Abstract
The fruit fly Drosophila melanogaster performs many behaviors, from simple motor actions to complex social interactions, that are of interest to neurobiologists studying how the brain controls behavior. Here, an undergraduate laboratory exercise uses cutting-edge methods to activate sets of neurons thermogenetically, triggering 60 different behaviors. Students learn how to recognize this large repertoire of behaviors from 16 fly strains that are publicly available, and from a large set of training videos provided here. A full protocol, timeline and handouts are included. Instructors need not have any experience working with flies. Student feedback is reported; in our experience, students are fascinated and highly engaged by watching animals perform such a broad array of behaviors. This exercise teaches fly husbandry and crossing, careful scientific observation, and principles of behavioral screening.
Keywords: Drosophila, behavior, genetics, screen, teaching, pedagogy, neuron, thermogenetic
The fruit or vinegar fly, Drosophila melanogaster, is one of the most widely used organisms in biological research. It has a fast generation time and is easy to raise for experiments with very little equipment (Greenspan, 2004), making it a popular organism for teaching purposes (Pulver and Berni, 2012). Drosophila has a large repertoire of behaviors. Behavior is of interest to the field of ethology for its own sake, and to the field of neuroscience for the investigation of how it is controlled by the nervous system. Behavior, neural circuits, and the genes that regulate these can all be studied in Drosophila.
Here, a laboratory exercise is presented that teaches students to recognize 60 behaviors that flies perform, from simple motor actions of single body parts, such as wing flapping, to more complicated social behaviors, such as courtship or aggression “dances.” Students can screen all of the fly strains in one session (of flexible timing, about 2–3 hours), and, if desired, can also assist the instructor in preparing the fly crosses in the weeks ahead of time. Full instructions and equipment lists are provided so that the instructor does not need to have any fly experience, or even to consult any other reference material unless desired. A detailed timeline of fly crosses is explained.
The instructor can tailor the exercise to students of any level. Undergraduates with a basic understanding of neurons and genes will emerge from this exercise with the knowledge of how to rear and cross Drosophila, how to conduct a behavioral screen, and how to recognize a large repertoire of actions that an organism can perform. Yet versions of the same exercise have been used for groups ranging from children as young as five years at community outreach events (by simply displaying the different fly behaviors), to Ph.D. level students (demonstrating the principles of behavioral screens and neural circuit mapping). The sophistication of the principles discussed can be tailored to undergraduates of different levels of experience. This exercise was initially developed for the Cold Spring Harbor Drosophila Neurobiology course, where it has been taught from 2012 to present. The exercise makes use of an extensive set of videos, provided here, that were originally recorded for training purposes for the Janelia Fly Olympiad team project.
To cause flies to perform the many behaviors demonstrated here, different sets of neurons in the fly brain are activated. A full understanding of the following principles is not necessary to teach or carry out the exercise, but they can be explained to more sophisticated students if desired. Transgenic flies have been made that contain an ion channel (TrpA1) that excites neurons in response to gentle heat (Hamada et al., 2008), under the control of a yeast “Upstream Activation Sequence” (UAS). The strain of flies containing UAS-TrpA1 is one part of a binary expression system that can be used to drive expression of a transgene of interest in particular tissues (Brand and Perrimon, 1993). This UAS strain can be crossed to flies containing the other part, GAL4, a yeast transcription factor that activates UAS, which here drives TrpA1. GAL4 has been cloned under the control of many different fly enhancer fragments in different fly strains, each of which drives expression in different sets of neurons. By crossing UAS-TrpA1 to several GAL4 lines, the experimenter can activate several different sets of neurons “thermogenetically” with heat. The GAL4 lines used are part of the Rubin collection (Pfeiffer et al., 2010), publicly available from the Bloomington Stock Center. They have been chosen because they each drive expression in several cell types, and were found to have many behavioral phenotypes in a screen carried out by author CM at Janelia Research Campus. For those accustomed to genetic screens, an important difference here is that no genes have been mutated; instead, neuronal activity is being manipulated, making this a “neuronal” screen.
MATERIALS AND METHODS
Equipment and reagents
Computer with an internet connection, able to play .mp4 videos with sound.
Stereomicroscope with light source (e.g., Carolina Biological Supply #597151, $73) or any means of magnifying flies 8–20×. (Optimal magnification is about 10×, but student stereomicroscopes often have a fixed magnification of 20×, which is acceptable. Professional fly-sorting microscopes can also be used, as they often have 10× oculars and a zoom range of 0.6–60, for a total magnification of 6–600×. A working distance of about 10cm is useful, so that a surface for fly sorting can fit underneath and a behavior chamber can be observed. If a stereomicroscope is not available, good quality magnifying glasses or other means of magnification may work; try this empirically by observing flies.)
Small room (approx. 100 ft2).
2–3 space heaters (available from home stores).
Thermometer.
Timer.
Fly morgue: fill a bottle part way with 70% ethanol (or vegetable oil or mineral oil), with a funnel in the opening for tapping in unwanted flies.
Small funnel (e.g., Carolina Biological Supply #734040: $11.60/pk of 10, 5cm diameter at top, 8mm diameter at bottom). Will be used to tap flies from vial into behavior chamber. Remove the funnel stem. A small funnel can also be made from common laboratory supplies such as the cut-off end of a conical tube. The large end must be wide enough to accommodate a fly vial and the small end should have an opening of 5–8mm, for transferring flies through the hole in the side of behavior chambers.
-
Behavior chamber material (see instructions below):
▪ 35×10mm tissue culture or petri dishes (e.g., Falcon #353001). Large lab supply companies sell by the case, but a single sleeve of 20 can often be ordered on eBay or other resellers.
▪ Index cards, file folders or other sturdy card stock.
▪ Pliers for making a hole in the petri dish.
▪ Tape.
Fly rearing supplies (food, vials, small paintbrushes for sorting). Carolina Biological Supply kit #173052 contains these supplies for 72 vials, $99.50. Follow instructions included for making food. Wash and reuse vials as needed.
Fly anesthesia system. Use a CO2-based fly sorting station if available. Otherwise, use cold anesthesia as follows. Tap flies into an empty vial and place in a bucket of ice until all flies stop moving. To keep flies anesthetized while sorting males and females with a paintbrush under a microscope, tap them onto an iced metal surface, about the size of a deck of cards, as a stage. For example, use an Altoids tin, painted white so flies can easily be seen, in a small container of ice (such as a food container) that can fit under the microscope.
Flies can be ordered, preferably from the Bloomington Stock Center http://flystocks.bio.indiana.edu/, or by request to the authors. Order all the lines listed in Table 1 ($169 for the full set, plus shipping and handling). For the exercise, each type of fly used for behavior will ultimately need to have two transgenes, UAS-TrpA1 to activate neurons and one of the GAL4s to target particular groups of neurons.
Table 1.
Drosophila stocks to order.
| Stock | Bloomington # |
|---|---|
| UAS-TrpA1 | 26263 |
| (will cross the above stock to the lines below:) | |
| Control (w1118) | 5905 |
| GMR10B03-GAL4 | 48241 |
| GMR13F07-GAL4 | 48575 |
| GMR15A01-GAL4 | 48670 |
| GMR15C01-GAL4 | 48682 |
| GMR15C05-GAL4 | 48683 |
| GMR18B07-GAL4 | 47476 |
| GMR19G09-GAL4 | 47886 |
| GMR21A03-GAL4 | 48920 |
| GMR21D07-GAL4 | 48943 |
| GMR23F03-GAL4 | 47496 |
| GMR24A06-GAL4 | 49056 |
| GMR26A02-GAL4 | 49149 |
| GMR26E01-GAL4 | 60510 |
| GMR27G01-GAL4 | 49233 |
| GMR41G04-GAL4 | 50136 |
Behavior chambers
If only one microscope is available, only one chamber is needed, but extras are useful if chambers become dirty or several students want to practice loading flies into chambers. Having 10–20 chambers is useful.
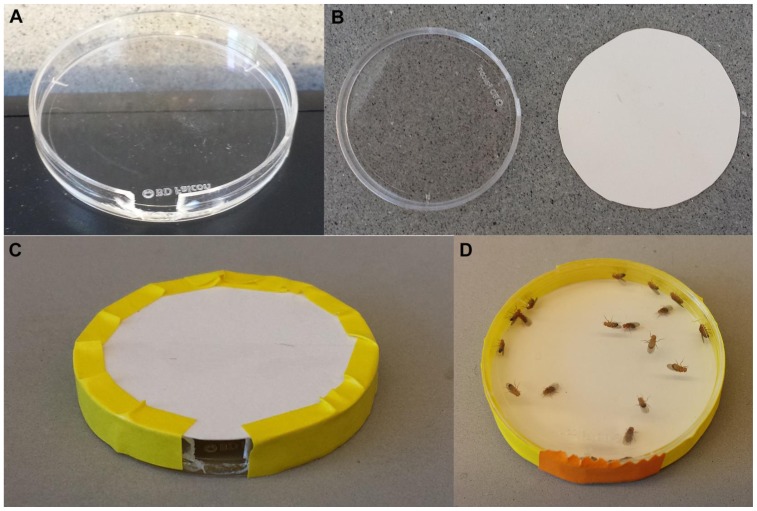
For each behavior chamber, use the lid of a 35×10mm petri dish, not the base, since the lower height of the lid (about 5.5mm) restricts the flies to a shallower depth of field for viewing. (1) Create a hole for loading flies into the behavior chamber: remove a small portion (about 8mm wide) of the side wall of the lid of the petri dish (Fig. 1A), by breaking the plastic with pliers. (2) Cut a circle of sturdy card stock to fit the bottom of the petri lid (Fig. 1B), and tape in place, without obscuring the loading hole or the clear top of the petri dish (Fig. 1C). (3) Cut a small piece of paper that will be used to tape over the hole once flies are loaded (Fig. 1D).
Figure 1.
Behavior chamber. (A) Lid of a 35mm petri or tissue culture dish, with ~8mm wide section removed from one side. (B) Circle of card stock cut to fit dish. (C) Card stock taped to dish, leaving loading hole free. (D) Once flies are loaded, seal the loading hole with a small piece of paper (here, hidden behind the orange tape).
Warm room
The most efficient way to activate neurons with TrpA1 is to simply carry out experiments in a warm room. A small room (approximately 100 ft2) can be warmed up sufficiently (to 30–32°C) with usually about two space heaters. Make sure nothing flammable is near the heaters. Space heaters frequently have an overheat safety shutoff mechanism, so rather than turn them on to full power, try medium power, and allow the room to come up to temperature over several hours. If the heaters cease to work, allow them to cool, then turn them on again at lower power. Alternatively, the heaters may have overloaded the power circuit; try turning the circuit breaker in the room’s panel back on. Place a thermometer in the room, monitor every few hours, and adjust the heaters as necessary to stabilize the temperature in the desired range of 30–32°C. Consider beginning this at least a day ahead of the class, so that enough time remains to obtain a third space heater if two are not sufficient to heat the room. If a small room is not available, a heat lamp (from a pet supply store) may serve as an alternative for heating the flies; test to determine whether it can heat the behavior chamber sufficiently.
Fly rearing and crossing
Eleven weeks before the exercise, order flies from the Bloomington Stock Center. Store flies at room temperature (18–22°C). Avoid exposing flies to higher temperatures while rearing, since TrpA1 is activated at 26°C (Hamada et al., 2008). After flies are put into an unused food vial, they will lay eggs, larvae will hatch, pupae will form on the walls of the vial, and the next generation of adults will emerge from the pupal cases. A single generation takes 12–13 days at 22°C, 13–14 days at 21°C, and 18–19 days at 18°C. Time the crosses accordingly (see Table 2). To keep fly stocks long term, flip onto fresh food every two weeks (at 22°C). Keep both old and new copies, discarding old copies once they are four weeks old.
Table 2.
Timeline (for crosses kept near 22°C).
| Week –11: Order fly stocks from Bloomington. |
| Week –8: Next generation of adults will have emerged from the vials that were shipped. Anesthetize the Trp vial and count the number of females available. Expand this stock: divide into as many vials as possible with at least 5 females each (and any number of males; most females will already have been fertilized). Flip all other lines onto fresh food and save old copy. |
| Week –6: When next generation emerges from Trp vials, divide into 20 vials of at least 5 females each (and any number of males). Flip all other lines onto fresh food as well, save one old copy, discard oldest copy. After 3–5 days, flip these 20 Trp vials into fresh vials to provide backup copies. |
| Week –4: Collect virgin females from 20 Trp vials (and backup copies, if necessary) for several days of this week. (See instructions under “Trp flies” section.) |
| Week –3: Cross Trp × all GAL4 lines and w1118 control. |
| Week –2: Discard parents from Trp × GAL4 crosses. |
| Week –1: Collect Trp × GAL4 progeny. |
| Week 0: Behavior exercise |
Trp flies
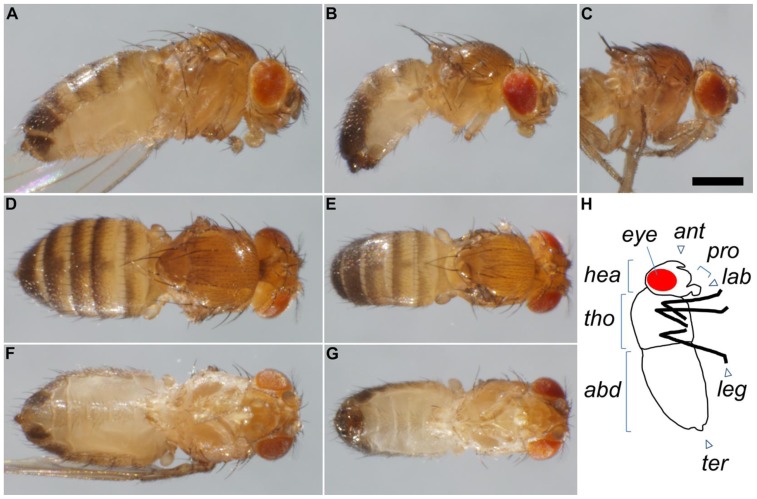
To count Trp flies or select males and females, anesthetize as described above. Females can be identified as having less black on the rear of their dorsal abdomen than males (Fig. 2). To maintain other lines until needed for crossing, simply tap vials down then flip onto fresh vials.
Figure 2.
Features of flies. Females (left column) and males (center column) viewed from side (A, B), top (D, E) and bottom (F, G), showing that males are smaller, have more black on the end of the abdomen, and have darker genital structures than females. (C) Some fly strains may contain the TM3 balancer, with a mutation causing a “stubble” phenotype. Note the shorter bristles on the back than in (B). After crossing a GAL4 strain carrying TM3 to UAS-TrpA1, the progeny will be sorted under anesthesia to discard any flies carrying the stubble marker. Scale bar: 0.5mm. (H) Diagram of fly body parts, viewed from side. Abbreviations, in alphabetical order: abd: abdomen, ant: antennae, eye: eye, hea: head, lab: labellum (lips), leg: legs, pro: proboscis, ter: terminalia (which include the anus and genitals, and in females, also the ovipositor, an egg-laying tube that can be extended), tho: thorax.
To collect virgin females from the Trp vials in week −4, clear the vials of all adults each morning, and collect all females before 8 hours have passed (as long as the room temperature is 25°C or below) to ensure that they are young enough to be virgin. House virgins in fresh vials in groups of up to 100. Continue this procedure daily until at least 80 virgins have been collected for every group of students that wishes to have their own set of 16 crosses for testing. (You can do one set of flies for whole class or let smaller groups of students each do their own set.)
Behavior crosses (3 weeks before exercise)
Have each group of students set up 16 crosses on unused food, each with 5 virgin females from the Trp stock and at least one (preferably about 5) male(s) from each of the 15 GAL4 stocks, plus another cross of Trp × w1118 as controls. Continue to store flies below 25°C.
Discard parents (2 weeks before exercise)
Tap all parents from the vials into the morgue and continue allowing the progeny to develop. Note the day that progeny begin to emerge as adults (see the approximate generation times, above).
Collect progeny (1 week before exercise)
Leave vials until 20 or more flies have emerged for the experiment, then tap flies into vials of unused food. Two of the GAL4 lines (GMR18B07 and GMR19G09) contain a balancer chromosome that must be discarded: when ready to collect progeny, anesthetize these two crosses for sorting. Discard those with abnormally short hairs on the back (“stubble”; Fig. 2C) and keep those with normal hairs.
[Optional: No additional knowledge of balancer chromosomes is necessary, but for more advanced students, mention that this balancer is called TM3. It is used because the GAL4 chromosome is homozygous lethal in these two stocks, probably because of a mutation in the genetic background. If the GAL4 chromosome were kept in the stock as a heterozygote, it would likely be lost over several generations. A balancer chromosome has several mutations and inversions, so it cannot recombine with a normal chromosome (Greenspan, 2004). It also contains mutations (such as the stubble phenotype) that allow for visual identification of flies that have this balancer. Since balancer chromosomes are homozygous lethal, flies can be stably maintained with one copy of each of the two different kinds of homozygous lethal chromosomes, the GAL4 and the balancer. When a cross is performed with these heterozygous flies, approximately half the progeny will have one of these chromosomes, and half will have the other. Since the GAL4 is needed for the experiments, the progeny that got the balancer are discarded.]
Also discard any flies with curly wings, if present. Allow all progeny to age approximately 1 week before the experiment, but this is flexible, as long as flies are at least 2 days old, and less than 10. Vials may get stickier over time; for longer wait times, transfer flies to fresh vials.
Two or more days before exercise
Turn on space heaters and begin stabilizing the temperature of the small room as described above. Suggest that students bring cool clothing such as shorts on the day of the exercise. Set up microscope in warm room.
At least one day before exercise
Place behavior chambers in the warm room. If it has not reached 30–32°C, add a third space heater. Print a copy of the handout (Supplement 1, Handout.docx), for each student. Its checklists include a section for each of the 16 crosses. Provide a computer nearby, with videos of fly behaviors (at https://www.youtube.com/playlist?list=PL2D0slyugueK6E9UKPqfe-fdQbiVHnlCz). Practice by loading some unneeded flies into chambers.
Behavior exercise
If only one microscope is available, each group will take turns observing flies on the day of the exercise. When not in the warm room, students can watch the videos demonstrating each behavior. There are videos for 60 behaviors; most but not all will be seen in the flies. Ensure that students don’t spend too long in the warm room and that drinking water is available.
Preheat behavior chambers in the warm room. Ask students to avoid getting fingerprints on the clear surface of the chamber. Focus the microscope above the chamber so that when the chamber is held by hand, it can be moved up and down to focus on flies on the floor or ceiling of the chamber. Bring checklist sheets into warm room.
Show students how to access the videos on the computer outside the warm room. Have them watch the video labeled “Normal.mp4,” to get a sense of the range of behaviors that control flies perform. Phenotypes observed when activating neurons will be behaviors that occur at higher frequency than they normally do in control flies. Phenotypes are explained in the handouts.
Provide unneeded extra flies for students to practice loading flies into behavior chambers (see Chambers.mp4). Remove vial cap and replace with small funnel, avoiding tapping flies down into the food. (Exposure to sticky food will cause flies to perform more grooming.) Instead, hold vial upside down to allow flies to run upward off the cap (due to gravitaxis), then replace cap with funnel. Tap about 20 flies (5 to 40 is acceptable) through the funnel into the behavior chamber and tape on a small piece of paper to seal opening. Discuss with students whether different densities of flies might affect behavior.
To observe fly behaviors, bring a preheated chamber out of warm room and load flies from one vial, then quickly return to warm room. Start by observing control flies (UAS-TrpA1 × w1118), but discuss the importance of screening blindly in a real screen. Start timer and observe flies under the microscope for 5 minutes. Instead of placing chamber on surface, hold it by hand, so that it can be moved to track individual flies as they walk, or to focus on floor or ceiling of chamber. Try to observe many different flies. Check off observed behaviors on checklist. Encourage students to try also to note penetrance of a phenotype (how often it is observed), and the sex in which it occurs.
Across the 16 lines, students should be able to observe a variety of normal and abnormal behaviors triggered by heat. Sixty of these are shown in the videos (linked below). Among them are: abdomen movement and posture changes, repetitive grooming (abdomen, antennae, head), head movement and posture (bent, nodding), locomotion (walking sideways, backwards, in circles), posture changes (narrow or wide stance, paralysis), proboscis movement (extension, spitting), terminalia movement (extension, egg extrusion), wing movement (flicking, flapping, extension, and social behavior (aggression, courtship, following). See Supplement 2, Phenotypes.pdf for behaviors seen by the first author in each of the 16 lines.
When finished with a chamber of flies, remove tape over hole, tap flies into morgue, and leave chamber in warm room for reuse. Alternatively, flies can be saved for retesting; many behaviors can be observed again if flies are allowed to cool then are re-warmed.
Screen as many of the 16 crosses as possible. When finished, ask students to compare the phenotypes they saw in each line. Points for discussion are outlined below.
DISCUSSION
This exercise gives students the opportunity to observe up to 60 different behaviors that flies perform. When defining a phenotype for scoring, an observer can create any kind of controlled vocabulary desired, and need not adhere to the definitions used here. The most important goal is internal consistency throughout a screen. Ask students if they observed any behaviors that were not on the list or would change anything about the behavioral definitions.
If desired, student results can be compared to the author’s observations (Supplement 2, Phenotypes.pdf). For many of these lines, the author screened them more than once, blindly. Students can check how reproducible the author’s observations were between different experiment entries and how consistent observations were between student groups. Ask students why observations may not always be consistent. Possible answers include biological variability, a change in experimental conditions, or a change in the experimenter’s observation skills over time. Point out that when designing screens, it is useful to incorporate a pilot phase to settle upon the methods or the phenotype definitions that will be used. Once the protocol has been decided upon, the lines from the pilot screen should be redone, but mixed into the larger screen blindly. Controls should also be mixed in blindly.
Discuss the difficulty of scoring behaviors that occur naturally in control flies, compared to behaviors that are not seen in controls in these conditions, and emerge only when turned on by Trp. For example, grooming is very common in controls, so detecting a grooming phenotype that is triggered by neuronal activation requires careful observation or quantification.
This is a high-throughput observational screen. If such a screen were carried out for research, a secondary assay would be developed to quantitatively study a phenotype of interest. Ask students to consider how behaviors could be quantified. Some phenotypes (such as subtle differences in locomotion speed or inter-fly distance) may be better scored by machine vision (Branson et al., 2009) than by human observation. Students could depict transitions between behaviors as ethograms (see Chen et al., 2002, for an example with Drosophila aggression).
This behavioral screening exercise complements other laboratory exercises that use thermal or optical activation of genetically modified Drosophila. For example, Berni et al. (2010) thermally activate TrpA1 to investigate neuro-anatomy and electrophysiology in larvae and locomotion in larvae and adults. Similarly, Pulver et al. (2011) investigate larval neurophysiology and locomotion by flashing light on flies that express channelrhodopsin in glutamatergic neurons. Titlow et al. (2015) use flies that express channel-rhodopsin in motor neurons and giant fibers to investigate components of escape behavior and the neurophysiology of flight muscles. Our exercise differs from these in concentrating on an earlier step in investigation of neural bases of behavior (behavioral screening) and examining a broad range of behaviors.
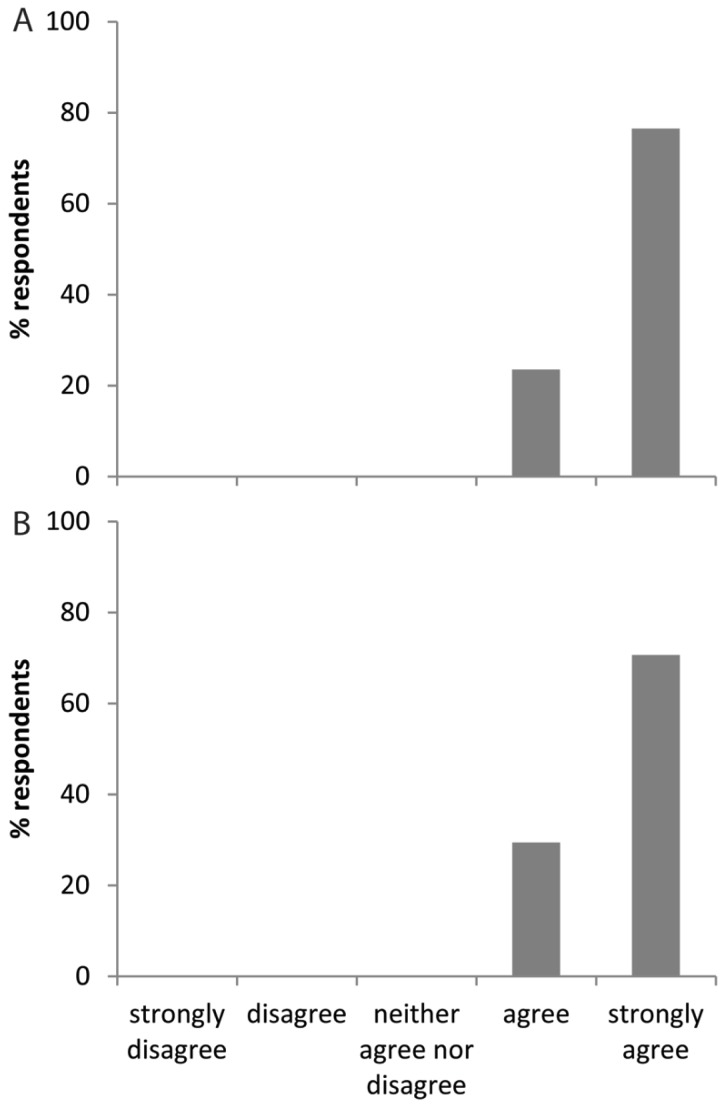
A survey was conducted of the students of the Cold Spring Harbor Drosophila Neurobiology course in 2015 and 2016, after they had carried out this exercise. Most strongly agreed that “The behavior observation lab activity was interesting” (Fig. 3A) and “The lesson made students more likely to consider using these tools and techniques in their own future work” (Fig. 3B). These classes were mostly composed of graduate students and postdocs, but an advanced science background is not required.
Figure 3.
Survey results. Responses of 17 students, from the Cold Spring Harbor Drosophila Neurobiology course in 2015 and 2016, to the statements (A) “The behavior observation lab activity was interesting” and (B) “The lesson made students more likely to consider using these tools and techniques in their future work.”
Discussion points for advanced students
The true purpose of screening GAL4 lines for behavioral phenotypes is not only to observe different behaviors that flies can perform, but to discover what neurons control those behaviors. Images of the cell types targeted in each GAL4 line are available online (http://flweb.janelia.org). The lines used here were chosen because they demonstrate many different behavior phenotypes, but they tend to have expression in large groups of neurons, not single cell types, so particular functions cannot be attributed to particular neurons. In a larger screen, however, multiple lines with a phenotype in common could be identified. If they contained any neurons in common, those neurons would be candidates for controlling that behavior. To determine whether those neurons are indeed involved, intersectional strategies such as split GAL4 (Luan et al., 2006) could be used to refine the expression pattern down to just the essential neurons.
Even if an expression pattern contained only one neuron type, triggering a behavioral phenotype does not prove that it is normally involved in that behavior. Additional experiments that can help to resolve that question include silencing those neurons to determine if the normal performance of that behavior is impaired, and recording or imaging those neurons during the behavior to determine if their activity is correlated with the movement (Venken et al., 2011). Finding that a group of cells shows some activation phenotype is just the beginning of exploring the functions of that cell group.
Each of the GAL4 lines used here is inserted in the same location in the genome, from the same strain of flies. Yet one line has wings that are deformed even before neurons are activated with Trp. Ask students to speculate about possible causes. One possibility is a background mutation in that line, unrelated to Trp function.
Finally, we defined behavior broadly for this exercise, ranging from simple movements (head nodding) through locomotion (walking in circles) to complex sequences (courtship). Each of these results from stimulating a particular set of neurons. We do not intend to suggest that stimulation of a set of neurons somehow defines a unit of behavior. Rather, these results may provide insight into the neural bases of behavior and the building blocks thereof. This exercise could be a springboard for class discussion of the meaning of “behavior” (Levitis et al., 2009).
CONCLUSION
With model organisms such as Drosophila, behaviors can be manipulated to discover how the nervous system controls those behaviors. The exercise presented here introduces students to cutting-edge genetic tools for the manipulation of fly behaviors and allows them to observe up to 60 of these behaviors first-hand, revealing principles about the design of behavioral screens.
Supplementary Information
Footnotes
SUPPLEMENTAL MATERIAL
Videos showing each behavior and showing how to load flies into the chamber are in a playlist on JUNE’s YouTube channel (https://www.youtube.com/playlist?list=PL2D0slyugueK6E9UKPqfe-fdQbiVHnlCz). The JUNE web site hosts a student handout and a table of phenotypes seen by the first author in each fly line as supplementary material for this article.
This work was supported by the Howard Hughes Medical Institute’s Janelia Research Campus, and a Janelia Teaching Fellowship (CM). Thank you to Drs. Julie Simpson and Wyatt Korff for providing leadership. The GAL4 lines were a gift of Drs. Barret Pfeiffer and Gerald Rubin. Other present and former Janelia colleagues contributed to the development and maintenance of that collection, participated in the Olympiad Observation Screen, and provided helpful discussions. Five years of students and instructors in the Drosophila Neurobiology course at Cold Spring Harbor participated in these exercises and improved them. The course is supported by Cold Spring Harbor Laboratory, the National Science Foundation (NSF grant IOS 1523125), and the National Institutes of Health (R13DA034437).
REFERENCES
- Berni J, Mudal A, Pulver SR. Using the warmth-gated ion channel TRPA1 to study the neural basis of behavior in Drosophila. J Undergrad Neurosci Educ. 2010;9:A5–A14. [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yeelin Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci USA. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan RJ. Fly pushing: the theory and practice of Drosophila genetics. 2nd edition. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2004. [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitis DA, Lidicker WZ, Freund G. Behavioural biologists don’t agree on what constitutes behaviour. Anim Behav. 2009;78:103–110. doi: 10.1016/j.anbehav.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo T-TB, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Berni J. The Fundamentals of flying: simple and inexpensive strategies for employing Drosophila genetics in neuroscience teaching laboratories. J Undergrad Neurosci Educ. 2012;11:A139–A148. [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Hornstein NJ, Land BL, Johnson BR. Optogenetics in the teaching laboratory: using channel-rhodopsin-2 to study the neural basis of behavior and synaptic physiology in Drosophila. Adv Physiol Educ. 2011;35:82–91. doi: 10.1152/advan.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titlow JS, Johnson BR, Pulver SR. Light activated escape circuits: a behavior and neurophysiology lab module using Drosophila optogenetics. J Undergrad Neurosci Educ. 2015;13:A166–A173. [PMC free article] [PubMed] [Google Scholar]
- Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.