hospitalized or critically ill children [28], [30], [32] or neonates [29], [31], and therefore lacked generalizability in an outpatient or emergency room setting. Additionally, sonographers read the LUS at time of clinical evaluation and therefore were not blinded to clinical information.
Our study has several strengths and contributes novel and important information. We enrolled >1000 children aged under five years and performed all testing in a busy tertiary care hospital in a resource-limited setting, suggesting that LUS can be realistically implemented in high-volume clinical settings without major disruptions to work flow. To our knowledge, this study is the first to examine reproducibility of LUS between multiple readers, using a standardized approach for the conduct of LUS. We provided specific details of sonographic findings, separating minimal pleural abnormalities and interstitial B-lines from large consolidations. Furthermore, we performed LUS evaluation on participants with a breadth of diverse respiratory pathologies including a group of children without respiratory symptoms. This differs from prior research that only focused on pneumonia without including healthy children or those with other respiratory disease processes, with the exception of Caiulo et al. who also studied bronchiolitis [33]. While not part of the reference standard for diagnosis, ancillary laboratory testing provided additional confidence to our diagnoses. Procalcitonin has previously been shown to be elevated in bacterial disease [34] and was highest in children with radiographically-confirmed pneumonia when compared to children who did not. The proportion of children with respiratory syncytial virus was greatest among children with bronchiolitis than among those with pneumonia, which is consistent with previous epidemiologic studies [24]. However, a limitation to the use of pharyngeal swabs is evident because there we did not find a no difference in the prevalence of S. pneumoniae between children with or without respiratory symptoms.
Our study has some potential shortcomings. First, we did not have computerized tomography (CT) scan on all children with respiratory findings but standard of care would have not directed the use of CT scans to diagnose pneumonia in children. We used radiographically-confirmed clinical pneumonia as our gold standard, despite its limitations, which we believe is the safest alternative to the current gold-standard. As such, we were unable to arbitrate differences between LUS and CXR findings. LUS cannot detect consolidations that do not reach the pleura, whereas small consolidations (<1 intercostal space) observed on LUS cannot be visualized on CXR [27]. Nonetheless, the clinical relevance of small consolidations of LUS remains uncertain. Moreover, retrocardiac opacifications or consolidations may not be easily visualized on antero-posterior CXRs when compared to LUS. Second, we did not have information on the time course or severity of illness. Specifically, we did not follow the clinical course and had imaging only at the time of enrollment. Third, LUS may not be able to differentiate between small alveolar abnormalities and atelectasis. While Lichtenstein et al. described that, in adults, dynamic air bronchograms were more likely to occur in pneumonia whereas static air bronchograms were more likely to occur in atelectasis [35], recognition of dynamic air bronchograms is difficult in children because of their body size. Fourth, overall sensitivity of LUS for the diagnosis of radiographically-confirmed pneumonia was lower than previously reported [15], [16]. However, this may be in part due to selection bias in previous studies where participants may have been more likely to have severe pneumonia. It might also be due to probe placement in our study: we did not consistently utilize transverse views, which may improve the diagnostic yield in children [17]. Fifth, the study physician conducting the ultrasound was not blinded to visible clinical symptoms, which may induce them to spend longer conducting LUS on children that appear more ill. Sixth, the kappa for agreement for CXR in our study was lower that what is previously found [36], [37]. This may be due lack of adherence to the WHO guidelines for reading CXRs, poor quality of the CXR and variability of respiratory illness in our study population.
LUS is a promising tool that offers portability and diagnostic accuracy in resource-limited settings. It has the added benefits of rapid testing and appears to be easily taught. These results offer promise and may help improve the specificity of case management algorithms for the diagnosis of pediatric pneumonia. Future research should focus on prospective randomized trials using LUS as part of the case management algorithm for acute lower respiratory infections. Moreover, with prospective follow-up, we may better understand the evolution of LUS findings over time and determine whether early diagnosis using LUS may translate into clinically meaningful outcomes. Through increased development and production of high performance, portable devices, and standardized training modules, LUS could be a cost-effective solution to improve the diagnosis and management of pediatric pneumonia in appropriate settings worldwide.
Funding
Funding for this study and support for Laura Ellington was provided in part by the Doris Duke Charitable Foundation Clinical Research Fellowship. William Checkley was further supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health.
Contributorship
Laura Ellington contributed equally to the study design, was responsible for the conduct of the study, supervision of data gathering and ultrasound, participated in sonographic grading, contributed to the analysis and interpretation, and was responsible for writing the manuscript. Robert Gilman contributed equally to the study design, contributed equally to the analysis and interpretation, and contributed to writing the manuscript. Miguel Chavez was responsible for data analysis, was equally responsible of supervision of data collection and conduct of lung ultrasound, participated in sonographic grading, contributed equally to interpretation of findings, and contributed to writing the manuscript. Farhan Pervaiz contributed equally to data analysis and writing of the manuscript. Julio Marin-Concha was equally responsible supervision of data gathering and ultrasound, participated in sonographic grading, and contributed to writing of manuscript. Patricia Compen-Chang was responsible for radiographic analysis for chest X-rays, and contributed to writing the manuscript. Stefan Riedel was responsible for procalcitonin expertise, and contributed to writing the manuscript. Shalim Rodriguez was responsible ultrasound protocol and training, and contributed to writing the manuscript. Charlotte Gaydos was responsible for etiological analysis and interpretation and contributed to writing the manuscript. Justin Hardick was equally responsible for etiological analysis and interpretation, and contributed to writing the manuscript. James Tielsch provided expert guidance during the conduct of the study, and contributed to writing the manuscript. Mark Steinhoff provided expert guidance during the conduct of the study, and contributed to writing the manuscript. Jane Benson was responsible for radiographic analysis for chest X-rays, and contributed to writing the manuscript. Evelyn May was responsible for radiographic analysis for chest X-rays, and contributed to writing the manuscript. Dante Figueroa-Quintanilla was responsible for the conduct of the study, and contributed to writing the manuscript. William Checkley conceived the study design, contributed equally to analysis and interpretation was also responsible for writing the manuscript, and had ultimate oversight over study conduct, analysis and interpretation of results.
Disclosures
Authors have no competing interests to disclose.
Acknowledgements
Additional support was provided by Asociacion Benefica PRISMA, Instituto Nacional de Salud del Niño, and collaborators at Johns Hopkins University, Cincinnati Children's Hospital and Hospital Nacional Eduardo Rebagliati Martins.
References
- 1.Liu L., Johnson H., Cousens S. Global, regional, and national causes of child mortality in 2000-2010: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Walker C.L., Rudan I., Liu L. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izadnegahdar R., Cohen A.L., Klugman K.P. Qazi S a. Childhood pneumonia in developing countries. Lancet Respir. Med. 2013;1(7):574–584. doi: 10.1016/S2213-2600(13)70075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch T., Bialy L., Kellner J.D. A systematic review on the diagnosis of pediatric bacterial pneumonia: when gold is bronze. PLoS One. 2010;5(8):e11989. doi: 10.1371/journal.pone.0011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pio A. Standard case management of pneumonia in children in developing countries: the cornerstone of the acute respiratory infection programme. Bull. World Health Organ. 2003;81(4):298–300. [PMC free article] [PubMed] [Google Scholar]
- 6.Sazawal S., Black R.E. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect. Dis. 2003;3(9):547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 7.Hazir T., Qazi S., Nisar Y.B. Assessment and management of children aged 1-59 months presenting with wheeze, fast breathing, and/or lower chest indrawing; results of a multicentre descriptive study in Pakistan. Arch. Dis. Child. 2004;89(11):1049–1054. doi: 10.1136/adc.2003.035741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazir T., Bin Nisar Y., Abbasi S. Comparison of oral amoxicillin with placebo for the treatment of world health organization-defined nonsevere pneumonia in children aged 2-59 months: a multicenter, double-blind, randomized, placebo-controlled trial in Pakistan. Clin. Infect. Dis. 2011;52(3):293–300. doi: 10.1093/cid/ciq142. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . 2004. Consultative Meeting to Review Evidence and Research Priorities in the Management of Acute Respiratory Infections (ARI): Meeting Report. [Google Scholar]
- 10.Bradley J.S., Byington C.L., Shah S.S. Executive summary: the management of community acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines the Pediatric Infectious Diseases Society and the infectious diseases society of America. Clin. Infect. Dis. 2011;53(7):617–630. doi: 10.1093/cid/cir625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madico G., Gilman R.H., Jabra A. The role of pulse oximetry: its use as an indicator of severe respiratory disease in Peruvian children living at sea level. Arch. Pediatr. Adolesc. Med. 1995;149(11):1259. doi: 10.1001/archpedi.1995.02170240077012. [DOI] [PubMed] [Google Scholar]
- 12.McCollum E.D., King C., Deula R. Pulse Oximetry for children with pneumonia treated as outpatients in rural Malawi. Bull. World Health Organ. 2016;94(12):893–902. doi: 10.2471/BLT.16.173401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley J.S., Byington C.L., Shah S.S. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011;53(7):e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherian T., Mulholland E.K., Carlin J.B. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ. 2005;83(5):353–359. [PMC free article] [PubMed] [Google Scholar]
- 15.Cao A., Choy J., Mohanakrishnan L., Bain R., Van Driel Ml. Chest radiographs for acute lower respiratory tract infections ( Review ) Cochrane Collab. 2013;12 doi: 10.1002/14651858.CD009119.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chavez M., Shams N., Ellington L.E. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir. Res. 2014;15(1):50. doi: 10.1186/1465-9921-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereda M.A., Chavez M.A., Hooper-Miele C. Lung ultrasound for diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135(4):714–722. doi: 10.1542/peds.2014-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah S., Noble V.E., Umulisa I. Development of an ultrasound training curriculum in a limited resource international setting: successes and challenges of ultrasound training in rural Rwanda. Int. J. Emerg. Med. 2008;1(3):193–196. doi: 10.1007/s12245-008-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah S.P., Epino H., Bukhman G. Impact of the introduction of ultrasound services in a limited resource setting: rural Rwanda 2008. BMC Int. Health Hum. Rights. 2009;9:4. doi: 10.1186/1472-698X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedetti G., Gargani L., Corbisiero a. Evaluation of ultrasound lung comets by hand-held echocardiography. Cardiovasc Ultrasound. 2006;4:34. doi: 10.1186/1476-7120-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellington L.E., Gilman R.H., Tielsch J.M. Computerised lung sound analysis to improve the specificity of paediatric pneumonia diagnosis in resource-poor settings: protocol and methods for an observational study. BMJ Open. 2012;2(1):e000506. doi: 10.1136/bmjopen-2011-000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bossuyt P.M., Reitsma J.B., Bruns D.E. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326(7379):41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Community Acquired Pneumonia Guideline Team . 2005. Cincinnati Children's Hospital Medical Center. Evidence-based Care Guideline for Children with Community Acquired Pneumonia in Children 60 Days to 17 Years of Age.http://www.cincinnatichildrens.org/WorkArea/DownloadAsset.aspx?id=87957 [Google Scholar]
- 24.Village G. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 25.Bateman E.D., Hurd S.S., Barnes P.J. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2008;31(1):143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 26.Volpicelli G., Elbarbary M., Blaivas M. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 27.Shah V.P., Tunik M.G., Tsung J.W. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013;167(2):1–7. doi: 10.1001/2013.jamapediatrics.107. [DOI] [PubMed] [Google Scholar]
- 28.Reali F., Sferrazza Papa G.F., Carlucci P. Can lung ultrasound replace chest radiography for the diagnosis of pneumonia in hospitalized children? Respiration. 2014;88:112–115. doi: 10.1159/000362692. [DOI] [PubMed] [Google Scholar]
- 29.Liu J., Liu F., Liu Y., Wang H.-W., Feng Z.-C. Lung ultrasound for the diagnosis of severe neonatal pneumonia. Chest. 2014;146(2):383–388. doi: 10.1378/chest.13-2852. [DOI] [PubMed] [Google Scholar]
- 30.Esposito S., Papa S.S., Borzani I. Performance of lung ultrasonography in children with community-acquired pneumonia. Ital. J. Pediatr. 2014;40:37. doi: 10.1186/1824-7288-40-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seif El, Dien H.M., Abd Ellatif D. a K. The value of bedside Lung Ultrasonography in diagnosis of neonatal pneumonia. Egypt J. Radiol. Nucl. Med. 2013;44(2):339–347. [Google Scholar]
- 32.Iuri D., De Candia a, Bazzocchi M. Evaluation of the lung in children with suspected pneumonia: usefulness of ultrasonography. Radiol. Med. 2009;114(2):321–330. doi: 10.1007/s11547-008-0336-8. [DOI] [PubMed] [Google Scholar]
- 33.Caiulo V.A., Gargani L., Caiulo S. Lung ultrasound in bronchiolitis: comparison with chest X-ray. Eur. J. Pediatr. 2011;170(11):1427–1433. doi: 10.1007/s00431-011-1461-2. [DOI] [PubMed] [Google Scholar]
- 34.Esposito S., Tagliabue C., Picciolli I. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respir. Med. 2011;105(12):1939–1945. doi: 10.1016/j.rmed.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Copetti R., Cattarossi L. Ultrasound diagnosis of pneumonia in children. Radiol. Med. 2008;113(2):190–198. doi: 10.1007/s11547-008-0247-8. [DOI] [PubMed] [Google Scholar]
- 36.Elemraid M.A., Muller M., Spencer D.A. Accuracy for the interpretation of chest radiographs for the diagnosis of paediatric pneumonia. PLoS One. 2014;9(8):e106051. doi: 10.1371/journal.pone.0106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben Shimol S., Dagan R., Givon-Lavi N. Evaluation of the World Health Organization criteria for chest radiographs for pneumonia diagnosis in children. Eur. J. Pediatr. 2012;171(2):369–374. doi: 10.1007/s00431-011-1543-1. [DOI] [PubMed] [Google Scholar]