SYNOPSIS
Limited literature exists pertaining to rehabilitation of ice hockey players seeking to return-to-sport after anterior cruciate ligament reconstruction (ACLR). The purpose of this clinical commentary is to present a criterion-based, return-to-ice hockey progression for athletes after ACLR. First, we review pertinent literature and provide previously published guidelines on general rehabilitation after ACLR. Then, we present a four-phase, on-ice skating progression with objective criteria to initiate each phase. During the early on-ice phase, the athlete is reintroduced to specific demands, including graded exposure to forward, backward, and crossover skating. In the intermediate on-ice phase, the emphasis shifts to developing power and introducing anticipated changes of direction within a controlled environment. During the late on-ice phase, the focus progresses to developing anaerobic endurance and introducing unanticipated changes of direction, but still without other players or contact. Finally, once objective return-to-sport criteria are met, non-contact team drills, outnumbered and even-numbered drills, practices, scrimmages, and games are progressively reintroduced during the return-to-sport phase. Recommendations for off-ice strength and conditioning exercises complement the on-ice progression. Additionally, we apply the return-to-hockey progression framework to a case report of a female collegiate defensive ice hockey player who returned to sport successfully after ACLR. This criterion-based return-to-hockey progression may guide rehabilitation specialists managing athletes returning to ice hockey after ACLR.
Keywords: knee, return-to-sport, rehabilitation, ice skating, clinical practice guidelines
Introduction
Approximately 250,000 anterior cruciate ligament (ACL) injuries occur each year in the United States,21 and around 130,000 individuals undergo ACL reconstruction (ACLR).30,34 After ACLR, only 55% of athletes return to their pre-injury competitive level of sport.2 Second ACL injury rates of 20-30% are reported,4,41,42 with younger athletes41,46,47,58 at especially high risk for re-injury. While ACL injuries are most prevalent in jumping, cutting, and pivoting sports (e.g., basketball and soccer), they also occur in other sports.36 ACL injuries are the third most common knee injury in international ice hockey,53,54 and account for 0.7% of all injuries in women’s collegiate ice hockey.25 Recent data from professional men’s ice hockey players indicate career length and performance are adversely affected after ACL injury and reconstruction.48 As such, determining optimal rehabilitation programs for returning athletes to sport following ACL injury and reconstruction is essential.
Limited evidence exists to guide physical therapists and other rehabilitation specialists managing hockey players returning to sport after ACLR. Tyler and McHugh suggested the importance of utilizing neuromuscular rehabilitation, particularly perturbation training, to facilitate dynamic knee stability in an Olympic women’s ice hockey player returning to sport after ACLR.55 However, a comprehensive on-ice skating progression was not described. Pierce and colleagues developed an on-ice, six-phase skating progression for a goaltender following arthroscopic hip surgery for femoracetabular impingement.44 This progression, however, did not address the specific impairments associated with ACLR nor the demands of a skating (i.e., non-goalie) ice hockey player. Additional guidance for returning athletes to ice hockey after hip adductor strain56 and other injuries60,61 provide some framework and drills to facilitate the on-ice progression; however, the on-ice phases are limited and do not specifically address deficits associated with ACLR.
Return-to-sport guidelines12 and graded exposures3 exist following ACLR for other sports. Both sports-specific drills and strength and conditioning exercises are essential to comprehensive rehabilitation. Given the limited availability of ice time experienced by many non-elite athletes (e.g., high school or collegiate club athletes), designing specific on- and off-ice rehabilitation training programs may be critical for returning to sport.
The purpose of this clinical commentary, therefore, is to present and describe a criterion-based return-to-hockey progression and accompanying off-ice strength and conditioning program. Additionally, we apply the return-to-hockey progression within a brief case report of a female collegiate club ice hockey defender following ACLR. Our goal is to provide clinicians a framework—including objective clinical measures—for the successful management of ice hockey players returning to sport after ACLR.
Early Rehabilitation after ACLR
Following ACLR, impairment resolution and biological healing must occur prior to initiating a return-to-sport progression. The focus of early rehabilitation is to restore full range of motion (ROM), resolve knee effusion, normalize gait, and promote quadriceps activation and neuromuscular control.1,28 Clinical commentaries1,28 draw heavily from systematic reviews and randomized controlled trials to provide detailed, criterion-based rehabilitation programs. These programs promote early weight-bearing, restoration of ROM, resolution of effusion and gait impairments, use of resistance training exercises, and use of high-intensity electrical stimulation to treat quadriceps strength and activation deficits.29,49,50 Progression is based on both clinical milestones and healing time frames. Readers may consult these criterion-based rehabilitation programs1,28 and the complimentary MOON guidelines63 for general ACLR rehabilitation.
Monitoring knee effusion52 and joint soreness may guide appropriate progression of activity throughout rehabilitation. Performance and progression of activities in the presence of knee effusion or joint soreness likely have deleterious effects on long-term knee health. Accordingly, we permit initiation and progression of activity only when minimal or no effusion is present and only in the absence of joint soreness. The soreness rules, initially developed for weight-training modifications after upper extremity injury,15 have since been adapted for use after ACLR.1 While we are unaware of any empirical evidence supporting the efficacy of using knee effusion or joint soreness rules, we believe following these principles is prudent given the high risk of knee osteoarthritis after ACLR.32,33,40
In addition to quadriceps strengthening, several other lower extremity muscles merit consideration for the ice-skating athlete. The forward stride in hockey combines hip extension, abduction, and external rotation, knee extension, and plantar flexion.7,43 Quadriceps muscle torque at both 90°/sec and 210°/sec is positively correlated with ice skating speed in 11 elite women.18 Higher peak activation and prolonged activation of the hip adductor magnus (relative to other thigh muscles) occurred at faster forward skating velocities in 7 collegiate players, highlighting the importance of hip adductor strength and abductor-adductor muscle balance.9 Exercises to address these muscles include: leg press with theraband around knees, single leg squats, single leg bridge, cable column hip abduction, adduction, and flexion, knee extensions (90°– 60° knee ROM initially, progressing gradually to 90° – 0°), and heel raises.
Lateral slide board training has been shown to be a beneficial adjunct to improving quadriceps strength following ACLR.5 Patients who performed slide board training as part of a home exercise program had greater peak isometric knee extension torque after training and a higher maximum lateral step height after training and compared to controls. While hip muscle strength was not assessed following this training, the similarity of the lateral slide board to ice skating makes it a potentially useful component of rehabilitation for hockey players. As such, we developed a hockey-specific lateral slide board progression (TABLE 1) that considers the short interval nature of ice hockey. This progression may commence when an athlete is ≥8 weeks post-operative, has full knee ROM, trace or no effusion, and can complete the activity without knee joint soreness or increased effusion.
TABLE 1.
The lateral slide board progression may be initiated once the athlete is ≥8 weeks after surgery, has full knee range of motion, has minimal (i.e., trace) or no effusion, and can complete the activity without pain or increase in effusion. (Note: Week number is based on when the sliding board progression is initiated, not weeks after surgery.)
| Week | Effort | Work Interval (min:sec) | Rest Interval (min:sec) | Repetitions |
|---|---|---|---|---|
| 1 | 25 – 50% | :20 | :40 | 6–8 |
| 2 | 50 – 75% | :30 | 1:00 | 8–10 |
| 3 | 75 – 100% | :45 | 1:30 | 10–12 |
| 4 | 100% | 1:00 | 2:00 | 12–16 |
Neuromuscular training, including perturbation training, is also an important consideration for rehabilitating hockey players after ACL injury16,17 and reconstruction.55 Perturbation training is a type of neuromuscular training designed to improve knee stability and function during which the athlete stands on an unstable surface (e.g., rollerboard or rockerboard) and activates lower extremity muscles in response to surface perturbations applied by the physical therapist. Once an athlete adapts to these demands, the physical therapist progressively challenges the athlete by providing more rapid and random perturbations and incorporating sport-specific activities.17,59 Pre-operative perturbation training17 improves muscle activation patterns,10,26 restores inter-limb symmetry during gait,10,13,23 and maintains strength and function after ACLR,23 but there is limited evidence on post-operative perturbation training. Tyler and McHugh adapted perturbation training to hockey-specific positions (e.g., forward stride) and surfaces (e.g., slide board for lower resistance) to improve knee stability in a female Olympic ice hockey player after ACLR.55 This case suggests that post-operative perturbation training may be a useful component of rehabilitation after ACLR and can be modified for the demands of ice hockey.
When an athlete is ≥12 weeks after ACLR and has surgeon clearance, he or she may initiate a running progression1 when objective criteria are met: full and symmetrical knee ROM, trace or less effusion, and ≥80% quadriceps strength index (QI). Given limitations of using manual resistance to assess quadriceps strength,6,37 we recommend using an electromechanical dynamometer; however, using a one-repetition maximum for knee extension is an acceptable alternative.28 Running progression advancement is based on the soreness rules1,15 and minimal or no effusion. Slide board training may continue on days when the athlete is not running.
Return-to-Hockey Progression
The return-to-hockey on-ice progression is broken down into four broad phases: early, intermediate, late, and return-to-sport. We present the purposes, criteria, and recommendations plus sample drills to progress the athlete back to sport.
Early On-Ice Phase
The purpose of the early on-ice phase is to gradually expose the athlete to the specific demands of skating. We recommend that objective criteria be met prior to initiating this and each subsequent phase of the return-to-hockey progression (TABLE 2). We also recommend following the soreness rules1,15 throughout rehabilitation to monitor potential symptom exacerbation and modifying accordingly.
TABLE 2.
General guiding principles for on-ice hockey progression.
| Phase | Purpose | Criteria for Initiating Phase | Recommendations |
|---|---|---|---|
| Early On-Ice Phase | Gradually expose the athlete to the specific demands of skating | ≥16 weeks after (primary) ACLR and physician clearance Trace or less effusion Full knee ROM ≥80% QI Running progression1 level 4* without increase in knee effusion or soreness |
Follow the soreness rules1,15 Stick is optional No more often than every other day Each sub-phase should last ≥1 week and be performed ≥2x prior to progression to the next sub-phase Off-ice strengthening (TABLE 4) 2–3x/week: muscle strength and hypertrophy emphasis |
| Intermediate On-Ice Phase | Develop power and initiate anticipated changes of direction | ≥20 weeks after (primary) ACLR and physician clearance Completion of early phase without increase in effusion or soreness ≥85% QI ≥75% LSI on all 4 hop tests |
Stick recommended Once able to complete drills in this phase with proper form, incorporate puck handling, then passing and eventually shooting Complete off-ice strengthening and agility program (TABLE 4) |
| Late On-Ice Phase | Develop anaerobic endurance and initiate unanticipated changes of direction without contact | ≥6 months after (primary) ACLR and physician clearance Completion of intermediate phase for ≥2–4 weeks without an increase in effusion or soreness |
Complete off-ice strengthening, agility, and power exercise program (TABLE 4) Progress on-ice drills to incorporate unanticipated changes in direction, slap shots and one-timers Running interval training56 |
| Return-to-Sport Phase | Return to non-contact and contact drills, scrimmages, and games | ≥9 months after (primary) ACLR and physician clearance ≥90% QI ≥90% Hip strength LSI for hip extension, external rotation, abduction, and adduction ≥90% LSI on all 4 hop tests ≥90% KOS-ADLS ≥90% Global Rating |
Initiate noncontact drills with teammates first, progressing to outnumbered situations, even-numbered situations, scrimmages, and finally games |
Level 4 Running Progression
alternate jogging 700 meters and walking 100 meters for 3.2 kilometers (2 miles)
Abbreviations: ROM=range of motion (knee flexion and extension); QI=quadriceps strength index; ACLR=anterior cruciate ligament reconstruction; LSI=limb symmetry index (involved/uninvolved); KOS-ADLS=Knee Outcome Survey-Activities of Daily Living Scale27
The early on-ice phase is divided into four sub-phases (TABLE 3), each at least one week in duration. Increased intensity and more challenging maneuvers are progressively introduced. For example, an athlete first uses the inside skate edges while forward skating during sub-phase A, and further incorporates this skill using C-cuts during drill sessions in sub-phase B (supplemental video 1, on-ice drills). Crossovers in both directions are introduced in a drill setting (e.g., half circles progressing to full circles). We recommend gradually increasing the total ice time during each sub-phase according to player level, while monitoring knee effusion and soreness. Initially, skating should not occur more frequently than every other day.
TABLE 3.
Representative on-ice activities and drills during the early, intermediate, late, and return-to-sport phases of the on-ice skating progression (supplemental video 1, on-ice drills). The drills are not meant to be all-encompassing but rather provide a framework for appropriate progression.
| Early* | Intermediate | Late | Return-to-Sport |
|---|---|---|---|
|
Sub-phase A: Forward skating: 25% effort with hockey turns (i.e., no crossovers) E.g., 8–10 × 60–90” skating with 20–30” rest (alternating direction each interval) Drills: none Duration: ≤20 minutes total (rests included) Sub-phase B: Forward skating: 50% effort with hockey turns Backwards skating: 25% effort Drills: Single, double, and alternating C-cuts (forward and backward); Half circles with crossovers at 25–50% effort Duration: 30 minutes total Sub-phase C: Forward skating: 75% effort Backwards skating and crossovers: 50% effort Drills: Full circles at 50–75% effort; 3 stride starts; Figure 8 drill; Incorporate puck-handling Duration: 30–45 minutes total Sub-phase D: Forward skating: 75–90% effort Backwards skating and crossovers: 50–75% effort Drills: 1 leg endurance drill; Increase effort with figure 8 drill, circles, etc. Duration: 30–60 minutes total |
Transitions and form: Between blue-lines transitions—pivoting forward/backward Half ice→full ice transition drills Transition Circles—always facing one end of the ice Tight circles around cones/dots Use both inside/outside edges Forwards/backwards/pivoting Power: Power strides with pauses (forward and backward) Increase depth and power Resisted partner push drill Resisted partner pull drill Puck Skills: Incorporate puck-handling with drills as athlete progresses (e.g., circles, figure 8 drill, puck-handling around cones, etc.) Introduce submaximal wrist and snap shots (e.g., circles with shot at end) |
Anaerobic training with direction changes: Between blue-line sprinting “Suicide” sprints Agilities: Crossovers with stops Crossovers with sprints and stops Iron cross drill Reaction drills Quick starts and stops Puck Skills: Passing Drills with Coach Break out routes Passing while skating forward/backward Shuttle passing Neutral zone regroup routes Shooting Drills with Coach Give and Go’s—corner, half-wall, blue line Catch and shoot Slap shots One-timers Blue line push/drag |
Non-contact team drills (e.g., passing drills, dump-ins, shooting drills, etc.) Outnumbered situations: 3 vs. 1, 2 vs. 1, 3 vs. 2 Progress to 1 vs. 1 and corner drills Progress to full participation in team practice Progress to scrimmages Progress to games |
The early phase should be completed on empty ice.
Off-ice rehabilitation should include continued strengthening and running as well as initiation of agility drills 2–3x/week. Developing muscle strength and hypertrophy are the primary focus in this phase of the strengthening program to provide a base to develop power in subsequent phases. If strength deficits persist (i.e., the involved limb strength is <90% of the uninvolved limb), an athlete may perform an additional set with the involved limb. Off-ice agility drills (TABLE 4) may commence at this time, gradually progressing in intensity. Drop jumps with proper form and landing technique should be completed59 prior to initiating higher level jumping drills (e.g., tuck jumps, split-squat plyometric jumps). The athlete should continue to perform the running progression (on alternate days as the on-ice skating progressing) using the soreness rules to guide progress. Continued lateral slide board training may complement on-ice skating, particularly if ice-time is limited. An ACL brace may be worn during running, agility drills, and (early) on-ice skating. While limited and conflicting evidence exists regarding the efficacy of wearing an ACL brace,11,19,31,35,51 many physicians prescribe its use for at least 9-12 months post-ACLR,11,19 thus acclimating to wearing it is essential for some athletes. The ACL brace presents a unique challenge to the rehabilitating hockey player, who is unable to wear it and traditional padding (i.e., shin pads) simultaneously. Therefore, we recommend on-ice use of an ACL brace only when prescribed by the physician.
TABLE 4.
Off-ice strength and conditioning program (supplemental video 2, agility drills and skater jumps).
| Strength* | Agility (2-3x/week)† | Power‡ | Endurance & Speed:56 |
|---|---|---|---|
| Triple Threat physio ball series (FIGURE 1) Single leg squat or split-squat Stride-length lunges (FIGURE 2) Single leg Romanian Deadlifts or Nordic Hamstrings Single leg heel raises Hip adduction, abduction, and flexion at cable column Core stability: planks, side planks, planks with alternating leg lifts, single-leg bridges |
Agility Drills/Agility Ladder: high knees, butt kicks, side shuffles, carioca, etc. § Lateral line hops § Jump Rope: two feet, one foot, double jumps, forward/backward, side-to-side, etc. |
Skater jumps Tuck jumps Split-squat plyometric jumps |
Track Intervals: 200 meter run at 90–100% effort with 200 meter recovery jog (8–12 intervals) Sprint Intervals: 4–6 sets of 6×30 meter sprints at max effort starting every 30 seconds with 2 minutes rest intervals between each set |
During the early phase, develop strength (e.g., 3–4 sets of 8–12 reps). During the intermediate, late, and return-to-sport phases, emphasize power (e.g., 4–5 sets of 4–6 reps).
Gradually progress from 50% to 100% effort over about 10 sessions per soreness rules.
Start with 2–3 sets of 8–10 reps and progress to 3–4 sets of 10–15 reps with increased intensity; 30”–60” rest intervals.
Start with 15–20” intervals with 15” rest, progressing to 30” to 45” intervals with 10–15” rest.
Intermediate On-Ice Phase
The purpose of the intermediate phase is to improve power and introduce quick changes of direction within a controlled and anticipated environment. The partner push drill, partner pull drill, and power strides are examples of drills designed to improve skating power. Blue line transitions, pivoting drills, and other skating drills (e.g., figure 8 drill) introduce changes of direction in an anticipated manner without exposing the athlete to risk of contact. When athletes demonstrate appropriate technique, we recommend incorporating puck handling into drills, gradually re-introducing the dual-tasking demands of the sport. Puck handling may progress to passing with a coach or single teammate and introducing light shooting (i.e., wrist and snap shots). We recommend athletes perform intermediate phase drills for ≥2-4 weeks without increase in effusion or soreness prior to moving to the next phase.
Athletes may concurrently perform the off-ice strength and conditioning program targeting strength, agility, power, and endurance (TABLE 4). This program (2–3x/week) complements the on-ice progression and provides sample exercises addressing the specific demands of hockey, including core stability (e.g., planks, side-planks, and triple-threat physio-ball series [FIGURE 1a–f]), squats, stride-length lunges (FIGURE 2), agilities, and plyometrics. Additionally, once the athlete is able to run three miles without knee symptoms, running interval training56 may commence, particularly if on-ice availability is limited.
FIGURE 1.
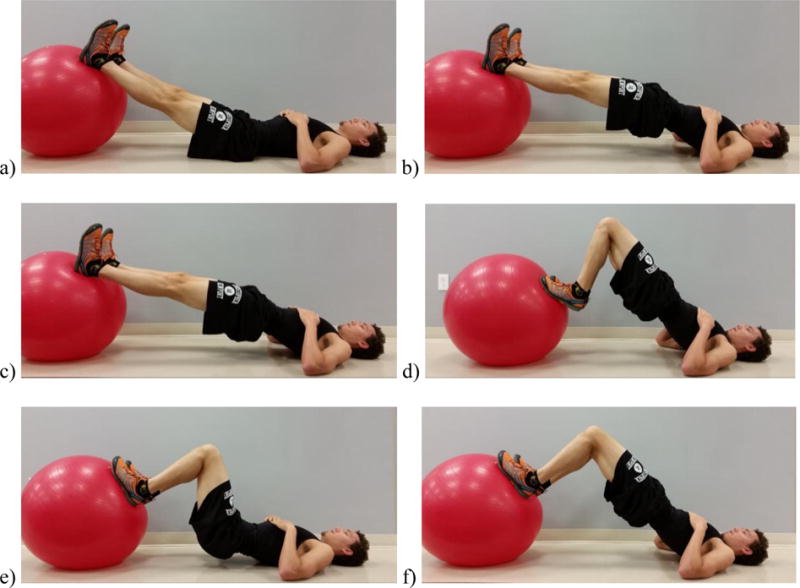
The Triple Threat physio ball series consists of straight leg hip extensions [(a) start and (b) end position], hamstring curls [(c) start and (d) end position], and flexed knee hip extensions [(e) start and (f) end position].
FIGURE 2.

Stride-length lunges combine the skating stride with a lunge position (note the wider stance and externally rotated left limb). A barbell, dumbbells, or medicine ball may be added for resistance.
Late On-Ice Phase
The purpose of the late on-ice phase is to improve anaerobic endurance and introduce unanticipated changes of direction without contact. During the late phase, progressively challenging drills such as blue line sprinting and “suicide” sprints are introduced to target anaerobic endurance. Unanticipated changes of direction—dictated by visual or audio stimuli—require the athlete to react and respond in a manner resembling competition. Passing and shooting drills progress in complexity to further simulate these unanticipated changes in direction. Shooting drills progress to incorporate slapshots and “one-timers” (i.e., shooting the puck without stopping it first). We recommend ≥2–4 weeks in this phase prior to initiating team drills or contact situations, which begin in the return-to-sport phase. However, if the athlete has progressed quickly through the phases up to this point, he or she should remain in the late phase and not progress to the return-to-sport phase until being ≥9 months post-operative. Our recommendation to delay return-to-sport clearance until ≥9 months after primary ACLR is based on evidence from Grindem and colleagues, who found a 51% increase in re-injury risk for each month an athlete returned to sport before nine months post-operatively.22 Recent evidence from a separate cohort of athletes after ACLR further supports delaying return-to-sport clearance until ≥9 months post-operatively even in the absence of clinical or biomechanical gait impairments.8 Further delay (i.e., ≥10-12 months post-op) is likely warranted for allografts or revision surgeries due to higher risk of graft rupture in allograft (versus autograft) ACLR38,57,62 and poorer outcomes and higher risk of re-injury in revision (versus primary) ACLR.20,62
Return-to-Sport Phase
Once objective criteria are met (TABLE 2), athletes may begin the return-to-sport phase, during which they gradually return to team drills, practices, and games. During this final phase, athletes first participate in noncontact team drills (e.g., passing drills, shooting drills, unopposed break-out drills). Outnumbered situations (i.e., three vs. one and two vs. one) are the first “live” drills for the athlete to initiate, with the rehabilitating player in either the majority or minority group, depending on position (i.e., offense vs. defense). While this may be counterintuitive to those unfamiliar with hockey, outnumbered drills have inherently less risk for contact, thus are initiated first. Next, graded exposure to contact continues through one-on-one and corner drills. An athlete next participates in full practice followed by scrimmages and finally games. Progression through the return-to-sport phase should take ≥4-6 weeks, although individual differences (e.g., longer time periods for allograft reconstruction28 or younger athletes42) must be considered. Successful completion of each step within this phase should occur before unrestricted return-to-sport clearance.
Case Description and Application
Informed consent was obtained from the patient, and her rights were protected. An 18-year-old female collegiate club ice hockey player sustained a left ACL injury after falling approximately 3 meters (10 feet) rock climbing. The patient was diagnosed via magnetic resonance imaging and physical exam with a full-thickness ACL rupture, medial and lateral meniscal tears, and a partial lateral collateral ligament sprain. The patient underwent ACLR (Bone Patellar-Tendon Bone), medial meniscal repair, and partial lateral meniscectomy 2 weeks after injury. The patient received post-operative physical therapy according to current concepts for ACLR rehabilitation.1,28 Timeframes were slightly prolonged in part due to the concomitant meniscal involvement and the patient’s initial toe-touch-weight-bearing status. Approximately 4.5 months post-ACLR, she met the criteria1 to begin running. The patient began the running progression and a strengthening program, including lateral slide board exercises, to perform on her own while away on summer break with periodic check-ins (1-2x/month). Progress was delayed due to limited time and intermittent gym access. Approximately 7 months post-operatively, the patient could run 2 miles symptom-free while maintaining full ROM and minimal effusion. At this time, she initiated 10 sessions (2x/week) of strengthening, agility, and neuromuscular training.
Following the first of these sessions and without increase in soreness or effusion, the patient began the early phase of the on-ice skating progression. She progressed through the early sub-phases while concurrently completing 10 strengthening, agility, and neuromuscular training sessions. During the early on-ice phase, the athlete skated on an open ice rink wearing her ACL brace; she did not carry her stick initially. She had no increase in knee joint soreness, pain, or effusion following her first time on the ice, although she did experience muscle soreness the next day. The athlete began doing crossovers during the second week of skating, which led to a mild increase in knee soreness the following day, thus leading to at least one day off and no increase in intensity or level as per the soreness rules.1,15 The athlete was performing three stride starts and skating for a total of 40 minutes by the end of her third week back on the ice, and added her stick and puck with drills by the fourth week, during which she logged 45 minutes on-ice per session. Throughout the early on-ice phase, she skated approximately 2x/week, which was limited by ice availability. She continued running, but did not progress beyond jogging two miles due to motivation.
Approximately 8.5 months post-ACLR (5-6 weeks after beginning to skate), we reassessed her lower extremity strength and functional symmetry and patient-report outcomes (TABLE 5). The athlete initiated on-ice transitions drills and power drills. Additionally, she began the off-ice strength program and additional hip strengthening exercises (given deficits in adduction and abduction), including hip adduction and abduction cable column exercises.
TABLE 5.
Case example of the objective measures obtained prior to initiating each phase; note that most, but not all, criteria were met as the formal hockey progression was developed alongside and after this athlete progressed through rehabilitation.
| Timeframe | Phase/Time | Selected Measures |
|---|---|---|
| 7 months post-op | Early |
|
| 8.5 months post-op | Intermediate |
|
| 9.5 months post-op | Late |
|
| 11 months post-op | Return-to-Sport Phase |
|
| 22 months post-op | Follow-up Outcome Measures |
|
Abbreviations: ROM=range of motion (knee flexion and extension); KOS-ADLS=Knee Outcome Survey—Activities of Daily Living Scale; LSI=limb symmetry index (involved/uninvolved); KOS-Sport=Knee Outcome Survey—Sports Activity Scale; KOOS=Knee Injury and Osteoarthritis Outcome Score
After approximately one month of progressing through the intermediate phase (9.5 months post-ACLR) and completing off-ice strength and conditioning, the athlete returned for follow-up testing. Noting improvements in inter-limb hip strength symmetry and progression through the intermediate phase without increase in effusion or soreness, we cleared her to initiate the late on-ice phase. Her on-ice sessions focused on addressing persistent challenges, including lateral crossovers, outside edge stopping, shooting and stabilizing shots on her involved limb, tight turns, fakes (i.e., “dekes”), and unanticipated movements. Drill emphases during this time included various reaction drills, stepping-up and crossing-over to alternate sides (e.g., Iron Cross drill), and dragging the puck into slapshots (given persistent challenge stabilizing with her involved limb during the shooting motion).
Approximately 11 months after ACLR, the athlete achieved all criteria to initiate the return-to-sport phase. At this time, she initiated non-contact team drills followed by out-numbered and then even-numbered situations. After one month, she was participating fully in practices. She played her first game approximately 13 months after ACLR. The athlete’s initial return to play was in a recreational league since it was the collegiate hockey off-season. During the following collegiate hockey season, she returned to her previous competitive level of sport. At 22 months post-operatively, she continued to play without re-injury and completed several outcome measures (TABLE 5). Her Knee Injury and Osteoarthritis Outcome Score45 subscale scores are similar to or higher than Delaware-Oslo ACL Cohort and Multicenter Orthopaedic Outcomes Network (MOON) scores among subjects two years after ACLR.14
Discussion
To our knowledge, this is the first article to describe a criterion-based on-ice skating progression for hockey players seeking to return-to-sport following ACLR. The return-to-hockey progression gradually exposes athletes to sport-specific demands using time- and criterion-based guidelines. Over the course of the four-phase progression, athletes acclimatize to skating, integrate anticipated and unanticipated changes of direction, and receive graded exposure to team drills, contact situations, practice, and ultimately games. The phases of the on-ice skating progression are accompanied by an off-ice strength and conditioning program to be performed with the latter phases of the on-ice progression. The return-to-hockey progression may provide clinicians with a criterion-based framework to guide ice hockey players back to sport following ACLR.
Our recommendations for return-to-sport criteria are based on previous literature utilizing a criterion-based approach,1,22,24,28,59 which has been validated recently.22 We also include the consideration of hip strength, which is essential to the ice hockey player. Notably, hip adductor strength is critical to the forward stride,9 and hip extension and external rotation are essential to propulsion.7,18 We suggest that an athlete have at least 90% hip strength symmetry based on other objective criterion-based approaches using 90% symmetry in other lower extremity muscle groups after ACLR.1,24,28 While hopping is not commonly performed during ice hockey, hop testing39 is used as an objective measure to evaluate functional limb symmetry after ACL injury or reconstruction, thus supporting its inclusion.
There are limitations to this clinical commentary, most notably that the return-to-hockey progression has not been rigorously tested. It is based on the best evidence from a variety of sources, but is still only expert opinion. Consequently, the implications of the return-to-hockey progression on future knee injury risk and long-term function, including the risk for knee osteoarthritis, are unknown. However, in the absence of higher-level evidence, we believe this return-to-hockey progression may be a useful tool to guide clinicians. The case study—which was the impetus in developing the progression—illustrates how an athlete may progress safely through the program and return to competitive sport successfully. While the case study athlete had co-morbidities and other factors that complicated her rehabilitation process, her 22-month outcomes compared favorably to recently published data from large cohorts.14 It is likely that athletes with isolated ACL injuries could initiate the early on-ice phase sooner (i.e., ≥16 weeks versus 7 months post-operatively) and complete the entire program earlier. Nevertheless, even for the uncomplicated athlete, we recommend not initiating the return-to-sport phase until at least 9 months post-operatively due to increased risk of re-injury for returning to sport before this time-frame, even in the absence of impairments.8,22 Finally, while the program was designed specifically for the athlete after ACLR, many aspects of the return-to-hockey progression could apply to athletes returning to hockey after other knee injuries (e.g., MCL sprains), just with different time-frames based on severity of injury and healing time.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants: R01-AR048212, P30-GM103333, and T32-HD00749. In addition, Amelia Arundale’s work was supported in part by a Promotion of Doctoral Studies (PODS) – Level I Scholarship from the Foundation for Physical Therapy. Thank you to Martha Callahan, the Delaware Rehabilitation Institute Research Core, and the University of Delaware Physical Therapy Clinic for their assistance with this project. Thank you to Hannah Beth Capin for her editorial assistance.
Funding: This work was supported by the National Institutes of Health: R01-AR048212, P30-GM103333, and T32-HD007490.
Footnotes
None of the authors have a conflict of interest or relevant financial relationship.
Statement of the IRB approval: Approval from the University of Delaware Institutional Review Board was obtained for the larger study in which the patient was enrolled. Informed consent was obtained from the patient regarding participation in this case report.
Public Trials Registry and registration number: n/a
Bibliography
- 1.Adams D, Logerstedt D, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42(7):601–614. doi: 10.2519/jospt.2012.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–1552. doi: 10.1136/bjsports-2013-093398. [DOI] [PubMed] [Google Scholar]
- 3.Arundale A, Silvers H, Logerstedt D, Rojas J, Snyder-Mackler L. An interval kicking progression for return to soccer following lower extremity injury. Int J Sports Phys Ther. 2015;10(1):114–127. [PMC free article] [PubMed] [Google Scholar]
- 4.Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthrosc - J Arthrosc Relat Surg. 2011;27(12):1697–1705. doi: 10.1016/j.arthro.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Blanpied P, Carroll R, Douglas T, Lyons M, Macalisang R, Pires L. Effectiveness of lateral slide exercise in an anterior cruciate ligament reconstruction rehabilitation home exercise program. J Orthop Sport Phys Ther. 2000;30(10):602–611. doi: 10.2519/jospt.2000.30.10.602. [DOI] [PubMed] [Google Scholar]
- 6.Bohannon RW. Manual muscle testing: Does it meet the standards of an adequate screening test? Clin Rehabil. 2005;19(6):662–667. doi: 10.1191/0269215505cr873oa. [DOI] [PubMed] [Google Scholar]
- 7.Buckeridge E, LeVangie MC, Stetter B, Nigg SR, Nigg BM. An on-ice measurement approach to analyse the biomechanics of ice hockey skating. PLoS One. 2015;10(5):1–16. doi: 10.1371/journal.pone.0127324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capin JJ, Khandha A, Zarzycki R, Manal K, Buchanan TS, Snyder-Mackler L. Gait mechanics and second ACL rupture: Implications for delaying return-to-sport. J Orthop Res. 2016 doi: 10.1002/jor.23476. Epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang R, Turcotte R, Pearsall D. Hip adductor muscle function in forward skating. Sport Biomech. 2009;8(3):212–222. doi: 10.1080/14763140903229534. [DOI] [PubMed] [Google Scholar]
- 10.Chmielewski TL, Hurd WJ, Rudolph KS, Axe MJ, Snyder-mackler L. Perturbation training improves knee kinematics and reduces muscle co-contraction after complete unilateral anterior cruciate ligament rupture. Phys Ther. 2005;85(8):740–754. [PubMed] [Google Scholar]
- 11.Decoster L, Vailas J. Functional anterior cruciate ligament bracing: a survey of current brace prescription patterns. Orthopedics. 2003;26(7):701–706. doi: 10.3928/0147-7447-20030701-14. [DOI] [PubMed] [Google Scholar]
- 12.Della Villa S, Boldrini L, Ricci M, et al. Clinical Outcomes and Return-to-Sports Participation of 50 Soccer Players After Anterior Cruciate Ligament Reconstruction Through a Sport-Specific Rehabilitation Protocol. Sports Health. 2012;4(1):17–24. doi: 10.1177/1941738111417564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Stasi SL, Snyder-Mackler L. The effects of neuromuscular training on the gait patterns of ACL-deficient men and women. Clin Biomech. 2012;27(4):360–365. doi: 10.1016/j.clinbiomech.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Failla MJ, Logerstedt DS, Grindem H, et al. Does Extended Preoperative Rehabilitation Influence Outcomes 2 Years After ACL Reconstruction?: A Comparative Effectiveness Study Between the MOON and Delaware-Oslo ACL Cohorts. Am J Sports Med. 2016;44(10):2608–2614. doi: 10.1177/0363546516652594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fees M, Decker T, Snyder-Mackler L, Axe MJ. Upper extremity weight-training modifications for the injured athlete: A clinical perspective. Am J Sports Med. 1998;26(5):732–742. doi: 10.1097/BLO.0b013e3180e79c6a. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald GK, Axe MJ, Snyder-Mackler L. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):76–82. doi: 10.1007/s001670050190. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald GK, Axe MJ, Snyder-Mackler L. The efficacy of perturbation training in nonoperative anterior cruciate ligament rehabilitation programs for physical active individuals. Phys Ther. 2000;80(2):128–140. doi: 10.2519/jospt.2000.30.4.194. [DOI] [PubMed] [Google Scholar]
- 18.Gilenstam KM, Thorsen K, Henriksson-Larsen KB. Physiological correlates of skating performance in women’s and men’s ice hockey. J Strength Cond Res. 2011;25(8):2133–2142. doi: 10.1519/JSC.0b013e3181ecd072. [DOI] [PubMed] [Google Scholar]
- 19.Goodstadt NM, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Functional testing to determine readiness to discontinue brace use, one year after ACL reconstruction. Int J Sports Phys Ther. 2013;8(2):91–96. [PMC free article] [PubMed] [Google Scholar]
- 20.Grassi A, Ardern CL, Muccioli G, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716–724. doi: 10.1136/bjsports-2015-094948. [DOI] [PubMed] [Google Scholar]
- 21.Griffin LY, Albohm MJ, Arendt Ea, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 22.Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804–808. doi: 10.1136/bjsports-2016-096031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartigan E, Axe MJ, Snyder-Mackler L. Perturbation training prior to ACL reconstruction improves gait asymmetries in non-copers. J Orthop Res. 2009;27(6):724–729. doi: 10.1002/jor.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartigan EH, Axe MJ, Snyder-Mackler L. Time Line for Noncopers to Pass Return-to-Sports Criteria After Anterior Cruciate Ligament Reconstruction. J Orthop Sport Phys Ther. 2010;40(3):141–154. doi: 10.2519/jospt.2010.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: Summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 26.Hurd WJ, Chmielewski TL, Snyder-Mackler L. Perturbation-enhanced neuromuscular training alters muscle activity in female athletes. Knee Surgery, Sport Traumatol Arthrosc. 2006;14(1):60–69. doi: 10.1007/s00167-005-0624-y. [DOI] [PubMed] [Google Scholar]
- 27.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Jt Surg Am. 1998;80(8):1132–1145. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Joreitz R, Lynch A, Rabuck S, Lynch B, Davin S, Irrgang J. Patient-specific and surgery-specific factors that affect return to sport after ACL reconstruction. Int J Sports Phys Ther. 2016;11(2):264–278. [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KM, Croy T, Hertel J, Saliba S. Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: a systematic review. J Orthop Sport Phys Ther. 2010;40(7):383–391. doi: 10.2519/jospt.2010.3184. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Jt Surg. 2011;93(11):994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 31.Kruse LM, Gray B, Wright RW. Rehabilitation after anterior cruciate ligament reconstruction: a systematic review. J Bone Jt Surg. 2012;94(19):1737–1748. doi: 10.2106/JBJS.K.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lohmander LS, Englund PM, Dahl LL, Roos EM. The Long-term Consequence of Anterior Cruciate Ligament and Meniscus Injuries: Osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 33.Lohmander LS, Östenberg a, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 34.Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363–2370. doi: 10.1177/0363546514542796. [DOI] [PubMed] [Google Scholar]
- 35.McDevitt E, Taylor D, Miller M, et al. Functional bracing after anterior cruciate ligament reconstruction: a prospective, randomized, multicenter study. Am J Sports Med. 2004;32(8):1887–1892. doi: 10.1177/0363546504265998. [DOI] [PubMed] [Google Scholar]
- 36.Mountcastle SB, Posner M, Kragh JF, Taylor DC. Gender differences in anterior cruciate ligament injury vary with activity: epidemiology of anterior cruciate ligament injuries in a young, athletic population. Am J Sports Med. 2007;35(10):1635–1642. doi: 10.1177/0363546507302917. [DOI] [PubMed] [Google Scholar]
- 37.Mulroy SJ, Lassen KD, Chambers SH, Perry J. The ability of male and female clinicians to effectively test knee extension strength using manual muscle testing. J Orthop Sports Phys Ther. 1997;26(4):192–199. doi: 10.2519/jospt.1997.26.4.192. [DOI] [PubMed] [Google Scholar]
- 38.Nelson IR, Chen J, Love R, Davis BR, Maletis GB, Funahashi TT. A comparison of revision and rerupture rates of ACL reconstruction between autografts and allografts in the skeletally immature. Knee Surgery, Sport Traumatol Arthrosc. 2016;24(3):773–779. doi: 10.1007/s00167-016-4020-6. [DOI] [PubMed] [Google Scholar]
- 39.Noyes F, Barber S, Mangine R. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19(5):513–518. doi: 10.1177/036354659101900518. [DOI] [PubMed] [Google Scholar]
- 40.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sport Med. 2009;37(7):1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 41.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22(2):116–121. doi: 10.1097/JSM.0b013e318246ef9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42(7):1567–1573. doi: 10.1177/0363546514530088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearsall DJ, Turcotte RA, Murphy SD. Biomechanics of ice hockey. In: Garrett WEJ, Kirkendall DT, editors. Exercise and Sport Science. Philadelphia, PA: Lippincott, Williams & Wilkins; 2000. pp. 675–692. [Google Scholar]
- 44.Pierce CM, LaPrade RF, Wahoff M, O’Brien L, Philippon MJ. Ice Hockey Goaltender Rehabilitation, Including On-Ice Progression, After Arthroscopic Hip Surgery for Femoroacetabular Impingement. J Orthop Sport Phys Ther. 2013;43(3):129–141. doi: 10.2519/jospt.2013.4430. [DOI] [PubMed] [Google Scholar]
- 45.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and osteoarthritis outcome score (KOOS) - Development of a self-administered outcome measure. J Orthop Sport Phys Ther. 1998;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 46.Shea KG, Pfeiffer R, Wang JH, Curtin M, Apel PJ. Anterior cruciate ligament injury in pediatric and adolescent soccer players: an analysis of insurance data. J Pediatr Orthop. 2004;24(6):623–628. doi: 10.1097/01241398-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 48.Sikka R, Kurtenbach C, Steubs JT, Boyd JL, Nelson BJ. Anterior Cruciate Ligament Injuries in Professional Hockey Players. Am J Sports Med. 2016;44(2):378–383. doi: 10.1177/0363546515616802. [DOI] [PubMed] [Google Scholar]
- 49.Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Jt Surg. 1995;77(8):1166–1173. doi: 10.2106/00004623-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Snyder-Mackler L, Delitto A, Stralka SW, Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74(10):901–907. doi: 10.1093/ptj/74.10.901. [DOI] [PubMed] [Google Scholar]
- 51.Sterett WI. Effect of functional bracing on knee injury in skiers with anterior cruciate ligament reconstruction: a prospective cohort study. Am J Sports Med. 2006;34(10):1581–1585. doi: 10.1177/0363546506289883. [DOI] [PubMed] [Google Scholar]
- 52.Sturgill LP, Snyder-Mackler L, Manal TJ, Axe MJ. Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sport Phys Ther. 2009;39(12):845–849. doi: 10.2519/jospt.2009.3143. [DOI] [PubMed] [Google Scholar]
- 53.Tuominen M, Stuart M, Aubry M, Kannus P, Takola K, Parkkari J. Injuries in women’s international ice hockey: an 8-year study of the World Championship tournaments and Olympic Winter Games. Br J Sport Med. 2016;50(22):1406–1412. doi: 10.1136/bjsports-2015-094647. [DOI] [PubMed] [Google Scholar]
- 54.Tuominen M, Stuart MJ, Aubry M, Kannus P, Parkkari J. Injuries in men’s international ice hockey: a 7-year study of the International Ice Hockey Federation Adult World Championship Tournaments and Olympic Winter Games. Br J Sports Med. 2015;49(1):30–36. doi: 10.1136/bjsports-2014-093688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tyler TF, McHugh MF. Neuromuscular rehabilitation of a female Olympic ice hockey player following anterior cruciate ligament reconstruction. J Orthop Sport Phys Ther. 2001;31(10):577–587. doi: 10.2519/jospt.2001.31.10.577. [DOI] [PubMed] [Google Scholar]
- 56.Tyler TF, Nicholas SJ. Beyond basic rehabilitation: return to hockey after adductor strain. In: Reider B, Davies G, Provencher M, editors. Orthopaedic Rehabilitation of the Athlete: Getting Back in the Game, 1st Edition. 1. Philadelphia, PA: Elsevier Saunders; 2015. pp. 785–789. [Google Scholar]
- 57.Wasserstein D, Sheth U, Cabrera A, Spindler KP. A systematic review of failed anterior cruciate ligament reconstruction with autograft compared with allograft in young patients. Sports Health. 2015;7(3):207–216. doi: 10.1177/1941738115579030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):641–647. doi: 10.1177/0363546513517540. [DOI] [PubMed] [Google Scholar]
- 59.White K, Di Stasi SL, Smith AH, Snyder-Mackler L. Anterior cruciate ligament-specialized post-operative return-to-sports (ACL-SPORTS) training: a randomized control trial. BMC Musculoskelet Disord. 2013;14(108) doi: 10.1186/1471-2474-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolfinger CR, Davenport TE. Physical therapy management of ice hockey athletes: From the rink to the clinic and back. Int J Sports Phys Ther. 2016;11(3):482–495. [PMC free article] [PubMed] [Google Scholar]
- 61.Woodward JS, Parker A, MacDonald RM. Non-surgical treatment of a professional hockey player with the signs and symptoms of sports hernia: a case report. Int J Sports Phys Ther. 2012;7(1):85–100. [PMC free article] [PubMed] [Google Scholar]
- 62.Wright R, Huston L, Haas A, et al. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301–2310. doi: 10.1177/0363546514549005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright RW, Haas AK, Anderson J, et al. Anterior cruciate ligament reconstruction rehabilitation: MOON guidelines. Sports Health. 2015;7(3):239–243. doi: 10.1177/1941738113517855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


