Abstract
The recent demonstration of modest single-agent activity of programmed death-ligand 1 (PD-L1) and programmed death receptor-1 (PD-1) antibodies in patients with breast cancer has generated hope that breast cancer can be made amenable to immunotherapy. Depending on the subtype of breast cancer, it is now clear in both primary and metastatic disease that the extent of tumor-infiltrating T cells is not only prognostic for survival but predictive of response to non-immune, standard therapies. Despite these findings, immune cytolytic activity in spontaneous breast tumors, the burden of nonsynonymous tumor mutations, and the predicted load of neo-epitopes – factors linked to response to checkpoint blockade in other malignancies – are all relatively modest in breast cancer, compared to melanoma or lung cancer. Thus, in breast cancer, combinations of immune agents with non-redundant mechanisms of action are the high-priority strategies. For most breast cancers that exhibit relatively modest T cell infiltration, major challenges include immune suppression in the tumor microenvironment as well as failed or suboptimal T cell priming. Agents that trigger de novo T cell responses may be critical for successful development of cancer immunotherapy and immune prevention in breast cancer. Success may also require reaching beyond nonsynonymous mutations as the T cell epitopes to target, especially as numerous unmutated proteins were validated as breast cancer associated antigens in the pre-checkpoint era. A deeper understanding of the immunobiology of breast cancer will be critical for immunotherapy to become broadly relevant in this disease.
Introduction
Stunning successes of cancer immunotherapy in melanoma, lung cancer, acute lymphoblastic leukemia and other cancers reflect the power of T cell immunity as an extrinsic tumor suppressor. After a lagging start, efforts to develop effective immune therapy for patients with breast cancer are beginning to bear fruit. Most promising among these strategies are PD-L1 or PD-1 monoclonal antibodies (mAb) for the treatment of patients with advanced breast cancer with triple negative disease. In these studies, the objective response rates (ORR) are in the 12%–19% range (1–3). On the other hand, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockade appears minimally active (4), and combinations of PD-1 and CTLA-4 mAb are still being studied. Chimeric antigen receptor-engineered T cells as adoptive therapy for breast cancer are at the earliest stages of development, and randomized studies of therapeutic vaccination in patients with local or advanced breast cancer as well as vaccination in the adjuvant setting have been negative (5, 6). Although the number of clinical trials testing immune therapy in breast cancer is rapidly increasing, there have been no FDA approvals of experimental immunotherapies as there has been in other tumor types (Figure 1).
Figure 1.
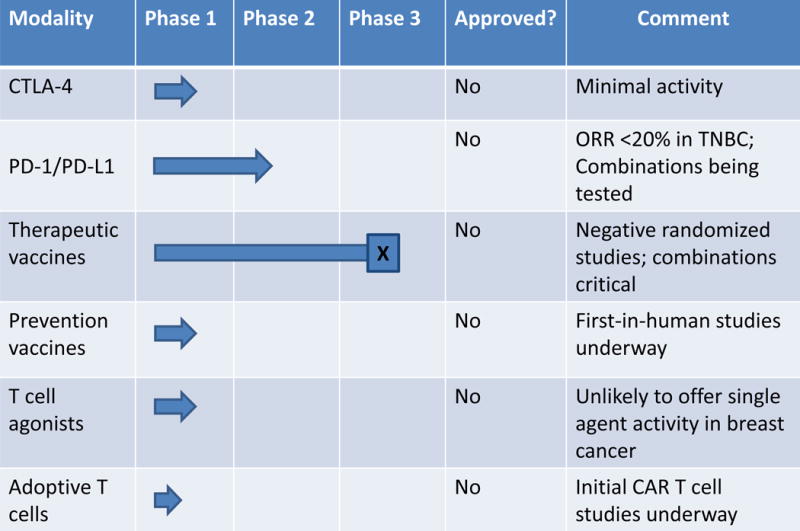
Summary of status of breast cancer immunotherapy investigational trials, by phase. (Anti-tumor antibodies such as trastuzumab are not listed.)
Advancing immune therapy to a more central and applicable role in breast cancer remains a high priority, particularly given the potential for high response durability that can be achieved, sometimes uniquely so, with immune therapy of advanced cancer. Successfully developing immunotherapy in breast cancer will be best achieved as part of a collaborative effort with investigators addressing other critical areas of this disease (7–10). A chief challenge for advancing immunotherapy for patients with advanced breast cancer is to move beyond PD-1/PD-L1 blockade alone and empiric combination of other agents with PD-1/PD-L1. A deeper understanding of the immunobiology of breast cancer is considered a critical step toward eventual clinical success of these therapies. In this article, we address what is known and our limitations of understanding of breast cancer immunology and immunotherapy.
Immunobiology of human breast cancer
Careful and comprehensive studies of human breast cancer tissue have provided a clear picture of the extent, variability, and clinical importance of leukocytes that infiltrate breast tumors, as extensively reviewed elsewhere (11–14). Tumor infiltrating lymphocytes (TIL) are found most extensively in triple negative breast cancer (TNBC) compared to other tumor subtypes. The extent of CD8+ T cells in primary TNBC – when rigorously demonstrated to be infiltrating into tumor nests, rather than only gathering in outer stroma – independently predicts survival in multivariate analyses (11, 15–17). Infiltration of follicular helper CD4+ T cells, perhaps an indication of organized tertiary lymphoid structures (TLS) in the tumor, is also predictive of better survival in TNBC (18, 19). Presence of TLS themselves predict better survival in TNBC (19). Conversely, the extent of immunosuppressive regulatory T cells (Tregs) predicts worse survival in TNBC (20). TILs in HER2+ breast cancer can also be robust and their extent similarly predicts overall survival (21). Moreover, the extent of TILs predicts improved response to certain therapies, including chemotherapy, particularly in TNBC, and trastuzumab in HER2+ disease. The association also holds true across multiple clinical scenarios including the pathological complete response rate to chemotherapy in the neoadjuvant setting and overall response in the metastatic setting (22–25). These predictive markers hold true for both anthracycline chemotherapy and trastuzumab (11). The predictive value of TILs in estrogen receptor (ER)-positive breast tumors is less clear (26). Recently, in patients with advanced HER2+ breast cancer treated with docetaxel, trastuzumab, and pertuzumab or placebo, increased TIL infiltration at baseline was associated with improved overall survival (comparing patients with >20% TILs vs. ≤20% TILs, median overall survival was 56.6 months vs. 44.5 months; HR 0.76, 95% CI 0.60-0.96, log rank p=0.021), suggesting that anti-tumor immunity is also found in advanced and metastatic disease (21, 27). A consensus, standardized method for evaluating TILs in breast cancer is available (28). Pathological analysis is able to reveal not just numbers of TILs but also the pattern of infiltration including TLS (19).
Hematoxylin/eosin stains and immunohistochemistry have been the primary tools for characterizing TILs in breast cancer, RNAseq was also used to examine immune signatures in TNBC and HER2+ disease in CALGB 40603, CALGB 40601, and NeoALTTO trials; in all cases, activated signatures were significantly and independently associated with pathological CR. Recently, RNAseq data from The Cancer Genome Atlas (TCGA) were used to quantitively examine immune cytolytic activity in 18 histologies of human cancer, including breast cancer, based on a two-gene signature that was proposed to represent T cell function (perforin 1 and granzyme A) (29). Cytolytic activity in breast cancer was reported to be modest, compared to other tumors such as kidney or lung cancer. Three driver mutations in breast cancer (beta-2 microglobulin, CUL4B, and ARID2) were significantly associated with cytolytic activity.
The burden of nonsynonymous DNA mutations in breast cancer is relatively low compared to other tumor types, especially carcinogen-related tumors such as sun-exposure associated melanoma and smoking-related lung and bladder carcinoma (30). In other tumors, the extent of nonsynonymous mutations correlates with response to checkpoint therapy (31, 32). In breast cancer, there is heterogeneity in this metric among subtypes and large variation from patient to patient. In a study of more than 700 tumors, TNBC tumors were found to exhibit more nonsynonymous mutations than non-TNBC (30).
The nonsynonymous mutational burden of cancer is important because it relates to a major molecular basis of anti-tumor T cell reactivity (33). Somatic gene mutations resulting in single changes in amino acids can ultimately lead to the expression of mutated peptides in the groove of MHC expressed on the surface of tumors cells, where these complexes can react with T cell receptors on infiltrating T cells. Because these “neo-epitopes” likely draw upon a T cell receptor (TCR) repertoire that has avoided classically defined central tolerance, a robust T cell response can manifest (33). Such T cell reactivity, which has been well-characterized in patients, can be specific for either driver or passenger mutations. Importantly, the mechanisms of actions of both CTLA-4 and PD-1/PD-L1 blocking mAb have been linked to activation or reinvigoration of neo-epitope specific T cells in melanoma and lung cancer (31, 32). Exceptions are well-described, however. For example, nivolumab has been approved for clear cell renal cell carcinoma, yet this is a cancer that exhibits modest levels of nonsynonymous mutations, at least in the primary tumor (34).
The load of classically defined neo-epitopes with high predicted affinity for major histocompatibility complex (MHC) in a given tumor is best predicted by the burden of nonsynonymous mutations, and as such, most breast cancers likely exhibit a relativity low number of predicted neo-epitopes (30). Still, it has been predicted that just for one class I MHC allele (HLA-A2), there is likely one neo-epitope for every 10 nonsynonymous mutations in breast cancer (35). Beyond the link to checkpoint therapy, neo-epitopes offer a therapeutic target in cancer for a variety of antigen-specific approaches including adoptive T cell therapy (36) and vaccines (37, 38).
PD-1/PD-L1 in human breast cancer
Expression of PD-L1 on breast tumors cells as well as on associated stromal cells has also been extensively studied by analyzing pathological specimens (2). This is important because the binding of PD-L1 to PD-1 on the surface of infiltrating T cells can lead to T cell inactivation or exhaustion. Overall, tumor cell expression of PD-L1 in breast cancer is modest and variable. For TNBC and ER+ HER2-negative tumors (2, 3, 27), only 20% of samples have been shown to exhibit more than 1% of tumor cells expressing PD-L1. In contrast, leukocytes in the stroma of TNBC, especially myeloid cells, are typically PD-L1+ (27). DCIS has very low levels of PD-L1 (39). These observations are important because PD-L1 expression in some tumors predicts, to some extent, improved clinical response to PD-1 or PD-L1 checkpoint mAb such as pembrolizumab and nivolumab (each anti-PD-1) or atezolizumab (anti-PD-L1). Pembrolizumab has an indication in the first-line therapy of non-small cell lung cancer expressing PD-L1 in more than 50% of tumor cells, based on a survival advantage of pembrolizumab compared to chemotherapy (40). On the other hand, a fraction of patients with PD-L1-negative tumors across a variety of histologies have also been shown to respond to these agents.
Clinical results of PD-1/PD-L1 checkpoint blockade in metastatic breast cancer are now emerging. In a small study of 27 evaluable patients with advanced TNBC, for which tumors had at least 1% PD-L1 expression, the ORR following pembrolizumab was 18.5% (1). Toxicities observed in this study were similar to those well-described for PD-1 blockade in other settings (41). Similarly, in 21 patients with metastatic TNBC, treatment with atezolizumab yielded a preliminary assessment of the ORR as 19% (2). Further investigation is required to understand if these patients with a clinical response were enriched for TNBC patients with detectable levels of PD-L1 in the tumor (2). In an unselected cohort of 168 breast cancer patients, the anti-PD-L1 antibody avelumab showed an ORR of only 4.8%; most of the responders were those with TNBC in this cohort (2, 42). In this study, known as JAVELIN, patients with PD-L1 expression on immune cells in the tumor showed greater overall response than patients with PD-L1-negative immune cells (4 of 12, or 33.3% vs. 3 of 124, or 2.4%); in TNBC patients, PD-L1 expression also appeared to be associated with response (4 of 9, or 44%, for PD-L1 positive patients vs. 1 of 39, or 2.0%, for PD-L1 negative patients) (42). Clinical trials combining PD-1/PD-L1 antibodies with other therapies such as trastuzumab or anti-estrogens are underway, including in the neoadjuvant and adjuvant settings.
CTLA-4 in breast cancer
We tested the CTLA-4 blocking antibody tremelimumab in combination with exemestane in 26 patients with metastatic ER+ breast cancer, but found only limited signs of clinical activity (4). Best overall response was stable disease for at least 12 weeks in 42% of patients. Interestingly, treatment was associated in most patients with increased peripheral CD4+ and CD8+ T cells expressing ICOS and a marked increase in the ratio of ICOS+ T cells to FoxP3+ Tregs – findings that likely signal immune activation secondary to CTLA-4. In a recent pilot study of 19 pre-surgical patients with breast cancer, the combination of cryoablation with single-dose ipilimumab (anti-CTLA-4) demonstrated favorable intratumoral and systemic immune effects (43).
Therapeutic vaccines in breast cancer
Extensive efforts have explored a role for therapeutic vaccination in breast cancer. Tumor associated antigens targeted in these clinical trials have included HER2, sialyl-TN-KLH, MUC1, telomerase, survivin, mammoglobin, mutant p53, and others (12, 44). Multiple formulations have been used including peptides, proteins, and recombinant or genetically engineered constructs. In some strategies, such as GVAX cell-based vaccines (irradiated autologous or allogeneic tumor cells engineered to express GM-CSF) (45), the antigen is not defined per se, but tumor antigen-specific T cell responses can be triggered. In some cases, an immunodominant antigen, such as mesothelin, has been discerned (46). For nearly every vaccine formulation, the generation of T cell immunity can be readily demonstrated, but positive clinical effects have been less clear. For example, in our studies in metastatic breast cancer using MHC-binding peptides derived from telomerase, we observed robust generation of functional effector CD8 T cells following vaccination, but the best response in any patient was stable disease (47). Presentation of telomerase-derived peptides on the surface of tumor cells has been demonstrated (48). However, in this small cohort of patients, those who demonstrated high-level immune response to telomerase peptides exhibited statistically significant improvement in overall survival compared to patients who failed to respond immunologically to vaccination (47). Nevertheless, two vaccination strategies (HER2 peptides or sialyl-Tn-KLH) that showed promise in early phase testing disappointingly failed to meet the primary clinical endpoints in randomized studies (5, 6), which dampened enthusiasm for vaccine-only strategies in breast cancer. Notably, checkpoint blocking antibodies were not used in these randomized vaccine trials.
The overall upshot of these findings is: although tolerance can be broken to breast cancer associated antigens, effector cells generated are either poorly potent or are subsequently suppressed by peripheral tolerance mechanisms in the tumor microenvironment. This hypothesis has prompted efforts to add other agents that block specific pathways of immune suppression to generate more effective T cell responses. In breast cancer, the negative role of intratumoral CD25+ FoxP3+ CD4+ Tregs is well-appreciated, and vaccination efforts in combination with strategies to deplete Tregs have been tested. Two examples include (i) adding low dose cyclophosphamide and other immunomodulatory agents to GVAX (49, 50), or (ii) adding CD25 blocking mAb daclizumab to telomerase and survivin peptide vaccination (51). The latter approach was shown to reduce systemic CD25+ CD4+ FoxP3+ and total CD4+ FoxP3+ T cells for up to 2 months following a single infusion of daclizumab. Only recently has the opportunity become available to combine breast cancer vaccination with PD-1 or PD-L1 antibodies, a high-priority potential strategy. Likewise, second generation vaccine approaches are molecularly defined and likely to be more potent. Thus, many strategies to simultaneously vaccinate and therapeutically block immune suppressive pathways are being developed. Recent preclinical data from breast cancer models point to a role for abrogating myeloid inflammation, such as by blockade of CSF1R or PI3K gamma (52, 53) in order to activate a T cell response.
CAR T cell and other therapies in breast cancer
In lymphoid leukemia and other hematological malignancies, impressive clinical results have been obtained with adoptive cell therapy using autologous T cells engineered to express chimeric antigen receptors (CAR) specific for CD19 (54). Progress of CAR T cells for breast cancer has been limited. Adoptive transfer of autologous HER2 specific polyclonal T cells generated from PBMC after vaccine-priming has been shown to be safe and with regressions observed in a few patients with advanced HER2+ cancers (55). However, respiratory distress and fatal cytokine storm acutely following therapy with ERBB2-specific CAR T cells in one patient with metastatic colon cancer (56) has underscored the importance of developing new CAR targets with scrutiny of “off-tumor, on-target” toxicity of organs expressing the CAR target. For example, based on wide expression of c-Met in TNBC, investigators at the Abramson Cancer Center have developed c-Met CAR T cells for breast cancer in which initial clinical trials involve the use of mRNA (for transient CAR expression), rather than integrating lentivirus, to accomplish gene transfer; route of administration is intratumoral rather than intravenous as another potential safety feature [NCT01837602].
What are we missing?
The way forward for cancer immunotherapy in breast cancer is tantalizing but challenging. Although there remain many critical areas of investigation, here are four areas we wish to highlight:
Turning cold tumors hot
As noted above, most breast cancers exhibit relatively low T cell infiltration, a feature that has been termed being immunologically “cold” (57); accordingly, single-agent checkpoint therapy is ineffective for the majority of patients with breast cancer. In contrast, immunologically “hot” tumors have marked tumor-infiltrating T cells that may be reinvigorated with single agent or dual checkpoint blockade (Figure 2). One hypothesis to explain cold tumors is that a network of immunosuppression develops in the tumor microenvironment as a reaction to a nascent anti-tumor T cell response and actively excludes further T cell infiltration. A recent study of TCGA data indicates that the lack of spontaneous immune infiltration in non-inflamed human melanoma tumors is unlikely due to lack of antigens per se (58). In patients with pancreas cancer, we have reported that the cytolytic index, defined by expression of granzyme A and perforin 1, is inversely proportional to the number of predicted neo-epitopes (59). In tumors with a high degrees of T cell exclusion, blocking only PD-1 or CTLA-4 may be insufficient to achieve a clinical response if other suppressive pathways such as IDO, CD73, TIGIT, VISTA and many others are also expressed and function in a non-redundant fashion regardless of PD-1 or CTLA-4 blockade. Tumor cells themselves can orchestrate T cell exclusion, for example by oncogene-driven alterations in chemokine production that recruit suppressive myeloid cells (60, 61). Oncogene-driven upregulation of tumor PD-L1 has also been describe and further contributes to immunosuppression (62).
Figure 2.
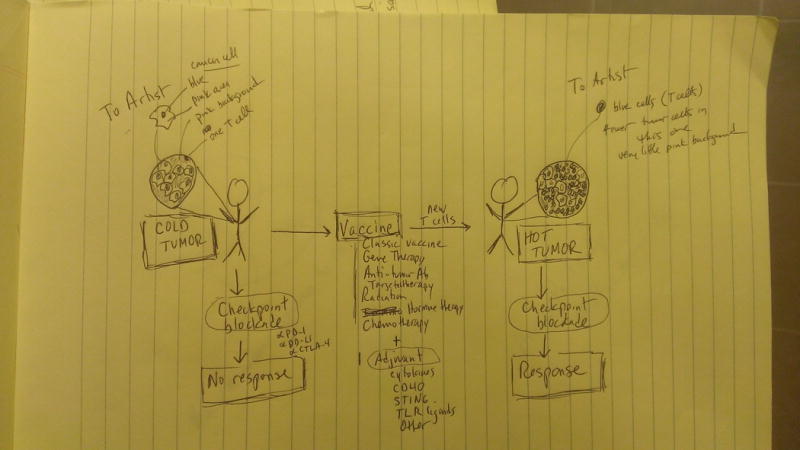
Cold to hot immunological conversion. Vaccination may be able to convert “cold” breast tumors, devoid of T cells, to “hot” tumors with robust T cell infiltration and responsiveness the checkpoint blockade. In addition to a classical experimental vaccine, many types of standard therapy may function to vaccinate the patient against tumor antigens released upon therapy-induced tumor cytotoxicity.
A second hypothesis is that the primary immunological deficit in cold tumors is poor T cell priming and expansion. Here, the problem is not exhausted T cells or T cells that do not penetrate tumor stroma, but T cells that have not been adequately generated in the first place. Thus, rather than checkpoint blockade, there are emerging efforts focused on “agonist therapy” to generate and amplify T cell responses. These include CAR T cells, second-generation cancer vaccines (such as prime boost GVAX/Listeria combinations), dendritic cell activators (such as agonist CD40 antibodies or Toll-like receptor ligands), and T cell agonists (such as OX40 or CD137 antibodies or cytokines including IL-7 and IL-15). Defining tumor-associated T cell antigens for which potent T cell responses can be generated will also be critical in converting cold breast cancer to hot and may require reaching beyond nonsynonymous mutations as the epitopes to rely on.
Advancing combination immunotherapy
Combining immune therapies with non-redundant mechanisms of action is considered crucial but complicated. Nivolumab in combination with ipilimumab is FDA-approved for melanoma, at the cost of increased toxicity and cost. In some cases, inhibiting multiple immune checkpoints may prove to be effective, whereas in other situations, a T cell response will need to be generated first and then necessarily protected from exhaustion with checkpoint blockade. Meaningful combinations are not likely to be restricted to only immune/immune combinations; many standard drugs – including chemotherapy, radiation therapy, targeted therapy or anti-tumor antibodies – may be immune modulatory, kill tumor cells in an immunogenic fashion, or otherwise synergize with approved immune therapies such as checkpoint blockade antibodies. However, issues of toxicity, dosing, and sequencing remain formidable, especially for agonists. The ultimate challenge in breast cancer “combination immunotherapy” is the vast number of potential combinations that likely overwhelm the number of patients and the capacity of the current clinical trial infrastructures to test them all. Priority combinations will be those with non-redundant mechanisms and those that are supported by preclinical work that includes a reliable immune pharmacodynamic biomarker.
Establishing routine immune profiling
Another opportunity is the development of methods for deep and routine immune profiling of patients and tumors. This approach can be built upon – and merged with – existing infrastructure established over the past years for next generation sequencing and mutation panel test of patient tumors. For example, tumor and normal whole exome sequencing and tumor RNA sequencing can establish a patient’s HLA type, mutational and neo-epitope burden, the tumor genome, and transcriptome from which the composition of the cellular infiltration as well as the constellation of primary suppressive pathways can be bioinformatically determined (59). If successful, based on the immune profile, patients could be matched to effective therapies (and similarly, guided away from costly or toxic ineffective therapies). We suggest immune precision medicine will become an important element of cancer therapeutics over time.
Developing immune prevention
Finally, as cancer immunotherapy continues to progress, we should look for ways to deploy this new art of immune cell activation for the prevention of cancer in the first place. This notion to develop vaccines and immunotherapies to prevent cancer was underscored by the Cancer Moonshot Blue Ribbon Panel in the 2016 report. For example, one of our goals is to develop a vaccine to prevent breast and other cancers in healthy individuals who carry a germline mutation in BRCA1 or BRCA2. A first-in-human study of a vaccine formulation that could be used one day for this purpose is under active clinical evaluation in the high-risk, post-adjuvant setting [NCT02960594]. Using DNA plasmid and in vivo electroporation for intramuscular delivery, this vaccine targets the human telomerase reverse transcriptase in concert with IL-12 as an adjuvant. Preclinical studies have shown marked resistance to tumor development in vaccinated vs. unvaccinated mice (63).
Acknowledgments
We thank Drs. Julia Tchou, Angela DeMichele, Kevin Fox, Jennifer Matro, Kate Nathanson, David Weiner, John Wherry, and Carl June for valuable discussions. This work was supported by the Breast Cancer Research Foundation (RHV, SMD), the Basser Research Center for BRCA (RHV, SMD), Susan G. Komen (ASC, SMD), and the Parker Institute for Cancer Immunotherapy (RHV).
Footnotes
Conflicts: Drs. Vonderheide and Domchek report a potential financial conflict of interest relating to inventorship on a patent for telomerase-specific cancer immunotherapy
References
- 1.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34:2460–7. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emens LA, Kok M, Ojalvo LS. Targeting the programmed cell death-1 pathway in breast and ovarian cancer. Curr Opin Obstet Gynecol. 2016;28:142–7. doi: 10.1097/GCO.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 3.Rugo H, DeLord J, Im S. Preliminary efficacy and safety of pembrolizumab (MK-3475) in patients with PD-L1-positive, ER-positive (ER+)/HER-2 negative breast cancer enrolled in Keynote 028. San Antonio Breast Cancer Symposium; San Antonio, TX. 2016. [Google Scholar]
- 4.Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, Dorazio P, Trosko JA, Ruter J, Mariani GL, Usari T, Domchek SM. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–94. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 5.Mittendorf EA, Clifton GT, Holmes JP, Schneble E, van Echo D, Ponniah S, Peoples GE. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014;25:1735–42. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miles D, Roche H, Martin M, Perren TJ, Cameron DA, Glaspy J, Dodwell D, Parker J, Mayordomo J, Tres A, Murray JL, Ibrahim NK, Theratope Study G Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16:1092–100. doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yates L, Desmedt C. Translational Genomics: Practical Applications of the Genomic Revolution. Clin Cancer Res. 2017;23:xxx–xxx. doi: 10.1158/1078-0432.CCR-16-2548. [DOI] [PubMed] [Google Scholar]
- 8.Freedman RA, Partridge AH. Breast Cancer in Special Populations: Where Are We Now and Where To Go From Here? Clin Cancer Res. 2017;23:xxx–xxx. [Google Scholar]
- 9.Nik-Zainal S. Mutational Signatures in Breast Cancer: The Problem at the DNA Level. Clin Cancer Res. 2017;23:xxx–xxx. doi: 10.1158/1078-0432.CCR-16-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeder-Hayes KE, Anderson BO. Breast Cancer Disparities at home and abroad: Review of the Problem and Preview of Interventions to Effect Change. Clin Cancer Res. 2017;23:xxx–xxx. doi: 10.1158/1078-0432.CCR-16-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, Loi S. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228–41. doi: 10.1038/nrclinonc.2015.215. [DOI] [PubMed] [Google Scholar]
- 12.Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12:1597–611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanton SE, Adams S, Disis ML. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016;2:1354–60. doi: 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 14.Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, Delorenzi M, Piccart M, Sotiriou C. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–65. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 15.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothe F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–92. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–87. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- 20.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, Kiermaier A, Swain SM, Baselga J, Michiels S, Loi S. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017;18:52–62. doi: 10.1016/S1470-2045(16)30631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Torne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 23.Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, Katsumata N, Kinoshita T, Takiguchi Y, Tanzawa H, Fujiwara Y. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132:793–805. doi: 10.1007/s10549-011-1554-7. [DOI] [PubMed] [Google Scholar]
- 24.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, Schem C, Fisch K, Darb-Esfahani S, Mehta K, Sotiriou C, Wienert S, Klare P, Andre F, Klauschen F, Blohmer JU, Krappmann K, Schmidt M, Tesch H, Kummel S, Sinn P, Jackisch C, Dietel M, Reimer T, Untch M, Loibl S. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–91. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 25.West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Research. 2011;13:R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–82. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 27.Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC, Cuka N, Argani P, Emens LA. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47:52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S, International TWG. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haricharan S, Bainbridge MN, Scheet P, Brown PH. Somatic mutation load of estrogen receptor-positive breast tumors predicts overall survival: an analysis of genome sequence data. Breast Cancer Res Treat. 2014;146:211–20. doi: 10.1007/s10549-014-2991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortes ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Lee RS, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CW, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, Allison JP. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–92. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 36.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vonderheide RH, Nathanson KL. Immunotherapy at large: the road to personalized cancer vaccines. Nat Med. 2013;19:1098–100. doi: 10.1038/nm.3317. [DOI] [PubMed] [Google Scholar]
- 38.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, Linette GP. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–8. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson E, Taube JM, Elwood H, Sharma R, Meeker A, Warzecha HN, Argani P, Cimino-Mathews A, Emens LA. The immune microenvironment of breast ductal carcinoma in situ. Mod Pathol. 2016;29:249–58. doi: 10.1038/modpathol.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, Investigators K Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 41.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11:91–9. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 42.Dirix L, Nikolinskos P, J G. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor trial. San Antonio Breast Cancer Symposium; San Antonio, TX. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McArthur HL, Diab A, Page DB, Yuan J, Solomon SB, Sacchini V, Comstock C, Durack JC, Maybody M, Sung J, Ginsberg A, Wong P, Barlas A, Dong Z, Zhao C, Blum B, Patil S, Neville D, Comen EA, Morris EA, Kotin A, Brogi E, Wen YH, Morrow M, Lacouture ME, Sharma P, Allison JP, Hudis CA, Wolchok JD, Norton L. A Pilot Study of Preoperative Single-Dose Ipilimumab and/or Cryoablation in Women with Early-Stage Breast Cancer with Comprehensive Immune Profiling. Clin Cancer Res. 2016;22:5729–37. doi: 10.1158/1078-0432.CCR-16-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coveler AL, Bates NE, Disis ML. Progress in the development of a therapeutic vaccine for breast cancer. Breast Cancer (Dove Med Press) 2010;2:25–36. doi: 10.2147/bctt.s6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis-Sproul JM, Harris MP, Davidson NE, Kobrin BJ, Jaffee EM, Emens LA. Cost-effective manufacture of an allogeneic GM-CSF-secreting breast tumor vaccine in an academic cGMP facility. Cytotherapy. 2005;7:46–56. doi: 10.1080/14653240510018082. [DOI] [PubMed] [Google Scholar]
- 46.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Domchek SM, Recio A, Mick R, Clark CE, Carpenter EL, Fox KR, DeMichele A, Schuchter LM, Leibowitz MS, Wexler MH, Vance BA, Beatty GL, Veloso E, Feldman MD, Vonderheide RH. Telomerase-specific T-cell immunity in breast cancer: effect of vaccination on tumor immunosurveillance. Cancer Res. 2007;67:10546–55. doi: 10.1158/0008-5472.CAN-07-2765. [DOI] [PubMed] [Google Scholar]
- 48.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–9. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 49.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, Stearns V, Disis ML, Ye X, Piantadosi S, Fetting JH, Davidson NE, Jaffee EM. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen G, Gupta R, Petrik S, Laiko M, Leatherman JM, Asquith JM, Daphtary MM, Garrett-Mayer E, Davidson NE, Hirt K, Berg M, Uram JN, Dauses T, Fetting J, Duus EM, Atay-Rosenthal S, Ye X, Wolff AC, Stearns V, Jaffee EM, Emens LA. A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER2+ metastatic breast cancer. Cancer Immunol Res. 2014;2:949–61. doi: 10.1158/2326-6066.CIR-14-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ, Jr, Colligon TA, Trosko JA, Leinbach LI, Pletcher CH, Tweed CK, DeMichele A, Fox KR, Domchek SM, Riley JL, Vonderheide RH. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:1324ra62. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J, Douglas M, Tibbitts T, Sharma S, Proctor J, Kosmider N, White K, Stern H, Soglia J, Adams J, Palombella VJ, McGovern K, Kutok JL, Wolchok JD, Merghoub T. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539:443–7. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gill S, June CH. Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015;263:68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- 55.Disis M, Salazar L, Coveler A, Waisman J, Higgins D, Childs J, Bates N, Dang Y. Phase I study of infusion of HER2/neu (HER2) specific T cells in patients with advanced-stage HER2 overexpressing cancers who have received a HER2 vaccine. 2009;27(15_suppl):3000. [Google Scholar]
- 56.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 58.Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, Gajewski AP, Andrade J, Gajewski TF. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A. 2016;113:E7759–E68. doi: 10.1073/pnas.1609376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balli D, Rech AJ, Stanger BZ, Vonderheide RH. Immune cytolytic activity stratifies molecular subsets of human pancreatic cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-Induced GM-CSF Production Promotes the Development of Pancreatic Neoplasia. Cancer Cell. 2012;21:836–47. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–35. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, Wilkerson MD, Fecci PE, Butaney M, Reibel JB, Soucheray M, Cohoon TJ, Janne PA, Meyerson M, Hayes DN, Shapiro GI, Shimamura T, Sholl LM, Rodig SJ, Freeman GJ, Hammerman PS, Dranoff G, Wong KK. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–63. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan J, Pankhong P, Shin TH, Obeng-Adjei N, Morrow MP, Walters JN, Khan AS, Sardesai NY, Weiner DB. Highly optimized DNA vaccine targeting human telomerase reverse transcriptase stimulates potent antitumor immunity. Cancer Immunol Res. 2013;1:179–89. doi: 10.1158/2326-6066.CIR-13-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]


