Abstract
Homologous recombination plays a central role in the repair of double-strand DNA breaks, the restart of stalled replication forks and the generation of genetic diversity. Regulation of recombination is essential since defects can lead to genome instability and chromosomal rearrangements. Strand exchange is a key step of recombination – it is catalysed by RecA in bacteria, Rad51/Dmc1 in eukaryotes and RadA in archaea. RadB, a paralogue of RadA, is present in many archaeal species. RadB has previously been proposed to function as a recombination mediator, assisting in RadA-mediated strand exchange. In this study, we use the archaeon Haloferax volcanii to provide evidence to support this hypothesis. We show that RadB is required for efficient recombination and survival following treatment with DNA-damaging agents, and we identify two point mutations in radA that suppress the ΔradB phenotype. Analysis of these point mutations leads us to propose that the role of RadB is to act as a recombination mediator, which it does by inducing a conformational change in RadA and thereby promoting its polymerisation on DNA.
Keywords: Homologous recombination, Archaea, RecA-family recombinase, Strand exchange, Recombination mediator
1. Introduction
Homologous recombination (HR) plays a central role in the repair of DNA double-strand breaks and the generation of genetic diversity in meiosis or conjugation – HR functions may also contribute to the restart of stalled DNA replication forks. Although HR is critical for cell viability, it can pose significant risks if improperly regulated. Suboptimal HR can result in inaccurate repair of DNA damage and accumulation of mutations. Conversely, excessive HR can result in DNA rearrangements. Several genetic diseases that are linked to increased cancer risk are associated with defects in HR regulation. These include Bloom’s syndrome and Werner’s syndrome, which are characterised by increased levels of recombination due to defective RecQ family helicases that function at different stages of HR [1], [2].
The central step of HR is strand exchange, which is catalysed by RecA-family recombinases: RecA in bacteria, Rad51/Dmc1 in eukaryotes and RadA in archaea. Deletion of recombinase genes leads to defects in HR and an increased sensitivity to DNA-damaging agents [3], [4]. The first stage of HR initiated at a DNA end is 5′-3′ end resection, which produces single-stranded DNA (ssDNA) onto which the recombinase protein polymerises. Activation of the recombinase is carried out by recombination mediators as described below. Nucleoprotein filaments consisting of recombinase and ssDNA then bind to double-strand DNA (dsDNA) molecules and search for a region of homology. When homology is found, the recombinases catalyse strand invasion and D-loop formation [5].
In vivo, ssDNA produced by end resection is coated with the single-strand DNA binding protein, termed SSB (in bacteria) or RPA (in eukaryotes and most archaea). The binding of SSB/RPA protects ssDNA from secondary structure formation and degradation, and is an important stage of HR. However, SSB/RPA poses a barrier to recombinase filament formation, since these proteins compete with recombinases for DNA binding.
Recombination mediators are a class of proteins required for efficient HR, which may assist in recombinase nucleoprotein filament formation by overcoming the inhibition imposed by SSB/RPA. They also play a role in stabilising nucleoprotein filaments. Deletion of mediator genes leads to defects in recombination and DNA repair. Examples of bacterial recombination mediators include Rec(F)OR, a complex that assists in the loading of RecA onto ssDNA [6], and RecX and DinI, which stabilise the RecA nucleoprotein filament [7], [8]; the bacterial Sms recombination modulator was recently shown to stimulate the branch migration phase of RecA-mediated strand transfer [9]. Eukaryotic recombination mediators include BRCA2 in humans, and Rad52 and Rad55-Rad57 heterodimer in yeast, all of which assist in the displacement of RPA and loading of Rad51 onto ssDNA [10], [11]. Rad55-Rad57 in yeast has also been shown to play a role in stabilising Rad51-DNA filaments from disassembly by the anti-recombinase Srs2 [12]. The balance between these two processes is thought to be a key regulatory step in controlling the initiation of HR. Recent work has shown that the Rad51 paralogue RFS-1 from Caenorhabditis elegans functions as a recombination mediator in combination with a partner protein, RIP-1 [13]. RFS-1/RIP-1 is proposed to stimulate HR by remodelling the Rad51 presynaptic filament into a more flexible structure that is less prone to disassembly by helicases.
Two archaeal recombination mediators have been identified, both are paralogues of RadA. SsoRal1 is found in Sulfolobus solfataricus and has been shown to stimulate RadA-mediated strand exchange in vitro by enhancing RadA binding to ssDNA [14]. RadB, which is found only in members of the phylum Euryarchaeota, has been proposed to function as a recombination mediator [15]. Genetic evidence has shown that deletion of radB increases the DNA damage sensitivity of Haloferax volcanii [16]. Furthermore, RadA and RadB from Pyrococcus furiosus have been shown to interact in vitro [17]. Therefore, RadB has been suggested to play a role in promoting HR, similar to the yeast Rad51 paralogue Rad55-57.
In this study, we elucidate the role of H. volcanii RadB in HR. We show that RadA and RadB interact in vivo, confirming previous in vitro results. We show that RadB is required for normal cellular growth, efficient HR and survival following treatment with DNA-damaging agents. Most significantly, we identify two point mutations in radA that suppress the ΔradB phenotype. The location and identity of these two amino acid substitutions leads us to propose that RadB induces a conformational change in RadA and thereby promotes its polymerisation on DNA.
2. Materials and methods
2.1. Strains and plasmids
Haloferax volcanii strains are shown in Table 1, plasmids used for gene deletion and protein overexpression in Table 2 and oligonucleotides in Table 3. Growth and transformation of H. volcanii, isolation of genomic and plasmid DNA, and construction of deletion mutants was carried out as described [18]. Protein over-expression strains were constructed by transformation with episomal overexpression plasmids as described [19]. Strains expressing tagged proteins at native levels were constructed by gene replacement as described [18].
Table 1.
H. volcanii strains.
| Strain | Genotype | Derivation | Use |
|---|---|---|---|
| H26 | ΔpyrE2 | [18] | Standard laboratory strain |
| H64 | ΔpyrE2 radBΔb/b | H26 pTA62 | Partial deletion of radB |
| H187 | ΔpyrE2 radBΔb/b Δhjc | H64 Δhjc | hjc deletion in radBΔb/b background |
| H188 | ΔpyrE2 radBΔb/b Δhjc radA-A196V | H187 radA-A196V | Spontaneous radA-A196V in radBΔb/b background |
| H195 | ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 | [16] | Background for recombination assays |
| H284 | ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 ΔradB | [16] | radB deletion strain |
| H388 | ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 ΔradA:trpA+ | H195 pTA324 | radA deletion, pTA411 also used |
| H724 | ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 ΔradB radA-A196V | H284 pTA769 | radA-A196V in a ΔradB background |
| H769 | ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 radA-A196V | H724 pTA311 | radA-A196V strain |
| H1309 | ΔpyrE2 radBΔb/b radA-S101P | H64 EMS | EMS-induced radA-S101P in radBΔb/b background |
| H1424 | ΔpyrE2 ΔhdrB Δmrr Nph-pit cdc48-Ct | [26] | Background for protein expression |
| H1428 | ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 ΔradB radA-S101P | H284 pTA1289 | radA-S101P in ΔradB background |
| H1439 | ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 radA-S101P | H195 pTA1289 | radA-S101P strain |
| H1450 |
ΔpyrE2 ΔhdrB Δmrr Nph-pit cdc48-Ct {p.tnaA:his6tag-radB+ pyrE2+ hdrB+} |
H1424 pTA1043 | Overexpression of His-tagged RadB |
| H1466 |
ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 ΔradB radA+:[radA-S101P-A196V pyrE2+] |
H284 pTA1314 | Integration of pTA1314, radA-S101P-A196V not viable |
| H1681 | ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 ΔradB ΔradA:trpA+ | H284 pTA324 | radA radB deletion, pTA411 also used |
| H2047 | ΔpyrE2 ΔtrpA Δmrr Nph-pit cdc48-Ct | H1424 pTA95 [18] | Protein expression strain, ΔtrpA |
| H2378 |
ΔpyrE2 ΔtrpA Δmrr Nph-pit cdc48-Ct ΔradB:trpA+ |
H2047 pTA1539 | radB deletion in protein expression strain |
| H3041 |
ΔpyrE2 ΔtrpA Δmrr Nph-pit cdc48-Ct his7tag-2xStrepIItag-radB+ |
H2378 pTA1847 | Expression of His-tagged RadB at native level |
| H3117 |
ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 ΔradB radA+:[radA-S101A pyrE2+] |
H284 pTA1868 | Integration of pTA1868, radA-S101A not viable in ΔradB background |
| H3231 | ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 radA-S101A | H195 pTA1868 | radA-S101A strain |
| H3264 |
ΔpyrE2 ΔhdrB ΔtrpA bgaHa-Bb leuB-Ag1 radA-S101A radB+:[ΔradB:trpA+ pyrE2+] |
H3231 pTA1539 | Integration of pTA1539, ΔradB not viable in radA-S101A background |
Table 2.
Plasmids.
| Plasmid | Relevant properties | Derivation |
|---|---|---|
| pGB70 | Integrative plasmid based on pUC19, with pyrE2 marker | [36] |
| pTA50 | pBluescript II with Eco47III-XmaI chromosomal fragment containing radB | [16] |
| pTA62 | pGB70 with radBΔb/b partial deletion, generated by excision of BstBI-BstEII fragment of radB from pTA50 | This study |
| pTA131 | Integrative plasmid based on pBluescript II, with pyrE2 marker | [18] |
| pTA163 | Integrative plasmid containing leuB-Aa2 allele, for use in recombination assay | [20] |
| pTA289 | pTA131 with ΔradB construct | [16] |
| pTA311 | pTA131 with radB+, generated by insertion of KpnI-BspEI fragment of pTA50 containing radB | This study |
| pTA324 | pTA131 with ΔradA:trpA+ construct | [25] |
| pTA409 | Shuttle vector based on pBluescript II, with pyrE2 and hdrB markers and ori-pHV1 origin | [25] |
| pTA411 | pTA409 with radA+ gene, for complementation of ΔradA | [25] |
| pTA769 | pTA131 with radA-A196V, generated by PCR of KpnI-BstBI radA-A196V fragment from H188 | This study |
| pTA963 | Overexpression vector with p.tnaA promoter, 6xHis tag, pyrE2 and hdrB markers, and pHV2 origin | [19] |
| pTA1043 | pTA963 with radB, for overexpression of 6xHis-tagged RadB | [19] |
| pTA1289 | pTA131 with radA-S101P, generated from pTA769 by replacement with AgeI-BstEII radA-S101P fragment from H1309 | This study |
| pTA1314 | pTA131 with radA-S101P-A196V, generated from pTA1289 by replacement with AflIII fragment from pTA769 | This study |
| pTA1539 | pTA131 with ΔradB:trpA+ construct, generated by PCR of XhoI-BamHI fragment of upstream flanking region and BamHI-XbaI fragment of downstream flanking region from pTA50, with insertion of trpA+ BamHI fragment of pTA298 [20] | This study |
| pTA1771 | pTA131 with insertion of his7tag-2xStrepIItag cassette at EcoRV site in multiple cloning site, features NdeI site upstream of 7xHis tag and PciI site downstream of 2xStrepII tag | This study |
| pTA1815 | pTA1771 with insertion of FatI-BamHI radB+ fragment of pTA1043, at PciI and BamHI sites | This study |
| pTA1847 | pTA1539 with replacement of ΔradB:trpA+ by NdeI-BamHI fragment of pTA1815 with his7tag-2xStrepIItag-radB+ allele | This study |
| pTA1868 | pTA131 with radA-S101A, generated from pTA1289 by replacement with AgeI-BstEII PCR fragment using radAS101Aint primers | This study |
Table 3.
Oligonucleotides
| Primer | Sequence (5′–3′) | Relevant properties | Plasmid |
|---|---|---|---|
| RADAF | GGggATCCGTGGGACTAACCGCGCTCGCCCGTCGTGCCTG | Amplification of radA | pTA769 pTA1289 |
| RADAR | CGTCGGAtcCCAGCGTTACCCCCACGTCGCCGTCG | Amplification of radA | pTA769 pTA1289 |
| pradAF | TATCGCCCTTGAATCTCCGCAC | Introduction of S101A point mutation in radA | pTA1868 |
| pradARTF | GACGATACGCTTGTCGCCC | Introduction of S101A point mutation in radA | pTA1868 |
| radAS101AintF | CGCAGgCgATCACCGAGGTGTACGG | Introduction of S101A point mutation in radA | pTA1868 |
| radAS101AintR | GTGATcGcCTGCGTTTCGAGACGCG | Introduction of S101A point mutation in radA | pTA1868 |
| dradBBamR | CGGTGGAtcCTGACTCTGTCACGTCAGG | radB deletion, upstream | pTA1539 |
| dradBXhoF | CGGTCTCGagGCGGACCGTTAGGCAGCCG | radB deletion, upstream | pTA1539 |
| dradBdsBamF | AAAAGGGaTCcACGCGGCCGGGGAGACG | radB deletion, downstream | pTA1539 |
| dradBdsXbaR | CCGGTCTAgaAGGGCGAAAAACAGTACGG | radB deletion, downstream | pTA1539 |
| 7His2xStrepF | caTATGCACCACCACCACCACCACCACGGCACGTCGGGCTGGTCGCACCCGCAGTTCGAGAAGGGCGGCTCGGGCTGGTCGCACCCGCAGTTCGAGAAGGGCGGCGAcatgt | his7tag-2xStrepIItag cassette | pTA1771 |
| 7His2xStrepR | aCATGTCGCCGCCCTTCTCGAACTGCGGGTGCGACCAGCCCGAGCCGCCCTTCTCGAACTGCGGGTGCGACCAGCCCGACGTGCCGTGGTGGTGGTGGTGGTGGTGCAtatg | his7tag-2xStrepIItag cassette | pTA1771 |
2.2. Growth curves
Growth curves of 250 μl cultures were performed in 48-well plates at 45 °C, with continuous double-orbital shaking at 425 rpm, using a BioTek Epoch2 microplate spectrophotometer. Optical density at 600 nm was measured every 15 min. Generation time was calculated between A600 values of 0.08–0.16.
2.3. Recombination assays
Plasmid × chromosome recombination assays were carried out as described [20].
2.4. Bioinformatic analyses
Primer design, and DNA and protein sequence analysis were performed using MacVector (MacVector Inc.). Predicted hydrophobicity indices were calculated using the Kyte-Doolittle scale [21]. Sequence alignments were performed using ClustalW [22] (Gonnet Series, open gap penalty of 10.0, extended gap penalty of 0.2, Delay Divergent value of 30%). Pfu RadA protein structure (1PZN) was obtained from Protein Data Bank (www.rcsb.org/pdb) and analysed using MacPyMOL (DeLano Scientific) [23].
2.5. Random mutagenesis
EMS (ethyl methane sulphonate, Sigma) was used for random mutagenesis as described [24] with the following modifications. Strains were grown in Hv-YPC broth to an A650 of 0.2, EMS was added to 3.5 μl/ml and mixed by gentle vortexing. Cells were incubated for 2 h at 45° C with rotation, washed twice with 18% salt water and resuspended in 1 ml of Hv-YPC broth. The culture was incubated overnight at 45° C with rotation, followed by plating on Hv-YPC agar. Plates were incubated for 5 days.
2.6. DNA damage assays
Assays for sensitivity to UV light and mitomycin C were carried out as described [20], [25].
2.7. Protein overexpression and purification
Protein (over)-expression and purification by metal-affinity chromatography (IMAC) was carried out as described previously [26] with the following modifications: cells were resuspended in buffer (2 M NaCl, 20 mM HEPES pH 7.5, 20 mM imidazole) containing 1× SigmaFAST protease inhibitor (Sigma) in replacement of 1 mM phenylmethanesulfonyl fluoride; lysate was incubated with the Ni2+ charged beads for 1 h at 4 °C; bound proteins were eluted in 4 column volumes (CV) of buffer containing 100 mM imidazole in place of 500 mM imidazole.
2.8. Mass spectrometry
Mass spectrometry of excised protein bands was carried out as described [19]. Details of protein identification are given in Supplementary Tables 1 and 2.
3. Results
3.1. RadA and RadB interact in vivo
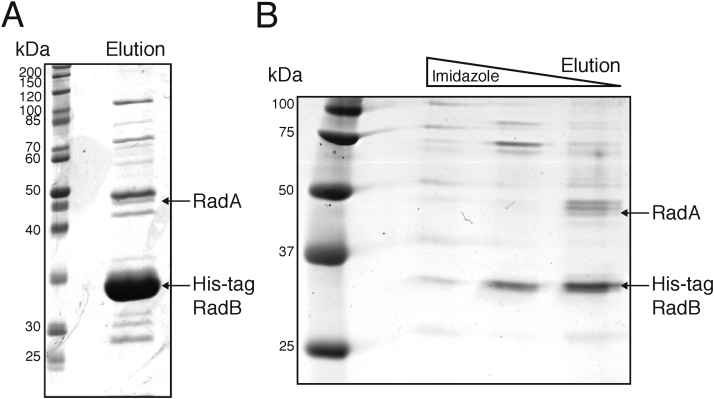
RadB from P. furiosus has been shown by co-immunoprecipitation to interact with RadA in vitro [17]. To test whether RadB and RadA from H. volcanii interact in vivo, cell lysate from strains over-expressing His-tagged RadB (or RadA) was purified by metal-affinity chromatography (IMAC). Tagged and co-purifying proteins were eluted and analysed by SDS-PAGE, and bands of interest identified by mass spectrometry (Supplementary Table 1). In agreement with previous studies, RadA was found to co-purify with His-tagged RadB (Fig. 1A). RadB was not found to co-purify with His-tagged RadA (data not shown), but intracellular levels of RadB are known to be low; in P. furiosus, levels of RadB have been shown to be approximately 200 times lower than the levels of RadA [17]. Therefore, the method used here may not be sensitive enough [17].
Fig. 1.
(A) RadA co-purifies with His-tagged RadB, which was over-expressed in H. volcanii (H1450) and purified by metal affinity chromatography (IMAC). Other proteins identified also purified from the parental strain H1424 containing an empty vector, which was used as a control for non-specific binding to the IMAC column [26]. (B) RadA also co-purifies with His-tagged RadB expressed in H. volcanii (H3041) at native levels. For mass spectrometry data, see Supplementary Tables 1 and 2.
To validate this interaction, a strain was generated where His-tagged RadB is expressed at native levels. A radB allele encoding His-tagged RadB was placed under control of the radB promoter and used to replace the wild-type (untagged) radB at its chromosomal locus. RadA was found by mass spectrometry to co-purify with natively-expressed His-tagged RadB, which had been purified by IMAC (Fig. 1B, Supplementary Table 2). Therefore, RadA and RadB interact in vivo, suggesting that they function together.
3.2. RadB is required for efficient DNA repair by HR
It has been shown that strains deleted for radA are completely deficient in recombination [4]. We examined the effect of radB deletion by carrying out a plasmid × chromosome recombination assay (Supplementary Fig. S1). The ΔradB mutant exhibited a recombination frequency of approximately 1.8% of wild-type (3.39 × 10−6 vs. 2.97 × 10−4 transformants per μg DNA per cell, respectively). Therefore, RadB is not essential for recombination (in contrast to RadA) but its presence dramatically improves the efficiency of this process.
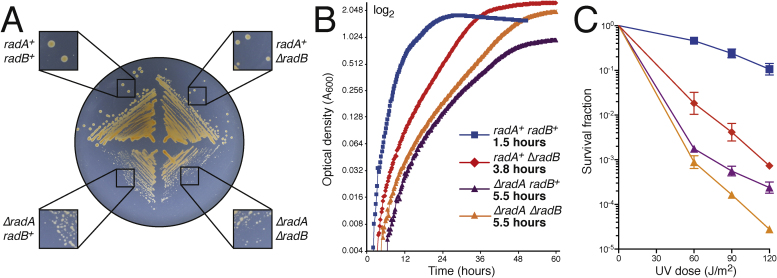
We have previously shown that strains deleted for radB have a growth defect and are sensitive to UV radiation [16]. Strains deleted for radA also show a growth defect and DNA-damage sensitivity [4]. To study the relationship between RadA and RadB, a ΔradA ΔradB strain was generated. We confirmed that strains deleted for either radA or radB have a growth defect (compared to wild-type), and that ΔradA strains have a more severe defect (Fig. 2A and B). The ΔradA ΔradB double mutant shows a similar growth defect to the ΔradA single mutant, therefore radA is epistatic to radB. This suggests that with respect to cellular growth, the primary role of RadB is in HR.
Fig. 2.
(A) Both ΔradA (H388) and ΔradB (H284) strains have a growth defect compared to wild-type (H195). The double mutant (H1681) has a similar growth defect to the ΔradA mutant. (B) The growth defect of ΔradA and ΔradB strains is also seen in broth; the double mutant has the same growth defect as the ΔradA mutant. Data was plotted on a log2 scale, generation time in exponential phase is shown in bold. (C) Both ΔradB and ΔradA strains are more sensitive to UV-irradiation than wild-type. The double mutant shows a similar sensitivity to the ΔradA mutant. Survival is relative to an unirradiated control. Each data point is an average of ≥3 independent repeats; standard error is shown.
Strains deleted for radA or radB have been shown to be sensitive to DNA-damaging agents [4], [16]. The ΔradA ΔradB double mutant was irradiated with UV light and its sensitivity compared to the single mutants (Fig. 2C). The ΔradA strain is more sensitive than the ΔradB strain, and at higher UV doses the ΔradA ΔradB strain is slightly more sensitive than the ΔradA mutant. This suggests that with respect to the repair of UV-induced lesions, RadB acts primarily in HR but may play a minor secondary role in another repair pathway.
3.3. Isolation of ΔradB suppressors
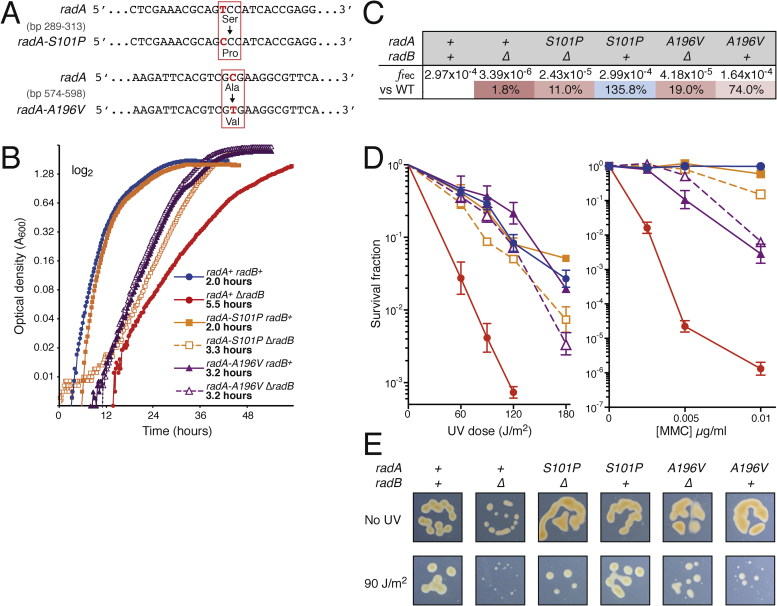
A significant insight into the role of RadB was gained from two point mutations that suppress the ΔradB phenotype; both are in the radA gene. The first of these to be identified, radA-A196V, was isolated as a spontaneous mutant based on improved growth of a ΔradB parent. Sequencing revealed a single point mutation in radA (Fig. 3A), a cytosine to thymidine transition at nucleotide 588 that results in an alanine to a valine substitution at amino acid 196. This mutation was confirmed as the ΔradB suppressor by replacing wild-type radA+ with radA-A196V in a ‘clean’ ΔradB background (Supplementary Fig. S2).
Fig. 3.
(A) Base substitutions in radA that result in radA-S101P and radA-A196V. (B) ΔradB strains have a growth defect in broth compared to wild-type, and radA-S101P (H1428) or radA-A196V (H724) suppress this defect. Data was plotted as in Fig. 2B. (C) Both radA-S101P and radA-A196V alleviate the recombination defect of ΔradB strains. Recombination frequency (frec) was measured using the assay shown in Supplemental Fig. S1. Transformants per μg DNA per cell was calculated as an average of ≥3 independent repeats; percentages indicate recombination frequency compared to wild-type. D) Both radA-S101P and radA-A196V suppress the DNA damage defect of ΔradB. Survival following DNA damage (UV, left. MMC, right) is calculated relative to an unirradiated control, see panel B for key. Each data point is an average of ≥3 independent repeats; standard error is shown. E) Strains expressing radA-A196V recover more slowly than strains expressing radA-S101P after UV-irradiation. Cultures were spotted onto complete media and treated with 90 J/m2 of UV (or no UV as a control); colony size was observed after 5 days. All spots are 10−5 dilution except for the irradiated ΔradB, which is 10−2.
The second point mutation was isolated by treating a ΔradB parent with the mutagen ethylmethane sulphonate (EMS) and screening for faster-growing colonies. This point mutation is a thymidine to cytosine transition at nucleotide 301 that results in a serine to proline substitution at amino acid 101 of RadA (Fig. 3A). The ΔradB suppressor was confirmed by introducing the same point mutation into a ‘clean’ ΔradB background that had not been subjected to mutagenesis (Supplementary Fig. S2).
3.4. RadA-S101P and RadA-A196V suppress ΔradB to differing degrees
Suppression of ΔradB by either radA-S101P or radA-A196V was measured by growth rate, recombination rate, and survival following DNA damage. Both radA-S101P and radA-A196V alleviate the growth defect associated with ΔradB to a considerable degree (Fig. 3B). The generation times of the ΔradB radA-S101P and ΔradB radA-A196V strains were 3.3 and 3.2 h, respectively, which is a marked improvement on ΔradB (5.5 h) but not as fast as wild-type (2.0 h). The presence of RadB in the radB+ radA-A196V strain did not lead to any further improvement in growth, but in the radB+ radA-S101P strain the presence of RadB restored the generation time to wild-type levels (2.0 h).
Both radA-S101P and radA-A196V alleles suppress the recombination defect associated with ΔradB (Fig. 3C), but the recombination frequencies were still lower than those seen in wild-type (11% of wild-type for radA-S101P and 19% for radA-A196V). For both alleles, the presence of RadB elevated the recombination frequency. Strains expressing both RadA-S101P and RadB have a recombination frequency above that of wild-type (135.8%), but in strains expressing RadA-A196V and RadB the level is below wild-type (74%). Therefore, radA-S101P and radA-A196V alleviate the recombination defect associated with ΔradB to differing extents.
Following irradiation with UV light, both radA-S101P and radA-A196V alleviate the DNA damage sensitivity conferred by ΔradB (Fig. 3D), and this was to the same extent for both mutations. When RadB is present in combination with these alleles, the UV-sensitivity was comparable to wild-type. After treatment with mitomycin C (MMC, DNA crosslinking agent), both radA-S101P and radA-A196V alleviate the DNA damage sensitivity conferred by ΔradB (Fig. 3D). In contrast to UV, survival of ΔradB strains after MMC treatment differ between the radA-S101P and radA-A196V alleles; these differences are also seen when RadB is present.
We noticed when monitoring UV sensitivity that ΔradB colonies are substantially smaller than those of unirradiated controls (Fig. 3E), indicating that the recovery of UV survivors is delayed. A delayed recovery from UV-induced damage was also seen in ΔradB strains expressing RadA-S101P or RadA-A196V. Strains expressing both RadA-A196V and RadB showed a greater delay in UV recovery than ΔradB radA-A196V strains, but this was not seen in strains expressing both RadA-S101P and RadB.
The suppression conferred by radA-S101P could be due to the presence of proline or the absence of serine (the latter is a common site of post-translational modification). To distinguish these possibilities, we attempted to generate a ΔradB strain containing a radA-S101A allele. This strain had a severe growth defect (worse than ΔradA) and could not be propagated. However, in a background containing wild-type RadB, the radA-S101A allele did not confer a growth defect. This indicates the absence of serine at residue 101 of RadA cannot suppress the growth defect associated with ΔradB (in fact, alanine it makes it worse), and suggests that the presence of a proline is most likely critical for suppression.
We also attempted to generate a ΔradB strain combining both radA-S101P and radA-A196V alleles. However, this strain had a severe growth defect (worse than ΔradA) and could not be propagated. This indicates that the presence of RadA-S101P-A196V is more detrimental than the complete absence of RadA.
3.5. Structural consequences of RadA-S101P and RadA-A196V
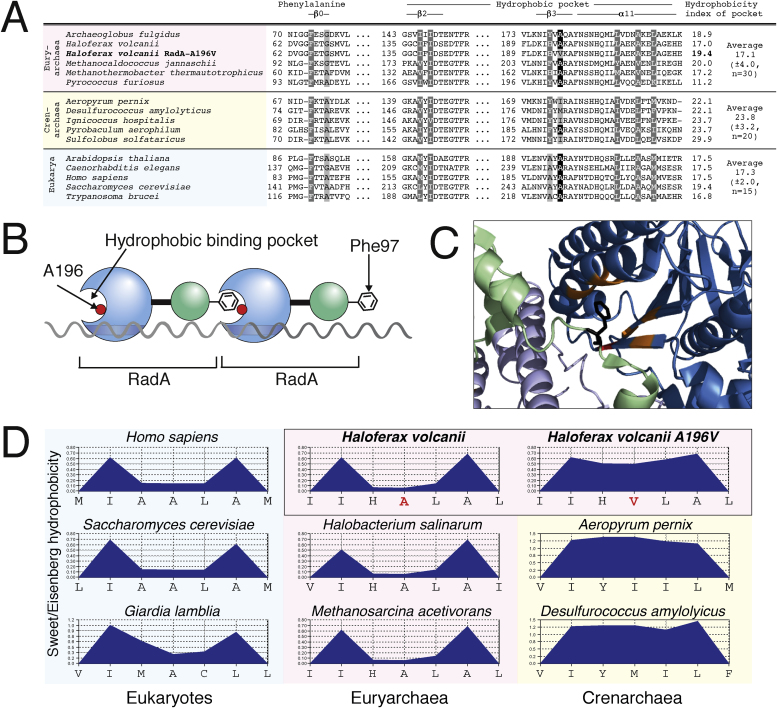
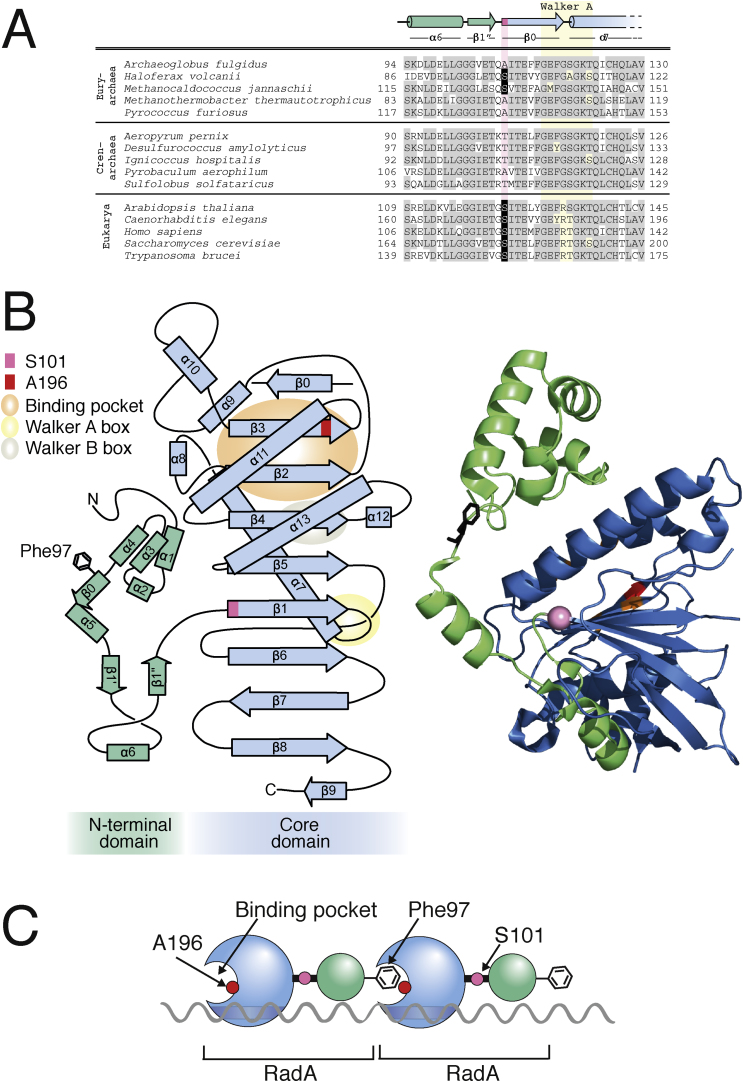
Amino acid residues corresponding to S101 and A196 in H. volcanii RadA are conserved in Euryarchaeota and eukaryotes, but not in Crenarchaeota. Since RadB is found only in Euryarchaeota (and not in Crenarchaeota), the conservation of H. volcanii RadA-S101 and A196 in archaea correlates with the presence of RadB. There is currently no crystal structure for H. volcanii RadA, therefore the corresponding residues were mapped onto P. furiosus RadA ([23], PDB number 1PZN). The equivalent residues to H. volcanii RadA-S101 and A196 in P. furiosus are RadA-A132 and A203, respectively (Figs. 4 A and 5 A ).
Fig. 4.
(A) Sequence alignment of the region of RadA/Rad51 containing the seven residues that comprise the hydrophobic socket (grey); shown are euryarchaeal (pink), crenarchaeal (yellow) and eukaryotic (blue) species. The residue equivalent to H. volcanii A196 is highlighted in black, it is conserved in euryarchaea and eukaryotes but not in crenarchaea. (B) RadA monomers polymerise by insertion of an invariant phenylalanine into a hydrophobic pocket of an adjacent monomer [23]. (C) Crystal structure of Pyrococcus furiosus (Pfu) RadA showing two RadA monomers (light green/blue and dark green/blue, respectively). Shown are the (core) ATPase domain (blue), N-terminal domain (green), hydrophobic binding pocket (orange), Pfu Ala203 (Hvo Ala 196) (red) and Pfu Phe97 (Hvo Phe66) (black). Crystal structure obtained from PDB (1PZN) [23]. D) Predicted hydrophobicity indices for the binding pocket of eukaryotic (blue), euryarchaeal (pink) and crenarchaeal (yellow) RadA/Rad51. Plots and overall average hydrophobicity were calculated using the Sweet/Eisenberg scale with a moving window of 3. The binding pocket of H. volcanii RadA-A196V (top row, right) has a higher predicted hydrophobicity than wild-type RadA (top row, centre), resembling the crenarchaeal binding pocket.
Fig. 5.
(A) H. volcanii RadA-S101 is conserved in euryarchaea and eukaryotes, but not crenarchaea. Sequence alignment of the RadA/Rad51 region containing H. volcanii RadA-S101. The equivalent residues for H. volcanii RadA-S101 are highlighted in pink and conserved serine highlighted in black. RadA from euryarchaea and crenarchaea, and Rad51 from eukaryotes are shown. Labelled at the top are the locations of conserved ß-sheets and α-helices [23]; the Walker A motif is shown in yellow. (B) Topology map (left) and crystal structure (right) of RadA monomer (adapted from Ref. [23]). The core domain is coloured blue and the N-terminal domain green. S101 is located at the joint between these two domains, just after a linker loop. (C) Schematic of RadA polymerisation via a ball and socket mechanism.
Archaeal RadA and eukaryotic Rad51 are conserved on a structural level. They consist of a core ATPase domain containing Walker A and B motifs for ATP binding and hydrolysis (respectively), and an N-terminal domain. Polymerisation occurs by the insertion of an invariant phenylalanine (Phe-96 in H. volcanii) located in the N-terminal domain of one monomer into a binding pocket of an adjacent monomer [23] (Fig. 4B). This binding pocket consists of seven surface-exposed hydrophobic residues (Fig. 4C), and monomer:monomer interactions are driven by hydrophobic interactions. Replacement of the invariant phenylalanine of human RAD51 with glutamic acid abolishes RAD51 polymerisation on ssDNA [27]. Hvo-RadA-A196 maps to the hydrophobic binding pocket implicated in RadA monomer:monomer interactions [23]. Valine and alanine are similar in size but valine is more hydrophobic. Substitution of valine for alanine in RadA-A196V increases the predicted hydrophobicity of the H. volcanii RadA pocket by 12% (Fig. 4D), which may result in stronger interactions between RadA monomers.
Hvo-RadA-S101 is located at the N-terminal end of the ß-1 sheet (Fig. 5B). This residue is at a joint between the two domains of RadA and this region has previously been described as an “elbow” [23]. Substitution of a proline in this position could induce a kink in this region of RadA – proline imposes constraints on the protein backbone and is commonly found in turns. This would alter the orientation of the N-terminal domain and may facilitate the polymerisation of RadA (Fig. 5C).
4. Discussion
4.1. RadA and RadB interact, and RadB acts in HR
By protein co-purification we show that H. volcanii RadA and RadB interact in vivo (Fig. 1). This is in agreement with previous observations in P. furiosus showing such an interaction in vitro [17]. As expected, RadB was found to play a critical role in HR. Deletion of radB leads to growth defects and sensitivity to DNA damage (Fig. 2), and reduces the level of recombination to 1.8% of wild-type. But in contrast to RadA [4], [16], RadB is not essential for HR. Therefore, RadA is able to carry out strand exchange by itself, but with greatly reduced efficiency. This supports the hypothesis that RadB functions as a recombination mediator [15].
The double ΔradA ΔradB mutant is slightly more sensitive to UV radiation than either single mutant. This synthetic defect suggests that RadB plays an additional role in DNA repair. Due to the complete abolition of HR in a ΔradA strain, it can be inferred that this additional role is not in recombination. In P. furiosus, RadB interacts with PolD1, the small subunit of DNA polymerase D [28] and in Pyrococcales, radB is located in an operon with polD1. Perhaps RadB plays a minor role in DNA replication.
4.2. radA-S101P and radA-A196V suppress ΔradB
Two suppressors of ΔradB were isolated. Both radA-S101P and radA-A196V mutations alleviate the ΔradB phenotype in terms of growth, recombination and DNA repair (Fig. 3). Since the extent of suppression differs between the two alleles, we propose that they act in different ways. For example, radA-S101P alleviates the ΔradB growth defect to a greater extent than radA-A196V. Both mutations suppress the UV sensitivity of a ΔradB strain equally, but there are minor differences in survival following MMC treatment. This suggests a difference in the ability of the RadA variants to repair inter-strand DNA crosslinks, or to restart stalled replication forks. Both mutations alleviate the recombination defect of a ΔradB strain, although only to 11% and 19% of wild-type (radA-S101P and radA-A196V, respectively). Since the growth rate of the ΔradB radA-S101P mutant is identical to wild-type, this suggests that only a limited level of HR is required for normal cellular growth.
4.3. Model for suppression by mutant RadA
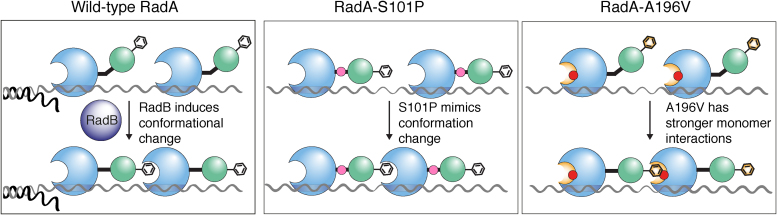
The two suppressor mutations are located in different regions of RadA (Fig. 5) and we propose that they act in different ways. Hvo-RadA-S101 is located at an “elbow” between the core ATPase domain and the N-terminal domain of RadA [23]. Substituting a proline for serine in RadA-S101P could induce a kink in the “elbow” region of RadA, altering the orientation of the N-terminal domain and thereby facilitating the polymerisation of RadA monomers. By contrast, substitution of alanine for serine in RadA-S101A was only possible in a strain containing RadB. This suggests that RadB stimulates HR by altering the conformation of RadA, and that RadA-S101P is already in an active conformation for polymerisation (Fig. 5); conversely, RadA-S101A is in a conformation that is refractory to polymerisation.
Hvo-RadA-A196 maps to the hydrophobic binding pocket implicated in RadA polymerisation, which is driven by hydrophobic interactions [23]. Substitution of valine for alanine in RadA-A196V increases the predicted hydrophobicity of the RadA pocket, which is likely to result in stronger RadA:RadA interactions. This suggests that RadB plays a role in stabilising RadA filaments, and that RadA-A196V no longer requires RadB due to greater filament stability (Fig. 6). It is noteworthy that euryarchaea and eukaryotes have a similar predicted hydrophobicity of their RadA/Rad51 binding pocket (Fig. 4D), but crenarchaea, which do not have RadB, have a higher predicted hydrophobicity. This suggests that recombination mediators in eukaryotes and euryarchaea, such as RadB, may have a common mode of action.
Fig. 6.
Model for RadB action. Wild-type RadA is not in the correct conformation for polymerisation and RadB is required. RadA-S101P is already in the correct conformation for polymerisation and does not require RadB. RadA-A196V has stronger hydrophobic interactions between monomers and does not require RadB.
If the two suppressors act in different ways, then a synergistic effect would be expected if they were combined. This would explain why we were unable to propagate a strain expressing radA-S101P-A196V. If RadA-S101P is already in an active conformation and RadA-A196V has stronger monomer:monomer interactions, then RadA-S101P-A196V filaments would polymerise rapidly due to S101P and be very stable due to A196V. This would result in slower dissociation from ssDNA, blocking the downstream processing of recombination intermediates. Kim et al. have recently shown that mutations that increase the recombination capacity of E. coli RecA have a detrimental effect on cellular growth [29]. This is due to the variant RecA filaments forming a barrier to replication and transcription.
4.4. Possible models for RadB activity
Previous studies have shown that mutant forms of the RecA-family recombinase can partially suppress defects associated with deletion of recombinase mediator genes, just as we have found for suppression of ΔradB defects by RadA-S101P or RadA-A196V. In E. coli, the mutant RecA730 protein can be loaded in the absence of RecFOR mediator because it is more proficient than the wild-type RecA in competition with SSB for ssDNA binding [30], [31]. In Saccharomyces cerevisiae, mutations in Rad51 that suppress the requirement for Rad55/57 mediators map to one of the DNA-binding sites of Rad51, thereby stabilising Rad51-DNA filaments and facilitating the displacement of RPA from ssDNA [32]. In both cases, the suppressor mutations improve ability of recombinase protein to bind DNA.
We propose that RadB induces a conformational change in RadA to facilitate efficient polymerisation. RadA polymerisation involves the insertion of an invariant phenylalanine into a hydrophobic socket of an adjacent monomer [23]. Based on our analysis of H. volcanii RadA-S101P, we propose that RadA monomers normally exist in an inactive form, where the phenylalanine is orientated away from the binding pocket of an adjacent monomer. RadB is required to alter the confirmation of RadA, thereby activating it (Fig. 6). Galkin et al. also proposed that the N-terminal domain of RadA/Rad51 undergoes a conformational change between the active (extended) and inactive (compressed) form, with only the extended form able to carry out strand exchange [33]. The authors propose that in the inactive form, the ATP binding site is rotated out of the filament, and that the N-terminal domain indirectly activates the filament by altering the conformation of the ATP binding site. By contrast, we propose that a conformation change directly activates RadA by relocating the phenylalanine in the correct position for polymerisation.
In naturally-competent bacteria, DprA binds cooperatively to ssDNA and activates RecA for nucleoprotein filament formation [34]. The ability to alter the conformation of recombinase filaments has also been observed for the recombination mediator RFS-1 from C. elegans [13]. Taylor et al. found that RFS-1/RIP-1 altered the conformation of RAD51, resulting in more flexible Rad51 filaments that facilitate the search for homologous sequences. RFS-1/RIP-1 does not appear to play a role in nucleating Rad51 filament formation in C. elegans (instead, BRCA2 plays this role). By contrast, we propose that RadB from H. volcanii can carry out this initial nucleation step. It is worth noting that no BRCA2 homologs or other recombination mediators have been identified in H. volcanii and therefore RadB may have evolved to perform multiple roles.
We expect RadA-A196V monomers to exist in a ‘wild-type’ conformation, since an alanine to a valine substitution in the binding pocket is unlikely to affect the structure of RadA. Instead, RadA-A196V is expected to have stronger hydrophobic monomer:monomer interactions (due to an increase in the hydrophobicity of the binding pocket), resulting in more stable RadA-A196V filaments. However, RadA filaments that are excessively stable would be unable to dissociate efficiently, and would block the downstream processing steps. This is consistent with our observation that strains expressing both RadA-A196V and RadB exhibit a delayed recovery from UV-irradiation, and suggests that RadB acts to stabilise RadA filaments. In yeast Rad55-Rad57 mediator complex has been shown to stabilise Rad51 filaments and counter the activity of the helicase Srs2 [12].
4.5. Comparison of genetic data on RadB with biochemical studies
Our genetic data shows that RadB is involved in HR and most probably assists in RadA polymerization. Biochemical evidence, however, does not necessarily support this hypothesis. RadB from P. furiosus was shown to inhibit RadA-mediated strand exchange [17] and the authors propose that this is due to RadB having a higher DNA binding affinity than RadA. We suggest an alternative explanation: P. furiosus RadB did not function as expected due to a missing protein co-factor. Eukaryotic recombination mediators such as human BRCA2 function as part of a multi-subunit complex [35], and C. elegans RFS-1 functions with its partner, RIP-1 [13]. Rad55 and Rad57 from S. cerevisiae function as a heterodimer, and Rad51 paralogues from higher eukaryotes function as heterodimers or tetramers.
We attempted to study the biochemical activity of wild-type and mutant H. volcanii RadA but were unable to purify RadA that is functional with respect to DNA binding and strand exchange (data not shown). This may be due to the difficulty of working with halophilic proteins, which require 2 M salt for activity. Alternatively, the reaction might be missing an essential protein co-factor. RadB was included in our strand exchange reactions but it might not be acting alone as a recombination mediator. To develop this study further, it will be necessary to identify all the interacting partner proteins of RadA and RadB in H. volcanii.
In conclusion, we provide evidence that RadB acts as a recombination mediator in H. volcanii. We propose that RadB induces a conformational change in RadA, allowing it to efficiently polymerise on ssDNA. Given the parallels between our findings and work in C. elegans [13], we expect that eukaryotic mediators might function in a similar manner.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Funding information
We are grateful to the Royal Society for a University Research Fellowship (516002.K5687) awarded to Thorsten Allers. This work was supported by the Wellcome Trust (grant number GR062124MF), and the Biotechnology and Biological Sciences Research Council (BBSRC) / Engineering and Physical Sciences Research Council (EPSRC) Synthetic Biology Research Centre Nottingham (grant number BB/L013940/1), through a PhD studentship awarded to Nathan Jones. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Bob Lloyd, Dasha Ausiannikava, Ed Bolt and Stéphane Delmas for helpful comments on the manuscript, and Laura Mitchell for help with strain construction.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dnarep.2017.04.005.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Bernstein K.A., Gangloff S., Rothstein R. The RecQ DNA helicases in DNA repair. Annu. Rev. Genet. 2010;44:393–417. doi: 10.1146/annurev-genet-102209-163602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monnat R.J., Jr. Human RECQ helicases: roles in DNA metabolism, mutagenesis and cancer biology. Semin. Cancer Biol. 2010;20(5):329–339. doi: 10.1016/j.semcancer.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark A.J., Margulies A.D. Isolation and characterization of recombination-deficient mutants of Escherichia coli K12. PNAS. 1965;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods W.G., Dyall-Smith M.L. Construction and analysis of a recombination-deficient (radA) mutant of Haloferax volcanii. Mol. Microbiol. 1997;23(4):791–797. doi: 10.1046/j.1365-2958.1997.2651626.x. [DOI] [PubMed] [Google Scholar]
- 5.McEntee K., Weinstock G.M., Lehman I.R. Initiation of general recombination catalyzed in vitro by the recA protein of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 1979;76(6):2615–2619. doi: 10.1073/pnas.76.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai A., Cox M.M. RecFOR and RecOR as distinct RecA loading pathways. J. Biol. Chem. 2009;284(5):3264–3272. doi: 10.1074/jbc.M807220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardenas P.P., Carrasco B., Defeu Soufo C., Cesar C.E., Herr K., Kaufenstein M. RecX facilitates homologous recombination by modulating RecA activities. PLoS Genet. 2012;8(12):e1003126. doi: 10.1371/journal.pgen.1003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renzette N., Gumlaw N., Sandler S.J. DinI and RecX modulate recA-DNA structures in Escherichia coli K-12. Mol. Microbiol. 2007;63(1):103–115. doi: 10.1111/j.1365-2958.2006.05496.x. [DOI] [PubMed] [Google Scholar]
- 9.Cooper D.L., Lovett S.T. Recombinational branch migration by the RadA/Sms paralog of RecA in Escherichia coli. Elife. 2016:5. doi: 10.7554/eLife.10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiyama T., Kowalczykowski S.C. Rad52 protein associates with replication protein A (RPA)-single-stranded DNA to accelerate Rad51-mediated displacement of RPA and presynaptic complex formation. J. Biol. Chem. 2002;277(35):31663–31672. doi: 10.1074/jbc.M203494200. [DOI] [PubMed] [Google Scholar]
- 11.Liu J., Doty T., Gibson B., Heyer W.D. Human BRCA2 protein promotes RAD51 filament formation on RPA-covered single-stranded DNA. Nat. Struct. Mol. Biol. 2010;17(10):1260–1262. doi: 10.1038/nsmb.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Renault L., Veaute X., Fabre F., Stahlberg H., Heyer W.D. Rad51 paralogues Rad55-Rad57 balance the antirecombinase Srs2 in Rad51 filament formation. Nature. 2011;479(7372):245–248. doi: 10.1038/nature10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor M.R., Spirek M., Chaurasiya K.R., Ward J.D., Carzaniga R., Yu X. Rad51 paralogs remodel pre-synaptic Rad51 filaments to stimulate homologous recombination. Cell. 2015;162(2):271–286. doi: 10.1016/j.cell.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham W.J., Rolfsmeier M.L., Haseltine C.A. An archaeal RadA paralog influences presynaptic filament formation. DNA Repair (Amst) 2013;12(6):403–413. doi: 10.1016/j.dnarep.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haldenby S., White M.F., Allers T. RecA family proteins in archaea: RadA and its cousins. Biochem. Soc. Trans. 2009;37(Pt. 1):102–107. doi: 10.1042/BST0370102. [DOI] [PubMed] [Google Scholar]
- 16.Guy C.P., Haldenby S., Brindley A., Walsh D.A., Briggs G.S., Warren M.J. Interactions of RadB, a DNA repair protein in archaea, with DNA and ATP. J. Mol. Biol. 2006;358(1):46–56. doi: 10.1016/j.jmb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Komori K., Miyata T., DiRuggiero J., Holley-Shanks R., Hayashi I., Cann I.K. Both RadA and RadB are involved in homologous recombination in Pyrococcus furiosus. J. Biol. Chem. 2000;275(43):33782–33790. doi: 10.1074/jbc.M004557200. [DOI] [PubMed] [Google Scholar]
- 18.Allers T., Ngo H.P., Mevarech M., Lloyd R.G. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl. Environ. Microbiol. 2004;70(2):943–953. doi: 10.1128/AEM.70.2.943-953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allers T., Barak S., Liddell S., Wardell K., Mevarech M. Improved strains and plasmid vectors for conditional overexpression of His-tagged proteins in Haloferax volcanii. Appl. Environ. Microbiol. 2010;76(6):1759–1769. doi: 10.1128/AEM.02670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lestini R., Duan Z., Allers T. The archaeal Xpf/Mus81/FANCM homolog Hef and the Holliday junction resolvase Hjc define alternative pathways that are essential for cell viability in Haloferax volcanii. DNA Repair (Amst) 2010;9(9):994–1002. doi: 10.1016/j.dnarep.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 22.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W. improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin D.S., Pellegrini L., Daniels D.S., Yelent B., Craig L., Bates D. Full-length archaeal Rad51 structure and mutants: mechanisms for RAD51 assembly and control by BRCA2. EMBO J. 2003;22(17):4566–4576. doi: 10.1093/emboj/cdg429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mevarech M., Werczberger R. Genetic transfer in Halobacterium volcanii. J. Bacteriol. 1985;162(1):461–462. doi: 10.1128/jb.162.1.461-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delmas S., Shunburne L., Ngo H.P., Allers T. Mre11-Rad50 promotes rapid repair of DNA damage in the polyploid archaeon Haloferax volcanii by restraining homologous recombination. PLoS Genet. 2009;5(7):e1000552. doi: 10.1371/journal.pgen.1000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroud A., Liddell S., Allers T. Genetic and biochemical identification of a novel single-stranded DNA-binding complex in Haloferax volcanii. Front. Microbiol. 2012;3:224. doi: 10.3389/fmicb.2012.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esashi F., Galkin V.E., Yu X., Egelman E.H., West S.C. Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nat. Struct. Mol. Biol. 2007;14(6):468–474. doi: 10.1038/nsmb1245. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi I., Morikawa K., Ishino Y. Specific interaction between DNA polymerase II (PolD) and RadB, a Rad51/Dmc1 homolog, in Pyrococcus furiosus. Nucleic Acids Res. 1999;27(24):4695–4702. doi: 10.1093/nar/27.24.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim T., Chitteni-Pattu S., Cox B.L., Wood E.A., Sandler S.J., Cox M.M. Directed evolution of RecA variants with enhanced capacity for conjugational recombination. PLoS Genet. 2015;11(6):e1005278. doi: 10.1371/journal.pgen.1005278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavery P.E., Kowalczykowski S.C. Biochemical basis of the constitutive repressor cleavage activity of recA730 protein: a comparison to recA441 and recA803 proteins. J. Biol. Chem. 1992;267(29):20648–20658. [PubMed] [Google Scholar]
- 31.Wang T.C., Chang H.Y., Hung J.L. Cosuppression of recF, recR and recO mutations by mutant recA alleles in Escherichia coli cells. Mutat. Res. 1993;294(2):157–166. doi: 10.1016/0921-8777(93)90024-b. [DOI] [PubMed] [Google Scholar]
- 32.Fortin G.S., Symington L.S. Mutations in yeast Rad51 that partially bypass the requirement for Rad55 and Rad57 in DNA repair by increasing the stability of Rad51-DNA complexes. EMBO J. 2002;21(12):3160–3170. doi: 10.1093/emboj/cdf293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galkin V.E., Wu Y., Zhang X.P., Qian X., He Y., Yu X. The Rad51/RadA N-terminal domain activates nucleoprotein filament ATPase activity. Structure. 2006;14(6):983–992. doi: 10.1016/j.str.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Mortier-Barriere I., Velten M., Dupaigne P., Mirouze N., Pietrement O., McGovern S. A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell. 2007;130(5):824–836. doi: 10.1016/j.cell.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 35.Marmorstein L.Y., Kinev A.V., Chan G.K., Bochar D.A., Beniya H., Epstein J.A. A human BRCA2 complex containing a structural DNA binding component influences cell cycle progression. Cell. 2001;104(2):247–257. doi: 10.1016/s0092-8674(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 36.Bitan-Banin G., Ortenberg R., Mevarech M. Development of a gene knockout system for the halophilic archaeon Haloferax volcanii by use of the pyrE gene. J. Bacteriol. 2003;185(3):772–778. doi: 10.1128/JB.185.3.772-778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.