Abstract
Background
Despite its potential for understanding tobacco dependence, behavioral discrimination of nicotine via smoking has not been formally examined as a function of nicotine dependence level.
Methods
Spectrum research cigarettes were used to compare non-dependent with dependent smokers on the lowest content of nicotine they could discriminate (i.e., “threshold”). Dependent (n=21; 16 M, 5 F) or non-dependent (n=7; 4 M, 3 F) smokers were tested on ability to discriminate between cigarettes with nicotine contents of 17, 11, 5, 2, and 1 mg/g, one per session, from an “ultra-low” cigarette with 0.4 mg/g (all had 9–10 mg “tar”). All abstained from smoking overnight prior to sessions, and number of sessions was determined by the lowest nicotine content they could reliably discriminate from the ultra-low on >80% of trials (i.e., ≥5 of 6). Subjective perceptions and cigarette choice behavior were also assessed and related to discrimination behavior.
Results
Discrimination thresholds (and most perceptions) did not differ between dependent and non-dependent smokers, with median thresholds of 11 mg/g for both subgroups. Yet, “liking” and puff choice for threshold cigarettes were greater in dependent but not non-dependent smokers, while cigarettes with nicotine contents below threshold did not support “liking” or choice in both groups.
Conclusions
In sum, this preliminary study suggests threshold for discriminating nicotine via smoking may not vary by dependence level, and further study is needed to confirm that cigarettes unable to be discriminated are also not reinforcing.
Keywords: Nicotine, Discrimination, Threshold, Dependence, Cigarette smoking
1. Introduction
Because nicotine intake is critical to tobacco smoking (Stolerman and Jarvis, 1995; USDHHS, 2010), it appears unlikely that a low-nicotine cigarette that smokers could not discriminate (i.e., “feel the effects”) from one virtually lacking in nicotine would support onset and maintenance of dependence (e.g., Kessler, 1994; Slade et al., 1995; Wayne and Carpenter, 2009). For this reason, cigarettes with nicotine content just above this amount, the minimum discriminable nicotine in tobacco (or “threshold”), likely have implications for public policy to reduce dependence risk (Hatsukami et al., 2010; Henningfield et al., 1998; Sofuoglu and LeSage, 2012). Specifically, the 2009 Tobacco Control Act allows FDA to regulate the nicotine content of tobacco products (U.S. govt, 2009). Therefore, restricting nicotine content in commercial cigarettes below this discrimination threshold could result in a maximum nicotine exposure from smoking that is inadequate for dependence onset or maintenance (Benowitz and Henningfield, 1994). With such regulation, anyone not already dependent may never become dependent, and dependent smokers might more easily quit.
As with all drugs (e.g., Bolin et al., 2016; Glennon and Young, 2011; Johanson, 1991), behavioral nicotine discrimination testing involves identifying if one dose of nicotine can be reliably detected from an identically-appearing substance containing a lower dose, or no nicotine. Nicotine discrimination testing has a long history with non-human animals (Smith and Stolerman, 2009) but not with humans (Perkins, 2009). Limited human study is due primarily to lack of control over nicotine dosing via tobacco smoking, as dose exposure can vary widely if smoking topography varies (Benowitz et al., 1983). Until recently, just a few discrimination studies in humans involved nicotine administration, and all controlled dosing by means other than tobacco smoking (nasal spray, see Perkins, 2011, or oral capsules in Duke et al., 2015).
However, Spectrum research cigarettes (from the National Institute on Drug Abuse) are specifically engineered to provide a narrow range of nicotine contents, with different versions available down to very low amounts (see Donny et al., 2015). In contrast with commercial brands, these research cigarettes essentially prevent smokers from obtaining nicotine exposure different from the cigarette’s contents by altering their puff topography (see Hatsukami et al., 2013; Marian et al., 2009). Using Spectrum cigarettes, we adapted methods from our prior studies on nicotine discrimination with nasal spray (reviewed in Perkins, 2011) to develop procedures testing nicotine discrimination via cigarette smoking (Perkins et al., 2016a). Subsequently, we evaluated procedures for determining the threshold “dose” for discriminating nicotine in dependent smokers (Perkins et al., 2016b), followed by comparison of dependent smokers who preferred menthol vs. non-menthol cigarette brands (Perkins et al., under review).
In the current study, we compared cigarette nicotine discrimination threshold between dependent and non-dependent smokers (i.e., those not meeting DSM criteria for dependence) to explore whether threshold may differ due to dependence. Low doses may be discriminable in non-dependent smokers but not discriminable in dependent smokers, reflecting their possibly higher threshold. Alternatively, based on our nasal spray nicotine discrimination threshold research with smokers and nonsmokers (Perkins et al., 2001), presence of dependence may not matter as both may be able to reliably discriminate the same nicotine doses via cigarettes. In any case, formal test of nicotine discrimination in humans differing in level of dependence may be needed to determine a discrimination threshold for all who would have access to those cigarettes.
Finally, similar to other drug discrimination research with humans (e.g., Johanson, 1991), secondary analyses also examined associations of nicotine discrimination behavior with concomitant subjective perceptions and subsequent choice behavior in response to these cigarettes. One goal here was to explore the notion that a cigarette with nicotine contents too low to be discriminated from one virtually lacking in nicotine would also be too low to support acute smoking reinforcement (i.e., choice).
2. Material and methods
2.1 Participants
Eligible were dependent (n=21; 16 M, 5 F) and non-dependent (n=7; 4 M, 3 F) smokers who preferred non-menthol cigarettes. (All compared here were those preferring non-menthol due to very few non-dependent menthol smokers available for testing; other research directly compares nicotine discrimination based on menthol preference in dependent smokers; Perkins et al., under review). Presence or absence of nicotine dependence was confirmed by DSM-V criteria (APA, 2013), which dependent smokers currently met and non-dependent smokers could never have met (i.e., no history of dependence). To further compare these subgroups on dependence, all also completed the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991), as well as the MNWS withdrawal scale (Hughes and Hatsukami 1986) upon arrival to the first session following overnight abstinence. As expected, dependent and non-dependent participants differed significantly on mean (SD) smoking history characteristics, respectively, of 14.8 (3.7) vs 1.4 (0.7) cigarettes per day, t(26) = 9.54, p<.001, and 4.2 (1.8) vs 0.1 (0.4) FTND score, t (26) = 5.73, p<.001, as well as on MNWS score after overnight abstinence of 40.7 (18.8) vs 19.0 (9.0), t (26) = 2.91, p<.01. Also as expected, they did not differ significantly on age, 32.1 (11.2) vs. 24.3 (7.0) years old, respectively, t (26) = 1.72, p<.10, and their self-identified ethnic representation was similar, with 90% vs 71% Caucasian, and the other 2 from each group comprising more than one race and either African American or Asian.
2.2 Research cigarettes
Spectrum investigational research cigarettes, manufactured by 22nd Century Group (Clarence NY; http://www.xxiicentury.com/), were obtained from NIDA’s Drug Supply Program (see Perkins et al., 2016a). Selected for the current study were all those cigarettes that differed in nicotine contents but were similar on “tar” yield (so differing only in nicotine per se). Nicotine contents of these cigarettes were 17, 11, 5, 2, 1, and 0.4 mg (averaging two Spectrum batches sent by NIDA), and all had about 9–10 mg “tar”. Not available is a true “placebo” Spectrum, with zero nicotine content. (Comparing their “yields” by FTC method with commercial brands, which is more familiar and estimates the inhaled portion of nicotine rather than total contents in the cigarette, these Spectrum research cigarettes correspond to approximately 0.8, 0.6, 0.26, 0.12, 0.07, and 0.03 mg nicotine; see http://grants.nih.gov/grants/guide/notice-files/NOT-DA-14-004.html. Most commercial brands yield about 0.9 mg nicotine of inhaled nicotine, with roughly 10 mg “tar”; USDHHS, 2010.) As described below in Procedures, all discrimination testing sessions involved comparing the very lowest content cigarette, 0.4 mg/g, with each of the higher nicotine content cigarettes. Just one of these “higher” nicotine cigarettes was compared with the 0.4 mg/g cigarette in a given session. To clearly differentiate this comparison cigarette with the others tested, this lowest is often called the “ultra-low” nicotine cigarette.
2.3 Smoke exposure control
Intake from all cigarettes during all sessions (see 2.4 Procedures, below) was fixed at 4 puffs per trial, just as in our prior discrimination testing via smoking (Perkins et al., 2016a; 2016b) as well as in the only prior controlled human test of discriminating any drug inhaled by smoking, on marijuana (Chait et al., 1988). All smoking in this study was done using the portable Clinical Research Support System (CReSS; Borgwaldt KC, Inc., Richmond VA), according to procedures common to our past research (e.g., Perkins et al., 2016a), with one puff every 30 sec and a new cigarette on each trial. Timing of each puff was determined by computer-displayed instructions, so that a 2-sec puff duration standardized smoke intake at approximately 60 ml per puff, which is similar to most ad lib puffing (Blank et al., 2009; Perkins et al., 2012). The CReSS assesses puff number, total puff volume (total intake across all puffs from a single cigarette), and average volume per puff, saving these data electronically and allowing puff topography to be compared with our 60 ml per puff target. This pattern of smoke exposure allowed intervals of 15 min between trials while minimizing smoking satiation or toxicity. Smoking 4 puffs is also typical exposure at the point a smoker forms expectations about a cigarette, which clearly impact the subsequent reinforcing and other effects of that cigarette (Gu et al., 2015; Hasenfratz et al., 1993; see also Perkins et al., 2001; Perkins et al., 1994; 1996).
2.4 Procedures
2.4.1. General
All study sessions involved testing ability to discriminate between the ultra-low (0.4 mg/g) nicotine content cigarette versus one of the higher nicotine content Spectrum cigarettes (≥1 mg/g). Thus, Spectrum cigarettes with previously described nicotine contents of 17, 11, 5, 2, and 1 mg/g were separately tested, one per session, on discriminability from the 0.4 mg/g cigarette. Behavioral discrimination was determined by reliable detection of which cigarette was which across the separate exposures to each under blind conditions during the session (e.g., Bolin et al., 2016; Perkins et al., 2011; see 2.4.2. Specific session procedures). Each participant’s total number of sessions depended on success of discrimination behavior, as participation ended when the lowest nicotine content cigarette he or she could reliably discriminate from the ultra-low (0.4 mg/g) was determined, identifying the “threshold” dose. The next lowest content cigarette below their threshold cigarette, which by definition they failed to discriminate, was labeled their “subthreshold” dose. These procedures were developed and evaluated (Perkins et al., 2016a) prior to studies of nicotine discrimination thresholds in dependent smokers via Spectrum cigarettes (Perkins et al., 2016b; under review 2017).
In the first session, participants learned the discrimination procedure and were tested on ability to discriminate the two most widely differing nicotine content cigarettes, 17 mg/g vs 0.4 mg/g. Those unable to discriminate repeated this identical testing procedure in a second session to verify their inability to discriminate. If again unable, their participation ended (but their data were included; see next paragraph), while those able to discriminate proceeded on to subsequent sessions (as described below in Specific session procedures). At that point, the order across sessions of the other nicotine content cigarettes, compared one per session with the ultra-low, differed systematically in either descending or ascending fashion. We have recently described this randomization of dose order in more detail elsewhere (Perkins et al., 2016b). Briefly, after the initial test of 17 mg/g (vs. 0.4 mg/g) in the first session, the order was progressively lower for the “descending” subgroup, to 11 mg/g for the next session, then 5 mg/g in the following, etc., but was progressively higher for the “ascending” subgroup, starting with 1 mg/g for the next session, then 2 mg/g in the following, and 5 mg/g, and so on. This approach allowed us to verify that discrimination thresholds did not differ as a function of order in which cigarettes were administered, increasing reliability of threshold determination (see Perkins et al., 2001; 2016b).
A last session involved repeat training and testing of the lowest nicotine content cigarette they were able to discriminate from the 0.4 mg/g ultra-low, to verify reliability of that discrimination (i.e., their “threshold” dose). As noted, those unable to discriminate the 17 mg/g cigarette did not continue on to further sessions, but they were included in analyses of discrimination responding so that tests of threshold were not biased by excluding smokers who may require an even higher nicotine content cigarette to discriminate it from the ultra-low. (Such Spectrum cigarettes exist but are not matched with the others on “tar”, which is why we did not include them in this discrimination threshold testing.) This study protocol was approved by the University of Pittsburgh Institutional Review Board.
2.4.2. Specific session procedures
All subjects were required to be abstinent from smoking overnight prior to each session, confirmed by CO≤10 ppm (SRNT, 2002) as assessed by BreathCO CO monitor (Vitalograph, Lenexa, KS). In each 3-hr session, subjects were initially “trained” to discriminate which cigarette was which, administered singly over 4 separate trials, and then “tested” on their ability to discriminate between them on 6 trials. Thus, the two cigarettes to be compared per session were presented individually in random order across a total of ten trials (5 per type of nicotine content cigarette), each 15-min in duration.
During the 4 training trials (two per cigarette), each cigarette was identified by letter code (e.g., “A” or “B” in session 1, “C” vs “D” in session 2, and so on, each code assigned randomly between cigarettes differing in nicotine content). Subjects were told that each correct cigarette identification during the subsequent 6 testing trials would result in $1 being added to their total participant payment so they all would be equally motivated to learn this discrimination. After the 4 puffs on the designated cigarette in each trial, subjects completed a brief self-report form on their perceptions of the cigarette (secondary measure), including how much “nicotine”, “liking”, “flavor”, and “similar to own brand” the cigarette was, each rated on a 0–100 visual analog scale (VAS, anchored by “not at all” to “very much”; see Perkins et al., 2012).
Following the 4 training trials were 6 testing trials, as detailed in our prior research (Perkins et al., 2016a; 2016b). In brief, procedures were the same during testing as during training trials, except subjects were uninformed of the administered cigarette’s letter code identification (i.e., to keep them blind) so we could assess their acquisition of discrimination. After the secondary measure on subjective perceptions, they circled the letter code (e.g., “A” or “B”, “C” or “D”) they believed identified the cigarette. “Successful” discrimination between cigarettes was defined a priori by greater than 80% correct identifications, i.e., ≥5 out of 6 trials, as with the rate of dichotomous drug-appropriate responding criterion used in most prior human drug discrimination research (see Takada, 1996; Perkins, 2011; Duke et al., 2015).
After the last testing trial were two additional trials, also 15 min apart, involving “choice” of puffs between the two cigarettes (see Perkins et al., 1996), now made available concurrently. During each of these two choice trials, both the ultra-low and the higher nicotine content cigarette were each identified by the letter code used in training trials. Subjects were instructed to smoke 4 puffs from some combination of the two cigarettes, (e.g., from a mix of the two, or all 4 from one or from the other), solely based on their preference. Of 8 total puffs over the two choice trials, the number of puffs from the higher nicotine cigarette relative to the 0.4 mg/g ultra-low was taken as the measure of nicotine’s relative reinforcement (with 4.0 being “chance”).
2.5 Data Analyses
This study’s goal was to determine whether nicotine discrimination threshold varied based on presence vs. absence of dependence. As described previously (Perkins et al., 2016b), uneven interval sizes between nicotine content levels of consecutive cigarettes necessitated coding the thresholds as an ordinal variable for analyses. The ordinal threshold variable ranged from 1 to 7, with 1 assigned to the ultra-low dose of 0.4 mg/g, ascending to 2 for 1 mg/g on up to 6 for 17 mg/g, while 7 was assigned to those unable to discriminate. Preliminary analyses used separate ordinal regressions to confirm no difference in thresholds due to sex, or between the ascending versus descending order subgroups. In the primary analyses, ordinal regression analyzed the pattern of nicotine thresholds between the dependent and non-dependent groups. This statistic modeled the probability of being able to discriminate at each ordinal threshold level or below as a function of presence vs. absence of dependence. Secondary analyses examined differences in subjective perceptions of the threshold and subthreshold cigarettes. These analyses compared responses to the higher nicotine content vs. ultra-low cigarette, collapsing all respective trials. Perceptions due to the difference in nicotine content between cigarettes were compared using multivariate analysis of variance (MANOVA), with follow-up univariate ANOVAs for each individual VAS response. The non-parametric Wilcoxon signed rank test (z) analyzed choice between the higher nicotine content and ultra-low cigarettes within each session.
3. Results
3.1 Preliminary comparisons
We first examined smoking exposure between dependent vs non-dependent participants by comparing topography per 4-puff trial in the first session (i.e., the only session all subjects completed), involving the two most widely varying nicotine content cigarettes, 17 mg/g and 0.4 mg/g. Dependent smokers took larger puffs than non-dependent smokers, F(1,26)= 8.19, p<.01. Means (SD) from the 4 puffs were 262 (54) and 257 (54) mls for the 17 mg/g nicotine versus ultra-low, respectively, among dependent, compared to 174 (56) and 210 (57) in the non-dependent smokers. (Intake per puff was about 65 ml for dependent, very close to the 60 ml per puff intended by our computerized puffing instructions and as shown in our prior research on dependent smokers [e.g., Perkins et al., 2012]. Slightly less intake by non-dependent smokers, 48 ml, is very consistent with the approximately 40 ml puff volume from acute use of these same Spectrum cigarettes under similar controlled conditions by vulnerable smokers [Higgins et al., 2017]. Yet, the engineered specific contents of these research cigarettes preclude much of a difference in nicotine intake due to these smaller puff volumes.)
We also compared dose order subgroup and sex on the odds of having higher nicotine discrimination thresholds. No differences were found due to ascending versus descending (reference group, or “ref grp”) subgroups, OR = 1.20, 95% CI [0.03, 4.52], Wald (1) = 0.07, ns, or between women and men (ref grp), OR = 0.82, 95% CI [0.19, 3.53], Wald (1) = 0.07, ns. Thus, results were combined for each subgroup to compare all dependent vs. non-dependent smokers on nicotine discrimination threshold.
3.2 Primary analyses—Behavioral discrimination threshold results
Thresholds did not differ between non-dependent and dependent smokers, with a median nicotine content threshold in cigarettes of 11 mg/g for both groups. Those unable to behaviorally discriminate the 17 mg/g vs. 0.4 mg/g ultra-low were 2 of the 7 non-dependent and 3 of the 21 dependent smokers. The individual thresholds (and frequencies) of those able to discriminate were 11 mg/g (3) and 5 mg/g (2) for the non-dependent smokers, and 17 mg/g (3), 11 mg/g (5), 5 mg/g (6), and 2 mg/g (4) for the dependent smokers.
3.3 Secondary analyses—Subjective perception and choice responses
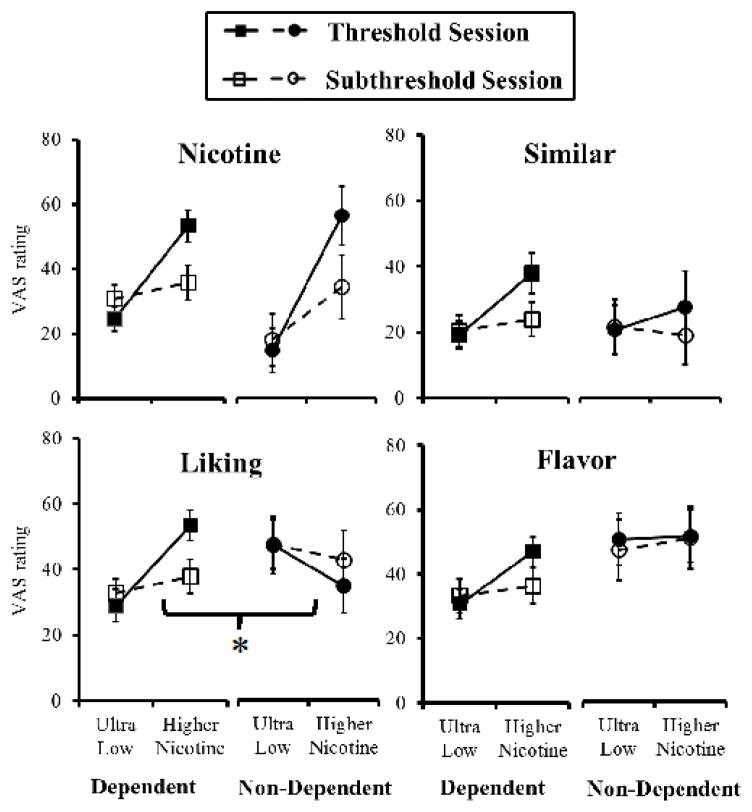
As noted, only those able to discriminate the highest nicotine content vs. ultra-low in the initial testing were included in the secondary analyses, as only they could proceed to subsequent sessions for determination of threshold and subthreshold cigarettes. The overall MANOVA showed perceptions were significantly greater in response to the threshold vs. subthreshold cigarettes (each compared with the ultra-low), F (8,12)=5.70, p<.005. In the between-groups comparison, the main effect of dependent vs. non-dependent was not significant, F(8,12)=1.59, p>.20, but the interaction of dependent/non-dependent x threshold/subthreshold was significant, F(8,12)=2.79, p=.05. However, in the follow-up univariate ANOVAs, only “liking” was significant for this interaction, F(1,19) = 6.90, p<.02, as no other items differentiated subjective responses to threshold vs. subthreshold as a function of dependence (see Figure 1). The only other significant ANOVA result was the main effect of threshold/subthreshold cigarettes for “how much nicotine”, F(1,19)=4.23, p=.05, as the subjective perception of “how much nicotine” (but no other perceptions) reflected the difference in actual nicotine contents between these cigarettes. This “nicotine” perception did not interact with dependent/non-dependent, F(1,19)<1, as clearly shown in Figure 1.
Figure 1.
Subjective perceptions of threshold vs. subthreshold cigarettes in non-dependent and dependent smokers.
Mean (SEM) perceptions (i.e., “How much….”, each on 0–100 VAS) among those dependent (n=16) and non-dependent (n=5) smokers able to discriminate, in response to smoking their threshold or their subthreshold nicotine Spectrum cigarettes. Each was tested on separate sessions vs. the ultra-low (0.4 mg/g) comparison. Relative to ultra-low, responses were greater due to threshold vs. subthreshold cigarette, but only “liking” differed due to non-dependent vs. dependent (* p<.05 for this difference).
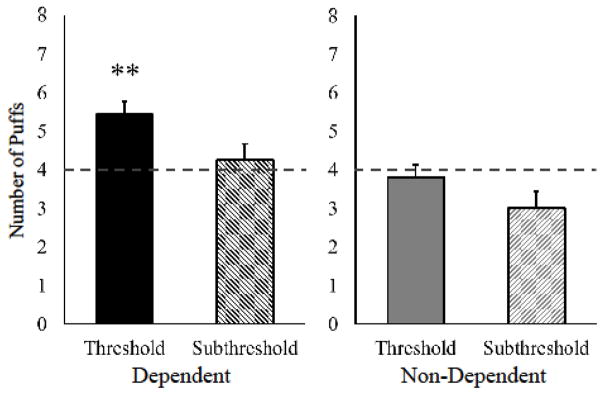
Yet, choice of puffs clearly differed due to dependence when each of the higher nicotine content cigarettes was available concurrently with the ultra-low, as shown in Figure 2. Among dependent smokers, puff choice (out of 8 total) was significantly greater for the threshold cigarette (vs. ultra-low), 5.4±0.3 vs. 2.6±0.3 (mean±SEM), z = 3.01, p<.01, but not for the subthreshold, 4.2±0.4 vs. 3.8±0.4 (with 4.0 being chance), z = 0.80, ns, and the difference in choice between threshold and subthreshold was significant, t(15)=2.19, p<.05. By contrast, puff choice by non-dependent smokers did not differ between cigarettes, with 3.8 ±1.4 vs. 4.2±1.4 for threshold (vs. ultra-low), z = 0.14, ns, and 3.0±0.9 vs. 5.0±0.9 for subthreshold, z = 1.00, ns. Thus, choice for the subthreshold nicotine cigarette was not significant in either group (Figure 2), even though it was higher in nicotine content than the concurrently available ultra-low.
Figure 2.
Threshold vs. subthreshold cigarette puff choice in non-dependent and dependent smokers.
Choice of puffs (out of 8 total) for participants’ discrimination threshold or subthreshold nicotine Spectrum cigarettes, each compared concurrently with the ultra-low (0.4 mg/g) cigarette on separate sessions, in dependent (n=16) and non-dependent (n=5) smokers. (4.0 dashed line signifies chance.) Relative to ultra-low comparison cigarette, choice was greater for the threshold but not for subthreshold cigarette in dependent smokers only (** p<.01).
4. Discussion
Although our results may be preliminary, this study suggests that the cigarette nicotine content for discrimination threshold does not differ due to presence or absence of the smoker’s dependence on nicotine. This finding, contrasting with the clear difference in typical cigarettes/day and abstinence withdrawal severity between these dependent and non-dependent smokers (see 2.1 Participants), is very consistent with our prior research showing no difference in nicotine discrimination threshold dose via nasal spray between dependent smokers and non-smokers (Perkins et al., 2001). Therefore, ability to discriminate nicotine content, whether via cigarette smoking or nasal spray, appears to be independent of the participant’s pre-existing dependence on nicotine. Supporting this notion is our observation that the subjective perceptions of “how much nicotine” differentiated threshold from subthreshold cigarettes comparably for dependent and non-dependent smokers. Another important similarity is that choice behavior (reinforcement) was not greater for the subthreshold vs. ultra-low nicotine cigarette in both dependent and non-dependent smokers (Fig 2). This finding is consistent with the theoretical notion that access only to very low nicotine cigarettes indistiguishable from those nearly devoid of nicotine would not support nicotine reinforcement in any smokers, although far more research is needed to confirm this point.
Sharply in contrast with dependent smokers, however, non-dependent smokers showed no increases in “liking” and puff choice for the threshold vs. ultra-low nicotine cigarettes. Thus, while non-dependent smokers may discriminate these cigarettes similarly to how dependent smokers discriminate them, they clearly experience less acute reinforcement (puff choice) and reward (“liking”). These very different reinforcing and rewarding responses to cigarettes matched on nicotine discrimination threshold may be as expected, based on the very presence vs. absence of dependence that define these groups of smokers. In sum, nicotine discrimination appears to be independent of nicotine dependence, and discriminability is associated with nicotine reinforcement and reward in dependent smokers but not in non-dependent smokers.
Strengths of this study include the fact that it is the first examination of discrimination threshold for nicotine content of cigarettes in non-dependent smokers (and one of the few such cigarette studies with any smokers to date). The study also carefully controlled smoking exposure to ensure sufficient intake by which to test for discriminability between cigarettes of different nicotine contents. Inclusion of the subjective perceptions and choice measures allowed for secondary comparisons of whether these responses may relate to discrimination threshold as a function of dependence. Among limitations of the study, the sample size of non-dependent smokers was modest, and replication of these findings in a larger sample is needed.
Results here also may depend on these specific procedures, in which cigarette exposure on each trial was restricted to just 4 puffs to minimize toxicity, which could lead non-dependent smokers to discriminate the cigarettes based on adverse responses. Our procedure, validated with dependent smokers in prior research (Perkins et al., 2016a; 2016b), may require more nicotine intake than non-dependent smokers usually consume over several hours, although we saw no evidence of avoidance of nicotine (e.g., choice of threshold was not below chance levels during the last 2 trials, in Fig. 2) or of smoking per se (e.g., liking of the ultra-low in Fig. 1), suggesting no toxicity. Also, participants took 4 puffs/trial on a total of 5 trials with the higher nicotine cigarette in each session (2 training and 3 testing, prior to the choice trials), which was always compared with the 0.4 mg/g “ultra-low”. Such intake means they took 20 puffs in all from the higher nicotine content cigarette over 2.5 hrs, or about 10 puffs from 2 regular nicotine cigarettes, less than one cigarette per hr. Because the 0.4 mg/g contained virtually no nicotine, as intended, this nicotine exposure is not more than would be expected from ad lib smoking by most smokers in the morning after overnight abstinence (e.g., Hatsukami et al., 1988).
5. Conclusions
With FDA authority to regulate the nicotine content in tobacco products, knowing the minimum discriminable nicotine content in tobacco (or “threshold”) likely has important implications for public policy to reduce dependence risk. The threshold content for discriminating nicotine in cigarettes appears to be similar regardless of whether or not a smoker is nicotine dependent. For all participants, acute reinforcement (puff choice) was not observed for cigarettes that could not be discriminated from the ultra-low (i.e., the subthreshold), suggesting inability to discriminate a cigarette’s nicotine may be associated with lack of reinforcement from smoking that cigarette. Although our results may be tentative, they suggest a policy to limit nicotine content of cigarettes below the discrimination threshold for most smokers may be sufficient to discourage dependence onset (i.e., non-dependent smokers) as well as maintenance (i.e., dependent smokers). While subjective perception of “how much nicotine” differentiated threshold versus subthreshold content cigarettes in both groups, subjective “liking” and puff choice did so only for dependent and not non-dependent smokers. The association of nicotine’s discriminability with these subjective and choice measures warrants further study. Finally, our study may help guide development of procedures to test behavioral discrimination of nicotine in cigarettes among other subgroups of smokers, such as vulnerable smokers (Higgins et al., 2017) or during onset of smoking in teens (e.g., Kandel et al., 2007), subject to ethical considerations.
Highlights.
Minimum discriminable nicotine in tobacco (“threshold”) may inform public policy
Nicotine discrimination threshold in cigarettes is similar regardless of dependence
Perceptions differentiated threshold vs subthreshold in both groups
Puff choice differentiated threshold only for dependent smokers
Limiting nicotine to below threshold may discourage dependence onset and maintenance
Acknowledgments
Role of Funding Source
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under grant award DA035968. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Contributors
Author KAP designed the study, oversaw protocol development and wrote the first draft of the manuscript. Author NK managed participant recruitment, data collection, and collected participant data during sessions. Author JLK oversaw statistical analyses. All authors contributed to and have approved the final manuscript.
Conflict of Interest
No author has any potential conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kenneth A. Perkins, Department of Psychiatry, University of Pittsburgh, Pittsburgh PA.
Nicole Kunkle, Department of Psychiatry, University of Pittsburgh, Pittsburgh PA.
Joshua L. Karelitz, Department of Psychiatry, University of Pittsburgh, Pittsburgh PA
References
- American Psychiatric Association (APA) Diagnostic and Statistical Manual-V. American Psychiatric Association; Washington DC: 2013. [DOI] [Google Scholar]
- Benowitz NL, Hall SM, Herning RI, Jacob P, Jones RT, Osman AL. Smokers of low-yield cigarettes do not consume less nicotine. New Engl J Med. 1983;309:139–142. doi: 10.1056/NEJM198307213090303. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. New Engl J Med. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: Mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11:896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin BL, Alcorn JL, Reynolds AR, Lile JA, Rush CR. Human drug discrimination: A primer and methodological review. Exper Clin Psychopharmacol. 2016;24:214–228. doi: 10.1037/pha0000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LE, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. The discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology. 1988;94:206–212. doi: 10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al’Absi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson JD, Stitzer Ml, Strasser AA, Tindle H, Hatsukami DK. Randomized trial of reduced-nicotine standards for cigarettes. New Engl J Med. 2015;373:1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AN, Johnson MW, Reissig CJ, Griffiths RR. Nicotine reinforcement in never-smokers. Psychopharmacology. 2015;232:4243–4252. doi: 10.1007/s00213-015-4053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R. Drug discrimination: Application to medicinal chemistry and drug studies. John Wiley and Sons; New York: 2011. [Google Scholar]
- Gu X, Lohrenz T, Salas R, Baldwin PR, Soltani A, Kirk U, Cinciripini PM, Montague PR. Belief about nicotine selectively modulates value and reward prediction error signals in smokers. Proceedings Nat Acad Sci. 2015;112:2539–2544. doi: 10.1073/pnas.1416639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfratz M, Jacober A, Battig K. Smoking-related subjective and physiological changes: pre- to postpuff and pre- to postcigarette. Pharmacol Biochem Behav. 1993;46:527–534. doi: 10.1016/0091-3057(93)90540-A. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, Roger-Batker AN, Mackowick KM, Jensen J, Murphy SE, Thomas BF, Donny E. Dose-response effects of Spectrum Research Cigarettes. Nicotine Tob Res. 2013;15:1113–1121. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, LeSage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger C, Zeller M. Nicotine reduction revisited: Science and future directions. Tob Control. 2010;19:436–445. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Pickens RW, Svikis DS, Hughes JR. Smoking topography and nicotine blood levels. Addict Behav. 1988;13:91–95. doi: 10.1016/0306-4603(88)90031-7. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD. Reducing the addictiveness of cigarettes. Tob Control. 1998;7:281–293. doi: 10.1136/tc.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Sigmon SC, Tidey JW, Gaalema DE, Stitzer ML, Durand H, Bunn JY, Priest JS, Arger CA, Miller ME, Bergeria CL, Davis DR, Streck JM, Zvorsky I, Redner R, Vandrey R, Pacek LR. Response to varying the nicotine content of cigarettes in vulnerable populations: An initial experimental examination of acute effects. Psychopharmacology. 2017;234:89–98. doi: 10.1007/s00213-016-4438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Johanson C-E. Discriminative stimulus effects of psychomotor stimulants and benzodiazepines in humans. In: Glennon RA, Jarbe TUC, Frankenheim J, editors. Drug Discrimination: Applications to Drug Abuse Research. U.S. Government Printing Office; Washington DC: 1991. pp. 181–196. NIDA Res Monograph 116. [PubMed] [Google Scholar]
- Kandel DB, Hu M-C, Griesler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug Alcohol Depend. 2007;91:26–30. doi: 10.1016/j.drugalcdep.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DA. Statement on nicotine-containing cigarettes. Tob Control. 1994;3:148–158. doi: 10.1136/tc.3.2.148. [DOI] [Google Scholar]
- Marian C, O’Connor RJ, Djordjevic MV, Rees VW, Hatsukami DK, Shields PG. Reconciling human smoking behavior and machine smoking patterns: Implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epid Biomarkers Prev. 2009;18:3305–3320. doi: 10.1158/1055-9965.EPI-09-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA. Discriminative stimulus effects of nicotine in humans. In: Henningfield JE, London E, Pogun S, editors. Nicotine Psychopharmacology. Springer-Verlag; New York: 2009. pp. 369–400. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Nicotine discrimination in humans. In: Glennon RA, Young R, editors. Drug Discrimination: Application to Medicinal Chemistry and Drug Studies. Chapter 15. John Wiley and Sons; New York: 2011. pp. 463–481. [Google Scholar]
- Perkins KA, DiMarco A, Grobe JE, Scierka A, Stiller RL. Nicotine discrimination in male and female smokers. Psychopharmacology. 1994;116:407–413. doi: 10.1007/BF02247470. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Sanders M, Meeker J, Wilson A. Threshold doses for nicotine discrimination in smokers and nonsmokers. Psychopharmacology. 2001;155:163–170. doi: 10.1007/s002130000660. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Weiss D, Fonte C, Caggiula A. Nicotine preference in smokers as a function of smoking abstinence. Pharmacol Biochem Behav. 1996;55:257–263. doi: 10.1016/S0091-3057(96)00079-2. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob Res. 2012;14:490–494. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Michael VC, Karelitz JL, Donny EC. Assessing discrimination of nicotine in humans via cigarette smoking. Nicotine Tob Res. 2016a;18:1830–1836. doi: 10.1093/ntr/ntw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Karelitz JL. Effects of menthol on threshold dose for behavioral discrimination of cigarette nicotine content. doi: 10.1007/s00213-017-4563-3. (under review) Manuscript under review. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Karelitz JL, Michael VC, Donny EC. Threshold dose for discrimination of nicotine via cigarette smoking. Psychopharmacology. 2016b;233:2309–2317. doi: 10.1007/s00213-016-4281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade J, Bero LA, Hanauer P, Barnes DE, Glantz SA. Nicotine and addiction: The Brown and Williamson documents. JAMA. 1995;274:225–233. doi: 10.1001/jama.1995.03530030045033. [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. In: Henningfield JE, London E, Pogun S, editors. Nicotine Psychopharmacology. Springer-Verlag; New York: 2009. pp. 295–333. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, LeSage MG. The reinforcement threshold for nicotine as a target for tobacco control. Drug Alcohol Depend. 2012;125:1–7. doi: 10.1016/j.drugalcdep.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT subcommittee. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Takada K. Drug discrimination studies in humans: A review of methodologies. Meth Find Exp Clin Pharmacol. 1996;18(suppl 1):187–196. [Google Scholar]
- U.S. Department of Health and Human Services (USDHHS) A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. [PubMed] [Google Scholar]
- U.S. Govt. Family Smoking Prevention and Tobacco Control Act. 2009 Pub. L. No. 111–31; http://www.gpo.gov/fdsys/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf.
- Wayne GF, Carpenter CM. Tobacco industry manipulation of nicotine dosing. In: Henningfield JE, London E, Pogun S, editors. Nicotine Psychopharmacology. Springer-Verlag; New York: 2009. pp. 457–485. [DOI] [PubMed] [Google Scholar]