Abstract
Background
A surgical site infection (SSI) is a frequent complication following gastrointestinal surgery, but the careful selection and administration of prophylactic antibiotics can reduce the risk. The aim of this study was to develop a package of interventions that could be used to improve surgical antibiotic prophylaxis (SAP) at our institution.
Methods
A pre-post quality improvement project at a private hospital in Saudi Arabia was conducted between January 2014 until July 2016. A multidisciplinary team was assembled to identify and overcome barriers that were responsible for patients receiving suboptimal antibiotic prophylaxis. Patients were included if they had undergone surgery on their appendix, colon, rectum, or small intestine. Compliance with use of an adapted order form, as well as appropriate antibiotic selection, dosing, timing, and timing of re-dosing, were measured. Data on the rates of SSI before and after the intervention were also obtained.
Results
Of the 269 patients included in the study, 161 (61.5%) had appendix surgery, 86 (32.8%) had colorectal surgery, and 15 (5.7%) had small bowel surgery. The surgery was performed laparoscopically in 218 (83.5%) of patients. Utilization of the adapted order form increased from 1.8% to 92.0% following the intervention (p < 0.001). Compliance with a bundle of appropriate antibiotic selection, dosing and timing improved from 47.3% to 82.2% after the intervention (p < 0.001). Additionally, there was a non-statistically significant reduction in SSI rate (9.1% vs 5.1%; p = 0.27).
Conclusions
Our quality improvement intervention was successful in improving SAP for patients undergoing gastrointestinal surgery at our institution.
Keywords: Quality improvement, Patient safety, Digestive system surgical procedures, Global surgery, Antibiotic prophylaxis
Highlights
-
•
A high rate of surgical site infections was identified at our institution.
-
•
A quality improvement intervention successfully increased the use of antibiotic prophylaxis.
-
•
A multidisciplinary team was a key enabling factor.
1. Introduction
According to the United States Centers for Disease Control and Prevention (CDC), Surgical Site Infections (SSIs) are infections that occur at or near the surgical incision within 30 days of the procedure [1]. SSIs occur in 2–5% of patients undergoing surgery in the United States, accounting for approximately 38% of hospital-acquired infections [1], [2], [3]. SSIs are associated with increased morbidity, mortality, length of stay, and cost [4], [5], [6].
The incidence of SSI following colorectal surgery remains much higher than for other types of surgery, likely due to exposure to gut flora. The National Healthcare Safety Network (NHSN), which facilitates the surveillance of healthcare-associated infections across a large network of hospitals in the United States, report SSI rates that are stratified by procedure type and risk index [7], [8]. In patients with the highest risk index score, the mean SSI rate following colon and rectal surgery was 9.47% and 26.67%, respectively [7]. Infection rates were not as high in patients requiring appendix surgery, with only 3.47% of high-risk patients developing an SSI [7]. These rates, while lower than they used to be in the 1990s [9], are still unacceptably high. The infectious organisms most frequently isolated from colorectal SSIs include staphylococci, enterococci, bacteroides thetaiotaomicron, clostridium innocuum, and eubacterium lentum [10].
Considering this problem, many hospitals have undertaken initiatives to reduce SSI rates following colorectal and appendix surgery [11], [12], [13], [14], [15], [16], [17], [18], [19]. Specifically, most studies have included surgical antibiotic prophylaxis (SAP) within a surgical care bundle to counter colorectal SSIs [20]. Performance measures regarding SAP have focused on appropriate antibiotic selection, dose, timing of administration, timing of re-dosing, and discontinuation [20]. In 2016, the World Health Organization published evidence-based recommendations for SSI prevention [21], [22].
At our hospital, general surgeons agreed that one of the biggest areas for improvement in the department was reducing the incidence of SSIs. For example, the incidence of SSI at our institution following colon surgery was approximately 20%, which was much higher than many other hospitals [16], [17]. Thus, to address this problem, a quality improvement project was initiated to understand why SSI rates were so high among patients undergoing gastrointestinal surgery at our institution and to design and implement interventions that would help address this issue. We hypothesized that a quality improvement intervention would decrease the SSI rate at our institution. We also examined factors that we thought may influence compliance with antibiotic prophylaxis for the purpose of informing future interventions.
2. Methods
2.1. Study information
This study was approved by the Institutional Review Board at Johns Hopkins Aramco Healthcare. This pre-post interventional study was conducted in Johns Hopkins Aramco Hospital.
2.2. Participants
Patients that underwent gastrointestinal surgery between January 2014 and July 2016 were included in the study. However, data was not collected on all patients meeting the inclusion criteria due to time and staffing limitations, and the patients that were included represented a convenience sample.
2.3. Process
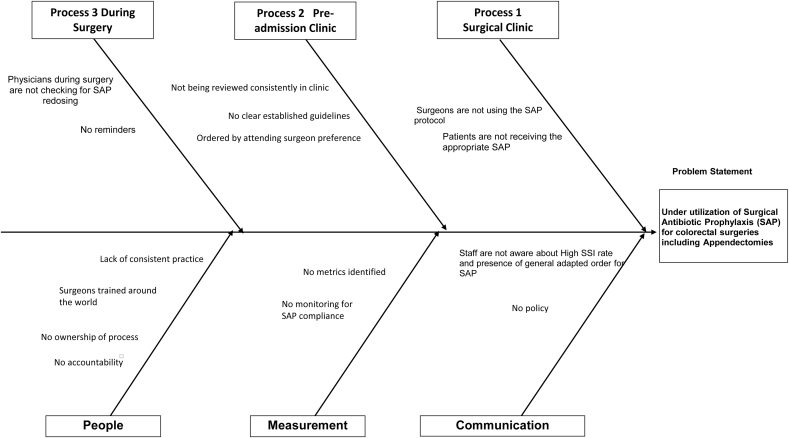
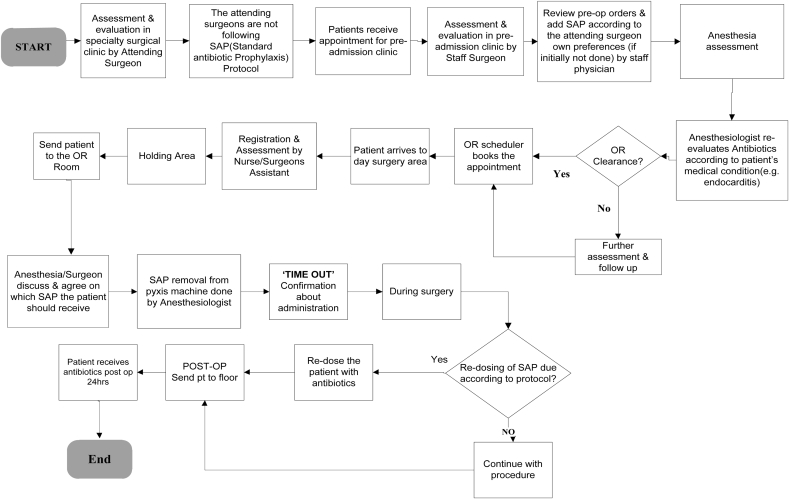
An interdisciplinary team was created to address a safety issue in the department of surgery. Physicians, nurses and quality improvement specialists (surgeons became involved 3 months later) came together and decided that SAP would be the target of a quality improvement intervention, as there was concern that this was not being done consistently. Thus, initial data was collected to assess the nature and the magnitude of the problem. An Ishikawa diagram (Figure A.1) was created by the team to brainstorm the various issues that might be preventing appropriate SAP and a process map (Figure A.2) was generated to identify opportunities for intervention. An action plan was then developed to implement a package of interventions designed to improve SAP for patients undergoing gastrointestinal surgery.
2.4. Measures
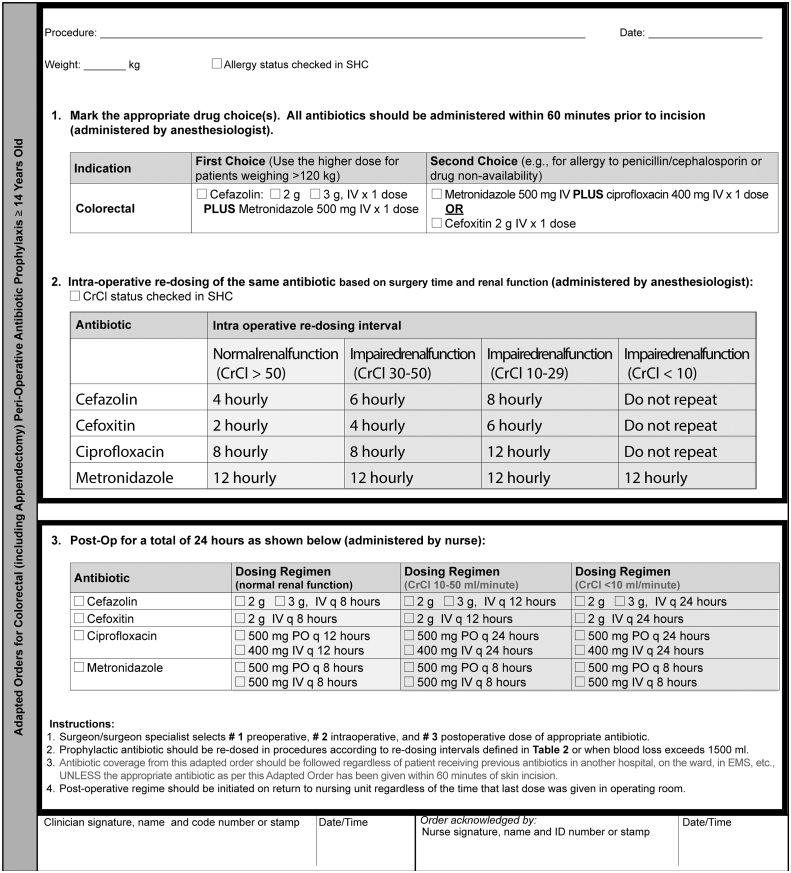
Eight process measures were tracked over the duration of the study. One of these measures was whether an adapted order form was completed on behalf of the patient by the surgeon prior to surgery. Other process measures that were also recorded included: whether SAP was given, appropriate type of antibiotic, appropriate dose, appropriate timing of administration, appropriate re-dosing and appropriate discontinuation. An additional process measure, named bundle, was created to capture whether compliance was achieved with three of the existing process measures: antibiotic type, dose, and timing. The criteria that determined whether the antibiotic type, dose, timing of administration, timing of re-dosing, and timing of discontinuation was appropriate are described in the adapted order form (Fig. 1).
Fig. 1.
Newly designed adapted order form for colorectal surgery (including appendectomy) peri-operative antibiotic prophylaxis for patients 14 years or older.
Other patient-related data were also collected, including patient demographics, type of procedure, and whether that procedure was laparoscopic or open. The type of procedure was coded using 4 categories: appendix surgery, colon surgery, rectal surgery, and small bowel surgery.
Baseline data was initially collected on patients undergoing the specified surgeries from January 1st, 2014 to September 30th, 2014. Following the implementation of the intervention, data collection resumed on July 1st, 2015 and continued until July 31st, 2016.
Furthermore, the rate of surgical site infection that occurred was recorded by the division for infection control throughout the duration of the study. A surgical site infection was reported if the CDC criteria for a surgical site infection was met [23]. Active surveillance was performed to follow up with patients up to 30 days following the surgery.
2.5. Interventions
Five interventions were implemented to help improve compliance with the correct administration of SAP. These were implemented during the period from April, 2015 to July, 2015. First, the adapted order form was modified and included in the hospital policy. Second, nursing staff were instructed to give SAP re-dosing reminders during operation. Third, interdisciplinary education and discussion sessions were held to draw awareness to the project. Fourth, providers that were not compliant with giving SAP per protocol were identified and given personal reminders. Fifth, monthly reports were given that highlighted the compliance with SAP for that month.
2.6. Analysis
Statistical analysis was performed using STATA 12 (StataCorp, College Station, Texas, USA). A p-value of <0.05 was considered statistically significant. The Student's t-test was used to compare age before and after the intervention while the chi square test was used to compare sex, procedure, and procedure type before and after the intervention. The chi square test was used to compare the compliance with the various process measures before and after the intervention. Univariate and multivariate logistic regression was used to model factors that predicted both compliance with a SAP bundle and SSI incidence, including age, sex, procedure type, whether the procedure was done laparoscopically or not, and intervention. All predictor variables were included in the multivariate analysis as they were all considered relevant.
3. Results
During the study period, data was collected on 269 patients that underwent gastrointestinal surgery. The breakdown by type of surgery is as follows: 161 (59.9%) had appendix surgery, 80 (29.7%) had colon surgery, 6 (2.2%) had rectal surgery, and 15 (5.6%) had small bowel surgery. The surgery was performed laparoscopically in 218 (83.5%) of patients. Baseline data stratified by intervention are presented in Table 1.
Table 1.
Baseline characteristics.
| Characteristic | Pre-Intervention (N = 55) | Post-Intervention (N = 214) | P-value |
|---|---|---|---|
| Age Mean, SD | 46.4 (19.6) | 40.5 (19.8) | 0.05 |
| Male N (%) | 29 (52.7) | 130 (61.9) | 0.22 |
| Procedure N (%) | – | – | 0.02 |
| Appendix surgery | 25 (47.2) | 136 (65.1) | – |
| Colon surgery | 19 (35.9) | 61 (29.2) | – |
| Rectal surgery | 3 (5.7) | 3 (1.4) | – |
| Small bowel surgery | 6 (11.3) | 9 (4.3) | – |
| Laparoscopic N (%) | 36 (67.9) | 182 (87.5) | 0.001 |
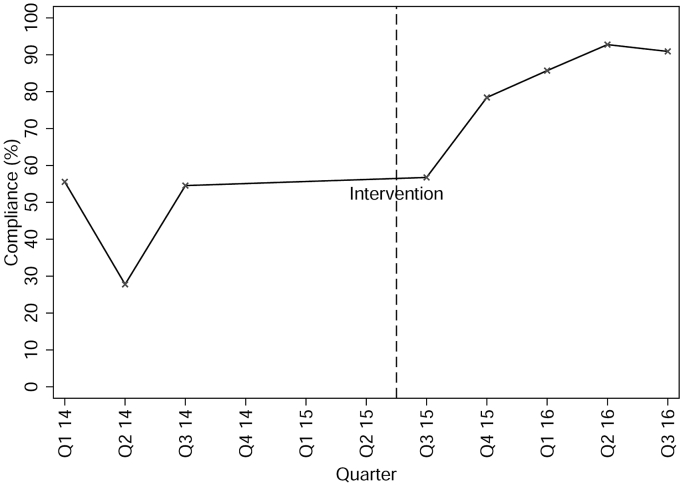
Table 2 illustrates how the proportion of patients that received appropriate SAP differed before and after the intervention. Appropriate dosing, timing of dosing, and timing of re-dosing all significantly improved following the intervention, but appropriate antibiotic selection did not. Fig. 2 demonstrates how compliance changed over time for the SAP bundle of antibiotic selection, dose, and timing per protocol. Bundle compliance improved significantly following the intervention (47.3% vs 82.2%; p < 0.001).
Table 2.
Compliance before and after the intervention on several different metrics.
| Compliance with antimicrobial prophylaxis | Pre-Intervention N (%) | Post-Intervention N (%) | P-value |
|---|---|---|---|
| Adapted order set used | 1 (1.8) | 196 (92.0) | <0.001 |
| Selection | 44 (80.0) | 185 (86.9) | 0.20 |
| Dose | 36 (65.5) | 188 (88.7) | <0.001 |
| Timing | 41 (74.6) | 200 (93.9) | <0.001 |
| Re-dosing (if indicated) | 0 (0.0) | 28 (90.3) | <0.001 |
| Bundle (SAP selection, dose, and timing per protocol) | 26 (47.3) | 176 (82.2) | <0.001 |
Fig. 2.
Change in compliance over time of the SAP bundle (antibiotic selection, dose, and timing all performed correctly per our protocol).
The results of the univariate and multivariate analysis examining factors that predicted bundle compliance are presented in Table 3. On multivariate analysis, the odds of bundle compliance were found to be 6.09 times higher following the intervention (p < 0.001) and 2.7 times higher in patients that had surgery on their colon as opposed to having surgery on their appendix (p = 0.05).
Table 3.
Univariate and multivariate analysis of compliance with a SAP bundle (antibiotic selection, dose, and timing given per protocol).
| Characteristic | Univariate Analysis (N = 269) |
Multivariate Analysis (N = 261) |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Intervention | 5.1 (2.7–9.4) | 0.00 | 6.1 (3.0–12.3) | 0.00 |
| Age | 1.0 (0.99–1.0) | 0.94 | 0.99 (0.97–1.0) | 0.36 |
| Sexa | 1.2 (0.67–2.0) | 0.59 | 0.97 (0.52–1.8) | 0.92 |
| Procedureb | – | – | – | – |
| Colon surgery | 1.4 (0.76–2.7) | 0.26 | 2.7 (0.99–7.2) | 0.05 |
| Rectal surgery | 1.9 (0.22–17.1) | 0.55 | 6.6 (0.55–79.2) | 0.14 |
| Small bowel surgery | 1.6 (0.42–5.8) | 0.51 | 5.1 (0.86–30.0) | 0.07 |
| Laparoscopic | 1.2 (0.61–2.5) | 0.55 | 1.5 (0.51–4.4) | 0.46 |
Reference group = Male.
Reference group = Appendix surgery.
Prior to the intervention, 5 (9.1%) patients suffered a SSI, compared to 11 (5.1%) following the intervention (p = 0.27). On multivariate analysis, older age (p = 0.03) was a risk factor for SSI and patients that had an appendectomy had a significantly lower chance of developing an SSI compared to patients who had colon (p = 0.00), rectal (p = 0.00), or small bowel (p = 0.00) surgery (Table 4).
Table 4.
Univariate and multivariate analysis of the factors that predicted development of a surgical site infection.
| Characteristic | Univariate Analysis (N = 269) |
Multivariate Analysis (N = 261) |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Intervention | 0.54 (0.18–1.6) | 0.28 | 0.87 (0.25–3.1) | 0.84 |
| Age | 1.0 (1.0–1.1) | 0.08 | 0.95 (0.91–0.99) | 0.03 |
| Sexa | 3.1 (0.85–11.0) | 0.09 | 3.6 (0.89–14.6) | 0.07 |
| Procedureb | – | – | – | – |
| Colon surgery | 25.5 (3.2–201.4) | 0.00 | 123.7 (9.8–1566.1) | 0.00 |
| Rectal surgery | 32.0 (1.7–588.1) | 0.02 | 204.7 (6.4–6527.6) | 0.00 |
| Small bowel surgery | 40.0 (3.9–414.4) | 0.00 | 68.2 (4.7–984.6) | 0.00 |
| Laparoscopic | 0.22 (0.08–0.63) | 0.01 | 0.60 (0.16–2.3) | 0.45 |
Reference group = Male.
Reference group = Appendix surgery.
4. Discussion
The findings from this study demonstrate that we substantially improved compliance to a bundle of interventions designed to reduce the incidence of an SSI following gastrointestinal surgery. Following the intervention, the adapted order form was used more frequently and there were clear improvements in the proportion of patients that received SAP that complied with the protocol that was developed in terms of antibiotic dose, timing, and timing of re-dosing. The rate of SSIs was also observed to have decreased after the intervention, although this was not statistically significant.
Interestingly, compliance with the bundle of appropriate antibiotic selection, dosing, and timing was lower for appendix surgery compared to the other types of surgeries. Perhaps this reflects that staff are less mindful of ensuring comprehensive SAP in patients undergoing appendectomies due to the lower risk of infection [7]. Patient age, sex, and whether the surgery was performed laparoscopically did not significantly influence compliance to the bundle.
Several barriers were encountered that threatened to prevent us from successfully implementing the intervention. Originally, when the SAP protocol was first developed, the surgeons were not involved in the process of deciding upon the specifics of the protocol and in designing the adapted order form. However, once they were involved in designing the adapted order form, they felt much more invested and subsequently were much more likely to complete the form. Furthermore, the surgeons did not actually realize that there was a high infection rate at our hospital, but once they were made aware of this data, they became more inclined to alter their practice. Additionally, given that surgeons at our hospital were trained at different centers across the world, they had their own, distinctive practice regarding SAP, and so it took time for the surgeons to embrace the institutional policy for SAP.
One of the strengths of this project was that it was multidisciplinary in nature, involving surgeons, anaesthesiologists, nurses, pharmacists, and staff from quality improvement/infection control. Other studies have commented on the importance of having multidisciplinary involvement in improving SAP compliance [13], [14], [15], [17], [18], [24], [25], [26]. Due to having a multidisciplinary team, a wide range of perspectives were available when deciding upon the hospital's SAP policy. Additionally, each member of the team had a role to play in ensuring compliance with the policy: a few surgeons and anaesthesiologists functioned effectively as change agents by convincing their colleagues to follow the protocol; pharmacists decided that only antibiotics listed in SAP adapted order form would be stocked in the electronic medication dispensing cabinets; nurses were responsible for giving reminders regarding time of re-dosing in the operating room; and quality improvement staff were responsible for providing monthly feedback to the team regarding compliance with SAP. Regular feedback has also been identified as an important aspect for improving SAP compliance [14], [25], [26], [27].
Our study had several limitations. First, because each of the interventions was implemented around the same time, it is not possible to determine which interventions had the biggest impact on improving compliance. Second, we did not collect data on all patients that met the inclusion criteria during the study period due to time and staffing constraints, thereby creating the possibility for sampling bias. Third, our study was insufficiently powered to detect small changes in SSI rates because of the intervention. This prohibited us from determining whether compliance with any of the process measures included in our study was predictive of the eventual development of a SSI.
In the future, we are planning to employ a more comprehensive approach to SSI prevention beyond ensuring compliance with SAP. The recent guidelines offered by the WHO for pre-operative, peri-operative, and post-operative strategies to reduce SSI's provide a framework to achieve this [21], [22]. In addition, we plan to expand our SSI prevention quality improvement initiative to other types of surgeries and other departments.
5. Conclusions
Our multidisciplinary quality improvement initiative successfully improved SAP compliance for patients undergoing gastrointestinal surgery. Keys to the success of the project included systematically identifying and addressing barriers that were impeding compliance, empowering all members of the multidisciplinary team, and ensuring that regular feedback was given regarding compliance.
Ethical approval
Ethical approval was received by Johns Hopkins Aramco Healthcare Hospital.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
Study design: Rabie Kilan, Wadie Binsaddiq, Corrine Quinn, Michael H. Johnson.
Data collection: Rabie Kilan, Iyad Eid, Christopher Okeahialam, Tammy Williams, Dane Moran.
Data analysis: Dane Moran.
Manuscript preparation: Dane Moran.
Critical revision: Rabie Kilan, Iyad Eid, Christopher Okeahialam, Corrine Quinn, Wadie Binsaddiq, Tammy Williams, Michael H. Johnson.
Conflicts of interest
Nothing to disclose.
Guarantor
Rabie Kilan.
Research registration unique identifying number (UIN)
N/A.
Acknowledgements
The authors would like to thank Johns Hopkins Aramco Healthcare, the Armstrong Institute, and Johns Hopkins International for supporting this research.
Appendix.
Fig. A.1.
Ishikawa diagram for categorising the barriers preventing adequate antibiotic prophylaxis.
Fig. A.2.
Process map detailing the steps involved with providing SAP.
References
- 1.Horan T.C., Gaynes R.P., Martone W.J., Jarvis W.R., Emori T.G. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect. Control Hosp. Epidemiol. 1992;13:606–608. [PubMed] [Google Scholar]
- 2.The Society for Hospital Epidemiology of America; The Association for Practitioners in Infection Control; The Centers for Disease Control; The Surgical Infection Society Consensus paper on the surveillance of surgical wound infections. Infect. Control Hosp. Epidemiol. 1992;13:599–605. [PubMed] [Google Scholar]
- 3.Lewis S.S., Moehring R.W., Chen L.F., Sexton D.J., Anderson D.J. Assessing the relative burden of hospital-acquired infections in a network of community hospitals. Infect. Control Hosp. Epidemiol. 2013;34:1229–1230. doi: 10.1086/673443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vegas A.A., Jodra V.M., Garcia M.L. Nosocomial infection in surgery wards: a controlled study of increased duration of hospital stays and direct cost of hospitalization. Eur. J. Epidemiol. 1993;9:504–510. [PubMed] [Google Scholar]
- 5.Poulsen K.B., Bremmelgaard A., Sorensen A.I., Raahave D., Petersen J.V. Estimated costs of postoperative wound infections. A case-control study of marginal hospital and social security costs. Epidemiol. Infect. 1994;113:283–295. doi: 10.1017/s0950268800051712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce J.M., Potter-Bynoe G., Dziobek L. Hospital reimbursement patterns among patients with surgical wound infections following open heart surgery. Infect. Control Hosp. Epidemiol. 1990;11:89–93. doi: 10.1086/646127. [DOI] [PubMed] [Google Scholar]
- 7.J.R. Edwards, K.D. Peterson, Y. Mu, S. Banerjee, K. Allen-Bridson, G. Morrell, M.A. Dudeck, D.A. Pollock, T.C. Horan, National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009, Am. J. Infect. Control. 37 (2009) 783–805. [DOI] [PubMed]
- 8.Gaynes R.P., Culver D.H., Horan T.C., Edwards J.R., Richards C., Tolson J.S. Surgical site infection (SSI) rates in the United States, 1992-1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Clin. Infect. Dis. 2001;33(2):S69–S77. doi: 10.1086/321860. [DOI] [PubMed] [Google Scholar]
- 9.Culver D.H., Horan T.C., Gaynes R.P., Martone W.J., Jarvis W.R., Emori T.G., Banerjee S.N., Edwards J.R., Tolson J.S., Henderson T.S. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am. J. Med. 1991;91:152S–157S. doi: 10.1016/0002-9343(91)90361-z. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein E.J., Citron D.M., Merriam C.V., Abramson M.A. Infection after elective colorectal surgery: bacteriological analysis of failures in a randomized trial of cefotetan vs. ertapenem prophylaxis. Surg. Infect. (Larchmt) 2009;10:111–118. doi: 10.1089/sur.2007.096. [DOI] [PubMed] [Google Scholar]
- 11.Guanche Garcell H., Villanueva Arias A., Pancorbo Sandoval C., Valle Gamboa M.E., Bode Sado A., Alfonso Serrano R.N. Impact of a focused antimicrobial stewardship program in adherence to antibiotic prophylaxis and antimicrobial consumption in appendectomies. J. Infect. Public. Health. 2016 doi: 10.1016/j.jiph.2016.06.006. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Bull A., Wilson J., Worth L.J., Stuart R.L., Gillespie E., Waxman B., Shearer W., Richards M. A bundle of care to reduce colorectal surgical infections: an Australian experience. J. Hosp. Infect. 2011;78:297–301. doi: 10.1016/j.jhin.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Keenan J.E., Speicher P.J., Thacker J.K., Walter M., Kuchibhatla M., Mantyh C.R. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. 2014;149:1045–1052. doi: 10.1001/jamasurg.2014.346. [DOI] [PubMed] [Google Scholar]
- 14.Wick E.C., Hobson D.B., Bennett J.L., Demski R., Maragakis L., Gearhart S.L., Efron J., Berenholtz S.M., Makary M.A. Implementation of a surgical comprehensive unit-based safety program to reduce surgical site infections. J. Am. Coll. Surg. 2012;215:193–200. doi: 10.1016/j.jamcollsurg.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Connolly T.M., Foppa C., Kazi E., Denoya P.I., Bergamaschi R. Impact of a surgical site infection reduction strategy after colorectal resection. Colorectal Dis. 2016;18:910–918. doi: 10.1111/codi.13145. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt D.B., Tannouri S.S., Burkhart R.A., Altmark R., Goldstein S.D., Isenberg G.A., Phillips B.R., Yeo C.J., Cowan S.W. Reducing colorectal surgical site infections: a novel, resident-driven, quality initiative. Am. J. Surg. 2016 doi: 10.1016/j.amjsurg.2016.04.009. (in press) [DOI] [PubMed] [Google Scholar]
- 17.Cima R., Dankbar E., Lovely J., Pendlimari R., Aronhalt K., Nehring S., Hyke R., Tyndale D., Rogers J., Quast L. Colorectal Surgical Site Infection Reduction Team, Colorectal surgery surgical site infection reduction program: a national surgical quality improvement program–driven multidisciplinary single-institution experience. J. Am. Coll. Surg. 2013;216:23–33. doi: 10.1016/j.jamcollsurg.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 18.DeHaas D., Aufderheide S., Gano J., Weigandt J., Ries J., Faust B. Colorectal surgical site infection reduction strategies. Am. J. Surg. 2016;212:175–177. doi: 10.1016/j.amjsurg.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Wick E.C., Gibbs L., Indorf L.A., Varma M.G., Garcia-Aguilar J. Implementation of quality measures to reduce surgical site infection in colorectal patients. Dis. Colon Rectum. 2008;51:1004–1009. doi: 10.1007/s10350-007-9142-y. [DOI] [PubMed] [Google Scholar]
- 20.Tanner J., Padley W., Assadian O., Leaper D., Kiernan M., Edmiston C. Do surgical care bundles reduce the risk of surgical site infections in patients undergoing colorectal surgery? A systematic review and cohort meta-analysis of 8,515 patients. Surgery. 2015;158:66–77. doi: 10.1016/j.surg.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Allegranzi B., Bischoff P., de Jonge S., Kubilay N.Z., Zayed B., Gomes S.M., Abbas M., Atema J.J., Gans S., van Rijen M., Boermeester M.A., Egger M., Kluytmans J., Pittet D., Solomkin J.S. WHO Guidelines Development Group, New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect. Dis. 2016 doi: 10.1016/S1473-3099(16)30398-X. (in press) [DOI] [PubMed] [Google Scholar]
- 22.Allegranzi B., Zayed B., Bischoff P., Kubilay N.Z., de Jonge S., de Vries F., Gomes S.M., Gans S., Wallert E.D., Wu X., Abbas M., Boermeester M.A., Dellinger E.P., Egger M., Gastmeier P., Guirao X., Ren J., Pittet D., Solomkin J.S. WHO Guidelines Development Group, New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect. Dis. 2016 doi: 10.1016/S1473-3099(16)30402-9. [DOI] [PubMed] [Google Scholar]
- 23.CDC . SSI; 2017. Procedure-associated Module. (in press) [Google Scholar]
- 24.Telfah S., Nazer L., Dirani M., Daoud F. Improvement in adherence to surgical antimicrobial prophylaxis guidelines after implementation of a multidisciplinary quality improvement project. Sultan Qaboos Univ. Med. J. 2015;15:e523–e527. doi: 10.18295/squmj.2015.15.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutherland T., Beloff J., Lightowler M., Liu X., Nascimben L., Kaye A.D., Urman R.D. Description of a multidisciplinary initiative to improve SCIP measures related to pre-operative antibiotic prophylaxis compliance: a single-center success story. Patient Saf. Surg. 2014;8 doi: 10.1186/s13037-014-0037-2. 37–014-0037-2. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putnam L.R., Chang C.M., Rogers N.B., Podolnick J.M., Sakhuja S., Matusczcak M., Austin M.T., Kao L.S., Lally K.P., Tsao K. Adherence to surgical antibiotic prophylaxis remains a challenge despite multifaceted interventions. Surgery. 2015;158:413–419. doi: 10.1016/j.surg.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Ozgun H., Ertugrul B.M., Soyder A., Ozturk B., Aydemir M. Peri-operative antibiotic prophylaxis: adherence to guidelines and effects of educational intervention. Int. J. Surg. 2010;8:159–163. doi: 10.1016/j.ijsu.2009.12.005. [DOI] [PubMed] [Google Scholar]