Abstract
In this manuscript, I review the current and relevant classical studies on properties of the Mollusca heart and their central nervous system including ganglia, neurons, and nerves involved in cardiomodulation. Similar to mammalian brain hemispheres, these invertebrates possess symmetrical pairs of ganglia albeit visceral (only one) ganglion and the parietal ganglia (the right ganglion is bigger than the left one). Furthermore, there are two major regulatory drives into the compartments (pericard, auricle, and ventricle) and cardiomyocytes of the heart. These are the excitatory and inhibitory signals that originate from a few designated neurons and their putative neurotransmitters. Many of these neurons are well-identified, their specific locations within the corresponding ganglion are mapped, and some are termed as either heart excitatory (HE) or inhibitory (HI) cells. The remaining neurons are classified as cardio-regulatory, and their direct and indirect actions on the heart’s function have been documented. The cardiovascular anatomy of frequently used experimental animals, Achatina, Aplysia, Helix, and Lymnaea is relatively simple. However, as in humans, it possesses all major components including even trabeculae and atrio-ventricular valves. Since the myocardial cells are enzymatically dispersible, multiple voltage dependent cationic currents in isolated cardiomyocytes are described. The latter include at least the A-type K+, delayed rectifier K+, TTX-sensitive Na+, and L-type Ca2+ channels.
Keywords: Excitatory neurons, Heart, Inhibitory neurons, Ion channels, Neurotransmitters
Snail’s heart — the muscle [is] composed of elongated spindle-shaped cells closely superimposed and very intimately attached to each other.
1. Introduction
The Mollusca is one of the biggest phyla, and therefore, a specifically dedicated field of science — malacology, encompasses these invertebrates. The structure and form of their shells (in those who possess such) alone are fascinating (Schilthuizen and Davison, 2005). However, their designated name was based on the feature of their body – mollis – soft, which has a Greek origin and suggestively was used already by Aristotle. The most commonly used English version Molluscs (or mollusks) was adopted from mollusque in French; in Latin, it is molluscus. Similar transcription and pronunciation is used in Russian: Moллюcкa (s) or Moллюcки (pl). The same meaning, which derives from their body constitution, is also kept in German —Weichtiere, Hebrew — תוכיכר, and Macedonian — Meкoтeли.
The Molluscs caught attention between XIX and XX centuries, because of their central nervous system (CNS) and giant-size neurons ranging up to 600 μm (Kerkut and Taylor, 1956; Sakharov, 1966; Epstein and Tauc, 1970; Jerelova et al., 1971). These “giant neurons” are surrounded by glia cells. These two types of cells interact (Gommerat and Gola, 1994), and the glia cells may invade the neuronal soma in some gastropods (Rosenbluth, 1963). The axons of neurons in Molluscs are also large. Therefore, attempts were made in order to isolate the soma from the axonal synaptic inputs by using ligatures (fine silk filament) before the electrophysiological recordings (Alving, 1968). The rate of spontaneous action potentials (APs) in neurons of Molluscs similar to mammals is temperature sensitive.
In this review, I would like to emphasize the properties of cardioregulatory neurons, their direct and indirect (via other neurons) influences on the heart’s function, and the effects of their putative neurotransmitters on the heart as a whole or its compartments/myocytes. Because of significant accumulation of knowledge on neuronal control of molluscan hearts, their myocardium and cardiomyocytes, it is timely to present established classical findings along with new insights.
2. Central nervous system of Molluscs
The function of the heart of Molluscs (Koester et al., 1974; Mayeri et al., 1974) is modulated by the CNS, which is composed of functionally connected distinct ganglion. Fig. 1 represents the CNS of snails with the dextral shell (Schilthuizen and Davison, 2005). The anatomy of CNS to some extent differs between genera; for example, in sea slug Aplysia, existence of visceral ganglion often is not clearly evidenced (Rosenbluth, 1963) and sometimes it is considered as a component of abdominal ganglion formed by the fusion of multiple ganglia (Kriegstein, 1977). Because of apparent fusion, the abdominal ganglion was designated as aberrant parieto-visceral ganglion in Aplysia (Jacklet et al., 1970). In Helix, the same ganglion bears dual names (abdominal or visceral). The right parietal ganglion of Achatina and parieto-visceral ganglion of Aplysia are homologues (Kerkut et al., 1975; Chase and Goodman, 1977).
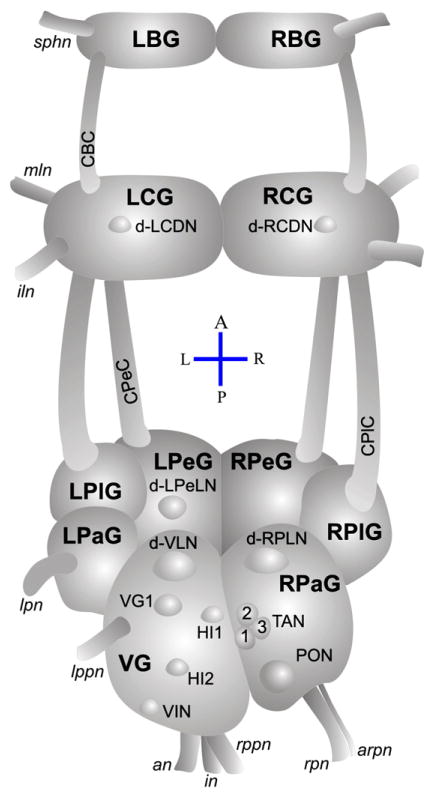
Fig. 1.
Simplified brain of snails, a dorsal view. The CNS of Mollusca is constituted from separate ganglion, a counterpart of distinct areas of mammalian brain. Ganglion: LBG/RBG, LCG/RCG, LPeG/RPeG, LPlG/RPlG, LPaG/RPaG and VG — left/right buccal, cerebral, pedal, pleural, parietal and visceral ganglion. Connectives: CBC, CPlC and CPeC —cerbrobuccal, cerebropleural and cerebropedal connectives; nerves: sphn, mln, iln, lpn, lppn, an, in, rppn, rpn and arpn — superficial pharangeal, medial labial, interior labial, left parietal, left posterior pallial, anal, intestinal, right posterior pallial, right parietal and accessory right parietal nerves. Neurons: d-LCDN/d-RCDN, dorsal left/right cerebral distinct neurons; d-LPeLN, dorsal left pedal large neuron; RPeD1, right pedal dorsal 1; d-VLN, dorsal visceral large neuron; d-RPLN, dorsal right parietal large neuron; VG1, visceral ganglion neuron; HI1/HI2, heart inhibitory neurons; TAN1, 2, 3, tonically autoactive neurons; PON, periodically oscillating neuron; VIN, visceral intermittent firing neuron. The relative size of neurons to each other is considered. Modified from the original studies (Jaeger et al., 1971; Kerkut et al., 1975; Chase and Goodman, 1977; Isao, 1980; Ku and Takeuchi, 1985; Furukawa and Kobayashi, 1987a; Croll, 1988; Buckett et al., 1990a; Zhuravlev et al., 1997; Zhuravlev et al., 2001; Zhuravlev et al., 2002).
3. Molluscan hearts
The anatomy and histology of the heart of Helix were already described in the XIX Century, as discussed by Darwin (Darwin, 1876).
3.1. Functional anatomy
The hearts of frequently used Helix, Aplysia, Lymnaea, and Achatina are composed of auricle and ventricle (Fig. 2). The myocardial, pericardial, and the whole heart functions in diverse Mollusca are widely studied (Kobayashi and Irisawa, 1961; S.-Rozsa and Zhuravlev, 1981; Buckett et al., 1990a; Weatherill and Chase, 2005; Bini et al., 2006a; Malyshev et al., 2008). Similar to vertebrates, each cardiac compartment is constituted of myocytes that contract simultaneously, thereby resulting in functional hemolymph pumping heart-beats. In Helix, the hydraulic property of hemolymph pressure is controlled by the heart, which drops suddenly and the snail collapses if the aorta close to the ventricle is damaged (Dale, 1973). Similar to mammals, values of blood pressure along the cardiovascular system of Molluscs are distinct with the highest in the ventricle. The systolic pressure (up to 35 cm of H2O) correlates with the intensity of actual activity in each animal, while the diastolic (6 cm) does not. Like human heart, the endocardial surface of the ventricle is constituted of trabeculae, although their fine structure is considered to be similar to those of smooth muscles in mammals (Collis et al., 2006), since the transverse tubule and intercalated disks were not discovered in Spisula. The heart of Mollusca also possesses auriculoventricular and semilunar valves (Fig. 2).
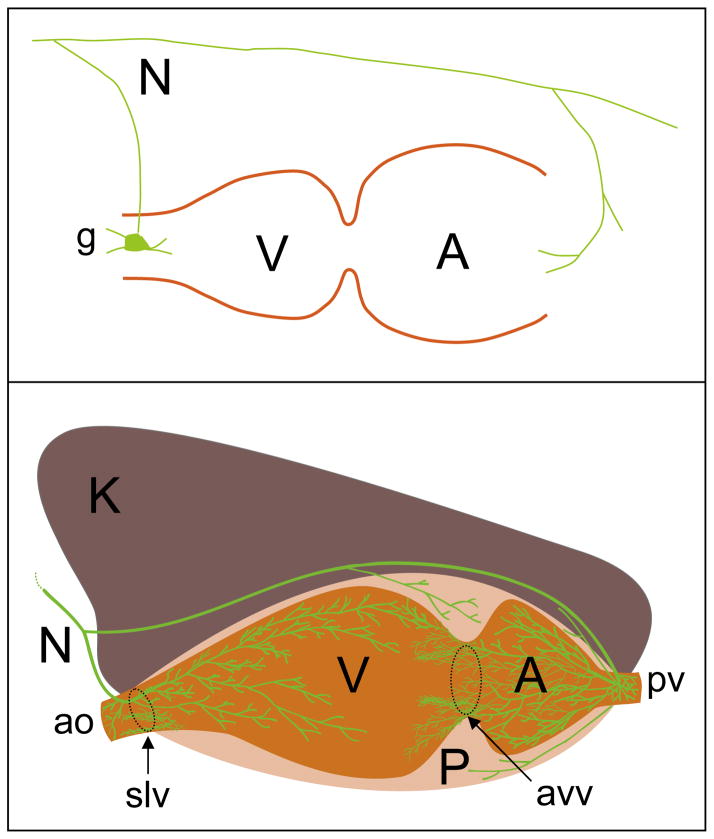
Fig. 2.
Cumulative knowledge on cardiac innervations during 106 years. Upper scheme illustrates how the branches of a single cardio-renal nerve (N) reach the atrium (A) and ventricle (V) of gastropod mollusk Archidoris (Carlson, 1904). Note the different termination of nerves in auricle and ventricle. In auricle, a branch of nerve terminates in a small ganglion from which further innervations will take place. Bottom, immunohistochemically supported schematic drawing of CNP2 positive nerve terminals in the heart of Helix (Aseyev et al., 2010). P — pericardium, K — kidney, ao — aorta, pv — pulmonary vein, avv — auriculoventricular valve, slv — semilunar valve.
3.2. Generation of the molluscan heartbeat
The heart rate in Mollusca can reach values comparable to humans, i.e. ~60 bpm depending on the degree of activity (Dale, 1973; Pandolfo et al., 2009). The heart is most suited for in vivo behavioral studies, since cardiac activity in many species can be observed through the shell (Orr et al., 2007). Similar to mammals, the heart rate alters in response to temperature changes and the latter may serve as a stress factor (Morley et al., 2009; Pandolfo et al., 2009). A gradual change from 15 to 25 °C increases the heart rate (20 vs. 50 bpm). Further increase up to 30 °C resulted in a sudden decrease in heart rate with a plateau at 15 bpm that is considerably less than the baseline value (Braby and Somero, 2006). Thus, the Mytilus senses the critical temperature (25 °C in this case) and responds adequately. Interestingly, the heart rate changed abruptly also in response to gradually descending temperature values and quickly stabilized back to the initial value. A similar sudden decrease in heart rate at 35 °C — Arrhenius break temperature, occurs in Tegula (Stenseng et al., 2005). The cardiac arrest occurred at 40 °C — flatline temperature for hot, which is not fatal, since it is reversed by gradual cooling. The cardiac activity of Mollusca can serve as adequate barometer for environmental pollutions, especially during a recent catastrophic oil spill, since heart frequency increases in response to oil derivates (Bakhmet et al., 2009). Also, the severe bradycardia in Patella is observed when the concentration of heavy metals including zinc increases compared to its regular content in the sea water (Marchán et al., 1999).
3.3. Properties of myocardial membrane and action potentials
Contraction of the atrium and ventricle in concert is derived by regular action potentials (APs; Fig. 3) triggered in cardiomyocytes (Elekes et al., 1973; Hill, 1974a, 1974b; Zhuravlev et al., 2002). Distinct ion channels contribute to the membrane potential and APs of cardiomyocytes in Molluscs (Wilkens, 1972a, 1972b; Curtis et al., 1999; Yeoman et al., 1999; Pereira Ferreira and Salomão, 2000; Kodirov et al., 2004), and their properties are to some extent comparable to mammals albeit resting membrane potential, −45 vs. −70 mV (Irisawa et al., 1967). The isolated ventricle of Mytilus contracts spontaneously despite the exclusion of Na+ after a transient blockade lasting up to 10 min. Also, tetrodotoxin (TTX) did not significantly influence the ventricular APs. In ventricular muscle fibers of Spisula APs and underling channels have been also studied (Filippov et al., 1988). However, amplitude of APs is small and, therefore, difficult to compare. Nevertheless, their properties differ from other studies. The input resistance of fiber was dramatically decreased by 1 mM 4-aminopyridine (4-AP). The toxic sapogenins obtained from starfishes facilitate contraction of isolated ventricle in Spisula (Gorshkov et al., 1998). The positive inotropic (force of heartbeats) effect presumably occurs via the Na+/K+-pump; however, 50 μM ouabain, a selective blocker alone (i.e. no 4-AP) did not affect the baseline contractions.
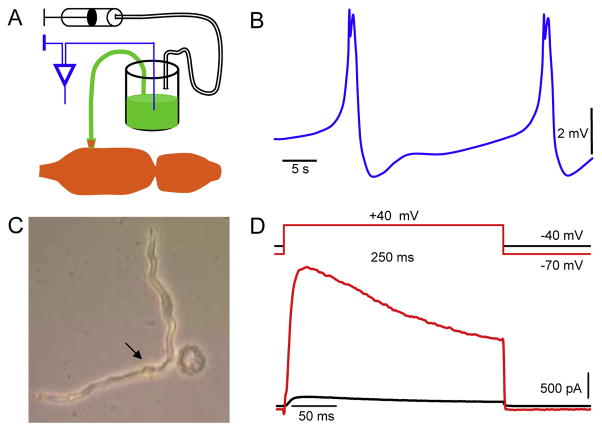
Fig. 3.
Whole heart and single cardiomyocyte recordings. A. The schematic drawings of experimental approach for the recording of cardiac activity using the suction electrode. Note that the electrode container is filled with 150 mM KCl solution supplemented with dye in order to facilitate the visual ablation and to prevent the myocardial damage. B. The ventricular APs that underlie each heartbeat. C. Snapshot of enzymatically isolated ventricular cardiomyocyte. The arrow points to the nucleus of the healthy cell, and the damaged cell is round. D. Family of outward K+ currents in cardiomyocyte activated from different holding potentials. Scale bars are shown.
3.4. The control of the heart by neurotransmitters
The effects of many neurotransmitters and psychotic drugs including LSD (lysergic acid diethylamide) were also first established using the heart and the CNS of Mollusca (Jaeger, 1961; 1962). Many curiosity driven experiments by the latter author include those elucidating effects of CNS and heart extracts on cardiac activity of Strophocheilos; even the effects of hemolymph collected before, during, and after the stimulation of cardiac nerve were tested (Jaeger, 1966). The terminals of the cardiac nerve do not only release neurotransmitters, but also uptake them. Electrical activity of cardiac nerve within the ventricle in Busycon is increased by ventricular stretch, which is sensed by visceral ganglion, since the simultaneous recordings show identical temporal effects also in nerve portion outside of the ventricle (Kuwasawa et al., 1975). Interestingly, between the two recording sites no latency was observed.
The network of neurons modulates the heart (S.-Rozsa, 1976). Neurons may possess inhibitory and excitatory effects on each other, and thereby they can either directly or indirectly modulate the heart. Neurons are identified by their neurotransmitters: cholin-, seroton-, peptid- and catecholaminergic (Cottrell et al., 1981; Boyd et al., 1984; Croll, 1988; Buckett et al., 1990a, 1990b; Brezden et al., 1991; Bright et al., 1993; Kuwasawa and Hill, 1997; Croll et al., 1999; Dyakonova et al., 2009). The role of some peptides for cardiac and neuronal functions overlaps between Molluscs and mammals (Walker et al., 2009). Some of neurotransmitters including dopamine are even found in the hemolymph (Santhanagopalan and Yoshino, 2000).
Serotonin (5-hydroxytryptamine, 5-HT) and their receptors impact cardiac functions (Leake et al., 1971; Kiss and Rózsa, 1978; Tierney, 2001; Swarowsky et al., 2005). In Acanthopleura, 5-HT exhibits positive inotropic and chronotropic (heart rate) effects (Matsumura et al., 1999). In isolated auricle of Sepia 5-HT increases the frequency of contractions, but decreases the amplitude (Lehr and Schipp, 2004). In auricle 5-HT is revealed throughout the myocardium, and next to nerve fibers. Positive inotropic and chronotropic effects of 5-HT on pericardium are also shown (Matsumura et al., 1999). The pericardium possesses distinct innervations (Fig. 2) and autonomous contractions (not correlated to those of the auricle and ventricle).
3.4.1. Role of nitric oxide in neurotransmission
Also, a newly established non-classical neurotransmitter, nitric oxide (NO), plays a role in Mollusca, since its donors inhibit the heartbeat in Achatina and Helix (Azatian et al., 1998; White et al., 2004). One of extensively studied NO donor is S-nitro-N-acetyl-penicillamine (SNAP). The effects of SNAP were completely reversible and more pronounced in Achatina than in Helix. Sodium nitrite, S-nitroso-L-glutathione, and sodium nitroprusside decrease, but L-arginine increases the heart rate in Lymnaea (Taylor et al., 2003). Concentration of NO in both heart and ganglia fluctuates around 1 nM, which is decreased by NO synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester (Mantione and Stefano, 2004). In the heart and CNS of Helix the NO release was also suppressed by a paradigm that mimics depression and fear (Gainutdinov et al., 2008). The endogenous sources of NO in Aplysia are L29 interneurons of left abdominal ganglion, since an in situ hybridization revealed expression of NOS (Antonov et al., 2007). Interestingly, only some of interneurons within the group were positive to neuronal NOS. The NOS, or at least the endothelial NOS, may interact with neuropeptides and thereby regulate the heart function (Springer et al., 2004). The blockade of NOS by 7-nitroindazole modulates the heart rate in Lymnaea revealing an increase in frequency at 250 μM and a decrease at 750 μM independent of either normoxia or hypoxia (Taylor et al., 2003). Interestingly, at 500 μM the heartbeat was facilitated under normoxia, while decreased under hypoxia.
In Helix, the NO donors increase the rate of APs and enable recurrent transitions into bursts in serotonergic neurons of left and right parietal ganglia (Dyakonova, 2002). The similar bursts under control conditions perhaps are attributed to the stress (increased 5-HT release in ganglia) occurring during preparation. This could be either observed or not depending on recovery time for given preparation. The nitroprusside – SNP (1 mM) also triggered the excitatory synaptic potentials in left pedal ganglion despite the slight hyperpolarization. The effects of SNP were observed even in those neurons prior treated with 5-HT; however, these experiments are difficult to interpret, since substances were not co-applied, and the influence of 5-HT was fully reversed by the time the neuron was exposed to SNP. The same shortcoming applies to the effects of 5-HTP and monomethylarginine (blocker of NOS) in this context.
3.5. Overview of innervation and control of the heart
Early observations established that “two tests must be applied to any theory of chemical mediation: the presence of the active substance should be demonstrated following nerve stimulation, and the effects of drugs should be combined with nerve stimulation” (Prosser, 1940).
The main branch of nerves (an, in, and rppn) that innervate the heart originate from visceral ganglion (Carlson, 1904), where several cardioregulatory neurons are located (Zhuravlev and Safonova, 1984). The left posterior pallial nerve (lppn) connects d-VLN/d-RPLN (dorsal visceral large neuron and dorsal right parietal large neuron) and the heart of Achatina. Stimulation of these neurons evokes junction potentials (JPs) in the myocardium (Zhuravlev et al., 2001). The d-VLN and d-RPLN (known also as r-PLN) are symmetrical giant neurons located in visceral and right parietal ganglia, respectively (Fig. 1). Since these neurons were comparably vulnerable to dopamine, serotonin, epinephrine, norepinephrine, octopamine, glycine, and histamine, their similar functions in Achatina were suggested (Takeuchi and Yamamoto, 1982). Interestingly, several of these agents have dual excitatory and inhibitory effects. The 5-HT (100 μM) depolarizes the quiescent d-RPLN and triggers burst of APs. Also, the intracellular cyclic adenosine monophosphate (cAMP) evokes such an activity, while cyclic guanosine monophosphate (cGMP) only depolarizes d-RPLN without triggering APs (Liu and Takeuchi, 1993). The somatic application of inositol trisphosphate (IP3) hyperpolarizes the membrane potential.
The heart is co-regulated by multiple CNS elements, which also control other physiological functions as in case of the network of interneurons – central pattern generator (CPG). The widely investigated CPG in Helisoma is composed of RPeD1–LPeD1–VD4, while in Lymnaea of RPeD1–IP3–VD4 (Syed et al., 1993; Lowe and Spencer, 2006); the input 3 (IP3) neuron is located in left parietal ganglion, while visceral dorsal 4 (VD4, known also as visceral white interneuron — VWI) in visceral one. In Clione the speed of swimming correlates with the cardiac rate, which are controlled by the network consisting of CPG interneurons, heart excitor neurons (HE), and the symmetrical anterior and posterior clusters of cells in right and left cerebral ganglia, which include cerebral serotonergic anterior (Cr-SA) and posterior (Cr-SP) neurons (Satterlie and Norekian, 1995). The serotonergic HE neuron of Clione is located in the left pedal ganglion (Arshavsky et al., 1990; Satterlie and Norekian, 1995). The axon of HE branches within the left pedal ganglion, and a single terminal passes through the left pleural ganglion without any arborization and reaches the left abdominal ganglion (Arshavsky et al., 1990). Here again the axonal branches are seen, which interestingly, overlap with those of HI and the terminals of both neurons are shown within the same medial abdominal nerve. There is a strong correlation between the high frequency discharges in HE (faster heart rate) and wing motoneuron 1A (increased locomotion). Similar coordinated activities are also observed between HE and Cr-SA (Satterlie and Norekian, 1995). However, these neurons are not coupled by the gap junctions.
Similar to mammals, no cells of neural origin are found within the heart of Mollusca. However, one of very first studies described the presence of small ganglion-like structure within the ventricular–aortic junction in Archidoris (Carlson, 1904); note that this structure is an extension of the cardiac nerve (Fig. 2). Sometimes, perhaps the varicosities of larger size can be mistaken for neurons (Aseyev et al., 2010). The heart evidently is controlled by the neuronal terminals located within compartments. In Nautilus, the density of fibers of neural origin in ventricle was revealed (by osmium–zinc iodide analysis) to be low than in auricle (Springer et al., 2005). The epicard, myocard, and endocard of the atrium possess axonal terminals.
4. Inhibition of heart
In earlier studies, the neuronal control of heart (Carlson, 1904) was manifested by targeted stimulation of distinct nerves (Fig. 1) in different Mollusca, which revealed either prevailing inhibitory (often), somewhat excitatory (rare) or dual effects (even seldom).
4.1. Inhibitory agents
The stimulation of visceral ganglion ceases the heartbeat at diastole (Carlson, 1905), and this effect is mimicked by ACh; therefore, the release mechanism directly into the myocardium was suggested (Prosser, 1940). In Helix, the inhibitory drive into the heart is transmitted by ACh, and its focal application (2 pM) decreases the amplitude of heartbeat (Lloyd, 1978). During the perfusion of the isolated heart, higher concentration of ACh (2 nM) is required. In contrast, in Strophocheilos 100 nM ACh led to both positive chrono-and inotropic effects and a complete blockade of heartbeat occurred only at 1 μM (Jaeger, 1961). ACh (5 μM) reversibly depolarizes the membrane potential of identified neurons in right parietal ganglion of Lymnaea, and the effect facilitates under acetylcholinesterase (AChE) blockade (Jurchenko et al., 1973b). The activity of AChE in pedal, parietal, and visceral ganglia of Lymnaea and Planorbarius varies (Jurchenko et al., 1973a).
Either the presence of α-bungarotoxin or the removal of the visceral ganglion eliminate the effect of Cu2+ – bradycardia in Mytilus demonstrating the role of cholinergic neurons and their vulnerability to heavy metals (Curtis et al., 2001). Cu2+ decreases the heart rate also in Patella and 1 μM TTX prevents it (Bini et al., 2006b). The activity dependent release of ACh, thus, was blocked by TTX. Interestingly, the same dose of TTX did not affect the cardiac performance.
4.2. Inhibitory neurons of heart
Despite the earlier studies (Carlson, 1904) and continuous research on neural modulation of heart, there are few articles on inhibitory control at neuronal level (Arshavsky et al., 1990; Buckett et al., 1990a; Zhuravlev et al., 1990; Bychkov et al., 1997). The majority of cardioregulatory neurons are identified in the right parietal and (depending on genera) visceral or abdominal ganglia. To the best of my knowledge, the heart inhibitory (HI) neurons of visceral ganglion were identified for the first time in Helix (S.-Rozsa, 1979). There are a few HI neurons and due to their locations in close proximity, they are often distinguished from each other only during the simultaneous recordings. In the left abdominal ganglion of Clione, three HI neurons were identified (Arshavsky et al., 1990). The rate of spontaneous APs in HI was high, which prevented the contraction of ventricle, but not auricle. The suppression of APs by hyperpolarization restituted the activity of ventricle; interestingly, upon rebound depolarization the contractions in both chambers were temporarily ceased. After about 5 s, the activity in atrium was restored. Neither electrical nor chemical couplings were observed among HI neurons. They did not affect the activity of heart excitor (HE) neuron, but a train of evoked APs in HE reversibly ceased the spontaneous spikes in HI. This occurs also during higher spontaneous discharges in HE derived by increased locomotion; HI and HE are not electrically coupled.
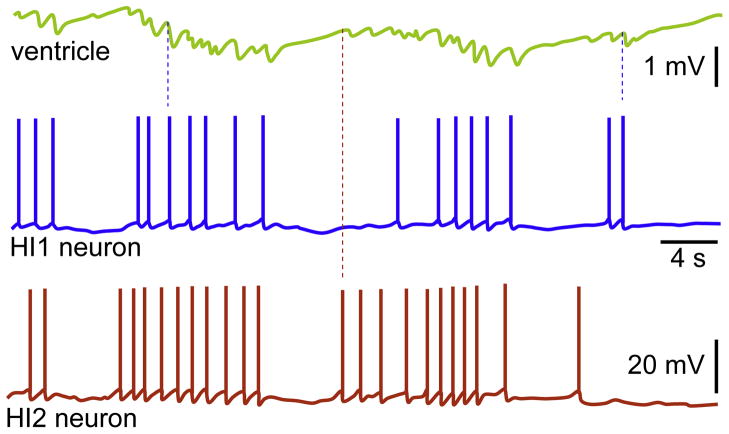
In visceral ganglion of Achatina (Bychkov et al., 1997) and Lymnaea (Buckett et al., 1990a) the heart inhibitory neuron 1 (HI1) and Khi were identified. These neurons possess direct inputs into the auricle and ventricle. Each AP of HI1 evokes a discrete inhibitory postsynaptic potential (IPSP) in the myocardium. Since in some experiments additional IPSPs in the myocardium were observed (Fig. 4), further investigations were undertaken. Subsequently, a second heart inhibitory neuron (HI2; Fig. 5) by Zhuravlev’s group was discovered (Bychkov et al., 1997). Both are unipolar and HI1 is located close to the septum in-between visceral and right parietal ganglia, while HI2 close to the medial part of visceral ganglion, but in closer proximity to the posterior edge (Fig. 1). Simultaneous recordings revealed that these neurons share overlapping effects on heart. This may explain why only a few cells could be powerful, since the combinatory effects are strong enough to significantly hyperpolarize the membrane potential. The magnitude of hyperpolarization during the high frequency APs is greater. Also in Khi, only the high frequency train of APs completely blocks the heartbeat (Buckett et al., 1990a); a slow recovery occurs upon the termination of stimulation. Interestingly, the APs in this inhibitory neuron, similar to excitatory ones in Lymnaea, evoke excitatory junction potentials (EJPs) in the auricle. The HI mediated IPSPs in the myocardium were completely blocked by Cd2+ (Fig. 6), and subsequently no significant hyperpolarization was observed (Bychkov et al., 1997). However, also under these conditions, the HI was able to slow down the ventricular beats, though only after the high frequency discharges; the inhibitory effects were to significant extent reversible. The few APs were ineffective independent of their rate. Only the amplitude of APs in auricle was decreased immediately after the Khi stimulation, and a complete and reversible inhibition was observed with a delay (Buckett et al., 1990a). In the presence of Co2+, there was a transformation in properties of Khi from the silent period (without APs) into the burst activity. The inhibition by Khi is reversed in the presence of α-bungarotoxin, thus, revealing the acetylcholine to be a neurotransmitter.
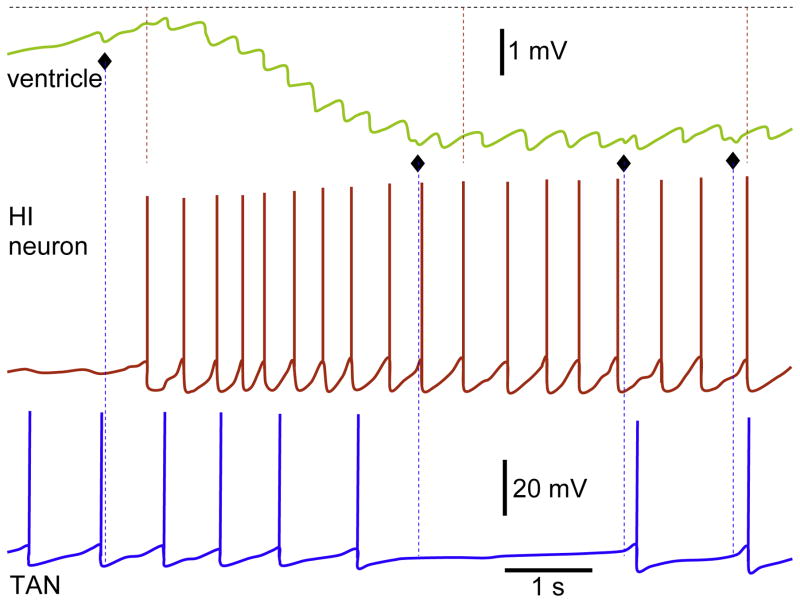
Fig. 4.
Simultaneous recordings from ventricle and two neurons. The IPSPs in ventricle are derived by APs in HI neuron. During recording, four additional IPSPs (屦) were observed, which were not mediated by HI and TAN. Note similar latencies between the APs in HI and corresponding IPSPs in the ventricle; these are shown with dashed red lines for randomly chosen responses. The amplitude scale bar for both neurons and time scale bar for all traces are identical.
Reproduced with permission and modified from Fig. 2 (Bychkov et al., 1997).
Fig. 5.
Spontaneous activity in heart inhibitory neurons. The properties of membrane potential and APs were simultaneously analyzed in ventricle and neurons, HI1 and HI2. Note that the burst activity to some extent coincides in HI1 and HI2, which subsequently results in summation of IPSPs. The dashed blue and red lines indicate randomly chosen APs in HI1 and HI2, respectively; they also refer to corresponding IPSPs and latencies after each APs.
Reproduced with permission (Bychkov et al., 1997).
Fig. 6.
Simultaneous recording from ventricle and HI neuron. Note that recordings were conceived in the presence of 1 mM Cd2+, and therefore the IPSPs in ventricle are completely blocked. Under these conditions, the APs in HI were able to slow down the heartbeat, but only at higher rates.
Reproduced with permission (Bychkov et al., 1997).
It should be emphasized that the acetylcholine binding protein (AChBP) release by glia cells was discovered in Lymnaea (Smit et al., 2001). The AChBP is an analog of the ligand-binding domain of nicotinic acetylcholine receptor (nAChR). The glia–neuron interaction is convenient to demonstrate in Mollusca, since the soma is large and satellite glia cells surround the neuron (Gommerat and Gola, 1994). The burst of APs in neuron VD4, and subsequently, acetylcholine release enables the cholinergic excitatory neurotransmission into the left pedal dorsal 1 neuron (LPeD1), which is inhibited if neuron is surrounded by glia cells (Smit et al., 2001). APs in VD4 evoke 1:1 cholinergic excitatory postsynaptic potentials (EPSP) in LPeD1 (Wiersma-Meems and Syed, 2006). Note that in Helisoma, LPeD1 excites the VD4 (Syed et al., 1993). The glia cell expresses the nAChR-like protein and responds to acetylcholine release triggered by neuronal activity. The response comprises a compound depolarization (with the slow rise and decay times) in membrane potential. However, the occasional spontaneous APs in VD4 do not influence the membrane potential of glia cell. The function of nAChRs and their similarities to mammalian counterparts are demonstrated also by exogenous acetylcholine and nicotine. Moreover, these receptors are sensitive to α-bungarotoxin, a selective blocker of α7 containing nAChRs in mammals. The latter results are also valuable for the elucidation of properties of vertebrate’s AChRs, including nicotinic one and subsequently smoking (Dougherty and Lester, 2001). In Venus, nicotine and ACh exhibit similar inotropic effects, which led to cardiac arrest at diastole (Prosser, 1940).
4.3. Cardioinhibitory peptides
Helix cardioinhibitory peptide — HCIP was first detected by radioimmunoassay test using antisomatostatin (Baud et al., 1998). Also the immunohistochemistry confirmed its presence in CNS, however, only in a few neurons of smaller (perhaps not previously identified) size in visceral and left/right parietal ganglia. The N-terminal of HCIP and FMRFamide are identical: H-Val-Phe-Gln-Asn-Gln-Phe-Lys-Gly-Ile-Gln-Gly-Arg-Phe-NH2. In isolated ventricle of Helix, 1 μM HCIP decreased the rate of contractions, but increased its amplitude. These effects were similar to those of 10 μM ACh, since both also caused first a transient diastolic arrest with the similar latencies (Baud et al., 1998). The latency increased by 10 nM HCIP and the ventricle continued to contract at the same pace and power after a short diastolic arrest.
4.4. Other inhibitory peptides
The FMRFamide related heptapeptide pQDPFLRI-amide was purified from CNS of Helix (Lesser and Greenberg, 1993). The pQDPFLRI-amide is also present in circulating hemolymph, which is perhaps the main source that influences the cardiac activity. Its inhibitory effects on isolated ventricle of Helix are seen already at 100 nM.
The APGWamide (Ala-Pro-Gly-Trp-NH2) was described as inhibitory tetrapeptide in Achatina, since it decreases the FMRFamide induced outward currents in d-RCDN (Han et al., 1997). The application of APGWamide alone also evokes outward currents in d-RCDN. However, if compared at identical 3 μM concentration to FMRFamide, the magnitude of currents was smaller.
Another biologically active substance was purified from ganglia of Achatina. Since its sequence contained the Trp (or W) residue at both C and N terminals, it was termed as WWamide (Minakata et al., 1993). This peptide was found in three forms; WWamide-1 selectively hyperpolarizes d-RCDN and inhibits spontaneous APs in reversible manner. The properties of tonically autoactive neuron (TAN), periodically oscillating neuron (PON), B1, and B4 remained unaffected by WWamide-1.
MIP — Mytilus inhibitory peptide was extracted from the pedal ganglia of Mytilus edulus, and therefore incorporated into the name (Hirata et al., 1988). Another part derives from its inhibitory effects on anterior byssus retractor muscle (ABRM). The MIP (10 nM) also reversibly inhibits the contraction of cardiac tissue obtained from Meretrix. A brief application of MIP evokes outward currents in RCDN of Achatina (Han et al., 1997).
Achatin-1 is a tetrapeptide and the name is derived from its presence in CNS of snail Achatina (Kamatani et al., 1989). Achatin-1 is composed of Gly-D-Phe-Ala-Asp sequence. Independent of intracellular levels of Ca2+, it hyperpolarizes the cardioregulatory neurons of Achatina. In PON, 10 μM Achatin-1 also induces inward currents.
Pedal peptide — Pep was suggested as possible neurotransmitter between the CNS and heart of Helix. Numerous neurons throughout the subesophageal ganglia positively immunoreact to Pep (Pavlova and Willows, 2005).
5. Excitation of heart
The cardiac excitation in Mollusca occurs mainly due to serotonergic and peptidergic neurons of the CNS.
5.1. Identified excitatory neurons
The direct stimulation of either the auricle or ventricle (S.-Rozsa and Salanki, 1973) evokes EPSPs in these compartments and related antidromic APs in visceral ganglion’s neuron 1 (VG1; Fig. 8). Also stimulation of nerves in Helix evokes antidromic APs in neurons of the left/right parietal and visceral ganglia (Kerkut et al., 1975). However, depending on the intensity, the antidromic stimulation of rpn may lead only to slow depolarization, as observed in neurons of right parietal ganglion with the white pigmentation in Achatina (Chase and Goodman, 1977). The antidromic stimulation targets axons and the response hints to the presence of neuronal terminals in certain nerves, which can be shown also by the injection of Co2+ into the soma.
Fig. 8.
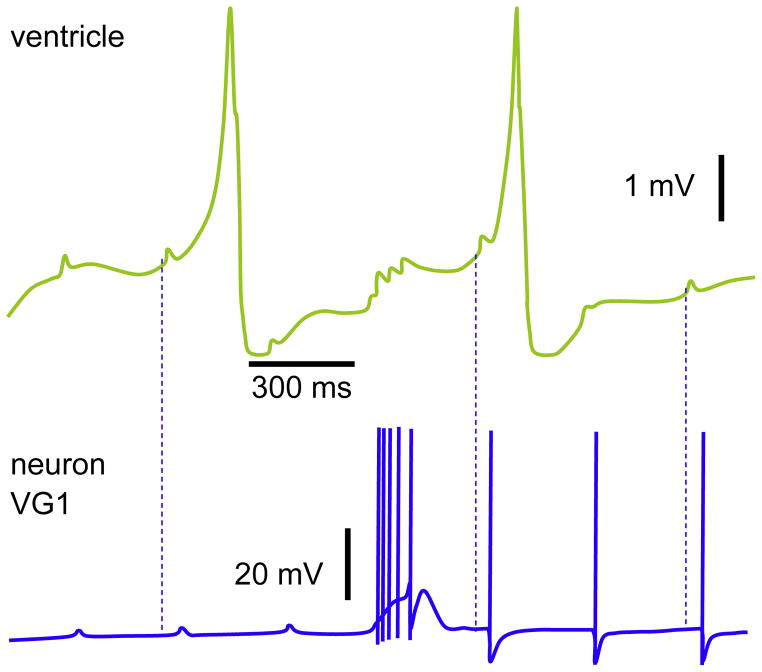
Cardioregulatory neuron VG1. The EPSPs in the ventricle and antidromic APs (the three last) were evoked by the direct stimulations of heart. The spontaneous burst coincides with the current injections applied to the ventricle. Note the resultant EPSPs in the ventricle after each AP within the burst. Dashed lines indicate the latency of VG1 response to EPSPs in ventricle; the corresponding pairs are randomly chosen.
Reproduced with permission (Zhuravlev et al., 1997).
The VG1 was for the first time described in two elegant studies (Furukawa and Kobayashi, 1987a, 1987b). VG1 was considered as interneuron based upon two distinct effects; namely, depending on stimulation’s duration/frequency, VG1 either increases or decreases the heart rate. The existence of VG1 and its role as an inhibitory interneuron in Achatina is confirmed (Zhuravlev et al., 1997). The inhibitory drive by VG1 evidently occurs not directly, but at least in part via the hyperpolarization of PON. Therefore, the question of whether or not the VG1 could be considered as a multimodal neuron remains open.
The basal activities of cardioregulatory neurons do not significantly influence the heartbeat; however, an evoked burst either increases or decreases the rate. The burst of APs in She was powerful enough to trigger the beat in quiescent heart. Each spike of neuron She evokes 1:1 excitatory junction potential (EJP) in the auricle (Buckett et al., 1990a). During prolonged burst, the rate and magnitude of heartbeat were dramatically increased; these effects lasted longer than the duration of burst and the inotropic effect reversed gradually, while the chronotropic one abruptly. In Achatina, the APs of d-VLN evoke inhibitory–excitatory junction potentials (I–EJPs) in the myocardium (Zhuravlev et al., 2001). Similar neuron in ventral surface of visceral ganglion is designated as v-VLN (Liu and Takeuchi, 1993). The v-VLN is either quiescent or generates APs, and somatic injection of 10 mM cGMP evokes a high frequency burst lasting up to 60 s, which is ceased by sudden repolarization. Interestingly, the prior baseline spontaneous activity is also blocked under these conditions, suggesting the depolarization induced suppression of APs. The cAMP evoked only giant depolarization without any APs, while IP3 caused hyperpolarization.
Since the locations and properties of d-VLN in Achatina and She in Lymnaea are similar, they could be homologues. The Lucifer-yellow staining revealed the single axon of She as a component of the intestinal nerve. The She is a heart motoneuron, since its cardioexcitatory influence remained unaffected in presence of Co2+, which blocks the synapses (Buckett et al., 1990a). In She, however, Co2+ decreased the amplitude of APs within the burst and resultant after hyperpolarization (AHP). Although Co2+ was applied only to CNS, the magnitude of heartbeat was also slightly decreased. Another type of motoneurons in Lymnaea include four heart excitatory neurons — Hhe (Buckett et al., 1990a). These serotoninergic neurons are spontaneously active, but their basal tone does not influence the heartbeat. The heart responds with arrhythmic activity only during evoked high frequency discharges. The spike in Hhe evokes EJP in auricle, and when the APs in two neurons are triggered at the same time, the magnitude of EJPs increases; but this does not depolarize the membrane potential up to the threshold. However, APs in Hhe during prior depolarization of myocardium led to AP-like response. In presence of Co2+, the amplitude of APs decreases and burst firing is observed; even the longest spontaneous burst had only a slight effect on heartbeat. Therefore, the parameters of evoked bursts are probably too intense and can remotely resemble those under physiological conditions. The lack of spontaneous burst’s effects could result because of Co2+. The synaptic inputs of motoneurons into the heart are conserved even in vitro, i.e. if the muscle fibers and these neurons are kept in culture in close proximity, they will develop functional connections (Lee et al., 2002). Interestingly, even neurites can survive and function without soma in vitro. The interneuron RPeD1 exerts excitatory effect on Hhe and inhibitory on Khi (Buckett et al., 1990a).
The visceral intermittent firing neuron (VIN) in Achatina is an interneuron and does not affect the heart activity directly. VIN along the heart motoneurons, PON and TAN1-3, receive excitatory inputs from d-LCDN, d-RCDN, and d-LPeLN (Furukawa and Kobayashi, 1987b). The serotonin is a neurotransmitter of d-LCDN and d-RCDN, since a) the train of APs in these neurons depolarized the PON, b) application of 3 μM 5-HT mimicked the latter effects, and c) the 5-HT receptor antagonist, methysergide, abolished the effects seen under both conditions (Furukawa and Kobayashi, 1988). The depolarization mainly occurs via inhibition of K+ channels in PON, but activation of Ca2+ and inward rectifier K+ channels are also involved. The synaptically released and acutely applied 5-HT either blocks or activates these channels, which also prolongs the APD (action potential duration). This is consistent with the decrease in outward K+ currents and their dominating role in repolarization of PON. In sensory neurons of pleural ganglia of Aplysia, an increase in APD by 5-HT has been shown to be mainly PKA dependent with some contribution of PKC (Goldsmith and Abrams, 1992). 5-HT (20 μM) depolarizes membrane potential and increases the number of APs. Antidromic stimulation of intestinal nerve evokes EPSP in PON. Since the higher intensity led to graded increase in amplitudes, PON may possess multiple axons within the intestinal nerve (Furukawa and Kobayashi, 1988). VIN and PON have common inhibitory inputs and their activities coincide, since both are coupled by gap junctions. VIN is depolarized by d-RCDN. The powerful inhibition of PON and VIN takes place even after a single AP in VG1, which is followed by evoked IPSP (Furukawa and Kobayashi, 1987b). The d-LCDN, d-RCDN, d-LPeLN, and VG1 are not connected.
5.2. Serotonergic neurotransmission
Many neurons in several ganglia of Mollusca release 5-HT, which modulates the heart. The amplitude of beats in isolated heart of Helix is increased by single application at <1 pM (Lloyd, 1978); during the perfusion, the threshold is considerably higher (~100 pM). In Nautilus, the bell shaped effects of 5-HT on isolated auricle are also documented, which is often quiescent in vitro (Springer et al., 2005). Under these conditions, 10 nM 5-HT was ineffective, 100 nM led to initial activity, and only at 1 μM adequate contraction was observed. A subsequent cumulative increase up to 10 μM resulted in an opposite effects. In this study, since the preparation was quiescent under baseline conditions, the initial positive and following negative ino-and chronotropic effects are not conclusive. After the wash out, the rhythmical activity was sustained; therefore, the preparation was used for further experiments (see Section 6). The support for the positive inotropic influence of 10 nM 5-HT was provided by using spontaneously contracting ventricular trabeculum (Collis et al., 2006). The underlying mechanism for 5-HT effects involve the increase in cAMP in cells (for review see Fabbri and Capuzzo, 2010). The cAMP with other neurotransmitters co-participates during the response to the stress factors.
The serotonergic neurons are easy to identify, since they exhibit a brown pigmentation after being exposed to exogenous dihydroxytryptamine (DHT) in vivo (Jahan-Parwar et al., 1987). Either 5,6-DHT or 5,7-DHT can be injected intrahemocoelly into the foot of Aplysia, Achatina, Helix, and Lymnaea and after 21 days to 2 months, the multiple (up to 100) serotonergic neurons are revealed in several ganglia (Kemenes et al., 1989; Kodirov et al., 1994; Zhuravlev et al., 1997; Zhurawlow et al., 1997; Marinesco et al., 2004a). Also p-chlorophenylalanine, another depletory agent of serotonin, is used for this purpose. The intensity of acquired brown pigmentations increases with time and could be traced up to 12 months (Dyakonova, 2002). Interestingly, such treatment, in general, does not influence the properties of neurons, but some toxic effects of 5,7-DHT is discussed previously (Baker et al., 1993). Pretreatments with these agents additionally resulted in axonal sprouting in Achatina. In Helix, the content of 5-HT in the single giant (~100 μm) serotoninergic neuron of cerebral ganglion is decreased by ~50 and 20% with 10 μM and 1 mM 5,7-DHT, respectively (Osborne and Pentreath, 1976). The putative neurotransmitter of d-LCDN and d-RCDN is 5-HT. However, their effects can be either excitatory or inhibitory depending on the postsynaptic cell (Gerschenfeld and Paupardin-Tritsch, 1974). The latter dual effects were also observed by application of 5-HT to neurons of Helix (Kerkut and Walker, 1961).
In the right parietal ganglion of Achatina, one of the widely studied serotoninergic type of cell is TAN that comprises three members (TAN1-3) with similar properties (Furukawa and Kobayashi, 1987a). The TAN1-3 are situated next to each other close to the visceral ganglion’s border either in a circle or one after the other (Furukawa and Kobayashi, 1987a; Zhuravlev et al., 1994; Zhuravlev et al., 2002). The somatic application of IP3 hyperpolarizes the TAN, but cAMP and cGMP reversibly depolarize it and evoke burst of APs (Liu and Takeuchi, 1993). The soma of TANs are located in right parietal ganglion; however, the neural branches of at least one of them reach the left parietal ganglion via visceral one and the axonal terminals are found in the following nerves: an, lpn, lppn, and rpn (Isao, 1980). There was no electrical coupling between the TAN and HI1 (Fig. 4). A direct synaptic connection was found between d-LCDN/d-RCDN and TAN (Furukawa and Kobayashi, 1987b). The evoked burst in VIN, but not its basal tone, hyperpolarizes the membrane potential and decreases the AP’s rate in TAN1-3. In turn, the burst of TAN1-3 inhibits VIN, and since the response did not occur upon cutting of intestinal nerve (in), they involve a complex loop within both the ganglia and the heart; most probably the connecting element (neuron/synapses) is located within the heart (Bychkov et al., 1994). The same is true if neurons are situated even within one ganglion!
The concentration of 5-HT during certain activity can reach up to ~40 nM (Marinesco et al., 2004b). Stimulation of the tail nerve evokes the serotonin release in Aplysia pretreated with 5-hydroxytryptophan (5-HTP), a precursor that promotes the 5-HT synthesis. This also moderately increases the heart rate that is influenced by the 5-HTP treatments. The direct application of 5-HTP onto the isolated ganglia increases the number of evoked and spontaneous APs in sensory neuron. The iontophoretic injections of 5-HT into the neurons of right parietal ganglion depolarized the membrane potential in some and hyperpolarized in others, thus, resulting either in increased rate or the blockade of spontaneous APs (Kerkut et al., 1975). Although, the pretreatment with 5-HTP consistently did not influence the properties of serotonergic cells, its acute application (200 μM) in vitro ceased the APs in neuron of visceral ganglion and hyperpolarized the membrane potential (Dyakonova, 2002). Note that the latter neuron was maximally excited by prior application of 5-HT. The potency of exogenous serotonin was also greatly diminished in the presence of 5-HTP.
The activity pattern of APs among serotonergic cells differs and exhibits also burst firing, e.g. parapodia-opener-phase-like neuron of pedal ganglion in Aplysia (Marinesco et al., 2004b). A similar pattern was observed in neurons of right parietal ganglion in Helix, but the burst in this case developed slowly by preceding APs of gradually increasing rate (Dyakonova, 2002). Interestingly, soon thereafter, APs are ceased for a couple of seconds and recurrent activities were observed. TANs exhibit very similar synchronous firings (not derived by either synaptic or electrical coupling), and intriguingly, the strong hyperpolarization of second neuron eliminated only preceding APs before the burst; synchronous bursts were retained in two neurons.
The shock applied to the tail nerve in Aplysia increases the firing rate of RBhe – heart exciter neuron in RB cluster of abdominal ganglion (Marinesco et al., 2004a). This was also accompanied by EPSPs and overlapping APs, and contraction of auricle. The terminals of serotonergic RBhe are found as a component of the genital–pericardial nerve. The RB is the follower neuron of L10 and L32 in abdominal ganglion of Aplysia (Kretz et al., 1986). The discrete neurotransmission from L10 into the RB is reversibly decreased by the stimulation of histaminergic L32 interneuron, which also significantly hyperpolarizes the L10 neuron without directly affecting other parameters. In this tripartite circuit, L32 targets the presynaptic site between L10 and RB. The amplitude of hyperpolarization – IPSP in L10 was dependent on the value of membrane potential and decreased upon the negative values. In Helix, application of 1–100 nM histamine excites the distinct type of neurons in parietal and visceral ganglia, while in other neurons an opposite effect starting at 10 nM was observed (Kerkut and Walker, 1961).
5.3. Peptidergic neurotransmission
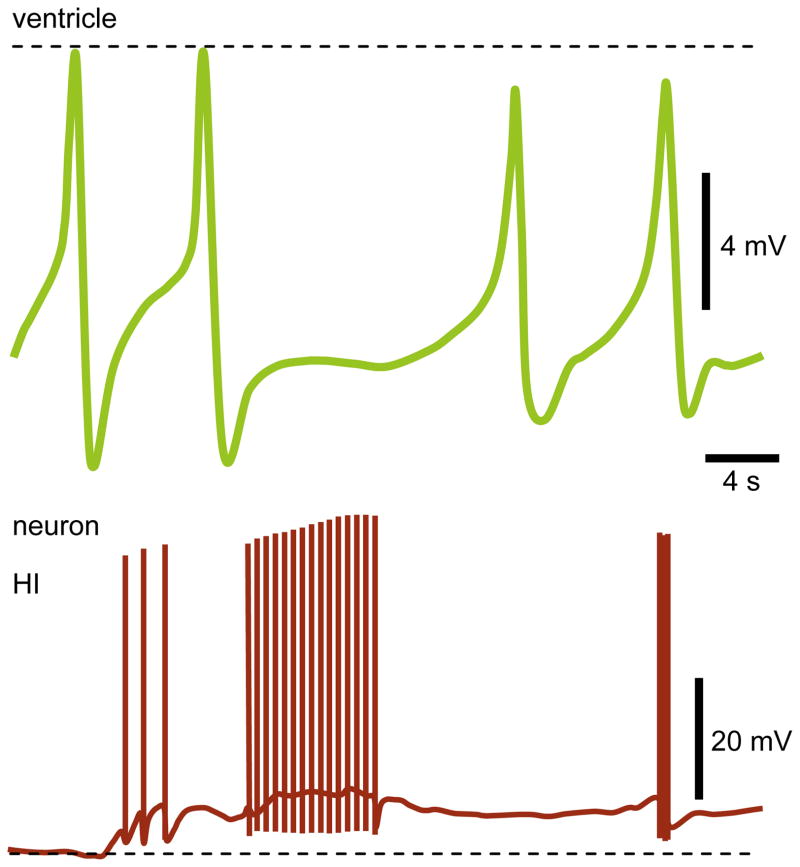
Takeuchi et al. (1976) discovered the PON in Achatina for the first time (to my knowledge); however, its homologues in different snails were described much earlier (Nikolic et al., 2008). PON facilitates heartbeat (Furukawa and Kobayashi, 1987a) and is located in right parietal ganglion (Fig. 1), but its axonal branches are extended up to visceral ganglion (Isao, 1980). PON’s terminal was found only within the intestinal nerve. PON is silent for a certain period of time at resting membrane potential, and bursts of APs occur superimposed on the onset of slow spontaneous depolarization. Although the recurrent oscillations are observed often, PON sometimes responds only with a single AP to each cycle, but not a burst (Takeuchi et al., 1975). In contrast to previous studies, PON was silent under experimental conditions employed by Zhuravlev et al. (1997). However, interestingly, in the presence of Cd2+ applied focally outside of CNS (which was separated from the internal organs by isolated compartments and perfusion systems), PON exhibited a typical spontaneous activity (Takeuchi et al., 1976; Furukawa and Kobayashi, 1987a). It is worthwhile to mention that the properties of PON to some extent resemble those of pacemaker cells, especially LPL2/3 and RPL2 in left and right pleural ganglia of Tritonia, respectively (Smith and Thompson, 1987). In Achatina, PON more powerfully excites the heart compared with TAN and VG1 (Zhuravlev et al., 1997). Even a single AP in PON triggered the contraction of prior quiescent auricle; four spikes led to summation of EJPs with the amplitude equal to overshoot value of atrial APs. A significant depolarization of atrium after a single AP in PON occurred even in the presence of Cd2+ in cardiac compartment of recording chamber (Fig. 7); subsequent APs in the burst further depolarized the membrane potential, which remained constant during interburst intervals. The second burst resulted in additional depolarization; each AP in PON evoked the EPSP in the myocardium. During analyses of PON and TAN1-3, additional EPSPs in the heart were recorded, which were not evoked by these neurons. Subsequent mapping of cells led to the discovery of cardioexcitatory neuron VG1 in visceral ganglion (Furukawa and Kobayashi, 1987a; Zhuravlev et al., 1997). The VG1 is more powerful than PON and both are synaptically coupled. These neurons are silent at resting membrane potential and the evoked APs in VG1 are able to hyperpolarize (by ~3 mV) the PON (Zhuravlev et al., 1997). The firing pattern of PON depends on the preparation’s type, and a typical activity is observed if the ganglia are separated (Takeuchi et al., 1976), while in semi-intact preparations (the ganglia and neural connections with organs are undisturbed) the properties of PON do not differ from many other neurons (Furukawa and Kobayashi, 1987b). The β-hydroxyglutamic acid inhibits PON by hyperpolarizing the membrane potential and subsequently (depending on the concentration) either by increasing the rate of periodic oscillations or blocking APs (Takeuchi et al., 1975). Using a double-barreled pipette for the simultaneous recording of neuronal activity and somatic injections, it was demonstrated that 10 mM cAMP triggers the burst of APs in quiescent PON (Liu and Takeuchi, 1993). The cGMP at identical concentration depolarized this neuron, but the threshold for APs was not reached; IP3 hyperpolarized the PON.
Fig. 7.
The excitatory action of PON on heart. Simultaneous recordings of atrial response to APs in PON. The cardiac compartment of the recording chamber was perfused with 1 mM Cd2+. Note a slight adaptation in spikes’ frequency in PON that closely correlates with the inter EPSP intervals. The membrane potential is gradually depolarized after each AP in PON and remained at this new level in-between of period of oscillations. Interestingly, the subsequent burst further depolarized the membrane potential, although not as strong as the preceding one.
Reproduced with permission (Zhuravlev et al., 1997).
5.3.1. FMRFamide and related peptides
To this family belong many different peptides based on several criteria: the C-terminal contains the RFamide sequence, the majority is encoded by the same precursor, they may be co-released, and have a structure similar to FMRFamide.
FMRFamide — was for the first time purified (Price and Greenberg, 1977) from the ganglia of marine Mollusca Macrocallista and is a tetrapeptide (Phe-Met-Arg-Phe). It is present in atrial granular cells of Achatina (Shabel’nikov et al., 2008). FMRFamide (1 μM) reversibly increases the amplitude of heartbeats in Helix and Lymnaea, which correlates with the increased levels of cAMP (Price et al., 1996; Willoughby et al., 1999). The identical dose hyperpolarizes the LPeD1 and RPeD1, and similar inhibitory effects are observed also after the stimulation of VD4 (Syed et al., 1993). The latter experiments and the immunoreactivity of VD4 led to conclude that FMRFamide might be a possible neurotransmitter. However, note that the homologues neuron in Lymnaea is cholinergic (Smit et al., 2001), suggesting that they might be co-transmitters.
FMRFamide-related peptides — FaRPs to this date comprise ~30 tetra-, penta-, hexa-, hepta-and decapeptides in Molluscs (López-Vera et al., 2008). FaRPs are encoded by the same precursor protein and some are known also as FMRFamide-like peptides — FLPs (Walker et al., 2009). FMRFamide and FaRPs are present during the development of CNS in Idiosepius (Wollesen et al., 2010) and in identified neurons of adult Helix (Cottrell et al., 1992).
FMRFamide similar to ACh gates the ion channels, thus, the amiloride-sensitive Na+ channel – FaNaC (or FaNaCh) was discovered (Lingueglia et al., 1995; Cottrell, 1997). FaNaC in neurons is selectively sensitive to FMRFamide, since other peptides from the same precursor do not evoke inward currents, e.g. pQFYRFamide (Price et al., 1996). In identified cerebral neurons of Helix pQFYRFamide evokes K+ outward currents. The isolated heart was more sensitive to pQFYRF compared to FMRFamide and FYRFamide and inotropic effects occurred at 100 nM, 1 μM, and 2 μM, respectively. In Helisoma, FMRFamide increases the unitary conductance in outside-out membrane patches of identified neurons of pedal ganglion (Jeziorski et al., 2000). The open probability of channels in the presence of FMRFamide follows exponential fit yielding fast and slow time constants. Similar to other ligands, upon the recurrent applications, the whole-cell inward currents significantly desensitize; these currents were blocked by 50 μM amiloride. Interestingly, an opposite effect was observed when single channel activities were recorded (i.e. 10 μM amiloride further increases the open probability in the presence of FMRFamide). The effect of amiloride (most likely) was not attributed to FMRFamide. The effects of FMRFamide on neurons were more pronounced compared to FLRFamide.
Some of FLPs modulate cardiac parameters concurrently with other neurotransmitters, e.g. ACh inhibits and 5-HT enhances their effects in isolated ventricles of Buccinum and Busycon (Moulis and Huddart, 2006). The pQFYRFamide was purified from CNS of Helix (Price et al., 1996). The inotropic effects of this pentapeptide on isolated hearts are significantly pronounced compared to FMRFamide. Another striking difference is that pQFYRFamide does not evoke the fast sodium currents in neurons. In Lymnaea, pQFLRIamide exhibits three distinct effects on heart: only inhibitory, only excitatory, or biphasic — the transient inhibition followed by a powerful excitation (Willoughby et al., 1999); the response of heart to EFLRIamide is also similar. Note that only the excitatory response was accompanied by increased adenylate cyclase (AC) levels in cardiac membrane homogenates; all these three amides also increase the cAMP. In line with these outcomes, also forskolin (AC agonist) and 8-bromo-cyclic AMP (a membrane-permeable analog of cAMP) increased the frequency and magnitude of beats with similar latencies.
SEEPLY — short form for SEQPDVDDYLRDVVLQSEEPLY, does not affect either the cardiac parameters or AC/cAMP levels even at 100 μM (Willoughby et al., 1999). In Lymnaea, SEEPLY is present in Ehe neuron of visceral ganglion (Santama and Benjamin, 2000). The evoked APs in Ehe increased the frequency and amplitude of heartbeat, which were reproduced by 1 μM FMRFamide. In isolated heart, 1 μM EFLRI also increased the frequency and magnitude of beats. Note that the latter effects were preceded by a transient inhibition.
5.3.2. Cardioactive peptides
These neuropeptides comprise two families with molecular weights of ~700 and ~7000, which were termed as a small and large cardioactive peptides, respectively (Lloyd, 1978).
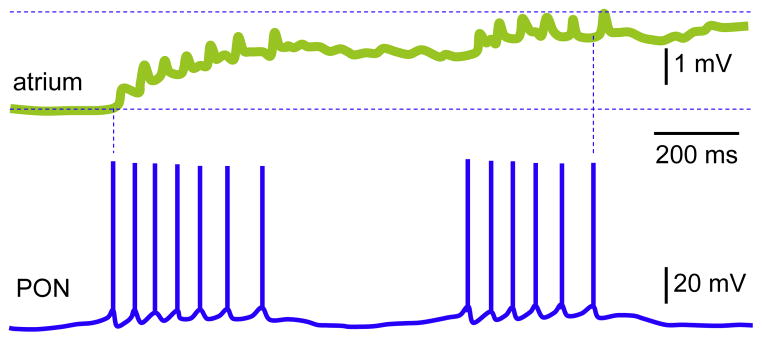
SCP — small cardioactive peptide was discovered in CNS of Helix. Initially, its participation in modulation of cardiac functions was excluded, since it was not present in the heart tissue and hemolymph (Lloyd, 1978). This conclusion persisted despite the similarity of SCP to FMRFamide and its positive inotropic effects on heart. The cardioactive peptide was then described also in Aplysia (Lloyd et al., 1985) and much later in visceral dorsal 1 (VD1) and right parietal dorsal 2 (RPD2) neurons of Lymnaea (Bogerd et al., 1994). Both peptidergic neurons sense the O2 fluctuations (Janse et al., 1985) and are coupled by reciprocal electrical contacts (Wildering et al., 1991). Because of strong electrical coupling they show regular spiking in intact CNS, but upon the isolation only VD1 retains such activity, which is consistent with its pacemaker role. VD1 and RPD2 modulate the heart by neuromuscular transmissions, since the axonal terminals were traced as a component of intestinal nerve in the pericardium and the auricle, though not in ventricle (Kerkhoven et al., 1991); the immunoreactivity of varicosities to cardioactive peptides was seen in atrial muscle. Each evoked single spike or train of APs in VD1 led to discrete EJPs in cardiac cells maintained in co-culture (Jimenez et al., 2006).
In Aplysia, the cardioactive peptide comprises SCPA and SCPB (Morris et al., 1982). The molecular weight (1140) of SCPB was estimated by fast atom bombardment mass spectrometry. The native and synthetic SCPB (25 fM) similarly and reversibly increased the amplitude of heartbeats; therefore, one could compare the potency of substances in the same preparation. Interestingly, the activity of SCP was also detected in following nerves: lppn>rppn>an>in. Note that the in is known also as visceral nerve (Osipenko and Kemenes, 1991) because of its functional pathways to internal organs.
LCP — large cardioactive peptide is predominantly present in subesophageal ganglia, auricle and hemolymph of Helix (Lloyd, 1978). Large granules containing LCP was found exclusively in the atrium and its release by the axonal terminals was suggested. In contrast to SCP, the activity of LCP in lppn, rppn, an, and in was similar. The LCP increases the amplitude of beats in isolated hearts.
In recent years, more peptidergic cells were discovered including the command neurons in right parietal and visceral ganglia, which express gene Helix Command-Specific 2 – HCS2. This term contains the name of snail, since it was discovered for the first time in Helix (Bogdanov et al., 1998). The HCS2 gene encodes the Command Neuron Peptide (CNP), which is selective to neurons and available in four splice variants (CNP1-4). The command neurons and their terminals in auricle and ventricle express the CNP2 (DYPRLamide), which excites the heart (Aseyev et al., 2010). The CNP2 is present not only in Helix lucorum, but also in Helix pomatia, and immunopositive fibers with multiple varicosities are found throughout the heart and pericardium (Fig. 2). Within the heart, the CNP2 positive nerves are equally revealed at both epi- and endocardial surfaces in both genera. A positive inotropic effect of CNP2 in comparison to FMRFamide was less pronounced. The initial responses of the heart to 10 μM CNP2 and 1 μM FMRFamide to some extent were similar. Nevertheless, FMRFamide exerted more persistent effects, while those of CNP2 decayed faster. The CNP2 restores the activity of isolated quiescent heart. Interestingly, when atrium and ventricle were separated, the influence of CNP2 was either predominantly chronotropic or inotropic in former and latter chambers.
There are additional bioactive substances, which may also regulate cardiac functions, e.g. the CNP-and enkaphalin-like peptides. The immunoreactivity to CNP-like peptide is shown in left cerebral (two neurons), right cerebral (2), left pleural (1), left buccal (1), and right buccal (1) ganglia of Clione (Malyshev and Balaban, 2009). The [Met5]-enkaphalin-like peptide was found in VD1 and RPD2 neurons and the neural terminals in atrium of Lymnaea (Ewadinger et al., 1996).
5.3.3. Achatina cardioexcitatory peptide
ACEP-1 (Achatina cardioexcitatory peptide) is present in the atrium of the snail Achatina fulica, and the purification revealed the following amino acid sequence: H-Ser-Gly-Gln-Ser-Trp-Arg-Pro-Gln-Gly-Arg-Phe-NH2 (Fujimoto et al., 1990). There are conserved regions in ACEP-1 and HCIP sequences (Baud et al., 1998), especially in C-termini (45% homology). A low dose of ACEP-1 reversibly excites identified neurons of Achatina. Interestingly, at 1–100 μM it does not influence the magnitude of contractions in isolated atrium, while the same concentrations exert positive inotropic effects in ventricle. Similar results were obtained also by using a synthetic ACEP-1.
6. Catecholamines
The catecholamines comprise epinephrine (adrenaline – A), norepinephrine (noradrenalin – NA), and dopamine (DA) and are released into the hemolymph as a consequence of stress similar to mammals.
In Venus, the chronotropic effects of A and subsequent arrest at systolic phase was mentioned previously (Prosser, 1940). In Mya, the rhythmic contraction of ventricular tissue at systolic phase was inhibited by A, NA and DA (Cottrell et al., 1970). However, NA and DA at lower concentration increased consistently the amplitude of contractions, while the effects on frequency not always were seen. Also, in Tapes the dual effects of NA and DA have been reported (Chong and Phillis, 1965). In Helix’s brain and heart NA, but not A was detected (Sloley et al., 1990). In Nautilus the A was absent in heart and NA was present only in auricle (Springer et al., 2005). The NA starting at 10 nM gradually increases the rate and amplitude of heartbeats. Strictly speaking, these experiments might not be adequate, since the same preparation had been used during the elucidation of serotonin effects. The level of NA in CNS is increased by mechanical stress, but is decreased in the heart (Lacoste et al., 2001).
In Helix, the DA level in brain is constant during hibernation in contrast to 5-HT (Michaelidis et al., 2002). During this time the DA content in heart decreases. The level of NA in CNS was low, absent in heart and no A was detected in these organs. In Crassostrea, the levels of NA and DA in hemolyph are increased abruptly during the stress (Lacoste et al., 2001). The NA gradually decreases despite the stress, while DA only upon the cessation of stimulus. Although the stress increases DA in CNS, the opposite effect was observed in the heart. In CNS and heart of bivalves DA and its metabolite are present (Pani and Croll, 1995). The activity of DA was also restricted mainly to auricle (Springer et al., 2005). The D1-like dopamine receptors with molecular weight of ~100 kDa are expressed in both cardiac and neural membranes of Aplysia (Barbas et al., 2006).
The embryonic Lymnaea at stage E15 contains DA (Voronezhskaya et al., 1999). The CNS of Lymnaea contains numerous catecholaminergic (CAergic) neurons (Croll et al., 1999) and majority of them react positively to tyrosine hydroxylase (TH+). CAergic cells of CNS emerge at ~E75 and their number and size increases accordingly during development. Among them, only RPeD1 and cerebral neuron (C7) are identifiable and remaining small neurons are in clusters. In Helisoma, LPeD1 contains 0.4 mM DA, but its homologues RPeD1 was described as serotonergic (Syed et al., 1993). Also, terminals of RPeD1 are TH+ as it is precisely shown already at hatchling stage within the pedal, pleural, parietal and visceral ganglia and nerves. In Sepia, some of CAergic neurons similarly react to TH and α-tubulin and their differentiation may occur at E16 (Baratte and Bonnaud, 2009). Also, in Aplysia, CAergic cells appear earlier in embryonic locations where the CNS develops (Dickinson et al., 2000).
7. Cardiac ion channels
The heart and subsequently the cardiac cells are vulnerable to multiple substances delivered by the axonal terminals of identified neurons and by hemolymph. Since the single neurons of Mollusca may release multiple neurotransmitters and several of them can modulate the identical channels in the heart, the convergent and divergent modes of neuromodulation were suggested.
Among Mollusca, the most detailed analyses of ion channels are performed on neurons of Helix (Kostyuk et al., 1972; Gola and Crest, 1993; Sotkis et al., 1998; Garateix et al., 2000; Bal et al., 2001; Kiss et al., 2002) and Aplysia (Furukawa et al., 1992; Goldsmith and Abrams, 1992; Vandorpe and Morris, 1992; Ma and Koester, 1996). Despite the fact that a significant body of work concerned the neuronal regulation of heart (Furukawa and Kobayashi, 1987b; Zhuravlev et al., 2001), there are scant studies on ion channels in the cardiomyocytes of pulmonate snails in particular, and Mollusca in general. Extensive studies on ionic currents including single channel recordings are available exclusively in the cardiac cells of pond snail Lymnaea (Brezden et al., 1986; Brezden and Gardner, 1992; Brezden et al., 1999; Yeoman et al., 1999). Another study was conducted at about the same time on blue mussel Mytilus revealing the presence of A-type K+, delayed rectifier K+, TTX-sensitive Na+, and L-type Ca2+ channels in cardiomyocytes (Curtis et al., 1999). Millimolar concentrations of tetraethylammonium (TEA) and 4-aminopyridine (4-AP) decrease K+ currents. In Lymnaea, the A-type K+ currents are characterized in the presence of 5 mM TEA (Yeoman and Benjamin, 1999). In ventricular myocytes of Aplysia brasiliana (Souza et al., 2002) two components of outward K+ currents, A-type and delayed rectifier, were readily recorded at holding potential of −80 mV; setting the HP to −40 mV eliminates the former component. In Aplysia kurodai, NdWFamide facilitates L-type Ca2+ currents (Kanemaru et al., 2002). In cardiomyocytes of Lymnaea, these channels are activated by α2 and SCPB peptides (Jimenez et al., 2006).
In cardiomyocytes of systemic and branchial hearts of squid Alloteuthis the Ca2+ activated K+ currents activate exponentially upon depolarization up to 40 mV (Odblom et al., 2000); thereafter, their amplitude suddenly decreases and at 60 mV again gradually increases. These currents are sensitive to Co2+ and operate in a window range of ±60 mV. In addition to the abovementioned four papers, the TEA-sensitive K+ channels have been described in Helix (Kodirov et al., 2004).
To my knowledge, these are the only reports on ion channels in cardiomyocytes using the whole-cell configuration of patch-clamp technique. In earlier studies, the high-resistance electrode was used (Yeoman et al., 1999). Nevertheless, also this classical method enabled a high quality analysis of ion channels in ventricular myocytes of Lymnaea. Note that the dissociated ventricular cells in Mytilus, Aplysia, and Helix (Fig. 3C) possess very similar morphology; they are distinct in Alloteuthis. Also note that the concentrations of TEA required to block the K+ channels among Mollusca are extremely apart with the highest affinity in Helix with an IC50 of ~300 μmol L−1. However, the sensitivity of K+ currents to 4-AP in millimolar range were comparable in all investigated species.
Finally, all the above described experiments were performed exclusively on ventricular myocytes, since atrial ones are more delicate and vulnerable to both mechanical and enzymatic dissociations (Zhuravlev, Brachmann, and Kodirov, unpublished results) and the whole-cell recordings in latter cells are less reproducible (Jimenez et al., 2006). Nevertheless, the atrial cells are viable after the dispersion in oysters Crassostrea (Pennec et al., 2004). Note that the enzymatic dissociation in latter study lasted 12 h. These cells contract spontaneously at resting membrane potential of −45 mV. Two major families of inward Na+ and outward K+ currents are present in auricular myocytes. The appearance of overlapping Na+ and K+ currents are similar to those pioneered in giant axon of squid Loligo (Hodgkin and Huxley, 1952). Similar to mammals, the outward currents in Crassostrea comprise two components resembling the A-type (known also as It0) and delayed rectifier currents. Both components mask each other as revealed by alternating blockade by 4-AP and TEA. Although, the A-type K+ currents are similar to those in rats (Kodirov et al., 2010), their high affinity to 4-AP (complete inhibition by 10 μM) is distinct. In mammals, 25 μM 4-AP does not block these channels (Kodirov et al., 2003; Kodirov, 2004). The waveform of outward currents in Crassostrea and their sensitivity to 4-AP also differ from those of Helix, which might or might not be related to the origin of cardiomyocytes (auricle vs. ventricle) and genus of animals (Kodirov et al., 2004). In the presence of 4-AP and TEA, the fast TTX sensitive (1 μM) Na+ currents in auricular myocytes of Crassostrea were observed (Pennec et al., 2004). The addition of all three substances to extracellular solution enables the selective measurement of L-type Ca2+ currents that were masked under control conditions and occasionally were seen upon the elimination of A-type currents by TEA. The L-type currents in Crassostrea are increased by 10 μM 5-HT and completely blocked by Co2+. The response of spontaneously contracting cultured cardiomyocytes to 5-HT were even dramatic exhibiting a 50 and 100% increase at 1 and 100 nM, respectively. In Loligo, relatively low expression of Cavβ1a and Cavβ1b were detected in systemic and branchial hearts (Kimura and Kubo, 2003). The expression of Cavβ1a was significantly lower compared to Cavβ1b. Their co-expression with mammalian Cav2.3 and α2/δ subunits equally led to the formation of functional channels with the current’s profile resembling those of L-type channels known also as high-voltage-activated (HVA) Ca2+ currents in Lymnaea (Yeoman et al., 1999). In this snail another component of inward currents activates readily at −70 mV and, therefore, was termed as low-voltage-activated (LVA) Ca2+ currents. The waveform of these currents was similar to those of T-type channels.
The indirect evidence for participation of ion channels in generation of cardiac action potentials is also provided for Modiolus (Wilkens, 1972a, 1972b), Perna (Pereira Ferreira and Salomão, 2000), and Aplysia (Souza et al., 2002). The ventricular APs in Perna possess three phenotypes: 1 — similarly fast depolarizing and repolarizing phases; 2 — relatively fast depolarization, rapid initial repolarization followed by plateau and final slow repolarization (similar to mammals albeit the duration); 3 — almost equally slow depolarization and repolarization. The depolarizing phase, unlike in mammals, was not derived by Na+ currents, since the APs persist in the absence of extracellular Na+. Interestingly, the APs were observed even in the absence of Ca2+. The non-selective L-type channel blocker cobalt inhibited the cardiac activity and similar blockers had complex effects depending on concentrations. The resting membrane potential in isolated ventricle was around −30 mV (by sucrose-gap method), and was dependent on potassium ion gradients; the AP’s waveforms were similar to those obtained by sharp microelectrodes (Wilkens, 1972b).
In Aplysia, the spontaneous APs were recorded by impaling the ventricular myocard with floating pipettes (Souza et al., 2002). This study revealed two types of APs: one did not possess the plateau phase that was clearly present in the second type. However, both did not reach the overshoot potential, and the depolarization phase terminated at −20 mV depending on resting membrane potential that ranged between −30 and −50 mV. The APs were not Na+–, but Ca2+-dependent. Interestingly, 4-AP shortened the duration of type 2 APs, unmasked the AHP, and enabled the appearance of overshoot potential. In Helix, only simultaneous exclusion of Ca2+ and Na+ from the extracellular solution eliminated spontaneous APs (Elekes et al., 1973).
The role of Na+–K+ pump in heart was defined by the exclusion of K+ ions from the extracellular solution in Helix (Ayrapetyan et al., 2008). The magnitude of beats in the absence of K+ (inactivation of pump) is increased, then upon inclusion is gradually decreased leading to final arrest at diastole. The latter was termed as TIHB-transient inhibition of heartbeat that is sensitive to hydrogen peroxide (H2O2). This compound in Helix exerts a bell shaped inotropic effects at 100 nM–100 μM. It was concluded that the H2O2 could be produced during the exposure to magnetic field. The latter modulates also the neuronal spike properties presumably via blockade of Ca2+ channels (Moghadam et al., 2011).
8. Conclusions
In conclusion, the heart of Mollusca is abundantly innervated directly or indirectly by neurons of different ganglion (Furukawa and Kobayashi, 1987a; Alevizos et al., 1989; Buckett et al., 1990a; Zhuravlev et al., 1991; Zhuravlev et al., 2002). The specific neurons in the CNS are easily distinguished by the distinct pigmentation of their soma, diameter, electrophysiological properties, and their neurotransmitters (Buckett et al., 1990a; Kodirov et al., 1994; Morishita et al., 2003). An increasing body of evidence suggests similarities between the Molluscs and mammals in regard to neurotransmitters and their receptors (Ha et al., 2006).
There is a dedicated neural network that tunes the cardiac properties, which are modulated also in overlap with other physiological functions including respiration and locomotion. However, the heart’s rhythm in Mollusca can also be maintained by myogenic drive, as supported by the APs recorded in isolated myocardium and cardiomyocytes. The heart, at least the auricle, possesses besides the myocytes also secretory granular cells (Erdelyi and Halasz, 1972; Shabelnikov et al., 2009), which to some extent modulate the overall cardiac activity. It should be emphasized that there are more similarities in basic cardiac properties in mammals and Molluscs. The negative ino- and chronotropic effects of mammalian ANF on systemic heart of Octopus in picomolar concentration also support this idea (Agnisola et al., 1989). In some cases, despite the absence of peptides/neurotransmitters in terminals innervating the heart, they still may modulate the cardiac activity when present in the hemolymph, and subsequently, in the circulation. The latter mechanism is described as humoral control of cardiac activity in gastropods (Zhuravlev, 1999). Similar pathway is also suggested for pQDPFLRI-amide and CNP2, in addition to those delivered by the nerve terminals (Lesser and Greenberg, 1993; Aseyev et al., 2010). Finally, although there is a common mechanism for cardiac pace in Molluscs and mammals (Irisawa, 1978), the detailed elucidation of the major pacemaker region for cardiac activity and underlying ion channels/subunits would be the next step to fulfill the comparability of their hearts.
Supplementary Material
Acknowledgments
I am grateful to Dr. Clara Downey-Adams (UTB) and Raul Consunji (MA) for proof reading the manuscript, administration of Business School for office space, Dr. Göran Nilsson for suggestion in regard to topic and directions of this manuscript, and anonymous reviewers for evaluation. The manuscript was in part supported by the National Institute of Health.
Appendix A. Supplementary data
Supplementary data to this article can be found online at doi:10.1016/j.cbpa.2011.06.014.
Footnotes
This paper is dedicated to the memory of Vladimir Leonidovich Zhuravlev — teacher, gifted scientist and mentor at Saint Petersburg University.
References
- Agnisola C, Cariello L, Santis A, Miralto A, Tota B. Chronotropic and inotropic effects of atrial peptides on the isolated systemic heart of Octopus vulgaris. J Comp Physiol B. 1989;158:637–641. doi: 10.1007/BF00693001. [DOI] [PubMed] [Google Scholar]
- Alevizos A, Bailey CH, Chen M, Koester J. Innervation of vascular and cardiac muscle of Aplysia by multimodal motoneuron L7. J Neurophysiol. 1989;61:1053–1063. doi: 10.1152/jn.1989.61.5.1053. [DOI] [PubMed] [Google Scholar]
- Alving BO. Spontaneous activity in isolated somata of Aplysia pacemaker naurons. J Gen Physiol. 1968;51:29–45. doi: 10.1085/jgp.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov I, Ha T, Antonova I, Moroz LL, Hawkins RD. Role of nitric oxide in classical conditioning of siphon withdrawal in Aplysia. J Neurosci. 2007;27:10993–11002. doi: 10.1523/JNEUROSCI.2357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky YI, Deliagina TG, Gelfand IM, Orlovsky GN, Panchin YV, Pavlova GA, Popova LB. Neural control of heart beat in the pteropod Mollusc Clione limacina: coordination of circulatory and locomotor systems. J Exp Biol. 1990;148:461–475. [Google Scholar]
- Aseyev N, Zakharov IS, Balaban PM. Morphology of neuropeptide CNP2 modulation of heart activity in terrestrial snail. Peptides. 2010;31:1301–1308. doi: 10.1016/j.peptides.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Ayrapetyan G, Dadasyan E, Hayrapetyan H, Ayrapetyan S. Exogenous hydrogen peroxide as a possible messenger for the stimulation effect of magnetized physiological solution on heart contractility. Bioelectromagnetics. 2008;29:549–558. doi: 10.1002/bem.20421. [DOI] [PubMed] [Google Scholar]
- Azatian KV, White AR, Walker RJ, Ayrapetyan SN. Cellular and molecular mechanisms of nitric oxide-induced heart muscle relaxation. Gen Pharmacol. 1998;30:543–553. doi: 10.1016/s0306-3623(97)00302-9. [DOI] [PubMed] [Google Scholar]
- Baker MW, Vohra MM, Croll RP. Serotonin depletors, 5,7-dihydroxytryptamine and p-chlorophenylalanine, cause sprouting in the CNS of the adult snail. Brain Res. 1993;623:311–315. doi: 10.1016/0006-8993(93)91444-w. [DOI] [PubMed] [Google Scholar]
- Bakhmet IN, Fokina NN, Nefedova ZA, Nemova NN. Physiological–biochemical properties of blue mussel Mytilus edulis adaptation to oil contamination. Environ Monit Assess. 2009;155:581–591. doi: 10.1007/s10661-008-0457-5. [DOI] [PubMed] [Google Scholar]
- Bal R, Janahmadi M, Green GG, Sanders DJ. Two kinds of transient outward currents, IA and IAdepol, in F76 and D1 soma membranes of the subesophageal ganglia of Helix aspersa. J Membr Biol. 2001;179:71–78. doi: 10.1007/s002320010038. [DOI] [PubMed] [Google Scholar]
- Baratte S, Bonnaud L. Evidence of early nervous differentiation and early catecholaminergic sensory system during Sepia officinalis embryogenesis. J Comp Neurol. 2009;517:539–549. doi: 10.1002/cne.22174. [DOI] [PubMed] [Google Scholar]
- Barbas D, Zappulla JP, Angers S, Bouvier M, Mohamed HA, Byrne JH, Castellucci VF, DesGroseillers L. An aplysia dopamine1-like receptor: molecular and functional characterization. J Neurochem. 2006;96:414–427. doi: 10.1111/j.1471-4159.2005.03561.x. [DOI] [PubMed] [Google Scholar]
- Baud C, Darbon P, Li KW, Marchand CR. Partial characterization of a novel cardioinhibitory peptide from the brain of the snail Helix aspersa. Cell Mol Neurobiol. 1998;18:413–424. doi: 10.1023/A:1022549515664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini G, Pugliese AM, Pepeu G, Chelazzi G. Neuronal control of the cardiac responses to osmotic stress in the gastropod limpet Patella caerulea. J Exp Zool A Comp Exp Biol. 2006a;305:472–479. doi: 10.1002/jez.a.275. [DOI] [PubMed] [Google Scholar]
- Bini G, Pugliese AM, Pepeu G, Chelazzi G. Tetrodotoxin prevents copper-induced bradycardia in gastropod limpets. Environ Pollut. 2006b;139:79–85. doi: 10.1016/j.envpol.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Bogdanov YD, Balaban PM, Poteryaev DA, Zakharov IS, Belyavsky AV. Putative neuropeptides and an EF-hand motif region are encoded by a novel gene expressed in the four giant interneurons of the terrestrial snail. Neuroscience. 1998;85:637–647. doi: 10.1016/s0306-4522(97)00561-7. [DOI] [PubMed] [Google Scholar]
- Bogerd J, Li KW, Jiménez CR, van der Schors RC, Ebberink RHM, Geraerts WPM. Processing, axonal transport and cardioregulatory functions of peptides derived from two related prohormones generated by alternative splicing of a single gene in identified neurons VD1 and RPD2 of Lymnaea. Mol Brain Res. 1994;23:66–72. doi: 10.1016/0169-328x(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Boyd PJ, Osborne NN, Walker RJ. The pharmacological actions of 5-HT, FMRFamide and substance P and their possible occurence in the heart of the snail Helix aspersa. Neurochem Int. 1984;6:633–640. doi: 10.1016/0197-0186(84)90044-5. [DOI] [PubMed] [Google Scholar]
- Braby CE, Somero GN. Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus) J Exp Biol. 2006;209:2554–2566. doi: 10.1242/jeb.02259. [DOI] [PubMed] [Google Scholar]
- Brezden BL, Benjamin PR, Gardner DR. The peptide FMRFamide activates a divalent cation-conducting channel in heart muscle cells of the snail Lymnaea stagnalis. J Physiol. 1991;443:727–738. doi: 10.1113/jphysiol.1991.sp018860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezden BL, Gardner DR. A review of the electrophysiological, pharmacological and single channel properties of heart ventricle muscle cells in the snail Lymnaea stagnalis. Experientia. 1992;48:841–852. doi: 10.1007/BF02118417. [DOI] [PubMed] [Google Scholar]
- Brezden BL, Gardner DR, Morris CE. A potassium-selective channel in isolated Lymnaea stagnalis heart muscle cells. J Exp Biol. 1986;123:175–189. [Google Scholar]
- Brezden BL, Yeoman MS, Gardner DR, Benjamin PR. FMRFamide-activated Ca2+ channels in Lymnaea heart cells are modulated by “SEEPLY,” a neuropeptide encoded on the same gene. J Neurophysiol. 1999;81:1818–1826. doi: 10.1152/jn.1999.81.4.1818. [DOI] [PubMed] [Google Scholar]
- Bright K, Kellett E, Saunders SE, Brierley M, Burke JF, Benjamin PR. Mutually exclusive expression of alternatively spliced FMRFamide transcripts in identified neuronal systems of the snail Lymnaea. J Neurosci. 1993;13:2719–2729. doi: 10.1523/JNEUROSCI.13-06-02719.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckett KJ, Peters M, Benjamin PR. Excitation and inhibition of the heart of the snail, Lymnaea, by non-FMRFamidergic motoneurons. J Neurophysiol. 1990a;63:1436–1447. doi: 10.1152/jn.1990.63.6.1436. [DOI] [PubMed] [Google Scholar]
- Buckett KJ, Peters M, Dockray GJ, Van Minnen J, Benjamin PR. Regulation of heartbeat in Lymnaea by motoneurons containing FMRFamide-like peptides. J Neurophysiol. 1990b;63:1426–1435. doi: 10.1152/jn.1990.63.6.1426. [DOI] [PubMed] [Google Scholar]
- Bychkov R, Zhuravlev V, Kodirov S, Safonova T. Cardiac inhibitory neurons in gastropod snail Achatina fulica. J Brain Res. 1997;38:263–278. [PubMed] [Google Scholar]
- Bychkov RE, Safonova TA, Zhuravlev VL. Viscerocardiac reflexes of the snail. Neurosci Behav Physiol. 1994;24:89–96. doi: 10.1007/BF02355657. [DOI] [PubMed] [Google Scholar]
- Carlson AJ. The rhythm produced in the resting heart of molluscs by the stimulation of the cardio-accelerator nerves. Am J Physiol. 1904;12:55–66. [Google Scholar]
- Carlson AJ. Comparative physiology of the invertebrate heart. II The function of the cardiac nerves in molluscs. Am J Physiol. 1905;13:396–426. [Google Scholar]
- Chase R, Goodman HE. Homologous neurosecretory cell groups in the land snail Achatina fulica and the sea slug Aplysia californica. Cell Tissue Res. 1977;176:109–120. doi: 10.1007/BF00220347. [DOI] [PubMed] [Google Scholar]
- Chong GC, Phillis JW. Pharmacological studies on the heart of Tapes watlingi: a mollusc of the family Veneridae. Br J Pharmacol Chemother. 1965;25:481–496. doi: 10.1111/j.1476-5381.1965.tb02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis LP, Sun Y, Hill RB. Length-dependent deactivation of ventricular trabeculae in the bivalve, Spisula solidissima. J Comp Physiol B. 2006;176:371–385. doi: 10.1007/s00360-005-0060-9. [DOI] [PubMed] [Google Scholar]
- Cottrell GA. The first peptide-gated ion channel. J Exp Biol. 1997;200:2377–2386. doi: 10.1242/jeb.200.18.2377. [DOI] [PubMed] [Google Scholar]
- Cottrell GA, Powell B, Stanton M. A simple method for measuring a picogram of acetylcholine using the clam (Mya arenaria) heart. Br J Pharmacol. 1970;40:866–870. doi: 10.1111/j.1476-5381.1970.tb10661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GA, Price DA, Doble KE, Hettle S, Sommerville J, MacDonald M. Identified Helix neurons: mutually exclusive expression of the tetrapeptide and heptapeptide members of the FMRFamide family. Biol Bull. 1992;183:113–122. doi: 10.2307/1542412. [DOI] [PubMed] [Google Scholar]
- Cottrell GA, Price DA, Greenberg MJ. FMRFamide-like activity in the ganglia and in a single identified neurone of Helix aspersa. Comp Biochem Physiol C. 1981;70:103–107. doi: 10.1016/0306-4492(81)90085-x. [DOI] [PubMed] [Google Scholar]
- Croll RP. Distribution of monoamines within the central nervous system of the juvenile pulmonate snail, Achatina fulica. Brain Res. 1988;460:29–49. doi: 10.1016/0006-8993(88)90427-1. [DOI] [PubMed] [Google Scholar]
- Croll RP, Voronezhskaya EE, Hiripi L, Elekes K. Development of catecholaminergic neurons in the pond snail, Lymnaea stagnalis: II. Postembryonic development of central and peripheral cells. J Comp Neurol. 1999;404:297–309. 297. doi: 10.1002/(sici)1096-9861(19990215)404:3<297::aid-cne2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Depledge MH, Williamson R. Voltage-activated currents in cardiac myocytes of the blue mussel, Mytilus edulis. Comp Biochem Physiol Part A. 1999;124:231–241. [Google Scholar]
- Curtis TM, Williamson R, Depledge MH. The initial mode of action of copper on the cardiac physiology of the blue mussel, Mytilus edulis. Aquat Toxicol. 2001;52:29–38. doi: 10.1016/s0166-445x(00)00135-1. [DOI] [PubMed] [Google Scholar]
- Dale B. Blood pressure and its hydraulic functions in Helix pomatia. L. J Exp Biol. 1973;59:477–490. [Google Scholar]
- Darwin F. On the structure of the snail’s heart. J Anat Physiol. 1876;10:506–510. [PMC free article] [PubMed] [Google Scholar]
- Dickinson AJ, Croll RP, Voronezhskaya EE. Development of embryonic cells containing serotonin, catecholamines, and FMRFamide-related peptides in Aplysia californica. Biol Bull. 2000;199:305–315. doi: 10.2307/1543187. [DOI] [PubMed] [Google Scholar]
- Dougherty DA, Lester HA. Neurobiology. Snails, synapses and smokers Nature. 2001;411:252–255. doi: 10.1038/35077192. [DOI] [PubMed] [Google Scholar]
- Dyakonova TL. Interaction between serotonin and nitric oxide (NO) in the activation of the serotoninergic system in the common snail. Neurosci Behav Physiol. 2002;32:275–282. doi: 10.1023/a:1015062324019. [DOI] [PubMed] [Google Scholar]
- Dyakonova VE, Chistopolsky IA, Dyakonova TL, Vorontsov DD, Sakharov DA. Direct and decarboxylation-dependent effects of neurotransmitter precursors on firing of isolated monoaminergic neurons. J Comp Physiol A. 2009;195:515–527. doi: 10.1007/s00359-009-0428-5. [DOI] [PubMed] [Google Scholar]
- Elekes K, Kiss T, S-Rózsa K. Effect of Ca-free medium on the ultrastructure and excitability of the myocardial cells of the snail, Helix pomatia. L. J Mol Cell Cardiol. 1973;5:133–136. doi: 10.1016/0022-2828(73)90046-1. [DOI] [PubMed] [Google Scholar]
- Epstein R, Tauc L. Heterosynaptic facilitation and post-tetanic potentiation in Aplysia nervous system. J Physiol. 1970;209:1–23. doi: 10.1113/jphysiol.1970.sp009152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdelyi L, Halasz N. Electron-microscopical observations on the auricle of snail heart (Helix pomatia L.) with special regard to the structure of granulated cells. Acta Biol. 1972;18:253–267. [Google Scholar]
- Ewadinger NM, Ridgway RL, Syed NI, Lukowiak K, Bulloch AG. Identification and localization of a [Met5]-enkephalin-like peptide in the mollusc, Lymnaea stagnalis. Brain Res. 1996;737:1–15. doi: 10.1016/0006-8993(96)00649-x. [DOI] [PubMed] [Google Scholar]
- Fabbri E, Capuzzo A. Cyclic AMP signaling in bivalve molluscs: an overview. J Exp Zool. 2010;313A:179–200. doi: 10.1002/jez.592. [DOI] [PubMed] [Google Scholar]
- Filippov AK, Porotikov VI, Novoselova VD, Bessonov BI, Butsuk SV. Action potential and ionic currents of myocardial fibers in the mollusc Spisula sachalinensis. Comp Biochem Physiol A. 1988;89:1–5. [Google Scholar]
- Fujimoto K, Ohta N, Yoshida M, Kubota I, Muneoka Y, Kobayashi M. A novel cardio-excitatory peptide isolated from the atria of the African giant snail, Achatina fulica. Biochem Biophys Res Commun. 1990;167:777–783. doi: 10.1016/0006-291x(90)92093-f. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Kandel ER, Pfaffinger P. Three types of early transient potassium currents in Aplysia neurons. J Neurosci. 1992;12:989–1000. doi: 10.1523/JNEUROSCI.12-03-00989.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Kobayashi M. Neural control of heart beat in the African giant snail, Achatina fulica Férussac. I Identification of the heart regulatory neurones J Exp Biol. 1987a;129:279–293. 279. [Google Scholar]
- Furukawa Y, Kobayashi M. Neural control of heart beat in the African giant snail. Achatina fulica Férussac II Interconnections among the heart regulatory neurones J Exp Biol. 1987b;129:294–307. [Google Scholar]
- Furukawa Y, Kobayashi M. Modulation of ionic currents by synaptic action and 5-HT application in the identified heart excitatory neurone of the African giant snail, Achatina fulica Ferussac. J Exp Biol. 1988;137:319–339. doi: 10.1007/BF01959145. [DOI] [PubMed] [Google Scholar]
- Gainutdinov K, Andrianov V, Gainutdinova T, Muranova L, Obynochny A, Timoshenko A, Shtark M, Epstein O, Yurtaeva S, Jafarova G. Investigation of changes in NO content during long-term sensitization in edible snail using EPR-spectroscopy: effects of antibodies to calcium-binding protein S-100. Bull Exp Biol Med. 2008;146:675–679. doi: 10.1007/s10517-009-0367-8. [DOI] [PubMed] [Google Scholar]
- Garateix A, Vega R, Salceda E, Cebada J, Aneiros A, Soto E. BgK anemone toxin inhibits outward K+ currents in snail neurons. Brain Res. 2000;864:312–314. doi: 10.1016/s0006-8993(00)02131-4. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld HM, Paupardin-Tritsch D. On the transmitter function of 5-hydroxytryptamine at excitatory and inhibitory monosynaptic junctions. J Physiol. 1974;243:457–481. doi: 10.1113/jphysiol.1974.sp010762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M, Crest M. Colocalization of active KCa channels and Ca2+ channels within Ca2+ domains in Helix neurons. Neuron. 1993;10:689–699. doi: 10.1016/0896-6273(93)90170-v. [DOI] [PubMed] [Google Scholar]
- Goldsmith BA, Abrams TW. cAMP modulates multiple K+ currents, increasing spike duration and excitability in Aplysia sensory neurons. Proc Natl Acad Sci USA. 1992;89:11481–11485. doi: 10.1073/pnas.89.23.11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommerat I, Gola M. Satellite glial cell responses to neuronal firing in the nervous system of Helix pomatia. J Membr Biol. 1994;138:209–219. doi: 10.1007/BF00232793. [DOI] [PubMed] [Google Scholar]
- Gorshkov BA, Kapustina II, Kicha AA, Aminin DL, Gorshkova IA. Stimulatory effects of starfish sapogenins (Asterias amurensis and Lethasterias nanimensis chelifera) on molluscan heart (Spisula sachalinensis) Comp Biochem Physiol C. 1998;120:235–239. doi: 10.1016/s0742-8413(98)10001-4. [DOI] [PubMed] [Google Scholar]
- Ha TJ, Kohn AB, Bobkova YV, Moroz LL. Molecular characterization of NMDA-like receptors in Aplysia and Lymnaea: relevance to memory mechanisms. Biol Bull. 2006;210:255–270. doi: 10.2307/4134562. [DOI] [PubMed] [Google Scholar]
- Han XY, Salunga TL, Zhang W, Takeuchi H, Matsunami K. Modulation by APGW-amide, an Achatina endogenous inhibitory tetrapeptide, of currents induced by neuroactive compounds on Achatina neurons: peptides. Gen Pharmacol. 1997;29:531–538. doi: 10.1016/s0306-3623(96)00579-4. [DOI] [PubMed] [Google Scholar]
- Hill RB. Effects of 5-hydroxytryptamine on action potentials and on contractile force in the ventricle of Dolabella auricularia. J Exp Biol. 1974a;61:529–539. doi: 10.1242/jeb.61.2.529. [DOI] [PubMed] [Google Scholar]
- Hill RB. Effects of acetylcholine on resting and action potentials, and on contractile force in the ventricle of Dolabella auricularia. J Exp Biol. 1974b;61:629–637. doi: 10.1242/jeb.61.3.629. [DOI] [PubMed] [Google Scholar]
- Hirata T, Kubota I, Iwasawa N, Takabatake I, Ikeda T, Muneoka Y. Structures and actions of Mytilus inhibitory peptides. Biochem Biophys Res Commun. 1988;152:1376–1382. doi: 10.1016/s0006-291x(88)80437-6. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952;116:449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiol Rev. 1978;58:461–498. doi: 10.1152/physrev.1978.58.2.461. [DOI] [PubMed] [Google Scholar]
- Irisawa H, Shigeto N, Otani M. Effect of Na+ and Ca2+ on the excitation of the Mytilus (bivalve) heart muscle. Comp Biochem Physiol. 1967;23:199–212. doi: 10.1016/0010-406x(67)90488-4. [DOI] [PubMed] [Google Scholar]
- Isao Y. Axonal pathways of two identifiable giant neurones of the African giant snail (Achatina fulica Férussac) Comp Biochem Physiol A. 1980;65:513–516. [Google Scholar]
- Jacklet JW, Peretz B, Strumwasser F. Synaptic influences on identified neurons in an aberrant parieto-visceral ganglion of Aplysia. J Comp Physiol A. 1970;66:318–325. [Google Scholar]
- Jaeger CP. Physiology of mollusca — I. Action of acetylcholine on the heart of Strophocheilos oblongus. Comp Biochem Physiol. 1961;4:30–32. doi: 10.1016/0010-406x(61)90043-3. [DOI] [PubMed] [Google Scholar]
- Jaeger CP. Physiology of mollusca — II. Action of serotonin and other amines on the heart of Strophocheilos oblongus. Comp Biochem Physiol. 1962;6:243–245. doi: 10.1016/0010-406x(62)90082-8. [DOI] [PubMed] [Google Scholar]
- Jaeger CP. Neuroendocrine regulation of cardiac activity in the snail Strophocheilus oblongus. Comp Biochem Physiol. 1966;17:409–415. doi: 10.1016/0010-406x(66)90577-9. [DOI] [PubMed] [Google Scholar]
- Jaeger CP, Jaeger EC, Welsh JH. Localization of monoamine-containing neurones in the nervous system of Strophocheilus oblongus (Gastropoda) Cell Tissue Res. 1971;112:54–68. doi: 10.1007/BF00665621. [DOI] [PubMed] [Google Scholar]
- Jahan-Parwar B, Rozsa KS, Salanki J, Evans ML, Carpenter DO. In vivo labeling of serotonin-containing neurons by 5,7-dihydroxytryptamine in Aplysia. Brain Res. 1987;426:173–178. doi: 10.1016/0006-8993(87)90438-0. [DOI] [PubMed] [Google Scholar]
- Janse C, van der Wilt GJ, van der Plas J, van der Roest M. Central and peripheral neurones involved in oxygen perception in the pulmonate snail Lymnaea stagnalis (Mollusca, Gastropoda) Comp Biochem Physiol A. 1985;82:459–469. [Google Scholar]
- Jerelova OM, Krasts IV, Veprintsev BN. The effect of sodium, calcium and magnesium on the amplitude of the action potential from giant neurons of Limnaea stagnalis. Comp Biochem Physiol A. 1971;40:281–293. doi: 10.1016/0300-9629(71)90168-x. [DOI] [PubMed] [Google Scholar]
- Jeziorski MC, Green KA, Sommerville J, Cottrell GA. Cloning and expression of a FMRFamide-gated Na+ channel from Helisoma trivolvis and comparison with the native neuronal channel. J Physiol. 2000;526:13–25. doi: 10.1111/j.1469-7793.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez CR, Spijker S, de Schipper S, Lodder JC, Janse CK, Geraerts WP, van Minnen J, Syed NI, Burlingame AL, Smit AB, Li K. Peptidomics of a single identified neuron reveals diversity of multiple neuropeptides with convergent actions on cellular excitability. J Neurosci. 2006;26:518–529. doi: 10.1523/JNEUROSCI.2566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurchenko OP, Kultas KN, Vulfius CA. Cholinesterase activity in ganglia of gastropoda, Lymnaea stagnalis and Planorbarius corneus — II. Histochemical investigation Comp Biochem Physiol A. 1973a;45:61–68. doi: 10.1016/0300-9629(73)90008-x. [DOI] [PubMed] [Google Scholar]
- Jurchenko OP, Vulfius CA, Zeimal EV. Cholinesterase activity of ganglia of gastropoda, Lymnaea stagnalis and Planorbarius corneus — I. Effect of anti-cholinesterase agents on giant neurone depolarization by acetylcholine and its analogues. Comp Biochem Physiol A. 1973b;45:45–60. doi: 10.1016/0300-9629(73)90007-8. [DOI] [PubMed] [Google Scholar]
- Kamatani Y, Minakata H, Kenny PTM, Iwashita T, Watanabe K, Funase K, Xia Ping S, Yongsiri A, Kim KH, Novales-Li P, Novales ET, Kanapi CG, Takeuchi H, Nomoto K. Achatin-I, an endogenous neuroexcitatory tetrapeptide from Achatina fulica Férussac containing a D-amino acid residue. Biochem Biophys Res Commun. 1989;160:1015–1020. doi: 10.1016/s0006-291x(89)80103-2. [DOI] [PubMed] [Google Scholar]
- Kanemaru K, Morishita F, Matsushima O, Furukawa Y. Aplysia cardioactive peptide (NdWFamide) enhances the L-type Ca2+ current of Aplysia ventricular myocytes. Peptides. 2002;23:1991–1998. doi: 10.1016/s0196-9781(02)00186-9. [DOI] [PubMed] [Google Scholar]
- Kemenes G, Elekes K, Hiripi L, Benjamin PR. A comparison of four techniques for mapping the distribution of serotonin and serotonin-containing neurons in fixed and living ganglia of the snail, Lymnaea. J Neurocytol. 1989;18:193–208. doi: 10.1007/BF01206662. [DOI] [PubMed] [Google Scholar]
- Kerkhoven RM, Croll RP, Van Minnen J, Bogerd J, Ramkema MD, Lodder H, Boer HH. Axonal mapping of the giant peptidergic neurons VD1 and RPD2 located in the CNS of the pond snail Lymnaea stagnalis, with particular reference to the innervation of the auricle of the heart. Brain Res. 1991;565:8–16. doi: 10.1016/0006-8993(91)91730-o. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Lambert JDC, Gayton RJ, Loker JE, Walker RJ. Mapping of nerve cells in the suboesophageal ganglia of Helix aspersa. Comp Biochem Physiol A. 1975;50:1–25. doi: 10.1016/s0010-406x(75)80194-0. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Taylor BJR. Effect of temperature on the spontaneous activity from the isolated ganglia of the slug, cockroach and crayfish. Nature. 1956;178:426. doi: 10.1038/178426a0. [DOI] [PubMed] [Google Scholar]
- Kerkut GA, Walker RJ. The effects of drugs on the neurones of the snail Helix aspersa. Comp Biochem Physiol. 1961;3:143–160. doi: 10.1016/0010-406x(61)90051-2. [DOI] [PubMed] [Google Scholar]
- Kimura T, Kubo T. Cloning and functional characterization of squid voltage-dependent Ca2+ channel βsubunits: involvement of N-terminal sequences in differential modulation of the current. Neurosci Res. 2003;46:105–117. doi: 10.1016/s0168-0102(03)00038-5. [DOI] [PubMed] [Google Scholar]
- Kiss T, Laszlo Z, Szabadics J. Mechanism of 4-aminopyridine block of the transient outward K+-current in identified Helix neuron. Brain Res. 2002;927:168–179. doi: 10.1016/s0006-8993(01)03351-0. [DOI] [PubMed] [Google Scholar]
- Kiss T, Rózsa KS. Pharmacological properties of 5-HT-receptors of the Helix pomatia L. (Gastropoda) heart muscle cells. Comp Biochem Physiol. 1978;61:41–46. doi: 10.1016/0306-4492(78)90109-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Irisawa H. Latent period of relaxation. Science. 1961;134:1365–1366. doi: 10.1126/science.134.3487.1365. [DOI] [PubMed] [Google Scholar]
- Kodirov SA. Comparative Study of Potassium Channels in Cardiomyocytes of Mammals and Molluscs. Saint Petersburg University; Saint Petersburg: 2004. p. 144. [Google Scholar]
- Kodirov SA, Brunner M, Busconi L, Koren G. Long-term restitution of 4-aminopyridine-sensitive currents in Kv1DN ventricular myocytes using adeno-associated virus-mediated delivery of Kv1.5. FEBS Lett. 2003;550:74–78. doi: 10.1016/s0014-5793(03)00822-6. [DOI] [PubMed] [Google Scholar]
- Kodirov SA, Zhuravlev VL, Bychkov RE, Safonova TA, Stepanov II. Electrical activity of serotonergic neurons of African giant snail, Achatina fulica Ferussac, before and after 5,6-dihydroxytriptamine treatment. Vestn Saint-Petersburg University. 1994;3:68–75. [Google Scholar]
- Kodirov SA, Zhuravlev VL, Pavlenko VK, Safonova TA, Brachmann J. K+ channels in cardiomyocytes of the pulmonate snail Helix. J Membr Biol. 2004;197:145–154. doi: 10.1007/s00232-004-0649-z. [DOI] [PubMed] [Google Scholar]
- Kodirov SA, Zhuravlev VL, Safonova TA, Kurilova LS, Krutetskaia ZI. Superfamily of voltage dependent K+ channels: structure, functions and pathology. Tsitologiia. 2010;52:697–714. [PubMed] [Google Scholar]
- Koester J, Mayeri E, Liebeswar G, Kandel ER. Neural control of circulation in Aplysia. II Interneurons J Neurophysiol. 1974;37:476–496. doi: 10.1152/jn.1974.37.3.476. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Krishtal OA, Pidoplichko VI. Potential-dependent membrane current during the active transport of ions in snail neurones. J Physiol. 1972;226:373–392. doi: 10.1113/jphysiol.1972.sp009989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz R, Shapiro E, Kandel ER. Presynaptic inhibition produced by an identified presynaptic inhibitory neuron. I Physiological mechanisms J Neurophysiol. 1986;55:113–130. doi: 10.1152/jn.1986.55.1.113. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR. Development of the nervous system of Aplysia californica. Proc Natl Acad Sci USA. 1977;74:375–378. doi: 10.1073/pnas.74.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku BS, Takeuchi H. Effects of catecholamines, monophenolamines and phenylamines on identifiable giant neurons of an African giant snail (Achatina fulica Férussac) Eur J Pharmacol. 1985;114:1–8. doi: 10.1016/0014-2999(85)90514-x. [DOI] [PubMed] [Google Scholar]
- Kuwasawa K, Hill R. Evidence for cholinergic inhibitory and serotonergic excitatory neuromuscular transmission in the heart of the bivalve Mercenaria mercenaria. J Exp Biol. 1997;200:2123–2135. doi: 10.1242/jeb.200.15.2123. [DOI] [PubMed] [Google Scholar]
- Kuwasawa K, Neal H, Hil RB. Afferent pathways in the innervation of the ventricle of a prosobranch gastropod Busycon canaliculatum. J Comp Physiol. 1975;96:73–83. [Google Scholar]
- Lacoste A, Malham SK, Cueff A, Jalabert F, Gelebart F, Poulet SA. Evidence for a form of adrenergic response to stress in the mollusc Crassostrea gigas. J Exp Biol. 2001;204:1247–1255. doi: 10.1242/jeb.204.7.1247. [DOI] [PubMed] [Google Scholar]
- Leake LD, Evans TG, Walker RJ. The role of catecholamines and 5-HT on the heart of Patella vulgata. Comp Gen Pharmacol. 1971;2:151–158. doi: 10.1016/0010-4035(71)90005-x. [DOI] [PubMed] [Google Scholar]
- Lee TK, Leung AA, Jr, Brezden BL, Lukowiak K, Syed NI. Specificity of synapse formation between Lymnaea heart motor neuron and muscle fiber is maintained in vitro in a soma-muscle configuration. Synapse. 2002;46:66–71. doi: 10.1002/syn.10125. [DOI] [PubMed] [Google Scholar]
- Lehr T, Schipp R. Serotonergic regulation of the central heart auricles of Sepia officinalis L. (Mollusca, Cephalopoda) Comp Biochem Physiol A. 2004;138:69–77. doi: 10.1016/j.cbpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Lesser W, Greenberg MJ. Cardiac regulation by endogenous small cardioactive peptides and FMRFamide-related peptides in the snail Helix aspersa. J Exp Biol. 1993;178:205–230. doi: 10.1242/jeb.178.1.205. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- Liu GJ, Takeuchi H. Effects of cyclic AMP, cyclic GMP and IP3 intracellularly injected into the identifiable Achatina giant neurones. Comp Biochem Physiol C. 1993;104:199–204. [Google Scholar]
- Lloyd PE. Distribution and molecular characteristics of cardioactive peptides in the snail, Helix aspersa. J Comp Physiol A. 1978;128:269–276. [Google Scholar]
- Lloyd PE, Mahon AC, Kupfermann I, Cohen JL, Scheller RH, Weiss KR. Biochemical and immunocytological localization of molluscan small cardioactive peptides in the nervous system of Aplysia californica. J Neurosci. 1985;5:1851–1861. doi: 10.1523/JNEUROSCI.05-07-01851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Vera E, Aguilar MB, Heimer de la Cotera EP. FMRFamide and related peptides in the phylum mollusca. Peptides. 2008;29:310–317. doi: 10.1016/j.peptides.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Spencer GE. Perturbation of the activity of a single identified neuron affects long-term memory formation in a molluscan semi-intact preparation. J Exp Biol. 2006;209:711–721. doi: 10.1242/jeb.02047. [DOI] [PubMed] [Google Scholar]
- Ma M, Koester J. The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis. J Neurosci. 1996;16:4089–4101. doi: 10.1523/JNEUROSCI.16-13-04089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyshev AY, Balaban PM. Buccal neurons activate ciliary beating in the foregut of the pteropod mollusk Clione limacina. J Exp Biol. 2009;212:2969–2976. doi: 10.1242/jeb.032227. [DOI] [PubMed] [Google Scholar]
- Malyshev AY, Norekian TP, Balaban PM. Neural control of heartbeat during two antagonistic behaviors: whole body withdrawal and escape swimming in the mollusk Clione limacina. J Comp Physiol A. 2008;194:899–906. doi: 10.1007/s00359-008-0362-y. [DOI] [PubMed] [Google Scholar]
- Mantione KJ, Stefano GB. A sub-nanomolar real-time nitric oxide probe: in vivo nitric oxide release in heart. Med Sci Monit. 2004;10:47–49. [PubMed] [Google Scholar]
- Marchán S, Davies MS, Fleming S, Jones HD. Effects of copper and zinc on the heart rate of the limpet Patella vulgata L. Comp. Biochem Physiol A. 1999;123:89–93. [Google Scholar]
- Marinesco S, Kolkman KE, Carew TJ. Serotonergic modulation in Aplysia I Distributed serotonergic network persistently activated by sensitizing stimuli. J Neurophysiol. 2004a;92:2468–2486. doi: 10.1152/jn.00209.2004. [DOI] [PubMed] [Google Scholar]
- Marinesco S, Wickremasinghe N, Kolkman KE, Carew TJ. Serotonergic modulation in Aplysia II Cellular and behavioral consequences of increased serotonergic tone. J Neurophysiol. 2004b;92:2487–2496. doi: 10.1152/jn.00210.2004. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Kurokawa M, Kuwasawa K, Hill RB, Ohsuga K. Serotonergic control of the heart and pericardium in the chiton Acanthopleura japonica. Comp Biochem Physiol A. 1999;124:561–567. [Google Scholar]
- Mayeri E, Koester J, Kupfermann I, Liebeswar G, Kandel ER. Neural control of circulation in Aplysia. I Motoneurons J Neurophysiol. 1974;37:458–475. doi: 10.1152/jn.1974.37.3.458. [DOI] [PubMed] [Google Scholar]
- Michaelidis B, Loumbourdis NS, Kapaki E. Analysis of monoamines, adenosine and GABA in tissues of the land snail Helix lucorum and lizard Agama stellio stellio during hibernation. J Exp Biol. 2002;205:1135–1143. doi: 10.1242/jeb.205.8.1135. [DOI] [PubMed] [Google Scholar]
- Minakata H, Ikeda T, Muneoka Y, Kobayashi M, Nomoto K. WWamide-1, -2 and -3: novel neuromodulatory peptides isolated from ganglia of the African giant snail, Achatina fulica. FEBS Lett. 1993;323:104–108. doi: 10.1016/0014-5793(93)81458-c. [DOI] [PubMed] [Google Scholar]
- Moghadam MK, Firoozabadi M, Janahmadi M. Effects of weak environmental magnetic fields on the spontaneous bioelectrical activity of snail neurons. J Membr Biol. 2011;240:63–71. doi: 10.1007/s00232-011-9344-z. [DOI] [PubMed] [Google Scholar]
- Morishita F, Minakata H, Sasaki K, Tada K, Furukawa Y, Matsushima O, Mukai ST, Saleuddin AS. Distribution and function of an Aplysia cardioexcitatory peptide, NdWFamide, in pulmonate snails. Peptides. 2003;24:1533–1544. doi: 10.1016/j.peptides.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Morley SA, Hirse T, Pörtner HO, Peck LS. Geographical variation in thermal tolerance within Southern Ocean marine ectotherms. Comp Biochem Physiol. 2009;153:154–161. doi: 10.1016/j.cbpa.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Morris HR, Panico M, Karplus A, Lloyd PE, Riniker B. Elucidation by FAB-MS of the structure of a new cardioactive peptide from Aplysia. Nature. 1982;300:643–645. doi: 10.1038/300643a0. [DOI] [PubMed] [Google Scholar]
- Moulis A, Huddart H. RFamide neuropeptide actions on the molluscan ventricle: interactions with primary neurotransmitters. Comp Biochem Physiol C. 2006;142:95–102. doi: 10.1016/j.cbpc.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Nikolic L, Kartelija G, Nedeljkovic M. Effect of static magnetic fields on bioelectric properties of the Br and N1 neurons of snail Helix pomatia. Comp Biochem Physiol A. 2008;151:657–663. doi: 10.1016/j.cbpa.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Odblom MP, Williamson R, Jones MB. Ionic currents in cardiac myocytes of squid, Alloteuthis subulata. J Comp Physiol B. 2000;170:11–20. doi: 10.1007/s003600050002. [DOI] [PubMed] [Google Scholar]
- Orr MV, El-Bekai M, Lui M, Watson K, Lukowiak K. Predator detection in Lymnaea stagnalis. J Exp Biol. 2007;210:4150–4158. doi: 10.1242/jeb.010173. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Pentreath VW. Effects of 5,7-dihydroxytryptamine on an identified 5-hydroxytryptamine-containing neurone in the central nervous system of the snail Helix pomatia. Br J Pharmacol. 1976;56:29–38. doi: 10.1111/j.1476-5381.1976.tb06955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipenko ON, Kemenes G. Interneuronal monosynaptic peptidergic contact responsible for the bursting activity generation in the rpal neuron of the snail Helix pomatia L. is axo-axonal. Comp Biochem Physiol A. 1991;99:371–373. [Google Scholar]
- Pandolfo TJ, Cope WG, Arellano C. Heart rate as a sublethal indicator of thermal stress in juvenile freshwater mussels. Comp Biochem Physiol A. 2009;154:347–352. doi: 10.1016/j.cbpa.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Pani AK, Croll RP. Distribution of catecholamines, indoleamines, and their precursors and metabolites in the scallop, Placopecten magellanicus (Bivalvia, Pectinidae) Cell Mol Neurobiol. 1995;15:371–386. doi: 10.1007/BF02089947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova GA, Willows AD. Immunological localization of Tritonia peptide in the central and peripheral nervous system of the terrestrial snail Helix aspersa. J Comp Neurol. 2005;491:15–26. doi: 10.1002/cne.20671. [DOI] [PubMed] [Google Scholar]
- Pennec JP, Talarmin H, Droguet M, Giroux-Metges MA, Gioux M, Dorange G. Characterization of the voltage-activated currents in cultured atrial myocytes isolated from the heart of the common oyster Crassostrea gigas. J Exp Biol. 2004;207:3935–3944. doi: 10.1242/jeb.01221. [DOI] [PubMed] [Google Scholar]
- Pereira Ferreira R, Salomão LC. The ionic basis of cardiac activity in the bivalve mollusc Perna perna. J Exp Mar Biol Ecol. 2000;249:1–12. doi: 10.1016/s0022-0981(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Price DA, Doble KE, Lesser W, Greenberg MJ, Swiderek KM, Lee TD, Lutz EM, Sommerville J, Falconer S, Cottrell GA. The peptide pQFYRFamide is encoded on the FMRFamide precursor of the snail Helix aspersa but does not activate the FMRFamide-gated sodium current. Biol Bull. 1996;191:341–352. doi: 10.2307/1543007. [DOI] [PubMed] [Google Scholar]
- Price DA, Greenberg MJ. Purification and characterization of a cardioexcitatory neuropeptide from the central ganglia of a bivalve mollusc. Prep Biochem. 1977;7:261–281. doi: 10.1080/00327487708061643. [DOI] [PubMed] [Google Scholar]
- Prosser CL. Acetylcholine and nervous inhibition in the heart of Venus mercenaria. Biol Bull. 1940;78:92–102. [Google Scholar]
- Rosenbluth J. The visceral ganglion of Aplysia californica. Cell Tissue Res. 1963;60:213–236. doi: 10.1007/BF00350477. [DOI] [PubMed] [Google Scholar]
- Rozsa SK. Neuronal network, underlying the regulation of heart beat in Helix pomatia L. In: Salanki J, editor. Neurobiology of Invertebrates. Akademiai Kiado; Budapest: 1976. pp. 597–613. [Google Scholar]
- S-Rozsa K. Analysis of the neural network regulating the cardio-renal system in the central nervous system of Helix pomatia L. Am. Zool. 1979;19:117–128. [Google Scholar]
- S-Rozsa K, Salanki J. Single neurone responses to tactile stimulation of the heart in the snail, Helix pomatia. J Comp Physiol. 1973;84:267–279. [Google Scholar]
- S-Rozsa K, Zhuravlev VL. Central regulation and coordination of activity of cardio-renal system and pneumostoma in the suboesophageal ganglia of Helix pomatia. Comp Biochem Physiol. 1981;69A:85–98. [Google Scholar]
- Sakharov DA. Recent studies of the properties of nudibranch nerve cells. Comp Biochem Physiol. 1966;18:957–959. doi: 10.1016/0010-406x(66)90226-x. [DOI] [PubMed] [Google Scholar]
- Santama N, Benjamin PR. Gene expression and function of FMRFamide-related neuropeptides in the snail Lymnaea. Microsc Res Tech. 2000;49:547–556. doi: 10.1002/1097-0029(20000615)49:6<547::AID-JEMT5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Santhanagopalan V, Yoshino TP. Monoamines and their metabolites in the freshwater snail Biomphalaria glabrata. Comp Biochem Physiol A. 2000;125:469–478. doi: 10.1016/s1095-6433(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Satterlie RA, Norekian TP. Serotonergic modulation of swimming speed in the pteropod mollusc Clione limacina III Cerebral neurons. J Exp Biol. 1995;198:917–930. doi: 10.1242/jeb.198.4.917. [DOI] [PubMed] [Google Scholar]
- Schilthuizen M, Davison A. The convoluted evolution of snail chirality. Naturwissenschaften. 2005;92:504–515. doi: 10.1007/s00114-05-0045-2. [DOI] [PubMed] [Google Scholar]
- Shabel’nikov SV, Bystrova OA, Martynova MG. Localization of substance P-and FMRFamide-like immunoreactivity in the atrium of the snail Achatina fulica. Tsitologiia. 2008;50:388–393. [PubMed] [Google Scholar]
- Shabelnikov SV, Bystrova OA, Ivanov VA, Margulis BA, Martynova M. Atrial granular cells of the snail Achatina fulica release proteins into hemolymph after stimulation of the heart nerve. J Exp Biol. 2009;212:3211–3220. doi: 10.1242/jeb.029108. [DOI] [PubMed] [Google Scholar]
- Sloley BD, Juorio AV, Durden DA. High-performance liquid chromatographic analysis of monoamines and some of their gamma-glutamyl conjugates produced by the brain and other tissues of Helix aspersa (Gastropoda) Cell Mol Neurobiol. 1990;10:175–192. doi: 10.1007/BF00734572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, Lodder H, van der Schors RC, van Elk R, Sorgedrager B, Brejc K, Sixma TK, Geraerts WP. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature. 2001;411:261–268. doi: 10.1038/35077000. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Thompson SH. Slow membrane currents in bursting pace-maker neurones of Tritonia. J Physiol. 1987;382:425–448. doi: 10.1113/jphysiol.1987.sp016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotkis AV, Kostyuk PG, Lukyanetz EA. Diversity of single potassium channels in isolated snail neurons. Neuroreport. 1998;9:1413–1417. doi: 10.1097/00001756-199805110-00030. [DOI] [PubMed] [Google Scholar]
- Souza MM, Stucchi-Zucchi A, Cassola AC, Scemes E. Electrophysiology of cardiac myocytes of Aplysia brasiliana. Comp Biochem Physiol A. 2002;133:161–168. doi: 10.1016/s1095-6433(02)00151-4. [DOI] [PubMed] [Google Scholar]
- Springer J, Ruth P, Beuerlein K, Palus S, Schipp R, Westermann B. Distribution and function of biogenic amines in the heart of Nautilus pompilius L. (Cephalopoda, Tetrabranchiata) J Mol Histol. 2005;36:345–353. doi: 10.1007/s10735-005-9006-5. [DOI] [PubMed] [Google Scholar]
- Springer J, Ruth P, Beuerlein K, Westermann B, Schipp R. Immunohistochemical localization of cardio-active neuropeptides in the heart of a living fossil, Nautilus pompilius L. (Cephalopoda, Tetrabranchiata) J Mol Histol. 2004;35:21–28. doi: 10.1023/b:hijo.0000020934.70110.0f. [DOI] [PubMed] [Google Scholar]
- Stenseng E, Braby CE, Somero GN. Evolutionary and acclimation-induced variation in the thermal limits of heart function in congeneric marine snails (genus Tegula): implications for vertical zonation. Biol Bull. 2005;208:138–144. doi: 10.2307/3593122. [DOI] [PubMed] [Google Scholar]
- Swarowsky A, Monteiro AF, Xavier LL, Zancan DM, Achaval M. Serotonergic immunoreactivity in the pedal ganglia of the pulmonate snail Megalobulimus abbreviatus after thermal stimulus: a semi-quantitative analysis. Comp Biochem Physiol. 2005;141:230–238. doi: 10.1016/j.cbpb.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Syed NI, Roger I, Ridgway RL, Bauce LG, Lukowiak K, Bulloch AG. Identification, characterisation and in vitro reconstruction of an interneuronal network of the snail Helisoma trivolvis. J Exp Biol. 1993;174:19–44. doi: 10.1242/jeb.174.1.19. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Yamamoto N. Pharmacological characteristics of the two largest neurons symmetrically situated in the suboesophageal ganglia of an African giant snail (Achatina fulica Férussac) Comp Biochem Physiol C. 1982;73:339–346. [Google Scholar]
- Takeuchi H, Yokoi I, Mori A, Ohmori S. Inhibitory effect of β-hydroxyglutamic acid on a molluscan giant neurone. Cell Mol Life Sci. 1975;31:1417–1418. doi: 10.1007/BF01923220. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Yokoi I, Mori A, Ohmori S. Effects of glutamic acid relatives on the electrical activity of an identified molluscan giant neurone (Achatina fulica Férussac) Brain Res. 1976;103:261–274. doi: 10.1016/0006-8993(76)90798-8. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Harris MB, Burk M, Smyth K, Lukowiak K, Remmers JE. Nitric oxide mediates metabolism as well as respiratory and cardiac responses to hypoxia in the snail Lymnaea stagnalis. J Exp Zool A Comp Exp Biol. 2003;295A:37–46. doi: 10.1002/jez.a.10174. [DOI] [PubMed] [Google Scholar]
- Tierney AJ. Structure and function of invertebrate 5-HT receptors: a review. Comp Biochem Physiol. 2001;128:791–804. doi: 10.1016/s1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- Vandorpe DH, Morris CE. Stretch activation of the Aplysia S-channel. J Membr Biol. 1992;127:205–214. doi: 10.1007/BF00231508. [DOI] [PubMed] [Google Scholar]
- Voronezhskaya EE, Hiripi L, Elekes K, Croll RP. Development of catechol-aminergic neurons in the pond snail, Lymnaea stagnalis: I. Embryonic development of dopamine-containing neurons and dopamine-dependent behaviors. J Comp Neurol. 1999;404:285–296. doi: 10.1002/(sici)1096-9861(19990215)404:3<285::aid-cne1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Walker RJ, Papaioannou S, Holden-Dye L. A review of FMRFamide- and RFamide-like peptides in metazoa. Invert Neurosci. 2009;9:111–153. doi: 10.1007/s10158-010-0097-7. [DOI] [PubMed] [Google Scholar]
- Weatherill D, Chase R. Modulation of heart activity during withdrawal reflexes in the snail Helix aspersa. J Comp Physiol A. 2005;191:355–362. doi: 10.1007/s00359-004-0590-8. [DOI] [PubMed] [Google Scholar]
- White AR, Curtis SA, Walker RJ. Evidence for a possible role for nitric oxide in the modulation of heart activity in Achatina fulica and Helix aspersa. Comp Biochem Physiol C. 2004;137:95–108. doi: 10.1016/j.cca.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Wiersma-Meems R, Syed NI. Synapse formation between identified Molluscan neurons: a model system approach. In: Dityatev A, El-Husseini A, editors. Molecular Mechanisms of Synaptogenesis. 2006. pp. 29–42. [Google Scholar]
- Wildering WC, Janse C, de Vlieger TA. The role of pacemaker properties and synaptic input in generation and modulation of spiking activity in a pair of electrically coupled peptidergic neurons. Brain Res. 1991;556:324–328. doi: 10.1016/0006-8993(91)90324-o. [DOI] [PubMed] [Google Scholar]
- Wilkens LA. Electrophysiological studies on the heart of the bivalve mollusc, Modiolus demissus I Ionic basis of the membrane potential. J Exp Biol. 1972a;56:273–291. doi: 10.1242/jeb.56.2.273. [DOI] [PubMed] [Google Scholar]
- Wilkens LA. Electrophysiological studies on the heart of the bivalve mollusc, Modiolus demissus II Ionic basis of the action potential. J Exp Biol. 1972b;56:293–310. doi: 10.1242/jeb.56.2.293. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Yeoman MS, Benjamin PR. Cyclic AMP is involved in cardioregulation by multiple neuropeptides encoded on the FMRFamide gene. J Exp Biol. 1999;202:2595–2607. doi: 10.1242/jeb.202.19.2595. [DOI] [PubMed] [Google Scholar]
- Wollesen T, Cummins SF, Degnan BM, Wanninger A. FMRFamide gene and peptide expression during central nervous system development of the cephalopod mollusk, Idiosepius notoides. Evol Dev. 2010;12:113–130. doi: 10.1111/j.1525-142X.2010.00398.x. [DOI] [PubMed] [Google Scholar]
- Yeoman MS, Benjamin PR. Two types of voltage-gated K+ currents in dissociated heart ventricular muscle cells of the snail Lymnaea stagnalis. J Neurophysiol. 1999;82:2415–2427. doi: 10.1152/jn.1999.82.5.2415. [DOI] [PubMed] [Google Scholar]
- Yeoman MS, Brezden BL, Benjamin PR. LVA and HVA Ca2+ currents in ventricular muscle cells of the Lymnaea heart. J Neurophysiol. 1999;82:2428–2440. doi: 10.1152/jn.1999.82.5.2428. [DOI] [PubMed] [Google Scholar]
- Zhuravlev V, Bugaj V, Kodirov S, Safonova T, Staruschenko A. Giant multimodal heart motoneurons of Achatina fulica: a new cardioregulatory input in pulmonates. Comp Biochem Physiol A. 2001;130:183–196. doi: 10.1016/s1095-6433(01)00384-1. [DOI] [PubMed] [Google Scholar]
- Zhuravlev V, Bychkov R, Kodirov S, Diakov A, Safonova T. Cardioexcitatory neurons in the snail Achatina fulica. J Brain Res. 1997;38:279–290. [PubMed] [Google Scholar]
- Zhuravlev V, Safonova T, Bugaj V, Kodirov S. Mechanisms of viscerocardial reflexes in land pulmonate mollusc, Achatina fulica F. Zool Poloniae. 2002;47:33–47. [Google Scholar]
- Zhuravlev VL. Mechanisms of neurohumoral control of the heart in gastropods. J Evol Biochem Physiol. 1999;35:85–103. [Google Scholar]
- Zhuravlev VL, Iniushin M, Safonova TA. The central neurons that inhibit the work of the heart in the edible snail. Nauchnye Doki Vyss Shkoly Biol Nauki. 1990:58–65. [PubMed] [Google Scholar]
- Zhuravlev VL, Kadyrov SA, Bychkov RE, Safonova TA, D’iakov AA. Heart-stimulating neurons in the subesophageal ganglia of the African snail Achatina fulica Ferussac. Fiziol Zh Im I M Sechenova. 1994;80:29–37. [PubMed] [Google Scholar]
- Zhuravlev VL, Safonova TA. Regulation of snail heart contractions by neurons of the visceral ganglion. Fiziol Zh SSSR Im I M Sechenova. 1984;70:425–429. [PubMed] [Google Scholar]
- Zhuravlev VL, Safonova TA, Bychkov RE. Neuronal control and coordination of the heartbeat in Helix pomatia. In: Sakharov D, Winlow W, editors. Simpler Nervous Systems, Studies in Neuroscience. Vol. 13. Manchester University Press; 1991. pp. 342–359. [Google Scholar]
- Zhurawlow WL, Safonowa TA, Ogorzalek A. Mikroiniekcja substancji do komórek. Wydawnictwo Uniwersytetu Wroclawskiego; 1997. p. 58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.