Abstract
Pseudomonas fluorescens strain 3JW1, which has a broad-spectrum antimicrobial activity, was studied to investigate whether it affects the amounts of aflatoxin B1 (AFB1) produced by Aspergillus flavus. It was found that the bacterium reduced the amounts of AFB1 in potato dextrose broth (PDB) and peanut medium by 97.8% and 99.4%, respectively. It also reduced AFB1 by ~183 μg/kg (55.8%) when applied onto peanut kernels. This strain reduced AFB1 via three mechanisms. First, it significantly inhibited A. flavus growth; second, our data showed that strain 3JW1 inhibits aflatoxin biosynthesis by A. flavus; and third, P. fluorescens strain 3JW1 is capable of degrading AFB1 at a rate as high as 88.3% in 96 hours. This is the first report demonstrating that Pseudomonas fluorescens can reduce toxin contamination caused by A. flavus on peanut kernels. Our findings indicate that P. fluorescens strain 3JW1 had multiple effects including reducing A. flavus infection and aflatoxin contamination. And the results also highlight the potential applications of the strain 3JW1 for the biological control of aflatoxin contamination in peanuts and other susceptible crops.
Introduction
Aspergillus flavus belongs to Aspergillus Section Flavi. The fungus is a common saprophyte that can infect susceptible crops, such as peanut, corn, cotton seeds and tree nuts under favorable conditions [1, 2]. The most hazardous effect of this type of fungi is the production of aflatoxins, including aflatoxin AFB1, AFB2, AFG1 and AFG2, a group of secondary metabolites produced mainly by A. flavus, A. parasiticus and several other species including A. nomius, A. pseudotamarii, A. parvisclerotigenus, and A. bombycis of section Flavi, A. ochraceoroseus and A. rambellii from section Ochraceorosei and Emericella astellata and E. venezuelensis from Nidulatans section [2–4]. AFB1 is regarded as the most carcinogenic biotoxin in nature [2–4]. Its toxicity is 10 times greater than that of potassium cyanide and 68 times greater than that of arsenic [5, 6]. This toxin has been shown to induce mutations, suppress immune function, reduce growth, increase human and animal liver diseases, promote cancer development and cause acute aflatoxicosis and even death [5–11]. Therefore, AFB1 has been classified as a class I human carcinogen by the International Agency for Research on Cancer (IARC) [8, 12]. As a result, many countries have enacted strict standards on allowable levels of aflatoxins in food and feed. For example, European Union regulations state that AFB1 content in crops shall not be greater than 2 μg/kg and that the total toxin content cannot be more than 4 μg/kg [13]. Thus, aflatoxin-contamination of food and feed not only poses serious health concerns, but also causes significant economic losses to farmers.
Peanuts (Arachis hypogaea) are rich in protein content and are considered a nutritional food for both animals and humans. In addition, they are a vital oilseed and food crop utilized in most areas of the world. However, peanuts are usually threatened by pre-harvest infection of A. flavus and A. parasiticus when their fruits are tender or wounded and encounter rainy days at about 22–35°C and by inadequate storage conditions with relatively high moisture (over 85%) and temperature (over 22°C), which are often two of the main contributing factors that lead to moldy peanuts, reduced seed viability and increased seed rot [11, 14].
For these reasons, determining how to effectively reduce A. flavus infection and subsequent aflatoxin contaminaiton has extremely important theoretical and practical significance. Currently, some measures have been taken to reduce the infection, including physical and chemical methods, such as good field management and pest control [15, 16]. However, the control of A. flavus and aflatoxin remains a global problem due to lack of effective control measures.
Recently, advances in green, environmental and health technologies have inspired renewed efforts to develop biological control strategies to reduce A. flavus infection and aflatoxin contamination. One of the major breakthroughs is the use of atoxigenic strains of A. flavus to compete with toxigenic A. flavus in the field, which has been shown to successfully reduce aflatoxin contamination in the U.S. and African countries [17–21]. Atehnheng et al. evaluated the abilities of eleven naturally occurring atoxigenic isolates in Nigeria to reduce aflatoxin contamination in corn in field studies during the 2005 and 2006 growing seasons and found relative levels of aflatoxin B1 + B2 reduction ranged from 70.1% to 99.9% [22].
However, the presence of partial toxin pathway gene cluster in the atoxigenic biocontrol strains is of special concern considering the recent reporting of possible recombination of A. flavus under natural conditions [23–24]. Therefore, the use of other antagonists in biocontrol of A. flavus has also been explored. For example, Palumbo et al. [25] found that strains of Pseudomonas chlororaphis and P. fluorescens from Mississippi maize field soil and maize rhizosphere samples could inhibit A. flavus growth in different media (i.e., liquid or agar media). Moreover, a strain of Bacillus pumilus isolated from Korean soybean sauce exhibited strong antifungal activity against the aflatoxin-producing fungi A. flavus and A. parasiticus [26]. A biocontrol yeast, Pichia anomala strain WRL-076, inhibited A. flavus spore germination and aflatoxin production [27]. Sangmanee and Hongpattarakere [28] reported that Lactobacillus plantarum K35 isolated from traditional Thai fermented rice noodles could effectively inhibit the growth and aflatoxin production of A. flavus TISTR304 and A. parasiticus TISTR3276.
The present study aimed to investigate the efficacy of a previously isolated Pseudomonas fluorescens strain 3JW1 in reducing AFB1 produced by A. flavus, and to explore the potential of applying P. fluorescens strain 3JW1 to reduce AFB1 in peanuts. P. fluorescens strain 3JW1, a non-pathogenic endophyte, was originally isolated from the stem of ginger and used safely to inhibit plant disease [29]. But there is no report of its efficacy against Aspergillus flavus. Therefore, how strain 3JW1 affects A. flavus growth and the subsequent aflatoxin production of the recovered A. flavus after being treated with the biocontrol agent were examined. In addition, the efficacy of this strain in suppressing aflatoxin contamination in peanut and on degrading AFB1 was also investigated.
Materials and methods
Materials
Strains
Aspergillus flavus strain 73 was isolated from an aflatoxin-contaminated peanut sample that was provided by the Oil Crops Research Institute of the Chinese Academy of Agricultural Sciences. Pseudomonas fluorescens 3JW1 was provided by the Biological Pesticides and Green Plant Protection Laboratory of Nanjing Agricultural University.
Media
Luria-Bertani (LB) medium (yeast extract 5 g/L, tryptone 10 g/L, NaCl 10 g/L, pH 7.0–7.2; autoclaved for 20 min at 121°C), Czapek medium (sodium nitrate 3 g/L, dipotassium hydrogen phosphate 1 g/L, magnesium sulphate 0.5 g/L, potassium chloride 0.5 g/L, ferrous sulfate 0.01 g/L, sucrose 30 g/L, and agar 20 g/L; autoclaved for 20 min at 121°C), Potato Dextrose Agar (PDA) medium (potato 200 g/L, dextrose 20 g/L, and agar 15 g/L; sterilized for 20 min at 121°C), Potato Dextrose Broth (PDB) medium (potato 200 g/L and dextrose 20 g/L; autoclaved for 20 min at 121°C) and peanut medium (potato 200 g/L, dextrose 20 g/L, and peanut flour 1.5 g/L; autoclaved for 20 minutes at 121°C) were prepared in house.
Methods
Preparation of the biocontrol strain
Pseudomonas fluorescens strain 3JW1 was grown in LB medium for 24 h at 28°C with shaking at 200 r/min. Escherichia coli TOP10F′was grown in LB medium for 24 h at 37°C with shaking at 200 r/min. Cells were counted using a plate counting method.
Preparation of an A. flavus spore suspension
A. flavus spore suspensions were prepared by flooding 10-day-old cultures of A. flavus on PDB with sterile distilled water (containing 0.1% Tween 80) in a biosafety hood. Spores were counted using a hemocytometer [30].
Effect of P. fluorescens strain 3JW1 on fungal AFB1 production in PDB
A. flavus was mixed with strain 3JW1 in a 100-ml flask containing 15 ml of PDB. The final concentrations of A. flavus and the biocontrol strain were 5×105 spores/ml and 1×107 colony-forming units (CFU)/ml according to the previous study [30]. Control treatments include A. flavus alone in PDB and A. flavus mixed with 1×107 CFU/ml E. coli. After 4 d (96 h) of incubation with constant shaking (200 r/min) at 28°C in an incubator shaker, the medium filtrate was collected (except mycelium), and the amount of AFB1 was determined by immune affinity column-high performance liquid chromatography (IAC-HPLC) [31]. In order to further determine whether any reduction on AFB1 was due to inhibition on fungal growth, A. flavus mycelium from PDB medium alone, PDB medium containing strain 3JW1 and PDB medium containing E. coli was harvested by filtration, followed by washing and drying at 80°C for 24 h. The mycelium dry weight obtained was recorded and compared. This study was repeated 3 times, each with 3 replications.
Effects of P. fluorescens strain 3JW1 on AFB1 production in peanut medium
In order to identify whether strain 3JW1 could play the same role in PDB with peanut powder, A. flavus was mixed with strain 3JW1 in a 100-ml flask containing 15 ml of peanut medium. The final concentrations of A. flavus and the biocontrol strain were 5×105 spores/ml and 1×107 colony-forming units (CFU)/ml, respectively. A control treatment with A. flavus in peanut medium alone and another control treatment of A. flavus mixed with E. coli at a final concentration of 1×107 CFU/ml were also included. After 96 h of incubation with constant shaking (200 r/min) at 28°C in an incubator shaker, the medium filtrates were collected, and the amount of AFB1 was determined by immune affinity column-high performance liquid chromatography (IAC-HPLC). This study was repeated 3 times, each with 3 replications.
Efficacy of P. fluorescens strain 3JW1 in reducing AFB1 contamination in peanuts
In our preliminary efficacy study, 20 peanut kernels were used in each assay. Peanut kernels were surfaced-disinfected with 0.1% sodium hypochlorite for 1 min, rinsed with sterile distilled water 3 times for 30 second each time, and then air-dried in a petri dish. One ml of biocontrol strain at 1.0×107 CFU/ml was added to the petri dish, and peanut kernels were dipped into petri dish containing the bacteria and shaken for 5 min to allow the bacteria to be absorbed by the peanuts. Then, 1 ml of A. flavus inoculum at 5×105 spores/ml was added to each petri dish four hours later and the dishes were then shaken as above for 5 min. All petri dishes were then placed in an artificial weather chamber to maintain high humidity (85%) and were incubated at 28°C [30]. The amount of AFB1 was determined by IAC-HPLC 7 days later. Each treatment was replicated three times and this study was repeated four times.
The ability of P. fluorescens strain 3JW1 in suppressing aflatoxin biosynthesis or degrading aflatoxins
To determine whether the reduced aflatoxin production by the biocontrol strain was due to its inhibitory effect on aflatoxin biosynthesis or due to degradation of the produced aflatoxins by the biocontrol agent, the following two studies were conducted. For the first study, 100 μl of A. flavus and strain 3JW1 mixed culture was collected at the end of 96 h co-incubation on PDB and plated on petri dishes containing PDA medium. After 4 days, a single colony of A. flavus was transferred onto a new PDA plate. Conidia of A. flavus on the new plate were collected 14 days later, and were used to inoculate into 15 ml of PDB in a 100-ml flask at a final concentration of 5×105 spores/ml. After 96 h of constantly shaking (200 r/min) the above culture at 28°C in an incubator shaker, the medium filtrate was collected and the amount of AFB1 was determined by IAC-HPLC. This study was conducted 3 times and each treatment was replicated three times.
For the second study, a 0.8 ml of 24 h old culture of strain 3JW1 in LB was added to a 1.5 ml microcentrifuge tube containing 0.2 ml of AFB1 standards (250 ng/ml). The tube was incubated on a shaker with constant shaking at 200 r/min and 28°C for 96 h at°Cbefore its AFB1 level was analyzed by IAC-HPLC. The fresh sterile LB and E. coli culture were included as controls, in which 0.8 ml of one day old E. coli TOP10F′ culture in LB or only LB was added to a 1.5 ml microcentrifuge tube containing 0.2 ml of AFB1 standards (250 ng/ml). The degradation rate was calculated using the formula [y = (x1 –x2) / x1×100%] [32]. Here, x1 represents the contents of AFB1 in the control treatment, x2 represents the contents of AFB1 in the treated group, and y represents the detoxification ratio. These studies were conducted 3 times and each treatment was replicated three times.
Data analysis
All data were analyzed for statistical significance by the least significant difference (LSD) test (p < 0.05) using the Data Processing System (DPS version 7.05; Hangzhou Rui Feng Information Technology Inc., Hangzhou, Zhejiang, China) statistical software package.
Results and discussions
Effects of the 3JW1 biocontrol strain on reducing AFB1 levels
Effects of P. fluorescens strain 3JW1 on AFB1 levels in PDB
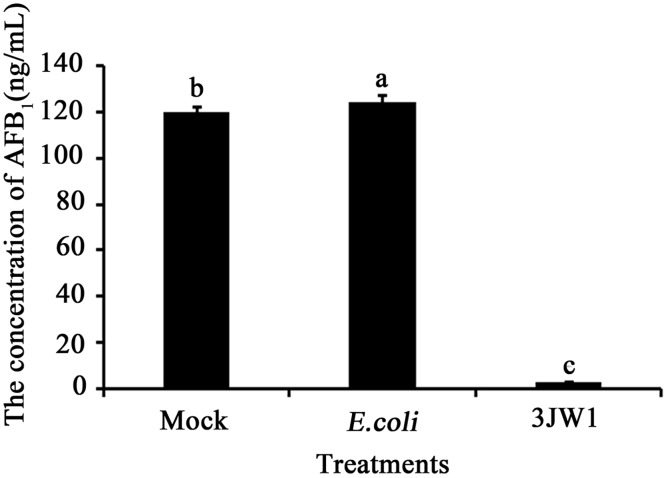
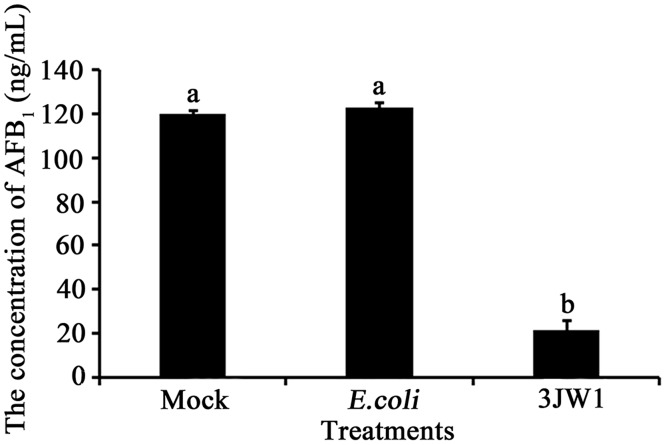
When A. flavus and other strains were cultured together in PDB, the results showed that E. coli in the culture had no effect on AFB1 production. However, cultures inoculated with biocontrol strain 3JW1 showed significant reductions in the amounts of AFB1 produced in PDB (Fig 1), with the inhibition rate reaching 97.8%. A previous study showed that Lactobacillus plantarum K35 could inhibit A. flavus growth and could reduce the amount of AFB1 produced by A. flavus by 69% at 8.8 mg/ml of the supernatant [28]. In comparison to this and other biocontrol agents used in earlier studies [30, 33] to suppress aflatoxin production (Table 1), this P fluorescen strain 3JW1 appears to have more potential as a new biocontrol agent in practical applications.
Fig 1. Effects of Pseudomonas fluorescens strain 3JW1 on AFB1 levels in PDB culture.
Aspergillus flavus was mixed with P. fluorescens strain 3JW1 in a 100-ml flask containing 15 ml of PDB. The final concentrations of A. flavus and P. fluorescens strain 3JW1 in PDB were 5×105 spores/ml and 1×107 CFU/ml, respectively. A mock control with PDB alone and another control inoculated with 1×107 CFU/ml E. coli TOP10F′ were used. After 4 d (96 h) of incubation with constant shaking (200 r/min, 28°C), the medium filtrate was collected (except mycelium), and the amount of AFB1 was determined by IAC-HPLC. This study was repeated 3 times, each with 3 replications.
Table 1. Mechanisms of aflatoxin suppression by various biocontrol agents against Aspergillus flavus.
| Strain | Inhibit growth of A. flavus | Degradation of AFB1 | Inhibit toxin production of the first generation | Inhibit toxin production of the later generation |
|---|---|---|---|---|
| Pseudomonas fluorescens 3JW1 | Y | Y | Y | Y |
| Lactobacillus plantarum K35 | Y | -- | Y | -- |
| Bacillus subtilis UTBSP1 | Y | Y | Y | -- |
| Bacillus cereus | Y | -- | Y | -- |
| Bacillus megaterium | Y | -- | Y | -- |
Note: Y means yes.
--means no results showed in the experiment.
Effects of P. fluorescens strain 3JW1 on AFB1 production in peanut medium
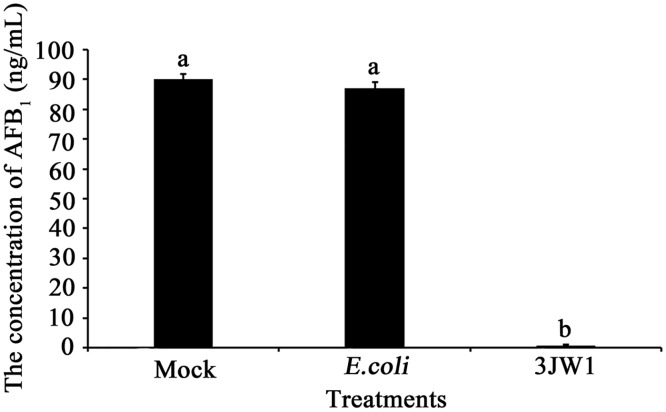
Using the same method, when A. flavus and other strains were co-cultured in peanut medium, the result indicated that AFB1 was produced at high amounts in both controls. In contrast, AFB1 was barely detected in cultures that contained the biocontrol strain 3JW1 (Fig 2).
Fig 2. Effects of Pseudomonas fluorescens strain 3JW1 on AFB1 production in peanut medium.
Aspergillus flavus was mixed with P. fluorescens strain 3JW1 in a 100-ml flask containing 15 ml of peanut medium. The final concentrations of Aspergillus flavus and P. fluorescens strain 3JW1 in PDB were 5×105 spores/ml and 1×107 CFU/ml, respectively. A mock control with PDB alone and another control inoculated with 1×107 CFU/ml E. coli were used. After 96 h of incubation with constant shaking (200 r/min) at 28°C in an incubator shaker, the medium filtrates were collected, and the amount of AFB1 was determined by IAC-HPLC. This study was repeated 3 times, each with 3 replications.
Efficacy of P. fluorescens strain 3JW1 on reducing AFB1 levels in peanuts
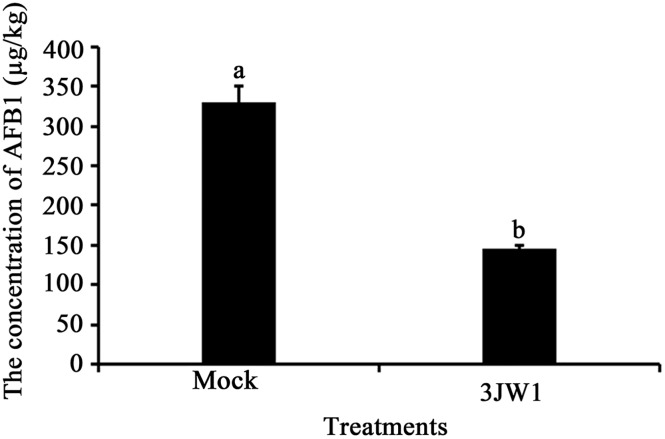
To evaluate the efficacy of strain 3JW1 in suppressing aflatoxin contamination in practical application, peanut kernels with or without precoating them with the P. fluorescens strain 3JW1 were inoculated with A. flavus. The result indicated that strain 3JW1 significantly reduced the amount of aflatoxin contamination in peanut kernels (Fig 3). The aflatoxin levels in the treated kernels were reduced by 55.8% on the average or by ~183 micrograms AFB1 per kilogram peanut kernels (183 μg/kg) compared with the controls. The data presented herein demonstrated for the first time that Pseudomonas fluorescens exhibited inhibitory effects on the amounts of AFB1 produced by A. flavus on peanut kernels. In addition to Lactobacillus plantarum K35 [28], previous reports found that Bacillus cereus and Bacillus megaterium could control kernel rot in peanut caused by A. flavus [30, 33]. Reddy et al. reported that Pseudomonas fluorescens treatment could lead to a 62.6% reduction of AFB1 in sorghum grains [34].
Fig 3. Effects of Pseudomonas fluorescens strain 3JW1 on AFB1 production in peanut kernels (μg/kg).
Peanut kernels were surfaced-disinfected with 0.1% sodium hypochlorite, rinsed with sterile distilled water 3 times, air-dried in a petri dish. Tewenty peanut kernels were used in each assay. One ml of biocontrol strain 3JW1 at 1.0×107 CFU/ml was added into petri dish, 4h later, 1 ml A. flavus at 1×107 CFU/ml was added into petri dish. All petri dishes were placed in an artificial weather chamber (humidity 85%, 28°C). The amount of AFB1 was determined by IAC-HPLC 7 days later. Each treatment was replicated three times and this study was repeated four times.
Effects of P. fluorescens strain 3JW1 on the growth of A. flavus
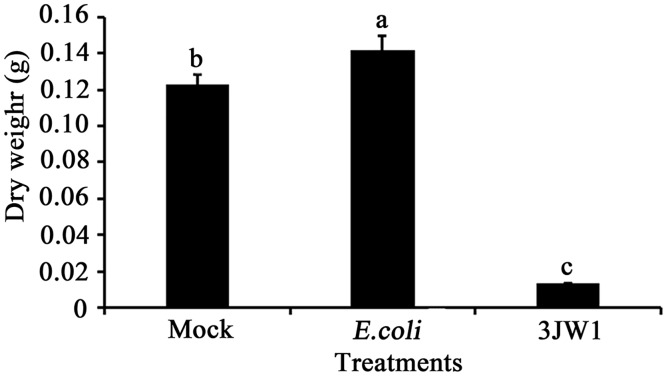
A. flavus (5×105 spores/ml) and strain 3JW1 (1×107 CFU/ml) were co-cultured in PDB to determine whether the biocontrol strain could inhibit fungal growth. A mock control with A. flavus in PDB alone and another control inoculated with 1×107 CFU/ml E. coli were used. Mycelia of A. flavus were collected, and the mycelium dry weights were compared. The result (Fig 4) showed that comparing to both mock control treatment and E. coli treatment, strain 3JW1 significantly suppressed mycelial growth (over 80%) of A. Flavus.
Fig 4. Effects of Pseudomonas fluorescens strain 3JW1 on the mycelial dry weight of Aspergillus flavus 73.
Aspergillus flavus was mixed with P. fluorescens strain 3JW1 in a 100-ml flask containing 15 ml of PDB. The final concentrations of A. flavus and strain 3JW1 in PDB were 5×105 spores/ml and 1×107 CFU/ml, respectively. A mock control with PDB alone and another control inoculated with 1×107 CFU/ml E. coli were used. After 4 d (96 h) of incubation with constant shaking (200 r/min, 28°C), the mycelium were collected, then washing and drying at 80°C for 24 h. The mycelium dry weight obtained was recorded. This study was repeated 3 times, each with 3 replications.
Aflatoxin production by A. flavus recovered from the P. fluorescens strain 3JW1-treated medium
The above result showed the biocontrol strain 3JW1 reduced aflatoxin accumulation, however, it was not clear whether it resulted from inhibiting aflatoxin biosynthesis or degrading the synthesized aflatoxin. Therefore, the A. flavus strain 73 was recovered again from the medium co-cultured with strain 3JW1, and its aflatoxin synthesis ability was examined in PDB medium. The result (Fig 5) showed the aflatoxin production of A. flavus was still inhibited after the first sequential sub-culturing on PDB medium, which indicated that the biocontrol strain had the ability to inhibit aflatoxin synthesis of A. Flavus.
Fig 5. Production of AFB1 in the first sequential sub-culturing of A. flavus on PDB medium.
One hundred microliter of A. flavus and strain 3JW1 mixed culture was collected at the end of 96 h co-incubation on PDB, then plated on petri dishes containing PDA medium. After 4 days, a single colony of A. flavus was transferred onto a new PDA plate. Conidia of A. flavus on the new plate were collected 14 days later, and were used to inoculate into 15 ml of PDB in a 100-ml flask at a final concentration of 5×105 spores/ml. After 96 h of constantly shaking (200 r/min) the above culture at 28°C in an incubator shaker, the medium filtrate was collected and the amount of AFB1 was determined by IAC-HPLC. This study was conducted 3 times and each treatment was replicated three times. The initial conidial concentration of A. flavus in the PDB medium was 5×105 spores/ml.
Effects of P. fluorescens strain 3JW1 on AFB1 degradation
To determine whether strain 3JW1 could also degrade aflatoxins, AFB1 was mixed with the biocontrol strain 3JW1, and the final amounts of AFB1 were compared to controls after 4 days. Compared with the controls, strain 3JW1 showed the ability to degrade AFB1, with the degradation rate reaching 88.3% in 4 days. In contrast, E. coli showed no ability to degrade AFB1. Previous study reported that AFB1 could be degraded into various other compounds (AFD1, AFD2, and AFD3) by Pseudomonas putida [35]. Bacillus subtilis UTBSP1 isolated from pistachio nuts from Iran can also degrade AFB1 [36].
In conclusion, through the series of experiments described above, the results showed that the Pseudomonas fluorescens strain 3JW1 could effectively reduce aflatoxin contamination on peanut kernels by not only suppress fungal growth and aflatoxin biosynthesis, but also breaking down the synthesized aflatoxin. As the field-isolated 3JW1 is relatively harmless, our findings suggest that it has great potential applications in both preventing pre-harvest aflatoxin contamination and degrading produced aflatoxins in the post-harvest agro-products.
Acknowledgments
This work was supported by the Special Fund for Agri-food-scientific Research in the Public Interest (201303088, 201513006), the National Key Project for Agro-product Quality & Safety Risk Assessment, PRC (GJFP2017001) and the International Science & Technology Cooperation Program of China (2016YFE0112900).
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Special Fund for Agri-food-scientific Research in the Public Interest (201303088, 201513006), the National Key Project for Agro-product Quality & Safety Risk Assessment, PRC (GJFP2017001) and the International Science & Technology Cooperation Program of China (2016YFE0112900). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153: 1677–1692. doi: 10.1099/mic.0.2007/007641-0 [DOI] [PubMed] [Google Scholar]
- 2.Farombi EO. Review-aflatoxin contamination of foods in developing countries:implications for hepatocellular carcinoma and chemopreventive strategies. Acad J. 2006;5: 1–14. [Google Scholar]
- 3.Mishra HN, Das C. A review on biological control and metabolism of aflatoxin. Crit Rev Food Sci Nutr. 2003;43: 245–264. doi: 10.1080/10408690390826518 [DOI] [PubMed] [Google Scholar]
- 4.Payne GA, Brown MP. Genetics and physiology of aflatoxin biosynthesis. Annu Rev Phytopathol. 1998;36: 329–362. doi: 10.1146/annurev.phyto.36.1.329 [DOI] [PubMed] [Google Scholar]
- 5.Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 2011;41: 740–755. doi: 10.3109/10408444.2011.575766 [DOI] [PubMed] [Google Scholar]
- 6.Henry SH, Bosch FX, Bowers JC. Aflatoxin, hepatitis and worldwide liver cancer risks. Adv Exp Med Biol. 2002;504: 229–233. doi: 10.1007/978-1-4615-0629-4_24 [DOI] [PubMed] [Google Scholar]
- 7.Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Annu Rev Pharmacol Toxicol. 1994;34: 135–172. doi: 10.1146/annurev.pa.34.040194.001031 [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect. 2010;118: 818–824. doi: 10.1289/ehp.0901388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31: 71–82. doi: 10.1093/carcin/bgp264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyikal J, Misore A, Nzioka C, Njuguna C, Muchiri E, Njau J, et al. Outbreak of aflatoxin poisoning-eastern and central provinces, Kenya, January-July 2004. Mmwr. Morb Mortal Wkly Rep. 2004;53: 790–793 [PubMed] [Google Scholar]
- 11.Kumar V, Basu MS, Rajendran TP. Mycotoxin research and mycoflora in some commercially important agricultural commodities. Crop Protect. 2008;27: 891–905. doi: 10.1016/j.cropro.2007.12.011 [Google Scholar]
- 12.Groopman JD, Wogan GN. Aflatoxin and hepatocellular carcinoma Chemical Carcinogenesis. Clifton: Humana Press; 2011. pp. 113–133. [Google Scholar]
- 13.Egmond HPV, Jonker MA. Worldwide regulations on aflatoxins—the situation in 2002 #. Toxin Rev. 2004;119: 92–102. [Google Scholar]
- 14.Horn BW, Dorner JW. Effect of nontoxigenic Aspergillus flavus and A. parasiticuson aflatoxin contamination of wounded peanut seeds inoculated with agricultural soil containing natural fungal populations. Biocontrol Sci Techn. 2009;19: 249–262. [Google Scholar]
- 15.Yazdanpanah H, Mohammadi T, Abouhossain G, Cheraghali AM. Effect of roasting on degradation of aflatoxins in contaminated pistachio nuts. Food Chem Toxicol. 2005;43: 1135–1139. doi: 10.1016/j.fct.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 16.Sindhu S, Chempakam B, Leela NK, Suseela Bhai R. Chemoprevention by essential oil of turmeric leaves (curcuma longa L.) on the growth of Aspergillus flavus and aflatoxin production. Food Chem Toxicol. 2011;49: 1188–1192. doi: 10.1016/j.fct.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 17.Bock CH, Cotty PJ. Wheat seed colonized with atoxigenic Aspergillus flavus: characterization and production of a biopesticide for aflatoxin control. Biocontrol Sci Techn. 1999;9: 529–543. doi: 10.1080/09583159929497 [Google Scholar]
- 18.Dharmaputra OS. Control of aflatoxigenic Aspergillus flavus in peanuts using nonaflatoxigenic A. flavus, A. niger and Trichoderma harzianum. Biotropia the Southeast Asian J of Tropi Biology. 2003; 21. [Google Scholar]
- 19.Cotty P J. Effect of atoxigenic strains of Aspergillus flavus on aflatoxin contamination of developing cottonseed. Plant Dis. 1990;74: 233–235. doi: 10.1094/PD-74-0233 [Google Scholar]
- 20.Probst C, Bandyopadhyay R, Price LE, Cotty PJ. Identification of atoxigenic Aspergillus flavus isolates to reduce aflatoxin contamination of maize in Kenya. Plant Dis. 2011; 95: 212–218. [DOI] [PubMed] [Google Scholar]
- 21.Wu F, Khlangwiset P. Health economic impacts and cost-effectiveness of aflatoxin-reduction strategies in Africa: case studies in biocontrol and post-harvest interventions. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010; 27: 496–509. doi: 10.1080/19440040903437865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atehnkeng J, Ojiambo PS, Ikotun T, Sikora RA, Cotty PJ, Bandyopadhyay R. Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008; 25: 1264–1271. doi: 10.1080/02652030802112635 [DOI] [PubMed] [Google Scholar]
- 23.Horn BW, Gell RM, Singh R, Sorensen RB, Carbone I. Sexual reproduction in Aspergillus flavussclerotia: acquisition of novel alleles from soil populations and uniparental mitochondrial inheritance. PLoS ONE. 2016; 11: e0146169 doi: 10.1371/journal.pone.0146169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore GG, Singh R, Horn BW, Carbone I. Recombination and lineage-specific gene loss in the aflatoxin gene cluster of Aspergillus flavus. Mol Ecol. 2009;18: 4870–4887. doi: 10.1111/j.1365-294X.2009.04414.x [DOI] [PubMed] [Google Scholar]
- 25.Palumbo JD, O'Keeffe TL, Abbas HK. Isolation of maize soil and rhizosphere bacteria with antagonistic activity against Aspergillus flavus and Fusarium verticillioides. J Food Prot. 2007;70: 1615–1621 [DOI] [PubMed] [Google Scholar]
- 26.Cho KM, Math RK, Hong SY, Asraful Islam SM, Mandanna DK, Cho JJ, et al. Iturin produced by Bacillus pumilus HY1 from Korean soybean sauce (kanjang) inhibits growth of aflatoxin producing fungi. Food Control. 2009;20: 402–406. doi: 10.1016/j.foodcont.2008.07.010 [Google Scholar]
- 27.Hua SS, Beck JJ, Sarreal SB, Gee W. The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 2014;30: 71–78. doi: 10.1007/s12550-014-0189-z [DOI] [PubMed] [Google Scholar]
- 28.Sangmanee P, Hongpattarakere T. Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control. 2014;40: 224–233. doi: 10.1016/j.foodcont.2013.12.005 [Google Scholar]
- 29.Yang W. Screening strategy of biocontrol agents against Ralstonia wilt on ginger and its impacts on the rhizosphere environment. Ph.D. Thesis. Nanjing agricultural university; 2011. http://auth.njau.edu.cn:2360/KCMS/detail/detail.aspx?dbcode=CDFD&dbname=CDFDLAST2015&filename=1015503161.nh&uid=WEEvREcwSlJHSldRa1FhdkJkcGkyNTdiZ0lGeEdJNzc2VmR1dnV2bzJXbz0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4ggI8Fm4gTkoUKaID8j8gFw!!&v=MjYwMzlBVkYyNkc3YTRIZERLcnBFYlBJUjhlWDFMdXhZUzdEaDFUM3FUcldNMUZyQ1VSTDJmWWVab0ZDbmtVNzM=
- 30.Kong Q, Shan S, Liu Q, Wang X, Yu F. Biocontrol of Aspergillus flavus on peanut kernels by use of a strain of marine Bacillus megaterium. Int J Food Microbiol. 2010;139: 31–35. doi: 10.1016/j.ijfoodmicro.2010.01.036 [DOI] [PubMed] [Google Scholar]
- 31.Chen R, Ma F, Li PW, Zhang W, Ding XX, Zhang Q, et al. Effect of ozone on aflatoxins detoxification and nutritional quality of peanuts. Food Chem. 2014;146: 284–288. doi: 10.1016/j.foodchem.2013.09.059 [DOI] [PubMed] [Google Scholar]
- 32.Liang Z, Li J, He Y, Guan S, Wang N, Ji C, et al. AFB1 Bio-Degradation by a New Strain—Stenotrophomonas. sp. Agric Sci China. 2008;7: 1433–1437. doi: 10.1016/S1671-2927(08)60399-5 [Google Scholar]
- 33.Kumar SN, Sreekala SR, Chandrasekaran D, Nambisan B, Anto RJ. Biocontrol of Aspergillus species on peanut kernels by antifungal diketopiperazine producing Bacillus cereus associated with Entomopathogenic nematode. PLoS ONE. 2014;9: e106041 doi: 10.1371/journal.pone.0106041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy KRN, Raghavender CR, Reddy BN, Salleh B. Biological control of Aspergillus flavus growth and subsequent aflatoxin B1 production in sorghum grains. Afr J Biotechnol. 2010;9: 4247–4250. [Google Scholar]
- 35.Samuel MS, Sivaramakrishna A, Mehta A. Degradation and detoxification of aflatoxin B1 by Pseudomonas putida. Int Biodeter Biodegr. 2014;86: 202–209. doi: 10.1016/j.ibiod.2013.08.026 [Google Scholar]
- 36.Farzaneh M, Shi Z, Ghassempour A, Sedaghat N, Ahmadzadeh M, Mirabolfathy M, et al. Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Control. 2012;23: 100–106. doi: 10.1016/j.foodcont.2011.06.018 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.