Abstract
Background
Methicillin- resistant Staphylococcus aureus (MRSA) is a major pathogen causing healthcare- and community- acquired infections. The purpose of this study was to characterize MRSA isolated at the Maternity Hospital between 2006 and 2011 for their genetic relatedness.
Materials and methods
The MRSA isolates were investigated using a combination of antibiogram, Staphylococcal chromosome cassette mec (SCCmec) and spa typing to determine their relatedness to MRSA isolated in other Kuwait hospitals. The isolates were also investigated for the carriage of genes for Pantone valentine Leukocidin (PVL).
Results
A total of 103 MRSA obtained from 64 neonates, 17 adult patients and 12 healthcare workers. The isolates were resistant to Kanamycin (46.6%), gentamicin (40.8%), trimethoprim (32%), ciprofloxacin (22.3%), fusidic acid (16.5%), tetracycline (19.4%), erythromycin (15.5%), clindamycin (15.5%), streptomycin (11.6%) high-level mupirocin (2.9%) and chloramphenicol (0.9%). Twenty (19.4%) of the isolates were multiresistant. Thirty-one (30.0%) isolates were positive for PVL. Molecular typing revealed the presence of 11 clonal complexes and 23 clones with ST5-V-t002, (N = 22), ST22-IV-t223 (N = 18), ST22-IV-t852 (N = 10), ST80-IV-t044 (N = 7), ST5-V-t688 (N = 5), ST772-V-t657 (N = 5) and ST239-III-t860 (N = 4) constituting 66.9% of the isolates. Other clones were isolated sporadically. The number of MRSA isolates increased from two in 2006 to 22 in 2011 with a peak of 43 in 2008.
Conclusion
The study revealed a high prevalence of community-associated MRSA Maternity hospital. The MRSA population consisted of known strains, such as ST239-III-t680, ST22-IV-t223/t852 and ST80-IV-t044, that were reported previously in Kuwait and novel strains such as ST5-V-t002, and several sporadic strains obtained for the first time in the Maternity hospital. This study has provided an initial data which will serve as a platform for future comparative studies on the distribution of MRSA clones in the Maternity hospital in Kuwait.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a major cause of healthcare- as well as community-acquired infections [1, 2].
Methicillin resistance is determined by the production of an altered low affinity penicillin-binding protein (PBP) called PBP2a (or PBP2’) [3, 4]. PBP2a is encoded by the mecA gene, which confers resistance to all beta-lactam antibiotics and is located on a mobile genetic island called staphylococcal cassette chromosome mec (SCCmec). In addition, to mecA, SCCmec contains site-specific cassette chromosome recombinases (ccr) that is responsible for the integration of the SCCmec into the S. aureus genome. The combination of SCCmec—and recombinase gene complexes defines SCCmec types, which differ in size and structural organizations [4,5]. The differences in SCCmec types have been used to type MRSA for epidemiological purposes and to distinguish healthcare-associated MRSA (HA-MRSA) from community-associated MRSA (CA-MRSA). Genetically, CA-MRSA harbor the staphylococcal cassette chromosome mec (SCCmec) type IV or type V [5], VI or VII [6] whereas HA-MRSA carries SCCmec types I, II and III [4, 5].
With the advent of CA-MRSA, infections caused by MRSA have increased in the community settings globally [7]. In addition, the prevalence of MRSA infections and colonization among pregnant women have increased [8, 9, 10]. Similarly, the number of reports linking MRSA with outbreaks in newborn nurseries and neonatal intensive care units are also increasing [11, 12, 13, 14, 15].
The Maternity hospital in Kuwait is a 500-bed tertiary hospital which handles 12000–14000 deliveries, accounting for approximately 30% of all deliveries, in the State of Kuwait [16]. MRSA was not reported in the Maternity Hospital in Kuwait prior to 2005 [17]. This was attributed to its select patient’s population. It was argued that pregnant women admitted to the hospital for child delivery were usually not sick, and spent few days in the hospital after delivery thereby eliminating the risk factor for the acquisition and transmission of healthcare—associated MRSA. However, the situation has since changed and the number of MRSA isolated at the Maternity hospital has been increasing gradually following the isolation of a single MRSA strain in 2005. Molecular typing of 22 MRSA isolates recovered from babies in the Special babies care unit of the Maternity hospital between 1 October and 31 December 2011, revealed the presence of CA-MRSA clones that were different from those usually isolated at other hospitals in Kuwait [16]. This finding revealed a lack of appreciation of the prevailing MRSA clones and their resistance profiles in other facilities of the hospital and demanded further investigations of MRSA isolated previously in the hospital. Consequently, to fully appreciate the epidemiology of MRSA in the Maternity hospital, MRSA that were isolated between 2006 and 2011 were characterized using a combination of molecular typing methods. Knowledge of the clonal types and their resistance patterns would provide useful background data which will be useful in assessing future changes in the MRSA population as well as assessing effectiveness of infection prevention and control measures designed to prevent MRSA becoming endemic in the hospital.
Materials and methods
MRSA isolates
The MRSA isolates were obtained as part of routine diagnostic microbiology testing and later submitted to the MRSA Reference Laboratory for molecular typing. In total, 103 MRSA isolates recovered between 2006 and 2011 from 64 neonates (72 isolates), 17 adult patients (18 isolates) and 12 healthcare workers (13 isolates) were investigated. The MRSA isolates were from 37 skin and soft tissues samples, 13 umbilical cord stumps, 18 nasal swabs, 11 eye swabs, 7 blood samples, 4 Vaginal swabs, and 13 miscellaneous sources. The MRSA isolates were identified using standard bacteriological methods including growth on Mannitol Salt Agar, Gram stain, tube coagulase and DNase testing and preserved in 40% glycerol (v/v) in nutrient broth at -80°C.
Antibiotic susceptibility testing
Susceptibility to benzyl penicillin, cefoxitin, kanamycin, gentamicin, erythromycin, clindamycin, chloramphenicol, tetracycline, minocycline, trimethoprim, fusidic acid, rifampicin, ciprofloxacin, mupirocin and linezolid was determined by the disk diffusion method [18]. Susceptibility to fusidic acid was interpreted following the British Society for antimicrobial chemotherapy guidelines [19]. Minimum inhibitory concentration (MIC) was determined for cefoxitin, mupirocin, vancomycin, and teicoplanin with Etest strips (BioMerieux, Marcey l’ Etoile, France) and interpreted following guidelines by the Clinical and Laboratory Standards Institute [18]. MIC for vancomycin was performed by the Etest macro method per the manufacturer’s protocol. S. aureus strain ATCC25923 was used as quality control strain for susceptibility testing. Methicillin resistance was confirmed by detecting PBP 2a in culture supernatants using a rapid latex agglutination kit (Denka-Seiken, Japan) following the manufacturer’s instruction. Multiple resistance was defined as resistance to three or more classes of antibacterial agents.
Detection of genes coding for Panton-Valentine leukocidin, (PVL)
The LukS-PV- lukF-PV gene which codes for PVL was amplified as described previously [20].
SCCmec typing and subtyping
SCCmec typing was performed by PCR assays as described previously [21, 22].
Spa typing
Spa typing was performed as described by Harmsen et al. [23] for all MRSA isolates. DNA sequencing was performed using a 3130x1 genetic analyzer (Applied Bio systems, Forster City, CA. USA) in accordance with the manufacturer protocol. Isolates were assigned to spa types using the spa typing website (http://www.spaserver.ridom.de) [24].
Multilocus sequence typing (MLST)
MLST was performed on all 37 isolates as described by Enright et al., [25]. Isolates were assigned a sequence type (ST) according to the MLST website (http://www.mlst.net) [26]. Clonal complexes were defined as group of sequence types (STs) that share at least five of seven identical alleles with at least one of the STs in the group [27].
Results
Antibiotic susceptibility
All MRSA isolates were susceptible to vancomycin, (MIC ≤ 2.0 mg/L), teicoplanin (MIC ≤ 2.0 mg/L), linezolid and rifampicin but were resistant to penicillin G (N = 93; 90.3%), gentamicin (N = 42; 40.8%), kanamycin (N = 48; 46.6%), trimethoprim (N = 33; 32.0%), ciprofloxacin (N = 23; 22.3%), fusidic acid (N = 17;16.5%), tetracycline (N = 20;19.4%), erythromycin (N = 16;15.5%), clindamycin (N = 16;15.5%), streptomycin (N = 12;11.6%), high-level mupirocin (N = 3; 2.9%) and chloramphenicol (N = 1; 0.9%).
Thirty-one (30.0%) isolates obtained from skin and soft tissue infections (13 isolates), nasal swabs (2 isolates), groin (2 isolates), HVS (3 isolates), and one each from blood, eye swab, catheter tip and unspecified source were positive for genes encoding PVL.
Molecular typing of MRSA isolates
Molecular typing yielded three SCCmec types (III, IV and V), 28 spa types, 19 sequence types (ST), 23 clones and 11 clonal complexes (CC) (Table 1). Fifty-seven (55.3%) of the isolates carried SCCmec IV genetic element, followed by SCCmec V (40 isolates, 38.8%) and SCCmec III (6 isolates, 5.8%). SCCmec types I and II were not detected.
Table 1. Characteristics of MRSA genotypes: 2006–2011.
| CC | MRSA Clones | # | PVL | Antibiotic resistance Profiles (# of resistant strains) |
|---|---|---|---|---|
| CC1 | ST772-V-t657 | 1 | - | - |
| ST772-V-t657 | 4 | + | Gm (4), Km (4), Tp (4), Cip (1) | |
| CC5 | ST5-V-t002 | 22 | - | Gm (22), Km (22), Tp (2), Fa (2) |
| ST5-V-688 | 5 | - | Em (2), Clin (2), Cm (1), Tet (5) | |
| ST5-IVa-t214 | 1 | - | Cip | |
| ST5-V-t985 | 1 | - | Em, Clin, LR-MUP | |
| ST5-IV-688 | 1 | - | - | |
| ST5-IV-t002 | 1 | + | Em, Clin, Cip | |
| ST194-IV-t002 | 1 | - | - | |
| ST627-IV-t105 | 1 | + | Tp | |
| ST1317-V-t311 | 1 | - | Em, Clin, Tp, Fa, Cip | |
| ST1462-IV-t690 | 2 | + | Tet | |
| ST1462-IV-t16184 | 1 | + | Em, Clin | |
| CC6 | ST932-IV-t304 | 7 | - | - |
| CC8 | ST239-III-t860 | 4 | - | Gm (4), Km (4), Sm (4), Em (4), Clin (4), Tet (4), Fa (4), Cip (4), LR-Mup (1), HLR-MUP (3) |
| ST239-III-t16202 | 1 | Gm, Km, Em, Clin, Tp, Cip | ||
| ST241-III-t037 | 1 | - | Gm, Km, Sm, Em, Clin, Tp, Cip | |
| CC9 | ST9-V-t267 | 1 | - | Gm, Km, Fa |
| CC22 | ST22-IV-t005 | 1 | + | Km, Em, Clin, Tet, Fa, Cip, LR-MUP |
| ST22-IVa-t223 | 18 | - | Te (3), Tp (12), Km (2), Fa (2), Gm (1), Em (1), Clin (1), Cip (1), Sm (1). | |
| ST22-IV-t15316 | 1 | - | Tp, Cip | |
| ST22-IV-t852 | 9 | + | Gm (6), Km (6), Tp (5), Cip (10) | |
| ST60-IV-t16202 | 1 | - | - | |
| CC30 | ST30-IVa-t318 | 1 | + | - |
| ST77-IV-t019 | 1 | + | - | |
| ST1055-IVa-t2051 | 1 | + | Tp | |
| CC80 | ST80-IV-t044 | 7 | + | Km (4), Sm (6), Tet (4), Fa (4), Tp (3), Em (1), Clin (1) |
| ST80-IV-t376 | 1 | - | Em, Clin | |
| CC88 | ST2148-IV-t6728 | 1 | - | Tet, Tp |
| CC97 | ST97-V-t1234 | 2 | - | Tet (1) |
| ST97-V-t359 | 2 | - | Gm (2), Km (2), Fa (2) | |
| CC121 | ST121-V-t314 | 1 | + | Tet |
The major spa types were t002 (N = 24), t223 (N = 18), t852 (N = 10), t044 (N = 7), t304 (N = 7), t688 (N = 6), t657 (N = 5), t860 (N = 4), t1234 (N = 2), t16202 (N = 2), t359 (N = 2) and t690 (N = 2). Spa types, t005, t019, t037, t105, t15316, t16184, t2051, t267, t311, t314, t318, t985, t376, t6728 occurred in single isolates. ST5 (N = 31) and ST22 (N = 29) were the dominant sequence types with both accounting for 58.2% of the isolates. Other common STs were ST80 (N = 8; 7.7%), ST932 (N = 7; 6.8%), ST772 (N = 5; 4.8%), ST239 (N = 5; 4.8%) and ST97 (N = 4; 3.8%).
The dominant clonal complexes were CC5 (N = 37), CC22 (N = 30), CC80 (N = 8), CC6 (N = 7), CC8 (N = 6) and CC1 (N = 5).
CC1 consisted of five ST772-V-t657 isolates. Four of these isolates were positive for PVL and were resistant to gentamicin, kanamycin, and trimethoprim. One isolate was negative for PVL and was susceptible to the non-beta-lactam antibiotics tested. The isolates were obtained from three babies (3 isolates) and adult patients (2 isolates) (Table 2).
Table 2. MRSA isolates obtained from adult patients.
| Strain No | Year | Sample | MRSA Genotype | |
|---|---|---|---|---|
| 1 | 6186 | 2007 | Wound | ST932-IVa-t304 |
| 2 | 6462 | 2007 | S/N | ST22-IVa-t223 |
| 3 | 6602 | 2007 | Nasal | ST241-III-t037 |
| 4 | 6708 | 2007 | Anal swab | ST932-IV-t304 |
| 5 | 7016 | 2008 | Wound | ST772-V-t657 |
| 6 | 7137 | 2008 | Sputum | ST5-IV-t688 |
| 7 | 7149 | 2008 | HVS | ST80-IV-t044 |
| 8 | 7231 | 2008 | Wound | ST22-IV-t223 |
| 9 | 7800 | 2008 | Wound | ST80-IV-t044 |
| 9 | 7858 | 2008 | HVS | ST80-IV-t044 |
| 10 | 7890 | 2008 | HVS | ST772-V-t657 |
| 11 | 7991 | 2008 | Abscess | ST1462-IV-t16184 |
| 12 | 7948 | 2008 | Nasal | ST5-V-t002* |
| 13 | 7969 | 2008 | Wound | ST1055-IV-t2051 |
| 14 | 7434 | 2008 | NS | ST97-V-t1234 |
| 15 | 7470 | 2008 | Wound | ST5-IV-t002 |
| 16 | 7509 | 2008 | Wound | ST22-IV-t15316 |
| 17 | 10561 | 2011 | NS | ST932-IV-t304* |
*; identical to isolates from their babies.
NS; not specified; HVS, high vaginal swab.
CC5 comprised diverse clones with ST5-V-t002 as the dominant clone, followed by ST5-V-t688. The ST5-V-t002 isolates were negative for PVL, but were resistant to gentamicin and kanamycin and variable resistance to other antibiotics (Table 1). ST5-V-t002 was widely distributed and was isolated from babies, adult patients (Table 2) and healthcare workers (Table 3). All five ST5-V-t688 isolates were resistant to tetracycline but expressed variable resistance to erythromycin, clindamycin and chloramphenicol. ST5-V-t688 isolates were recovered from babies, adult patients (Table 2) and healthcare workers (Table 3).
Table 3. MRSA isolates obtained from healthcare workers.
| Occupation | Source | MRSA clone |
|---|---|---|
| Receptionist 1 | Wound | ST80-IV-t044 |
| Lab. Technologist 1 | Nasal | ST22-IVa-t223 |
| Respiratory Technician | Wound | ST22-IVa-t223 |
| Lab. Technologist 2 | HVS | ST80-IV-t044 |
| Receptionist 2 | Wound | ST1462-IV-t690 |
| Receptionist 2 | HVS | ST1462-IV-t690 |
| Receptionist 3 | Wound | ST22-IV-t223 |
| Receptionist 4 | Wound | ST932-IV-t304 |
| Nursing Staff1 | Nasal | ST5-V-t002 |
| Nursing Staff3 | Nasal | ST5-V-t688 |
| Nursing Staff7 | Nasal | ST5-V-t002 |
| Nursing Staff 19 | Nasal | ST22-IVa-t223 |
| Nursing Staff 26 | Nasal | ST5-V-t002 |
CC6 consisted of seven ST932-IV-t304 isolates that were negative for PVL and uniformly susceptible to the non-beta-lactam antibiotics tested. The isolates were obtained from babies, one healthcare worker and three adult patients including a mother and her baby (Table 2).
CC8 consisted of six isolates that belonged to ST239-III-t860 (4 isolates), ST239-III-t16202 (1 isolate) and ST241-III-t037 (1 isolate). The CC8 isolates were negative for PVL and expressed multi antibiotic resistance. The t860 isolates were resistant to high-level mupirocin. ST239-III isolates were recovered only from babies while the ST241-III-t037 isolates was obtained from an adult patient (Table 2).
CC22 consisted of ST22- MRSA isolates carrying SCCmec IV genetic elements, and belonged to four different spa types, t005, t223, t15316 and t852 with t223, detected in 18 isolates, as the dominant spa type. The ST22 isolates differed in the carriage of genes for PVL and antibiotic resistance (Table 1). The ST22-IV-t005 and most of the ST22-IV-t852 isolates expressed multi antibiotic resistance whereas the ST22-IVa-t223 isolates were susceptible to most of the non-beta-lactam antibiotics. Whereas the t005 and t852 isolates were positive for PVL and negative for toxic shock syndrome toxin gene, the t223 isolates were negative for PVL but positive for toxic shock syndrome toxin gene. The ST22-IVa-t223 isolates were obtained from colonization as well as infection sites of babies, adult patients (Table 2) and healthcare workers (Table 3). The t852 isolates were isolated from babies and a baby cot.
CC30 was represented by three single clones, ST30-IVa-t318, ST77-IV-t019 and ST1055-IV-t2051 that were positive for PVL and susceptible to most non-beta-lactam antibiotics.
CC80 consisted of ST80-IV-t044 (7 isolates) that were positive for PVL and variably resistant to kanamycin, tetracycline, fusidic acid, trimethoprim, erythromycin and clindamycin, and ST80-IV-t376 (1isolate) that was negative for PVL and resistant to erythromycin and clindamycin only. ST80-IV-t044 isolates were obtained from babies, adult patients (Table 2) and healthcare workers (Table 3). CC9, CC88 and CC121 consisted of single isolates while CC97 consisted of four isolates comprising two ST97-V-t1234 and ST97-V-t359 (Table 1).
Distribution of MRSA isolates by year
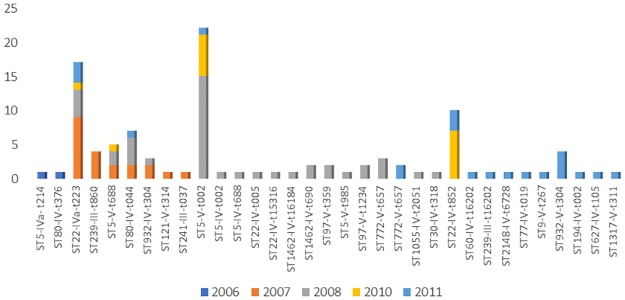
The isolates were analyzed by year of isolation to ascertain if any of the clones persisted in any units of the hospital. Fig 1 presents the MRSA isolates by year of isolation. Number of isolates increased from two in 2006 to a peak of 43 in 2008 with most clones occurring sporadically. The dominant clones, ST5-V-t002 and ST22-IVa-t223, were isolated in multiple years in clusters. Fifteen of the 22 ST5-V-t002 isolates were obtained in 2008. Eleven of these isolates were obtained from babies and a mother in the same ward depicting local transmission. The remaining seven isolates were obtained in 2010 (6 isolates) and 2011 (1 isolate) from four different wards. The six ST5-V-t002 isolates obtained in 2010 were all from babies in one ward. ST5-V-t688 isolates were obtained in 2007 (2 isolates), 2008 (2 isolates), and 2010 (1 isolate).
Fig 1. Distribution of MRSA clones by year: 2006–2011.
The ST22-IV isolates, obtained in 2007, 2008, 2010 and 2011, carried different spa types. Most (9/18) of the ST22-IVa-t223 isolates were obtained in 2007 while four and three of the isolates were obtained in 2008 and 2011 respectively. Six of the 10 ST22-IV-t852 isolates were recovered in 2010 from five babies and a baby cot in the same ward. The other three isolates were obtained in 2011. The ST80-IV-t044 isolates were all obtained in 2007 (2 isolates), 2008 (4 isolates) and 2011 (1 isolate).
ST932-IV-t304 were isolated in 2007 (2 isolates), 2008 (1 isolate) and 2011 (4 isolates). The ST772-V-t657 were isolated in 2008 (3 isolates) and 2011 (2 isolates).
Discussion
As the epidemiology of MRSA continue to change, the emergence of novel clones with novel virulence factors necessitates regular surveillance for better infection control and management of infection. Molecular typing revealed that the MRSA population in the Maternity hospital belonged to diverse genetic backgrounds dominated by internationally recognized clonal complexes CC5, CC22, CC80, CC6, CC8, CC1 and CC97. The major clones were ST5-V-t002 (21.3%), ST22-IVa-t223 (16.5%), ST22-IV-t852 (9.7%), ST932-IV-t304 (6.7%), ST80-IV- t044/t376 (7.7%), ST772-V-657 (4.8%) and ST239-III-t860 (3.8%). Apart from ST239-III/ST241-III and ST22-IV, which are healthcare- associated MRSA clones, the majority (66.0%) of the isolates had community-associated MRSA (CA-MRSA) genotypes. The high prevalence of CA-MRSA observed in this study mirrors recent observations in other centers where CA-MRSA are the dominant MRSA isolates obtained from neonatal units [12, 28, 29, 30, 31, 32].
This study also revealed that the MRSA population consisted of strains that have been reported previously in other hospitals in Kuwait, and those reported for the first time at the Maternity hospital. Isolates reported previously at other hospitals include ST239-III-t860, ST241-III-t037, ST5-IV-t688, ST22-IV-t223/t852, ST80-IV-t044/t376, ST772-V-657, ST121-V-t314, ST5-IV-t002 and ST97-V-t1234 [33, 34]. Unexpectedly, ST239-III-MRSA, which is the dominant MRSA clone encountered in other Kuwait hospitals [33], was present in small numbers in this study. Secondly, the ST239-III-t860 isolates, obtained only in 2007, did not persist in the Maternity hospital in contrast to their persistence in other hospitals in Kuwait [33, 35]. Most of the ST22-IVa-t223 isolates were obtained from samples of skin and soft tissue infections as well colonization sites as was the case in other hospitals in Kuwait [36] supporting their ability to be involved in different types of infections. In contrast, the UK-EMRSA-15/Barnim epidemic MRSA clone is mostly associated with bloodstream infections [37]. The detection of the tst-positive ST22-IVa-t223 isolates among different patients’ populations in this hospital suggests that it is becoming an important epidemic healthcare- associated pathogen in Kuwait hospitals. In addition to its growing prominence in Kuwait hospitals, tst-positive ST22-IVa-t223 isolates have also been reported widely in the Gaza strip [38], Jordan [39], Saudi Arabia and Egypt [40] and is becoming the leading variant of the UK Epidemic MRSA-15 clone in the Middle East affecting adults and babies. Besides the tst-positive ST22-IVa-t223 isolates, PVL-positive -ST22-IV-t852 MRSA also contributed to the MRSA burden among the neonates. In addition, a t16202 variant of ST22-IV-MRSA was also detected for the first time in this study adding to the ongoing diversification of CC22 strains observed in recent years [36, 41, 42].
The isolation of ST80-IV-MRSA in eight (7.7%) of the isolates in this study is comparable to the prevalence of 7.5% prevalence that was reported recently in other hospitals in Kuwait [33]. However, with only two of the eight isolates obtained from neonates, the results suggest that ST80-IV-MRSA had limited role in infections among the neonates. In contrast, ST80-IV-t044 clone was recently reported as the leading cause of infections among neonates in a hospital in Algeria [29].
Two of the four ST97-V-MRSA isolate, ST97-V-t359 isolates, were resistant to gentamicin, kanamycin and fusidic acid like the resistance profiles of ST97-V-MRSA isolates that caused an outbreak in a neonatal intensive care unit in another Kuwait hospital in 2007 [12]. The other two ST97-V-t1234 isolates were resistant to tetracycline and resembled isolates reported previously in another Kuwait hospital [33]. ST97-V-MRSA isolates have also been reported sporadically from Jordan [39], Saudi Arabia [43], Lebanon [44] and countries outside the Middle East including Australia [45], USA [46], UK [47], and Brazil [48] underlying their growing importance as a human pathogen in addition to their roles as veterinary pathogens [49, 50].
Novel MRSA clones isolated at the Maternity hospital included the dominant clone, ST5-V-t002, recovered from healthcare workers, adult patients and neonates, and sporadic clones such as ST77-IV-t019, and ST9-V-t267. Although newly isolated in Kuwait, ST5-V-t002 isolates have been reported to cause infections in other countries [42, 51].
Prior to the report of the ST9-V-t267 isolate in this study, CC9 isolates were recovered from animals or farm workers [42]. The present isolate was obtained from a blood sample of a baby admitted to the Special Care unit. However, history of parental contact with animals could not be established.
The isolation of the common MRSA clones, ST5-V-t002, ST22-IVa-t223 and ST80-IV-t044 from neonates, healthcare workers and adult patients in the hospital is suggestive of nosocomial transmission of these clones. The presence of colonized healthcare workers, neonates and their mothers, and adult patients in other wards of the hospital have previously been documented to provide opportunities for nosocomial transmissions of MRSA in a neonatal ward [52]. Furthermore, the isolation of ST5-V-t002 from a mother, her baby and nine other babies in the same unit in 2008 is suggestive of an outbreak with the mother as the likely source of the organism [53]. Likewise, the detection of ST22-IV-t852 from a baby’s cot and five different babies in the same unit suggests that the environment also contributed to the nosocomial transmission [54]. Therefore, multiple sources including colonized mothers, adult patients, healthcare workers and the environment contributed to the MRSA population in the Maternity hospital.
The study further sought to establish whether any of the clones persisted in the hospital overtime. The results indicated that although ST5-V-t002, ST22-IVa-t223 and ST80-IV-t044 were isolated in more than one year, the numbers isolated per year were small which suggested multiple acquisition rather than persistence. The other isolates occurred sporadically supporting their transient acquisition.
Although the isolates were susceptible to vancomycin, teicoplanin, linezolid and rifampicin, their susceptibility patterns followed predictable patterns with HA-MRSA isolates, ST239/ST241 –III-MRSA, ST22-IV-t005 and ST22-IV-t852 MRSA expressing multi resistance whereas the CA-MRSA isolates belonging to CC6 (ST6-IV-t6269, ST932-IV-t304 and ST932-IV-t657) were susceptible to all non-beta-lactam antibiotics and the other CA-MRSA clones were susceptible to most of the non- beta-lactam antibiotics.
In conclusion, the MRSA isolates belonged to diverse genetic backgrounds with ST5-V-t002 and ST22-IVa-t223 as the dominant clones. Multiple sources including healthcare workers, colonized or infected patients, colonized mothers and the environment contributed to the MRSA population which consisted of MRSA clones that have been isolated widely in other Kuwait hospitals and sporadic novel clones. This study has provided an initial data which will serve as a platform for future comparative studies on the distribution of MRSA clones in the Maternity hospital in Kuwait.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by Research Sector, Kuwait University, grant MI 01/11.
References
- 1.Chambers HF (2001) The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis 7: 178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maltezou H, Giamarellou H (2005) Community-acquired methicillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob Agents 27: 87–96. [DOI] [PubMed] [Google Scholar]
- 3.Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitoro C, et al. (2001) Structural comparison of three types of cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 45: 1323–1336. doi: 10.1128/AAC.45.5.1323-1336.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra, et al. (2002) Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother 46: 1147–1152. doi: 10.1128/AAC.46.4.1147-1152.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito T, Ma XX, Takeuchi F, Okuma K, Yuxawa H, Hiramatsu K (2004) Novel Type V Staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrb Agents Chemother 48: 2637–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higuchi W, Takano T, Teng LI, Yamamoto T (2008) Structure and specific detection of staphylococcal cassette chromosome mec type VII. Biochem Biophys Res Commun 377: 752–756. doi: 10.1016/j.bbrc.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 7.David M Z, Daum RS (2010) Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev 23:616–687. doi: 10.1128/CMR.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bratu S, Eramo A, Kopec R, Coughlin E, Ghitan M, Yost R, et al. (2005) Community-associated methicillin-resistant Staphylococcus aureus in hospital nursery and maternity units. Emerg Infect Dis 11: 808–813. doi: 10.3201/eid1106.040885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey AJ, Duchon J, Della-Latta P, Saiman L (2010) The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. J Perinatol 30: 135–139. doi: 10.1038/jp.2009.119 [DOI] [PubMed] [Google Scholar]
- 10.Creech CB, Litzner B, Talbot TR, Schaffner W (2010) Frequency of detection of methicillin-resistant Staphylococcus aureus from rectovaginal swabs in pregnant women. Am J Infect Control 38:72–74. doi: 10.1016/j.ajic.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 11.Kamath S, Mallaya S, Shenoy S (2010) Nosocomial infections in neonatal intensive care units: profile, risk factor assessment and antibiogram. Indian J Pediatr 77: 37–39. doi: 10.1007/s12098-010-0005-5 [DOI] [PubMed] [Google Scholar]
- 12.Udo EE, Aly NY, Sarkhoo E, Al-Sawan R, Al-Asar AS (2011) Detection and characterization of an ST97-SCCmec-V community-associated methicillin-resistant Staphylococcus aureus clone in a neonatal intensive care unit and special care baby unit. J Med Microbiol 60: 600–604. doi: 10.1099/jmm.0.028381-0 [DOI] [PubMed] [Google Scholar]
- 13.McAdams RM., Ellis MW, Trevino S, Rajnik M (2008) Spread of methicillin-resistant Staphylococcus aureus USA300 in a neonatal intensive care unit. Pediatr Int. 6: 810–815. [DOI] [PubMed] [Google Scholar]
- 14.Garcia CP, Rosa JF, Cursino MA, Lobo RD, Mollaco CH, Gobara S, et al. (2014) Non-multidrug-resistant- methicillin-resistant Staphylococcus aureus in a neonatal unit. Peditr Infect Dis 33: e252–9. [DOI] [PubMed] [Google Scholar]
- 15.Geraci DM, Giuffre M, Bonura C, Matranga D, Aleo O, Saporito L, et al. (2014) Methicillin-resistant Staphylococcus aureus colonization: a three-year prospective study in a neonatal intensive care unit in Italy. PloS One 9: e87760 doi: 10.1371/journal.pone.0087760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udo EE, Al-Sweih N (2013) Emergence of methicillin-resistant Staphylococcus aureus in the Maternity hospital, Kuwait. Med Princ Pract 22: 535–539. doi: 10.1159/000350526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Udo EE, Al-Sweih N, Dhar R, Dimitrov T, Mokaddas EM, Johny M, et al. (2008) Surveillance of antibacterial resistance in Staphylococcus aureus isolated in Kuwait hospitals. Med Princ Pract 17: 71–75. doi: 10.1159/000109594 [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standard Institute (2012) Performance standards for antimicrobial susceptibility testing; Twenty-Second Informational Supplement. CLSI; document M100-S22, Wayne, PA. [Google Scholar]
- 19.BSAC: BSAC methods for susceptibility testing. Version 9.1. March 2010.
- 20.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter M-O, Gauduchon V, et al. (1999) Involvement of Panton-Valentine leukocidin producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 29: 1128–1132. doi: 10.1086/313461 [DOI] [PubMed] [Google Scholar]
- 21.Oliveira DC, de Lencastre H (2002) Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 46: 2155–2161. doi: 10.1128/AAC.46.7.2155-2161.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K, McClure J-A, Elsayed S, Louie T, Conly JM (2005) Novel Multiplex PCR Assay for Characterization and Concomitant Subtyping of Staphylococcal Cassette Chromosome mec Types I to V in Methicillin-Resistant Staphylococcus aureus. J Clin Microbiol 43: 5026–5033. doi: 10.1128/JCM.43.10.5026-5033.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harmsen D, Claus H, Witte W, Rothanger J, Claus H, Turnwald D, et al. (2003) Typing of methicillin-resistant Staphylococcus aureus in a University Hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 41: 5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.http://www.spaserver.ridom.de
- 25.Enright MC, Day NPJ, Davies CE, Peacock SJ, Spratt BG (2000) Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.http://www.mlst.net
- 27.Enright MC, Robinson DA, Randle G, Feil EJ, Grundman H, Spratt BG (2002) The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci USA 99: 7687–7692. doi: 10.1073/pnas.122108599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machuca MA, Sosa LM, Gonzalez CL (2013) Molecular typing and virulence characteristics of methicillin-resistant Staphylococcus aureus isolates from pediatric patients in Bucaramanga, Columbia. PLoS One 8: e73434 doi: 10.1371/journal.pone.0073434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djoudi F, Bonura C, Benallaoua S, Touati A, Touati D, Aleo A, et al. (2013) Panton-Valentine leukocidin positive sequence type 80 methicillin-resistant Staphylococcus aureus carrying a staphylococcal cassette chromosome mec type IVc is dominant in neonates and children in an Algiers hospital. New Microbiol 36: 49–55. [PubMed] [Google Scholar]
- 30.Schaumburg F, Alabi AS, Mombo-Ngoma G, Kaba H, Zoleko RM, Diop DA, et al. (2014) Transmission of Staphylococcus aureus between mothers and infants in an African setting. Clin Microbiol Infect 20: 390–396. [DOI] [PubMed] [Google Scholar]
- 31.Alsubaie S, Bahkali K, Somily AM, Alzamil F, Alrabiaah A, Alaska A, et al. (2012) Nosocomial transmission of community-acquired methicillin-resistant Staphylococcus aureus in a well-infant nursery of a teaching hospital. Pediatr Int. 54: 786–792. doi: 10.1111/j.1442-200X.2012.03673.x [DOI] [PubMed] [Google Scholar]
- 32.Ali H, Nash JQ, Kearns AM, Pichon B, Vasu V, Nixon Z, et al. (2012) Outbreak of a South West Pacific clone Panton-Valentine leucocidin-positive meticillin-resistant Staphylococcus aureus infection in a UK neonatal intensive care unit. J Hosp Infect 80: 293–298. doi: 10.1016/j.jhin.2011.12.019 [DOI] [PubMed] [Google Scholar]
- 33.Boswihi SS, Udo EE, Al-Sweih N (2016) Shifts in the clonal distribution of methicillin-resistant Staphylococcus aureus in Kuwait hospitals: 1992–2010. PloS ONE 11: e0162744 doi: 10.1371/journal.pone.0162744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Udo EE, Al-Sweih N, Noronha B (2006) Characterization of Non multiresistant methicillin—resistant Staphylococcus aureus (including EMRSA-15) in Kuwait Hospitals. Clin Microbiol Infect 12: 262–269. doi: 10.1111/j.1469-0691.2005.01350.x [DOI] [PubMed] [Google Scholar]
- 35.Al-Haddad AM, Udo EE, Mokadas EM, Sanyal SC, Grubb WB (2001) Persistence of a clone of methicillin-resistant Staphylococcus aureus in a burns unit. J Med Microbiol 50: 558–564. doi: 10.1099/0022-1317-50-6-558 [DOI] [PubMed] [Google Scholar]
- 36.Udo EE, Boswihi SS, Al-Sweih N (2016) High prevalence of toxic shock syndrome toxin- producing methicillin-resistant Staphylococcus aureus 15 (EMRSA-15) strains in Kuwait hospitals. New Microbe and New Infect 12: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson AP, Aucken HM, Cavendish S, Ganner M, Wale MC, Warner M, et al. (2001) Dominance of EMRSA-15 and -16 among MRSA causing nosocomial bacteremia in the UK. Analysis of isolates from the European Antimicrobial Resistance Surveillance System (EARSS). J Antimicrob Chemother 48: 143–144. [DOI] [PubMed] [Google Scholar]
- 38.Biber A, Abuelaish I, Rahav G, Raz M, Cohen L, Valinsky L, et al. (2012) A typical hospital—acquired methicillin-resistant Staphylococcus aureus clone is widespread in the community in the Gaza strip. PLoS One 7: e42864 doi: 10.1371/journal.pone.0042864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aqel AA, Alzoubi HM, Vickers A, Pichon B, Kearns AM (2015) Molecular epidemiology of nasal isolates of methicillin-resistant Staphylococcus aureus from Jordan. J Infect Public Health 8: 90–97. doi: 10.1016/j.jiph.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 40.Abou Shady HH, Baker A.E.A, Hashad ME, Alzohairy MA (2014) Staphylococcus aureus nasal carriage among outpatients attending primary health care centers: a comparative study of two cities in Saudi Arabia and Egypt. Braz J Infect Dis 19: 68–76. doi: 10.1016/j.bjid.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senok A, Somily A, Raji A, Gawlik D, Al-Shahrani F, Baqi S, et al. (2016) Diversity of methicillin-resistant Staphylococcus aureus CC22-MRSA-IV from Saudi Arabia and the Gulf region. Int J Infect Dis 51: 31–35. doi: 10.1016/j.ijid.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 42.Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, et al. (2011) A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One 6: e17936 doi: 10.1371/journal.pone.0017936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monecke S, Skakni L, Hasan R, Ruppelt A, Ghazal SS, Hakawi A, et al. (2012) Characterization of MRSA strains isolated from patients in a hospital in Riyadh, Kingdom of Saudi Arabia. BMC Microbiol 12: 146 doi: 10.1186/1471-2180-12-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tokajian ST, Khalil PA, Jabbour D, Rizk M, Farah MJ, Hashwa FA, et al. (2010) Molecular characterization of Staphylococcus aureus in Lebanon. Epidemiol Infect 138: 707–712. doi: 10.1017/S0950268810000440 [DOI] [PubMed] [Google Scholar]
- 45.Coombs GW, Monecke S, Pearson JC, Tan HL, Chew YK, Wilson L, et al. (2011) Evolution and diversity of community-associated methicillin-resistant Staphylococcus aureus in a geographical region. BMC Microbiol. 11: 215 doi: 10.1186/1471-2180-11-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung M, Dickson G, de Lencastre H, Tomasz A (2004) International clones of methicillin-resistant Staphylococcus aureus in two hospitals in Miami, Florida. J Clin Microbiol 42: 542–547. doi: 10.1128/JCM.42.2.542-547.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellington MJ, Yearwood L, Ganner M, East C, Kearns AM (2008) Distribution of the ACME-arcA gene among methicillin-resistant Staphylococcus aureus from England and Wales. J Antimicrob Chemother 61:73–77. doi: 10.1093/jac/dkm422 [DOI] [PubMed] [Google Scholar]
- 48.Schuenck RP, Nouer SA, Einter Cge O, Cavalcante FS, Scotti TD, Ferreira AL, et al. (2009) Polyclonal presence of non-multiresistant methicillin-resistant Staphylococcus aureus isolates carrying SCCmec IV in healthcare-associated infections in a hospital in Rio de Janeiro, Brazil. Diagn Microbiol Infect Dis 64: 434–441. doi: 10.1016/j.diagmicrobio.2009.04.007 [DOI] [PubMed] [Google Scholar]
- 49.Smyth DS, Feil EJ, Meaney WJ, Hartigan PJ, Tollersrud T, Fitzgerald JR., et al. (2009) Molecular genetic typing reveals further insights into the diversity of animal-associated Staphylococcus aureus. J Med Microbiol 58: 1343–1353. doi: 10.1099/jmm.0.009837-0 [DOI] [PubMed] [Google Scholar]
- 50.Sung JM-L, Lloyd DH, Lindsay JA (2008) Staphylococcus aureus host specificity: comparative genomics of human versus animal isolates by multi-strain microarray. Microbiol 154: 1949–1959 [DOI] [PubMed] [Google Scholar]
- 51.Seidl K, Leimer N, Marques MP, Furrer A, Holzmann-Burgel A, Senn G, et al. (2015) Clonality and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus at the University hospital Zurich, Switzerland between 2012 and 2014. Annals of Clinical Microbiology and Antimicrobials 14: 14 doi: 10.1186/s12941-015-0075-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nubel U, Nachtnebel M, Falkenhorst G, Benzler J, Hecht J, Kube M, et al. (2013) MRSA transmission on a neonatal intensive care unit: epidemiological and genome-based phylogenetic analyses. PLoS One 8: e54898 doi: 10.1371/journal.pone.0054898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams K, Hopkins S, Turbitt D, Seng C, Cookson B, Patel BC, et al. (2014) Survey of neonatal unit outbreaks in North London: Identifying causes and risk factors. J Hosp Infect 88: 149–155. doi: 10.1016/j.jhin.2014.06.012 [DOI] [PubMed] [Google Scholar]
- 54.Takei Y, Yokoyama K, Katano H, Tsukiji M, Ezaki T (2010) Molecular epidemiological analysis of methicillin-resistant staphylococci in a neonatal intensive care unit. Biocontrol Sci. 15: 129–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.