Abstract
Sialadenitis is an inflammation or infection of the salivary glands that may affect the parotid, submandibular and small salivary glands. Imaging findings vary among unilateral or bilateral salivary gland enlargement, atrophy, abscess, ductal dilation, cysts, stones and calcification. Imaging can detect abscess in acute bacterial suppurative sialadenitis, ductal changes with cysts in chronic adult and juvenile recurrent parotitis. Imaging is sensitive for detection of salivary stones and stricture in obstructive sialadenitis. Immunoglobulin G4-sialadenitis appears as bilateral submandibular gland enlargement. Imaging is helpful in staging and surveillance of patients with Sjögren’s syndrome. Correlation of imaging findings with clinical presentation can aid diagnosis of granulomatous sialadenitis. Post-treatment sialadenitis can occur after radiotherapy, radioactive iodine or surgery.
Keywords: Parotid, salivary, submandibular, sialadenitis, Sjögren’s, stone
Introduction
Sialadenitis is the most common pathology affecting the salivary glands. Patients present with swelling of the salivary gland, pain and foul taste in the mouth or xerostomia. The presentation may be acute, chronic or acute on top of chronic. Causes of sialadenitis include acute or chronic infective, obstructive, immunoglobulin G4-related sialadenitis (IgG4-RS), lympho-epithelial, granulomatous and post-treatment sialadenitis.1–6 Table 1 shows the causes of sialadenitis and Table 2 shows the imaging appearance of different causes of sialadenitis.
Table 1.
Causes of sialadenitis.
| Acute infective sialadenitis | Acute bacterial suppurative sialadenitis Acute viral sialadenitis |
| Chronic infective sialadenitis | Chronic adult sialadenitis Chronic juvenile recurrent parotitis |
| Obstructive sialadenitis | Stone Stricture |
| Immunoglobulin G4-related sialadenitis | |
| Lymphoepithelial sialadenitis | Human immunodeficiency virus (HIV) sialopathy Sjögren’s syndrome |
| Granulomatous sialadenitis | Tuberculosis, cat scratch disease, actinomycosis, sarcoidosis, amyloidosis, granulomatosis with polyangiitis |
| Post-treatment sialadenitis | Radiation-induced sialadenitis Radioactive iodine-induced sialadenitis Anesthesia-induced sialadenitis |
| Others causes of sialadenitis | Sialadenosis Pneumoparotidis Necrotizing sialometaplasia Subacute necrotizing sialadenitis |
Table 2.
Imaging findings of sialadenitis.
| Causes | Imaging findings |
|---|---|
| Acute infective sialadenitis | |
| Acute bacterial sialadenitis | –Diffuse homogenous enlargement (early) and abscess (late). |
| Acute viral sialadenitis | –Bilateral diffuse enlargement of parotid glands in children. |
| Chronic infective | |
| Chronic adult sialadenitis | –Irregularly enlarged main duct and central ductal dilatation. |
| juvenile recurrent parotitis | –Sialography shows punctate sialectasis with no evidence of obstruction. |
| Obstructive | |
| Stone | –Common submandibular gland or duct that appears as dense calcified lesion. |
| Stricture | –Stenosis of the main salivary ducts detected with magnetic resonance (MR) sialography. |
| Ig G4-related sialadenitis | –Bilateral symmetric enlargement of salivary gland with homogenous pattern and enhancement. |
| Lymphoepithelial sialadenitis | |
| Human immunodeficiency virus (HIV)-sialopathy | –Bilateral or unilateral cystic, partially cystic and solid parotid lesions. |
| Sjögren’s sialadenitis | –Diffusely enlarged homogenous (early), enlarged with multiple cystic regions (intermediate), atrophic with hypoechoic regions (late). |
| Granulomatous | |
| Tuberculous | –Diffuse enlarged glands with abscess and enlarged intra-parotid lymph nodes. |
| Cat scratch disease | –Enlarged intra-parotid lymph node in children, self-limited disorder. |
| Actinomycosis | –Ill-defined inflammatory lesion extends into skin with sinuses and fistulae. |
| Sarcoidosis | –Bilateral foamy appearance parotid lesions with cervical lymphadenopathy. |
| Amyloidosis | –Well defined parotid masses with slight calcification and mild enhancement. |
| Post treatment | |
| Radiation-induced sialadenitis | –Enlarged with enhancement(early) and atrophic with fatty changes (late). |
| Radioactive iodine-sialadenitis | –Scintigraphy used for follow-up of salivary gland dysfunction. |
| Anesthesia-sialadenitis | –Enlarged and intense enhancement of salivary gland five to seven days post-operative. |
| Others causes | |
| Sialadenosis | –Bilateral enlarged parotid glands in alcoholics, diabetics and malnourished. |
| Pneumoparotidis | –Air within the duct and glands. |
| Necrotizing sialometaplasia | –Focal parotid mass that simulates malignancy. |
| Subacute necrotizing sialadenitis | –Focal mass in hard palate or floor of the mouth that simulates malignancy. |
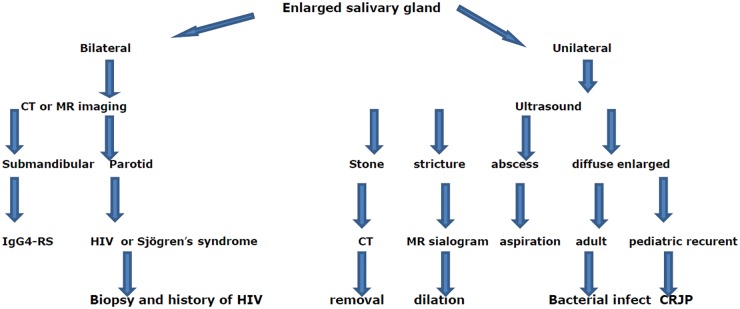
Different imaging modalities are used in the diagnosis of sialadenitis. Selection of imaging modality depends on the suspected cause of sialadenitis. Radiographs are of limited value and can be used in suspected radiolucent stones. It is a cheap method that is associated with radiation exposure.5,6 Ultrasound is a cheap, noninvasive imaging modality that is used as the first diagnostic imaging tool in assessment of sialadenitis. It is a tolerable imaging tool, especially for young patients, but this technique is clearly operator dependent. It can detect diffuse and focal lesions and dilated ducts, areas of calcifications and stones within the parotid glands.4–7 Sialography delineates the ductal system down to fourth-order branches that help in diagnosis of obstructive salivary disease and demonstrate the extent of salivary involvement in Sjögren’s syndrome; however, it is an invasive procedure.5–7 Non-enhanced computed tomography (CT) is particularly useful when there is a suspicion of calculi and contrast-enhanced CT is indicated when there is an associated abscess and Sjögren’s syndrome.6,9 The magnetic resonance (MR) imaging is an expensive and sophisticated technique that is used mainly for assessment of the ductal system of the salivary glands by MR sialogram. Short tau inversion recovery (STIR) increases lesion conspicuity over routine spin-echo images by lowering the high T1-signal intensity. It is an excellent pulse sequence that is used for early detection of sialadenitis. MR sialography depicts the main ductal system and first- and second-order branches. MR sialography is used for diagnosis of stricture, grading of Sjögren’s syndrome and follow-up after radiotherapy. Diffusion-weighted MR imaging has a role in grading of Sjögren’s syndrome and diagnosis of post-radiation sialadenitis.10,11 Radionuclide imaging uses 99mTc-pertechnetate applied as an objective technique to assess salivary gland function as it measures the degree of radioisotope uptake and excretion for major salivary glands separately; however, it is unavailable and is an expensive procedure.12 Intially, ultrasound is used for assessment of patients with a unilateral enlarged parotid gland and cross-sectional imaging including CT and MR imaging for patients with bilateral enlarged parotid and submandibular glands. Figure 1 is a flowchart algorithm showing the imaging approach for reaching a specific diagnosis of sialadenitis.
Figure 1.
Flowchart algorithm showing the imaging approach for reaching a specific diagnosis of sialadenitis.
CT: computed tomography; MR: magnetic resonance; IgG4-RS: immunoglobulin G4-related sialadenitis; HIV: human immunodeficiency virus; CRJP: chronic recurrent juvenile parotitis.
The aim of this article is to review the imaging appearance of sialadenitis.
Acute infective sialadenitis
Acute bacterial suppurative sialadenitis
The predisposing factors of acute bacterial suppurative sialadenitis are salivary stasis in patients with severe dehydration (terminally ill, postoperative, neonates). The incidence of acute bacterial suppurative sialadenitis is higher in patients with poor dental hygiene. Staphylococcus aureus is the most common pathogen. However, gram-negative organisms often are seen in neonates and in hospitalized, debilitated patients. It involves the parotid more often than the submandibular glands. Suppurative infections present with an exquisitely painful parotid swelling and purulent discharge from the duct.13,14
Early changes on ultrasound include enlarged hypoechoic salivary glands associated with ductal dilatation; abscess results in a relatively anechoic focus with fine and coarse echoes in the late stage. Peri-glandular inflammatory changes and reactive cervical lymphadenopathy are usually present. On CT, the gland is enlarged with diffuse inhomogeneous pattern of contrast enhancement; abscess formation results in a focal area of fluid attenuation with an enhancing rim (Figure 2). MR imaging typically demonstrates asymmetric diffuse enlargement of the parotid glands with high T2-signal intensity that may be associated with a dilated duct on STIR images. The gland shows inhomogeneous enhancement with non-enhanced regions representing the dilated ducts. A parotid abscess appears as a marginally enhanced lesion with characteristic restricted diffusion and low diffusivity of the abscess on the apparent diffusion coefficient (ADC) map.13,14
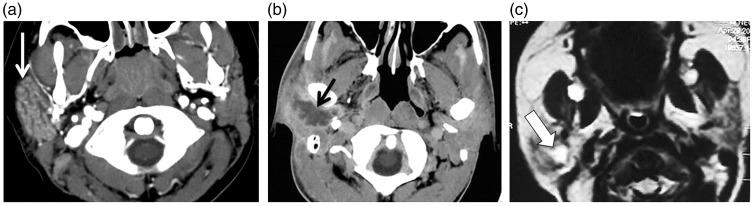
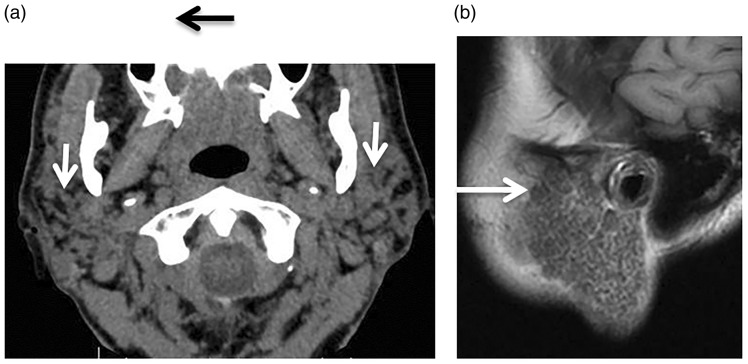
Figure 2.
Acute sialadenitis. (a) Axial contrast computed tomography (CT) scan shows enlarged right parotid gland with intense enhancement (arrow). (b) Axial contrast CT scan of another patient shows a well-defined marginal enhanced abscess (arrow) in the right parotid gland. (c) Axial T2-weighted image shows hyperintense fluid with thick wall of abscess in the right parotid gland of a neonate (arrow).
Imaging has an impact on patient management and selection of treatment method of patients with acute bacterial suppurative sialadenitis. Early stages are treated with antibiotics, and patients with abscess require surgical management with drainage of the abscess.
Acute viral sialadenitis
Mumps is the most common viral infection of the salivary glands, presenting with bilateral (90%) or unilateral (10%) swelling of the parotid glands. In 85% of cases, it affects children under the age of 15 years. It can also affect adults—mainly elderly patients. Mumps affects the parotid glands in 90% and the submandibular and sublingual glands may also be involved in 10% of cases. The diagnosis of a viral sialedenitis is usually made clinically, sometimes with the aid of serology. There is bilateral involvement in 90%, although the swelling may not be seen at the same time.1–5
Chronic sialadenitis
Chronic adult sialadenitis
Chronic adult sialadenitis is often associated with episodes of acute inflammation with subsequent glandular destruction. The chronic inflammatory process stimulates alterations in salivary chemistry and enzyme, which leads to sialectasis, ductal ectasia and acinar atrophy accompanied by a lymphocytic infiltrate. This condition occurs more often in the parotid glands.6 Chronic sialadenitis has a variable imaging depending on the severity and duration of inflammation. The echo-texture is often heterogeneous and hypoechoic because of fibrous scarring with small cystic foci due to minor ductal ectasia. Occasionally, intraglandular microcalcification is visible as an echogenic area with posterior acoustic shadowing. Hallmarks of chronic recurrent sialadenitis are an irregularly enlarged (sausage-shaped) main duct and central ductal dilation tapering to normal peripheral ducts. Diffuse enlargement with or without dystrophic calcifications associated with low-density areas are frequent findings. Fatty infiltration, duct ectasia, and volume loss are features of the late stage3,15 (Figure 3).
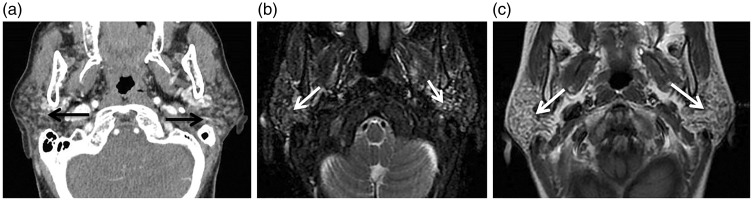
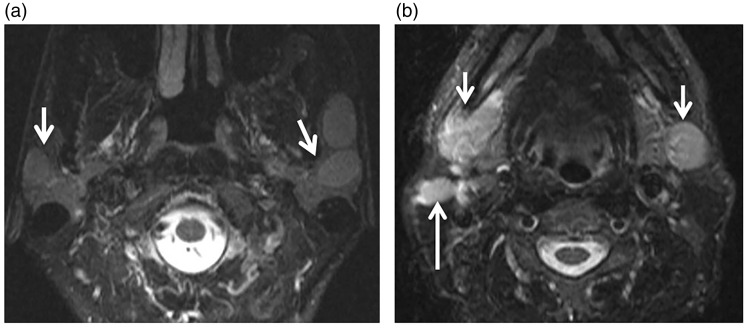
Figure 3.
Chronic adult sialadenitis. (a) Axial computed tomography (CT) shows granular appearance of both parotid glands (arrows). (b) Axial T2-weighted image shows multiple small cysts in both parotid glands (arrows). (c) Axial contrast T1-weighted image shows mild inhomogeneous pattern of enhancement of both parotid glands (arrows).
Chronic juvenile recurrent parotitis (CJRP)
CJRP is 10 times more common than adult chronic parotitis and mainly affects children between the ages of 3 and 6 years, with males being more commonly affected. The symptoms peak in the first year of school, and usually begin to subside around the mid-teens. The cause is still unclear (genetic, congenital duct ectasia, immunological). Painful swelling of one or both parotid glands recurs with acute purulent episodes at varying intervals and, in the later clinical course, painless swelling.15
Ultrasound demonstrates inhomogeneous texture of parotid glands with multiple minute hypoechoic or anechoic nodules that can represent ductal ectasia, congested parenchyma, or enlarged intraparotid lymph nodes. Very often both glands are affected to a different extent with lymph node enlargement. CT and MR imaging demonstrate nonspecific enlargement of the parotid glands, with heterogeneous density and signal intensity; later, the glands become atrophic. Sialography shows punctate sialectasis with no evidence of obstruction2,6,16 (Figure 4).
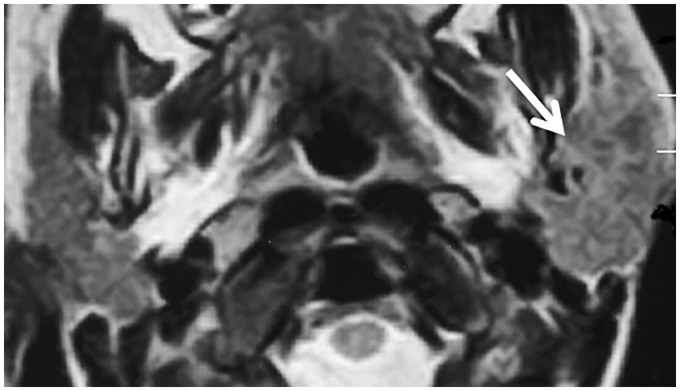
Figure 4.
Chronic juvenile recurrent sialadenitis. Axial T2-weighted image shows an enlarged left parotid gland with mixed pattern of signal intensity (arrow).
Conservative treatments of acute episodes include analgesics, antipyretics, massage, sialogogues, and hydration. Intraductal injection therapy can be performed during sialography. Sialoendoscopy has been advocated for the management of those with severe symptoms.
Obstructive sialadenitis
Stones
Stones are the most common major salivary gland disease. The classical presentation is an intermittent postprandial salivary gland swelling that gradually subsides over the next two to three hours. Recurrent and chronic obstruction causes stasis, inflammation, and infection, which causes persistent swelling. Eighty percent to 90% of salivary calculi occur in the submandibular gland. Most of the remaining stones occur in the parotid gland. Sublingual stones are uncommon. The exact localization of the stone (intraglandular, extraglandular, or intraductal) is important for treatment planning.6 The long, tortuous, upward path of the submandibular duct and the thicker, mucoid alkali secretions of the submandibular gland may be the reason for its greater tendency to form calculi. The size of the salivary stones can range from a few millimeters to 40 mm. The stones are often multiple in 13% of patients, and bilateral in 1% of patients.3–6
Treatment of patients with sialolithiasis is dependent on the size and location of the stones. Imaging can detect the exact site and size of the stones. Small stones (less than 3 mm) are removed by interventional salivary endoscopy, intermediate stones (3 mm–6 mm) are removed by endoscopy with fragmentation of the stone and large stones (more than 8 mm) are treated by diagnostic sialoendoscopy for stone localization and external or intraoral approaches for assisted removal of the stone.3–6
Ultrasound successfully diagnoses the majority of stones. Stones measuring 2 mm or larger are easily detected as echogenic foci, which cast distal acoustic shadows. Stones can also easily be detected with non-enhanced CT, which is the most sensitive technique for detecting stones. Dilatation of the obstructed duct results in a linear hypodense structure that abruptly terminates. The obstructed duct appears bright and dilated on MR sialography and a “black dot” represents the underlying stone. The gland may be enlarged with an indistinct margin and may have high-intensity streaks in the periphery of the gland on T2-weighted images4–6,17,18 (Figure 5).
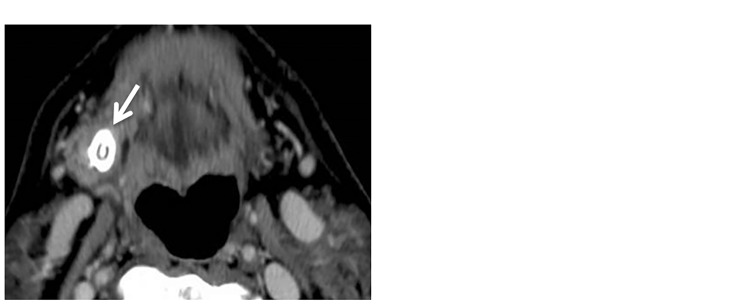
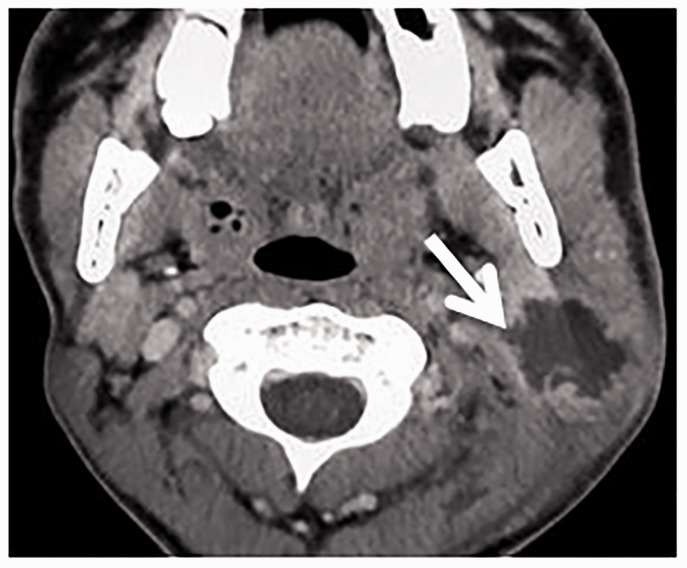
Figure 5.
Stone of submandibular duct. (a) Axial contrast computed tomography (CT) scan shows a stone (arrow) in the right submandibular gland region. (b) Axial T2-weighted image shows a signal void of a stone (arrow) in the right submandibular gland.
Stricture
Obstructive sialadenitis is the most frequent non-neoplastic cause of salivary gland swelling. Stricture of the salivary ducts is most often caused by a stone and less often by duct stenosis or webs of inflammatory origin. Occasionally, there are foreign bodies or the excretory duct suffers iatrogenic injury, leading to obstructive sialadenitis. Stenosis of the main salivary ducts can be accurately detected with MR sialography with a sensitivity of 100% and a specificity of 93%18 (Figure 6). Management of strictures includes endoscopic balloon dilation, steroid irrigation, and placement of a stent within the parotid duct. Imaging is important to detect the degree and exact location of stenosis of the main and branches of the salivary ducts.
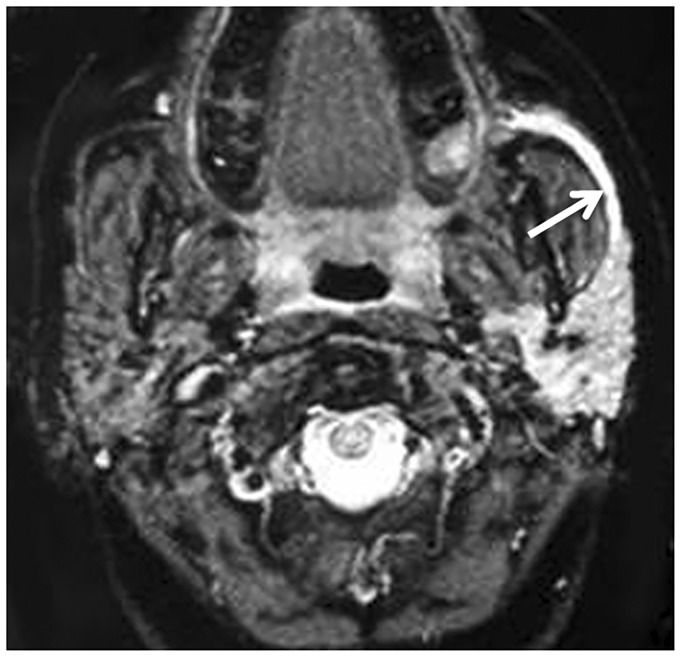
Figure 6.
Obstructive sialadenitis. Axial gradient-recalled echo magnetic resonance (MR) image shows a dilated duct (long arrow) of the left parotid gland that exhibits high-signal intensity of the retained fluid.
IgG4-RS
IgG4-RS is a systemic disease that is characterized by abundant infiltration of IgG4-positive plasma cells and lymphocytes with associated fibrosis, leading to organ dysfunction. Pathologic conditions such as Mikulicz disease and chronic sclerosing sialadenitis (Küttner tumor) are now considered to be part of the spectrum of IgG4-related disease. It occurs predominantly in elderly men and is frequently associated with elevated serum IgG4 levels with excellent response to corticosteroid therapy. It is mostly affects the submandibular gland.19
Cross-sectional imaging of IgG4-RS shows bilateral symmetric enlargement of the submandibular glands. On ultrasound, there is a hypoechoic, rather poorly demarcated mass-like appearance. At CT, the lesions usually demonstrate homogeneous attenuation and enhancement. At MR imaging, the lesions typically demonstrate homogeneous low to intermediate signal intensity on T2-weighted images and low signal intensity on T1-weighted images, with homogeneous enhancement. It may occur unilaterally with areas of calcification that may simulate malignancy. Lymphoma and the acute phase of Sjögren’s syndrome should be included in the differential diagnosis19,29 (Figure 7).
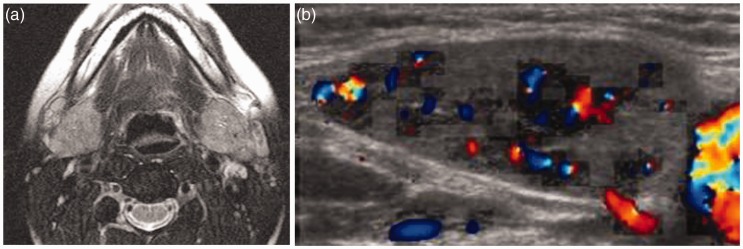
Figure 7.
Immunoglobulin G4-related sialadenitis (IgG4-RS). (a) Axial T2-weighted image shows both enlarged submandibular glands (arrow) that exhibit high signal intensity. (b) Color Duplex examination shows enlarged submandibular glands gland with increased color signal (arrow) and vascularity.
Lymphoepithelial sialadenitis
Human immunodeficiency virus (HIV) sialopathy
Parotid enlargement is seen in 5% of HIV-positive patients in the form of persistent lymphadenopathy, benign lymphoepithelial lesions (BLELs) and benign lympho-epithelial cysts (BLECs).2 Clinically, patients classically present with unilateral or bilateral painless enlarging parotid masses. On imaging, there are bilateral or unilateral cystic, partially cystic and solid parotid lesions. Solid lesions may simulate benign or malignant tumors. In HIV patients, the incidence of malignancy in cystic parotid lesions is about 1%. The incidence in the solid lesions may be as high as 40%.5,6,13 Cervical lymphadenopathy and nasopharyngeal lymphoid enlargement helps to differentiate HIV sialopathy from BLEBs in Sjögren’s syndrome13 (Figure 8).
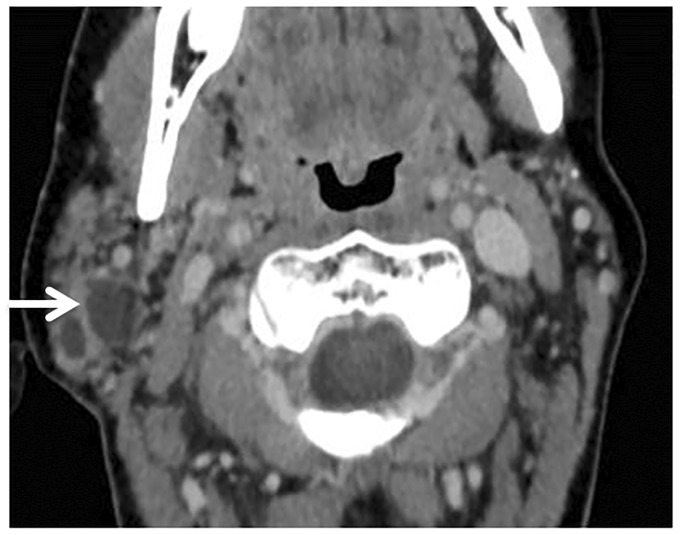
Figure 8.
Human immunodeficiency virus (HIV) sialadenitis. Axial contrast computed tomography (CT) scan shows benign lymphoepithelial lesions (BLEBs) (arrow) of the right parotid gland in a patient with HIV.
Sjögren’s syndrome
Sjögren’s syndrome is an autoimmune disorder of the salivary and lacrimal glands that occurs alone (primary) or associated with connective tissue diseases (secondary). It is commonly seen in female patients (90%) in the fourth through sixth decades of life. The parotid glands are affected in the vast majority, whereas isolated submandibular gland enlargement is seen in only 2.5% of patients. Clinically, patients present with symptoms and signs of keratoconjunctivitis sicca, xerostomia, and/or a connective tissue disease, usually rheumatoid arthritis. There is diffuse lymphocytic infiltration leading to destruction of acinar cells. Ducts become blocked by inspissated material resulting in pseudocystic acini formation. In advanced cases, parenchymal destruction and lymphocyte accumulation may result in large cysts with solid mass-like components.4–6 Imaging is important for staging of the disease, and to detect associated infection and surveillance for lymphomas as patients with Sjögren’s syndrome are at a higher risk (44 times) of developing lymphomas (5%–6% B-cell lymphoma, mucosa-associated lymphoid tissue (MALT)-lymphoma).1,19–22
The imaging appearance of Sjögren’s syndrome varies with stage of the disease. In the early phase, the parotid glands may be normal or diffusely enlarged with a normal parenchymal pattern. In the intermediate phase, the salivary glands are diffusely enlarged with multiple, scattered cysts and solid masses. The cysts are typically similar in size, ranging from <1 mm (microcysts) to macrocysts and mixed solid-cystic masses >2 cm with honey-comb or salt and pepper appearance. In the chronic phase, there is atrophy of the glands with low signal intensity (Figure 9). Using STIR, Sjögren’s syndrome is associated with premature fat deposition in the parotid gland and the degree of fat deposition correlates with loss of secretory function. The ADC value of the parotid glands in Sjögren’s syndrome correlates with the salivary flow rate. The ADC value of the parotid is lower in the glands at higher grades. MR sialogram shows punctate, globular, cavitary, or destructive appearance of primary branching ducts of the parotid glands, so it helps in grading of the disease. The sialographic staging of Sjögren’s syndrome is stage 1, punctuate contrast collections (<1 mm); stage 2, globular contrast collection (1–2 mm); stage 3, cavitary-contrast collection (>2 mm); and stage 4, destruction of the gland parenchyma. Scintigraphy using Gallium-67 citrate may produce the classic “panda sign” due to bilateral increased uptake by lacrimal and parotid glands; mediastinal lymphadenopathy results in the “lambda” sign.4–7,21–23
Figure 9.
Sjögren’s syndrome. (a) Axial contrast computed tomography (CT) scan shows granular appearance (arrow) of both parotid glands. (b) Sagittal T1-weighted image shows honey-comb appearance (arrow) of both parotid glands.
Granulomatous sialadenitis
Tuberculous sialadentitis
Tuberculosis is a chronic caseating granuloma caused by Mycobacterium tuberculosis. The disease occurs in adults, most commonly in the second and third decades of life. Seventy percent of tuberculosis affects the parotid glands, 27% the submandibular glands, and only 3% the sublingual glands. The presentation may be acute (similar to pyogenic infections) or chronic (which may mimic tumor or involve the overlying skin with fistula formation). The imaging findings are similar to those in bacterial parotitis, and necrotic nodes or focal abscesses within the gland (Figure 10). Enlarged intraparotid and peri-parotid lymph nodes are also reported. Calcification may be present within the gland or the affected nodes. Atypical mycobacterial infection of the parotid gland tends to represent extension from cervical adenitis and is radiologically indistinguishable from tuberculosis.24,25
Figure 10.
Tuberculous sialadenitis. Axial contrast computed tomography (CT) scan shows an enlarged right parotid gland with small marginally enhanced abscess (arrow).
Cat scratch disease
Cat scratch disease is a chronic granulomatous disease commonly seen in children and young adults. The causative agent is a rickettsia-like micro-organism called Bartonella henselae. Only one-third of patients have high fever. The disease is self-limited with a duration of two to four months. The disease may involve intra-parotid lymph nodes and may mimic a salivary gland tumor. Early, the intraparotid lymph nodes appear as a sharply marginated oval mass. Later, lymph node abscesses are typically seen as confluent nodes with poorly defined borders simulating tuberculous sialadenitis.26
Actinomycosis
Actinomycosis is an indolent chronic infection caused by Actinomyces Israelii that usually arises in the oral cavity of patients with poor dentition. The disease invades the parotid glands from a focus usually in the mandible. Rarely, the disease can spread to the parotid glands in a retrograde manner from the mouth. Actinomycosis has a predilection for male patients with a male-to-female ratio of 3:1 to 4:1. The lesion has an ill-defined margin with surrounding soft tissue inflammatory reaction that extends into the skin. The presence of infected soft tissue fistula/sinus and gas within the lesion, masticator space infection and cervical lymphadenopathy in and around the parotid gland suggest diagnosis of actinomycosis.24,27
Sarcoidosis
Sarcoidosis is a rare multisystem chronic granulomatous disease of unknown origin characterized by non-caseating granuloma affecting multiple systems. This disorder is reported in patients at the second through fourth decade of life. It is 10–20 times more common in black than in white people. The diagnosis is based on elevated levels of angiotensin-converting enzyme. The parotid glands are involved in 10%–30% of patients with sarcoidosis, most often bilaterally (83%). There is non-tender parotid gland enlargement, which can be nodular. On imaging, multiple, benign-appearing non-cavitating masses with a foamy appearance are seen in both parotid glands that often are associated with cervical adenopathy (Figure 11). Heerfordt syndrome is defined as a triad of uveitis, facial nerve palsy and parotid enlargement in patients with sarcoidosis.24,28
Figure 11.
Sarcoidosis. (a) Axial T2-weighted image shows well-defined hyperintense focal lesions (arrows) in both parotid glands more on the left side. (b) Axial T2-weighted image at another level shows enlarged both submandibular glands (short arrows) more on the right side with enlarged adjacent cervical lymph (long arrow).
Amyloidosis
Amyloidosis is a granulomatous disease characterized by idiopathic extracellular accumulation of amyloid in tissues. Amyloidosis is classified as systemic (81%) or localized (19%) amyloidosis, with the former being neoplastic and fatal and the latter being a benign and mild form. There is infiltration and replacement of the functional cells of the salivary glands with amyloidogenous stroma, which causes xerostomia. On imaging, the parotid amyloid deposits appear as well-defined, soft tissue masses, with mild contrast enhancement and variable degree of calcification.24,29
Granulomatosis with polyangiitis
Granulomatosis with polyangiitis, formerly Wegener’s granulomatosis, is anti-cytoplasmic autoantibodies (ANCA)-associated vasculitides characterized by a triad of necrotizing granuloma of the upper and lower respiratory tract, necrotizing vasculitis and glomerulonephritis. The peak incidence of the disorder is the fifth decade of life and men are affected twice as often as women. Sialadenitis may constitute the initial presentation of granulomatosis with polyangiitis, although it is a relatively rare manifestation. The submandibular or parotid glands on one or both sides may become enlarged. Salivary gland involvement typically occurs early in the course of the disease and may be seen in limited forms of the disease.20–31
Scleroma
Scleroma is a chronic granulomatous disease affecting the upper respiratory airway. The causative organism is Klebsiella rhinosclermatis, which is gram-negative bacteria. Scleroma usually begins at the nose and may progress to involve the larynx, pharynx or other regions of the neck such as the parotid glands. It is commonly seen in the second through fourth decade of life.32,33 Imaging shows diffuse enlargement of the parotid gland or as a focal lesion in the salivary gland simulating other parotid tumors.
Post-treatment sialadenitis
Radiation-induced sialadenitis
Radiation-induced sialadenitis occurs during radiotherapy for the oral cavity or pharyngeal tumors. The parotid gland is more severely affected by radiation than the submandibular glands. Radiotherapy initially results in xerostomia, but salivary flow rates partially recover after six to 12 months because of hyperplasia of the surviving acini.1,3,5 Ultrasound of radiation-induced sialadenitis shows features of acute or chronic sialadenitis depending on the time elapsed following radiotherapy. CT shows decrease volume and increase attenuation values of the parotid glands. On MR imaging, the parotid glands are smaller and of lower signal intensity on all sequences that show intense contrast enhancement. The ADCs of radiation-induced sialadenitis decrease significantly after radiotherapy.34,35
Radioactive iodine (RAI)-induced sialadenitis
RAI-induced sialadenitis occurs in 20%–67% of thyroid cancer patients who have received RAI therapy to ablate remnant thyroid tissues after thyroidectomy. RAI accumulates 30–40 times more in the salivary gland than in plasma. RAI accumulates in the submandibular glands through the sodium-iodide symporter, and damages ductal epithelium and vascular endothelium in the submandibular glands. Patients with RAI-induced sialadenitis present with swelling and pain in the salivary glands and dryness of the mouth.1,3,5 There is reduction in the volume of the parotid and submandibular glands and increase in attenuation of the parotid gland on non-enhanced CT scan.36 MR sialography in RAI-induced sialadenitis depicts reduced duct visualization, sialectasis, gland volume reduction, and main duct stenosis. Qualitative and quantitative salivary gland scintigraphy are used for assessment and follow-up of salivary gland dysfunction after RAI.37
Anesthesia (surgical)-induced sialadenitis
Anesthesia-induced parotitis reported in 0.02%–0.04% after surgery with peak incidence of between postoperative days 5 and 7. Straining, coughing, and sneezing of the patient, during a difficult anesthetic procedure or post-anesthetic period, serve to increase positive pressure in the oral cavity. On CT, there is mild enhancement of the parotid and submandibular glands on the affected side.38
Others lesions
Sialadenosis
Sialadenosis (sialosis) is defined as an asymptomatic, non-inflammatory, non-neoplastic salivary disorder characterized by persistent painless bilateral swelling of the salivary glands, most commonly involving the parotid glands. There is no sex predilection, and the peak age incidence is the third through seventh decades of life. Sialosis most commonly reported in alcoholics, diabetics and the malnourished patients.2–5 Ultrasound demonstrates a diffuse homogeneous, enlargement of the salivary glands with no focal lesions. CT and MR findings shows bilateral symmetrically enlarged salivary glands with increased density (acinar hypertrophy, fibrosis) or decreased density (fatty infiltration) and corresponding signal changes on MR.4,5
Pneumoparotitis
Pneumoparotitis is a rare disease that occurs through air insufflation into the excretory duct of the parotid gland (up to 150 mm Hg). There is a painful recurrent unilateral or bilateral swelling of the parotid glands. This disorder is most commonly seen in glassblowers and brass instrumentalists and can also occur in children through strong nose-blowing or blowing up balloons or playing wind instruments. On CT, the air inclusions are seen in the salivary duct, the gland, and, occasionally, in the surroundings.1,3
Necrotizing sialometaplasia
Necrotizing sialometaplasia is a spontaneously resolving inflammatory disease often found in the minor salivary gland and seldom observed in major salivary glands. Although the pathogenesis of lesions is thought to be owing to ischemic changes in the salivary glands arising from causes such as traumatic injury and medical procedures, the precise etiology has not been fully elucidated. Histologically, necrotizing sialometaplasia is characterized by acinar necrosis in early lesions with concomitant squamous metaplasia of the salivary ducts at the late stages. At imaging, it appears as a focal mass that mimics malignant tumors.39
Subacute necrotizing sialadenitis
Subacute necrotizing sialadenitis is a self-limiting, inflammatory condition of the minor salivary glands of unknown etiology. The lesion typically presents as a localized, palatal swelling with abrupt onset of pain. The etiology of subacute necrotizing sialadenitis is unknown, but traumatic, infectious, and allergic etiologies have been suggested. It appears as a mass in the hard palate or may be in the floor of the mouth that may simulate malignancy.40 Advanced MR imaging sequences such as diffusion-weighted MR imaging, dynamic susceptibility perfusion-weighted MR imaging and MR spectroscopy help to differentiate these lesions from malignancy.41,42
Conclusion
Different imaging modalities are important for diagnosis of different causes of sialadenitis. Correlation of the imaging findings at different imaging modalities with the clinical presentation and laboratory tests helps to establish the specific cause of sialadenitis and helps the clinician to select the best method of treatment.
Acknowledgment
This work was presented as an educational exhibit at the Radiologic Society of North America (RSNA) 2014 held in Chicago, IL, USA.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mandel L. Salivary gland disorders. Med Clin North Am 2014; 98: 1407–1449. [DOI] [PubMed] [Google Scholar]
- 2.Francis CL, Larsen C. Pediatric sialadenitis. Otolaryngol Clin North Am 2014; 47: 763–778. [DOI] [PubMed] [Google Scholar]
- 3.Carlson ER. Diagnosis and management of salivary gland infections. Oral Maxillofac Surg Clin North Am 2009; 21: 293–312. [DOI] [PubMed] [Google Scholar]
- 4.Thomas BL, Brown JE, McGurk M. Salivary gland disease. Front Oral Bio 2010; 14: 129–146. [DOI] [PubMed] [Google Scholar]
- 5.Zenk J, Iro H, Klintworth N, et al. Diagnostic imaging in sialadenitis. Oral Maxillofacial Surg Clin North Am 2009; 21: 275–292. [DOI] [PubMed] [Google Scholar]
- 6.Madani G, Beale T. Inflammatory conditions of the salivary glands. Semin Ultrasound CT MRI 2006; 27: 440–451. [DOI] [PubMed] [Google Scholar]
- 7.Horsburgh A, Massoud TF. Lessons learned from unintended sublingual sialography: Imaging anatomy, technical considerations, and diagnostic implications. AJR Am J Roentgenol 2013; 200: 879–883. [DOI] [PubMed] [Google Scholar]
- 8.Orlandi MA, Pistorio V, Guerra PA. Ultrasound in sialadenitis. J Ultrasound 2013; 16: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel Razek AA. Computed tomography and magnetic resonance imaging of lesions at masticator space. Jpn J Radiol 2014; 32: 123–137. [DOI] [PubMed] [Google Scholar]
- 10.Karaca Erdoğan N, Altay C, Ozenler N, et al. Magnetic resonance sialography findings of submandibular ducts imaging. Biomed Res Int 2013; 2013: 417052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razek AA. Diffusion-weighted magnetic resonance imaging of head and neck. J Comput Assist Tomogr 2010; 34: 808–815. [DOI] [PubMed] [Google Scholar]
- 12.Konstantinidis I, Tsakiropoulou E, Chatziavramidis A, et al. Scintigraphic detection of a parotid salivary gland malfunction, in chronic sialolithiasis and fat infiltration with no risk factors. Hell J Nucl Med 2014; 17: 49–51. [DOI] [PubMed] [Google Scholar]
- 13.Viselner G, van der Byl G, Maira A, et al. Parotid abscess: mini-pictorial essay. J Ultrasound 2013; 16: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel Razek AA, Ashmalla MD AA, Gaballa G, et al. Pilot study of Ultrasound Parotid Imaging Reporting and Data System (PIRADS): Inter-observer agreement. Eur J Radiol 2015; 84: 2533–2538. [DOI] [PubMed] [Google Scholar]
- 15.Vashishta R, Gillespie MB. Salivary endoscopy for idiopathic chronic sialadenitis. Laryngoscope 2013; 123: 3016–3020. [DOI] [PubMed] [Google Scholar]
- 16.Gadodia A, Seith A, Sharma R, et al. MRI and MR sialography of juvenile recurrent parotitis. Pediatr Radiol 2010; 40: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 17.Terraz S, Poletti PA, Dulguerov P, et al. How reliable is sonography in the assessment of sialolithiasis? AJR Am J Roentgenol 2013; 201: W104–W109. [DOI] [PubMed] [Google Scholar]
- 18.Capaccio P, Cuccarini V, Ottaviani F, et al. Comparative ultrasonographic, magnetic resonance sialographic, and videoendoscopic assessment of salivary duct disorders. Ann Otol Rhinol Laryngol 2008; 117: 245–252. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu M, Okamura K, Kise Y, et al. Effectiveness of imaging modalities for screening IgG4-related dacryoadenitis and sialadenitis (Mikulicz’s disease) and for differentiating it from Sjögren’s syndrome (SS), with an emphasis on sonography. Arthritis Res Ther 2015; 17: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabenge C, Ng S, Muyinda Z, et al. Diagnostic ultrasound patterns of parotid glands in human immunodeficiency virus-positive patients in Mulago, Uganda. Dentomaxillofac Radiol 2010; 39: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel Razek AA. Imaging of connective tissue diseases of the head and neck. Neuroradiol J 2016; 29: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokosawa M, Tsuboi H, Nasu K, et al. Usefulness of MR imaging of the parotid glands in patients with secondary Sjögren’s syndrome associated with rheumatoid arthritis. Mod Rheumatol 2015; 25: 415–420. [DOI] [PubMed] [Google Scholar]
- 23.Ding C, Xing X, Guo Q, et al. Diffusion-weighted MRI findings in Sjögren’s syndrome: A preliminary study. Acta Radio 2016; 57: 691–700. [DOI] [PubMed] [Google Scholar]
- 24.Razek AA, Castillo M. Imaging appearance of granulomatous lesions of head and neck. Eur J Radiol 2010; 76: 52–60. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Xiao J, Pui M, et al. Tuberculosis of the parotid gland: Computed tomographic findings. Acta Radiol 2008; 49: 458–461. [DOI] [PubMed] [Google Scholar]
- 26.Petrogiannopoulos C, Valla K, Mikelis A, et al. Parotid mass due to cat scratch disease. Int J Clin Pract 2006; 60: 1679–1680. [DOI] [PubMed] [Google Scholar]
- 27.Razek AA, Huang BY. Lesions of the petrous apex: Classification and findings at CT and MR imaging. Radiographics 2012; 32: 151–173. [DOI] [PubMed] [Google Scholar]
- 28.Banks GC, Kirse DJ, Anthony E, et al. Bilateral parotitis as the initial presentation of childhood sarcoidosis. Am J Otolaryngol 2013; 34: 142–144. [DOI] [PubMed] [Google Scholar]
- 29.Finkel KJ, Kolansky DM, Giorgadze T, et al. Amyloid infiltration of the salivary glands in the setting of primary systemic amyloidosis without multiple myeloma. Otolaryngol Head Neck Surg 2006; 135: 471–472. [DOI] [PubMed] [Google Scholar]
- 30.Ceylan A, Asal K, Çelenk F, et al. Parotid gland involvement as a presenting feature of Wegener’s granulomatosis. Singapore Med J 2013; 54: e196–e198. [DOI] [PubMed] [Google Scholar]
- 31.Abdel Razek AA, Alvarez H, Bagg S, et al. Imaging spectrum of CNS vasculitis. Radiographics 2014; 34: 873–894. [DOI] [PubMed] [Google Scholar]
- 32.Razek AA, Elasfour AA. MR appearance of rhinoscleroma. AJNR Am J Neuroradiol 1999; 20: 575–578. [PMC free article] [PubMed] [Google Scholar]
- 33.Abdel Razek AA. Imaging of scleroma in the head and neck. Br J Radiol 2012; 85: 1551–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng SC, Ying MT, Kwong DL, et al. Sonographic appearance of submandibular glands in patients treated with external beam radiotherapy for nasopharyngeal carcinoma. J Clin Ultrasound 2013; 41: 472–478. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Ou D, Gu Y, et al. Diffusion-weighted MR imaging of salivary glands with gustatory stimulation: Comparison before and after radiotherapy. Acta Radiol 2013; 54: 928–933. [DOI] [PubMed] [Google Scholar]
- 36.Choi JS, Lim HG, Kim YM, et al. Usefulness of magnetic resonance sialography for the evaluation of radioactive iodine-induced sialadenitis. Ann Surg Oncol 2015; 22(Suppl 3): S1007–S1013. [DOI] [PubMed] [Google Scholar]
- 37.Nabaa B, Takahashi K, Sasaki T, et al. Assessment of salivary gland dysfunction after radioiodine therapy for thyroid carcinoma using non-contrast-enhanced CT: The significance of changes in volume and attenuation of the glands. AJNR Am J Neuroradiol 2012; 33: 1964–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinn ME, Quinn TD, Mian AZ, et al. Imaging findings of “anesthesia mumps” (acute postoperative sialadenitis) after general anesthesia. J Comput Assist Tomogr 2012; 36: 745–748. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji T, Nishide Y, Nakano H, et al. Imaging findings of necrotizing sialometaplasia of the parotid gland: Case report and literature review. Dentomaxillofac Radiol 2014; 43: 20140127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eyibilen A, Ozkan NC, Aladağ I, et al. An unusual giant subacute necrotizing sialadenitis as an emergency case of otolaryngology. Kulak Burun Bogaz Ihtis Derg 2009; 19: 216–219. [PubMed] [Google Scholar]
- 41.Abdel Razek AA, Samir S and Ashmalla GA. Characterization of parotid tumors with dynamic susceptibility contrast perfusion-weighted magnetic resonance imaging and diffusion-weighted MR imaging. J Comput Assist Tomogr. Epub ahead of print 16 September 2016. DOI: 10.1097/RCT.0000000000000486. [DOI] [PubMed]
- 42.Abdel Razek AA, Poptani H. MR spectroscopy of head and neck cancer. Eur J Radiol 2013; 82: 982–989. [DOI] [PubMed] [Google Scholar]
- 43.Vogl TJ, Al-Nawas B, Beutner D, et al. Updated S2K AWMF guideline for the diagnosis and follow-up of obstructive sialadenitis—relevance for radiologic imaging. Rofo 2014; 186: 843–846. [DOI] [PubMed] [Google Scholar]
- 44.Zengel P, Schrötzlmair F, Schwarz F, et al. Elastography: A new diagnostic tool for evaluation of obstructive diseases of the salivary glands; primary results. Clin Hemorheol Microcirc 2012; 50: 91–99. [DOI] [PubMed] [Google Scholar]
- 45.Fujita A, Sakai O, Chapman MN, et al. IgG4-related disease of the head and neck: CT and MR imaging manifestations. Radiographics 2012; 32: 1945–1958. [DOI] [PubMed] [Google Scholar]
- 46.De Cocker LJ, D‘Arco F, De Beule T, et al. IgG4-related systemic disease affecting the parotid and submandibular glands: Magnetic resonance imaging features of IgG4-related chronic sclerosing sialadenitis and concomitant lymphadenitis. Clin Imaging 2014; 38: 195–198. [DOI] [PubMed] [Google Scholar]