Abstract
We have developed and optimized a 96-well microtiter plate assay, based on the reduction of alamarBlue, to assess the efficacies of much needed new antimicrobials against Acanthamoeba species. This assay has been optimized for determination of drug efficacy against two potentially pathogenic species, Acanthamoeba castellanii and Acanthamoeba polyphaga, and has been validated by comparison of their relative susceptibilities to chlorhexidine, a drug widely used to treat Acanthamoeba keratitis. The results demonstrate that the assay is comparable to a manual counting assay and that A. polyphaga is more resistant to chlorhexidine than A. castellanii. Thus, by use of the manual counting assay, 3.125 μM chlorohexidine was almost completely effective against A. castellanii, whereas this concentration was less than 20% effective against A. polyphaga. Similar results were obtained by the alamarBlue assay. The new assay was used to determine the relative susceptibilities of A. castellanii and A. polyphaga to the alkylphosphocholines (APCs) hexadecylphosphocholine (hexadecyl-PC; miltefosine) and octadecylphosphocholine (octadecyl-PC) as well as an alkylgycerolphosphocholine, edelfosine. Both APCs studied were equally effective against A. castellanii, but octadecyl-PC was less effective than hexadecyl-PC against A. polyphaga. Both APCs were more effective than edelfosine against both Acanthamoeba species. A. polyphaga was found to be significantly less susceptible to each of the phosphocholine analogues. The newly described assay offers a number of advantages over those described previously. It is less labor-intensive than previously described assays and is sensitive and rapid, and the results can be read in a nonsubjective manner. As it is based on a standard 96-well, microtiter plate, it is amenable to automation and high throughput.
Acanthamoeba species are predominantly free-living protozoa found ubiquitously throughout the environment. Acanthamoeba species are now recognized as the cause of Acanthamoeba keratitis and granulomatous Acanthamoeba encephalitis (GAE) in humans (7, 11). Acanthamoeba keratitis is an increasingly common and severe corneal infection. It is closely associated with contact lens wear and can affect immunocompetent individuals (2, 17, 20). In contrast, GAE is a disease of immunocompromised individuals, including those with AIDS, in whom it is invariably fatal.
Present therapeutic regimens for Acanthamoeba keratitis rely on topical applications of antimicrobials, including a combination of propamidine isethionate and neomycin or chlorhexidine. The need for these drugs to be applied every 15 to 60 min for a period of weeks makes treatment arduous. Corneal transplantation is often necessary due to the extensive damage caused by the parasites. Moreover, as present treatments are poorly effective against the cystic stages of the parasite, residual infection often remains following treatment and can even result in infection of transplanted corneas (18). No effective antimicrobial treatment for GAE has been described, although such therapy has been used with apparent effect as an adjunct to surgery (16, 19). This exemplifies the urgent need for new and effective antimicrobials. A group of compounds that shows some promise for the treatment of Acanthamoeba infection are the phospholipid analogues alkylphosphocholines (APCs) (10, 22). These were originally designed as anticancer agents but have recently proven extremely effective for the treatment of leishmaniasis, prompting studies with a number of parasites, including Trypanosoma cruzi, Entamoeba histolytica, and Acanthamoeba, against which they demonstrate some efficacy (10). A further class of phospholipid analogues, the alkylglycerolphospocholines (AGPCs), has also shown some efficacy as antineoplastic and antiparasitic agents (10).
A major obstacle in the discovery and development of new inhibitors is the lack of a microtiter plate assay suitable for testing their efficiencies against axenic Acanthamoeba and applicable for high throughput. The assays used at present to determine the efficiency of potential Acanthamoeba inhibitors include manual counting with a hemocytometer (9) or staining with fluorescent viability dyes and flow cytometric analysis (14, 5). Other quantitative methods include the standard plaque assay (14, 13) and a quantitative microtiter method for the enumeration of track-forming units (6). The number of viable organisms has also been estimated by most probable number enumeration methods (4). An ideal assay would measure only live parasites, the results would be read in a nonsubjective manner, and the assay would not rely on manual counting. Furthermore, if it were based on a standard microtiter plate, it would be amenable to scaling for high-throughput analysis.
The alamarBlue assay has been used to quantitatively evaluate the proliferation of mammalian cell lines (1, 24), fungi (21, 23), and bacteria (3, 8, 12). The assay measures innate cellular metabolic activity, which reduces the alamarBlue dye and changes its color as a measurable indicator of the amount of viable cells that are present in a test sample. Specifically, alamarBlue is reduced by NADPH, reduced flavin adenine dinucleotide, reduced flavin mononucleotide, and the cytochromes produced inside the cells.
Here we show that the alamarBlue assay can be designed to measure quantitatively the proliferation and viability of Acanthamoeba trophozoites. We determined the relative cytotoxicity of chlorhexidine to Acanthamoeba castellani and Acanthamoeba polyphaga and demonstrate that the results of the alamarBlue assay and the manual counting assay are comparable. Furthermore, we determined the susceptibilities of both Acanthamoeba species to the AGPC edelfosine in comparison to those to the APCs hexadecylphosphocholine (hexadecyl-PC) and octadecylphosphocholine (octadecyl-PC).
MATERIALS AND METHODS
A. castellanii and A. polyphaga.
A. polyphaga (strain 1501/18) was obtained from Culture Collection of Algae and Protozoa (Cumbria, United Kingdom). A. castellanii was kindly obtained from Keith Vickerman (Glasgow, United Kingdom). A. castellanii and A. polyphaga trophozoites were grown in medium containing 20% mycological peptone (Sigma, Poole, United Kingdom) and 0.9% maltose (Sigma, Poole, United Kingdom) and supplemented with 1% penicillin, streptomycin, and amphotericin B (all from Sigma). The Acanthamoeba spp. were incubated at room temperature in 75-cm2 tissue culture flasks until they reached 90 to 95% confluence and were then harvested or subcultured by using Accutase enzyme cell detachment medium (Sigma).
Determination of optimal culture volume for assays.
A. castellanii and A. polyphaga cells were seeded in triplicate on a 96-well tissue culture plate with 100 μl of serial dilutions from a stock solution with 8 × 105 cells per ml to generate a calibration curve. Further wells were filled only with 100 μl of medium for use as a blank. This plating protocol was repeated by the addition of a further 100 μl of medium to all wells to allow comparison of the effects of medium volumes of 100 and 200 μl on metabolic activity and, thus, assay sensitivity. The alamarBlue assay reagent (Biosource, Europe, Nivelles, Belgium) was placed into each test well, which contained medium and cells at 100 or 200 μl, at an amount equal to 10% of the medium volume. Test plates containing the alamarBlue reagent were then incubated for 6 h. The well contents were assayed for alamarBlue reduction by measuring the absorbance of the wells at 570 and 600 nm on a Spectromax 190 plate reader (Molecular Devices, Wokingham, United Kingdom).
The percent reduction of alamarBlue was calculated by the formula
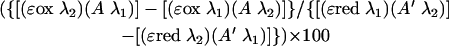 |
where ɛred λ1 is 155,677 (molar extinction coefficient of reduced alamarBlue at 570 nm); ɛred λ2 is 14,652 (molar extinction coefficient of reduced alamarBlue at 600 nm); ɛox λ1 is 80,586 (molar extinction coefficient of oxidized alamarBlue at 570 nm); ɛox λ2 is 117,216 (molar extinction coefficient of oxidized alamarBlue at 600 nm); A λ1 is the absorbance of the test wells at 570 nm; A λ2 is the absorbance of the test wells at 600 nm; A′ λ1 is the absorbance at 570 nm of the negative control wells, which contained medium and alamarBlue but to which Acanthamoeba was not added; and A λ′2 is the absorbance at 600 nm of the negative control wells, which contained medium and alamarBlue but to which Acanthamoeba was not added. The results are expressed as the mean for each triplicate culture ± the standard error (SE).
Determination of optimal seeding densities of Acanthamoeba.
A. castellanii and A. polyphaga cells were grown in 75-cm2 flasks until they reached 90 to 95% confluence and were then harvested by using Accutase (Sigma). A stock of 106 cells per ml was produced for each organism. The cells were seeded in triplicate on the 96-well plate with 100 μl of serial dilutions of the stock to generate a calibration curve. Further wells were filled only with 100 μl of medium and were used as blanks. This cell standard curve protocol was repeated on four plates to determine alamarBlue reagent reduction after 24, 48, 72, and 96 h of cell incubation under normal culture conditions. At 6 h prior to the end of incubation, 10 μl of alamarBlue reagent was added to each test well, and the test plate was incubated for 6 h at room temperature in the dark. The absorbance of each well was read at 570 and 600 nm by using a Spectromax 190 plate reader (Molecular Devices).
The percent reduction of alamarBlue was calculated by the formula
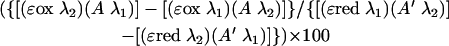 |
where the constants and variables are as defined above. The results are expressed as the mean for each triplicate culture ± SE.
Comparison of the relative susceptibilities of A. polyphaga and A. castellanii to chlorhexidine, as assessed by the alamarBlue assay and manual counting methods.
A. castellanii and A. polyphaga cells were grown in 75-cm2 flasks until they reached 90 to 95% confluence and were harvested by using Accutase (Sigma). A. castellanii and A. polyphaga cells were seeded in triplicate at 1,250 cells per well in 50 μl of medium in 96-well tissue culture plates (Greiner, Bio-One, United Kingdom) or 5,000 cells per well in 200 μl of medium in 24-well tissue culture plates (Greiner) and were allowed to adhere for 3 h. Medium containing chlorhexidine (Sigma) at a concentration range of 100 to 1.56 μM was freshly prepared to volumes of 50 and 200 μl and was added to the wells containing cells. The test plates were incubated for 96 h under normal culture conditions. At 6 h prior to the end of incubation, 10 μl of alamarBlue reagent was added to each of the test wells of the 96-well plate. The test plates were incubated for 6 h at room temperature in the dark. AlamarBlue reduction was assessed as described above. The percent inhibition of alamarBlue reduction was calculated by the formula
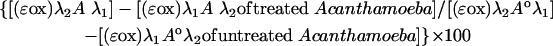 |
where the constants and variables are as defined above, Aoλ1 is the absorbance of the untreated control with alamarBlue at 570 nm, and Aoλ2 is the absorbance of the untreated control with alamarBlue at 600 nm.
This number was subtracted from 100 to give the percent alamarBlue reduction relative to that for the untreated control cultures. The results are expressed as the mean for each triplicate culture ± SE.
For manual counting, Acanthamoeba cells treated with chlorhexidine in 24-well tissue culture plates were detached by repeated pipetting, and 100 μl of the cell suspension was removed from each well and mixed with 100 μl of trypan blue vital stain (Sigma). Each treatment was done in triplicate test wells. Differential viable and nonviable cell counts were performed for each test well by counting the cells in a hemocytometer at ×100 magnification under a light microscope. The results are expressed as the mean for each triplicate culture ± SE.
Susceptibilities of A. castellanii and A. polyphaga to hexadecyl-PC, octadecyl-PC and edelfosine by alamarBlue assay.
The effects of hexadecyl-PC, octadecyl-PC, and edelfosine (Alexis Biochemicals, Nottingham, United Kingdom) on each species of Acanthamoeba were determined precisely as described above for chlorhexidine over 96 h. Each of the phospholipid analogues was initially dissolved in ethanol and diluted so that the final concentration of ethanol was less than 0.5% at the highest concentration used. For an ethanol control assayed in parallel, this or a lower concentration of ethanol had no significant effect on parasite multiplication, as assessed by alamarBlue reduction.
Statistics and experimental design.
All experiments were performed at least twice, with similar findings each time. Comparison of the susceptibilities of A. castellanii and A. polyphaga to chlorhexidine, hexadecyl-PC, octadecyl-PC, and edelfosine were performed by the Mann-Whitney U test. Regression analyses were performed with Cricket Graph software (Computer Associates International, Islandia, N.Y.).
RESULTS
Determination of optimal culture volume for assays.
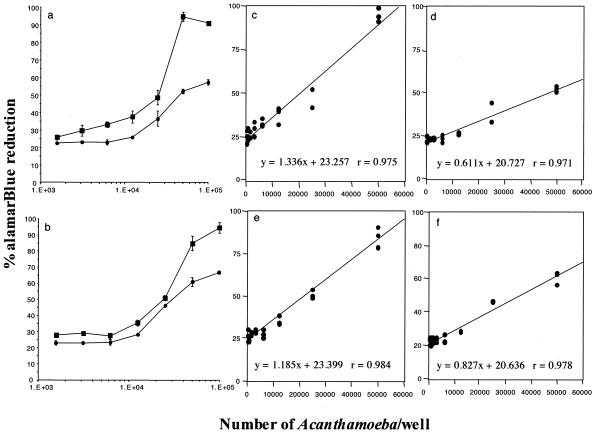
For both Acanthamoeba species, metabolic activity measured over a 6-h period was higher when they were cultured in 100 μl of medium (Fig. 1a and b). When alamarBlue was incubated in 200 μl of culture medium at a concentration of 5.0 × 104 cells/well, approximately 53 and 60% of the alamarBlue was reduced by A. castellanii and A. polyphaga, respectively. In contrast, the alamarBlue reduction by A. castellanii and A. polyphaga incubated in 100 μl of culture medium at this cell concentration measured approximately 95 and 88%, respectively. At 1.56 × 103 cells well, the lowest concentration of Acanthamoeba plated, the alamarBlue reduction was between 20 and 30% for both species in both culture volumes. This indicates that a culture volume of 100 μl provides a larger dynamic range of alamarBlue reduction and thus allows the detection of small differences in Acanthamoeba cell number and effectively increases the resolution of the assay. This culture volume was used in all further studies.
FIG. 1.
Reduction of alamarBlue by different plating densities of A. castellanii (a) and A. polyphaga (b) cultures in 100 μl (closed squares) and 200 μl (closed circles) of medium over 6 h. In the 100-μl cultures, 95 and 88% alamarBlue reductions were achieved over this time period by 5.0 × 104 A. castellani and A. polyphaga cells per well, respectively. In comparison, with the 200-μl cultures, maximum reductions of 53 and 60% alamarBlue were achieved by 5.0 × 104 A. castellani and A. polyphaga cells per well, respectively. The results are expressed as the means for triplicate cultures ± SEs. (c to f) AlamarBlue reduction had a linear correlation with Acanthamoeba cell number, irrespective of the species or the culture volume, up to 5 × 104 parasites per well (P < 0.05).
Regression analyses of the data obtained for A. castellanii and A. polyphaga cultured in both 100- and 200-μl volumes demonstrated that the parasite number correlated with percent alamarBlue reduction in a linear manner up to 5 × 104 parasites per well (P < 0.001 for both A. castellanii and A. polyphaga at both culture volumes) (Fig. 1c to f).
Determination of optimal seeding densities for Acanthamoeba.
The seeding densities of A. castellanii and A. polyphaga that attained close to 100% alamarBlue reduction were determined in assays conducted for total periods of 24, 48, 72, and 96 h. As anticipated for any given seeding concentration, the percentage of alamarBlue reduced increased with the length of incubation and correlated with the parasite number due to parasite proliferation (Fig. 2a and b). The optimum seeding densities for each time period, i.e., the density that reduced the maximum levels of alamarBlue before the curves plateaued, were 1.25 × 103, 1.0 × 104, 2.0 × 104, and 8.0 × 104 at 96, 72, 48, and 24 h, respectively, for both A. castellanii and A. polyphaga (Fig. 2a and b). The remainder of the studies reported were carried out over 96 h and thus used 1.25 × 103 cells of both A. castellanii and A. polyphaga.
FIG. 2.
Reduction of alamarBlue by different plating densities of A. castellanii (a) and A. polyphaga (b) cultured in 100 μl of medium for 96 h (open squares), 72 h (closed squares), 48 h (open circles), and 24 h (closed circles) prior to the addition of alamarBlue. The optimum plating densities, i.e., those that gave the highest alamarBlue reduction before the plateau, were determined to be 1.25 × 103, 1.0 × 104, 2.0 × 104, and 8.0 × 104 for 96, 72, 48, and 24 h, respectively, for both A. castellani and A. polyphaga. The results are expressed as the means for triplicate cultures ± SEs.
Comparison of the relative susceptibilities of A. polyphaga and A. castellanii to chlorhexidine, as assessed by the alamarBlue assay and manual counting methods.
The effects of chlorhexidine on A. castallanii and A. polyphaga were consistent between the alamarBlue assay and the manual counting method, with the percent reduction of alamarBlue correlating with the Acanthamoeba parasite numbers (Fig. 3). Regression analyses of the data obtained for both A. castellanii and A. polyphaga demonstrated that live parasite numbers (the numbers of parasites except those that were stained with trypan blue) assessed by manual counting correlated with the percent alamarBlue reduction in a linear manner for both A. castellanii (y = 13,090 + 26,827; R = 0.985; P < 0.001) and A. polyphaga (y = 5,685 − 4,004; R = 0.981; P < 0.001) (results not shown).
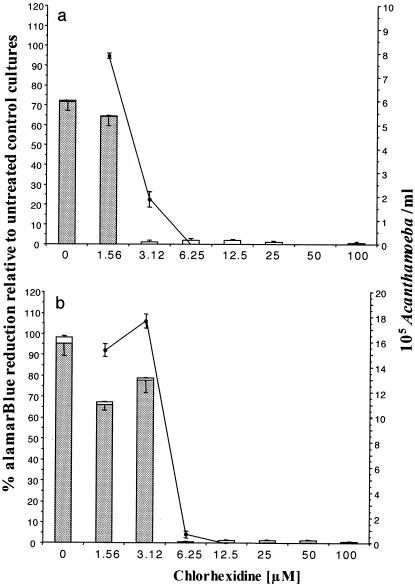
FIG. 3.
Comparison of alamarBlue assay with manual counting assay for determination of the relative susceptibilities of A. castellanii (a) and A. polyphaga (b) to chlorhexidine over 96 h. Live cells (grey bars) and dead cells (white bars) were determined by trypan blue staining. The effect of chlorhexidine was measured in parallel cultures by determining the percent alamarBlue reduction relative to that for the untreated control cultures (closed circles). Note the comparable results obtained by both methods, which showed a linear correlation for both A. castellanii and A. polyphaga (P < 0.05). The results are expressed as the means for triplicate cultures ± SEs.
A. castellanii was significantly more susceptible to chlorhexidine than A. polyphaga. Thus, complete inhibition of A. castellanii was achieved by 3.125 μM chlorhexidine, whereas this concentration of chlorhexidine inhibited multiplication of less than 20% of the A. polyphaga parasites (P < 0.05 by using data derived from both the alamarBlue assay and the manual counting assay). A chlorhexidine concentration of 6.25 μM was, however, completely effective (the 100% inhibitory concentration [IC100]) against A. polyphaga. Irrespective of the assay used, IC50s were between 1.5625 and 3.125 μM for A. castellanii and 3.125 and 6.25 μM for A. polyphaga.
Susceptibilities of A. castellanii and A. polyphaga to hexadecyl-PC, octadecyl-PC, and edelfosine by alamarBlue assay.
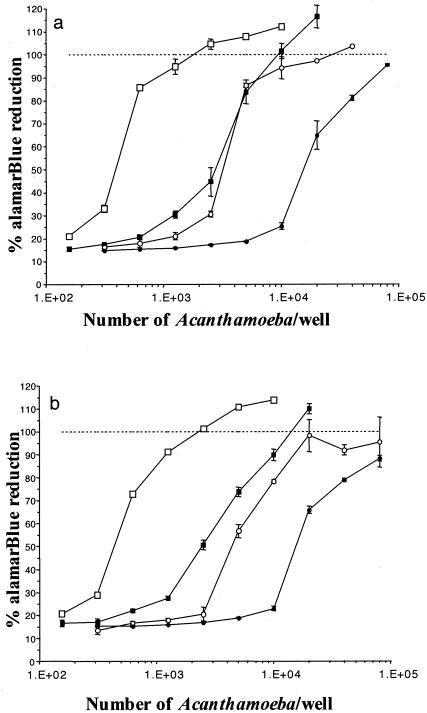
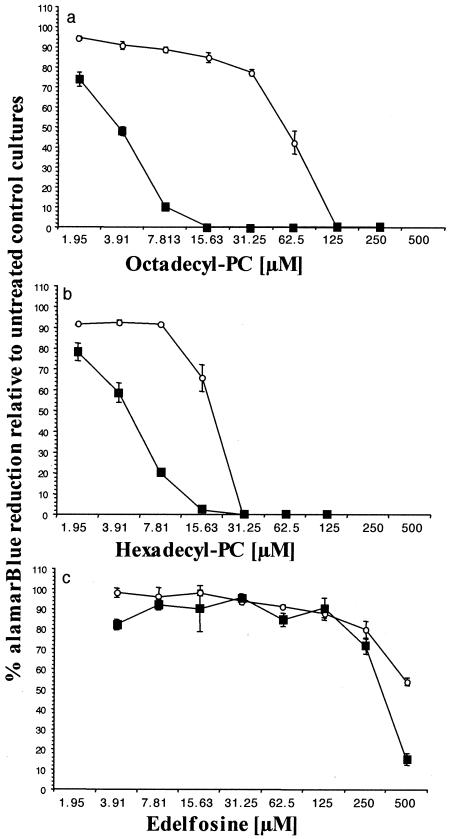
A. castellanii was found to be significantly (P < 0.05) more susceptible to octadecyl-PC than A. polyphaga at all concentrations used up to 15.63 μM, which was completely effective against A. castellanii but which was only approximately 20% effective against A. polyphaga. IC50s were between 3.906 and 7.813 μM for A. castellanii and between 31.25 and 62.5 μM for A. polyphaga (Fig. 4a). A similar pattern of relative susceptibility to hexadecyl-PC was observed, with A. castellanii being significantly (P < 0.05) more susceptible than A. polyphaga at all concentrations used up to 15.63 μM, which was almost completely effective against A. castellanii but which was only approximately 35% effective against A. polyphaga. IC50s were between 3.906 and 7.813 μM for A. castellanii and between 15.63 and 31.25 μM for A. polyphaga (Fig. 4b).
FIG. 4.
Relative susceptibilities of A. castellanii (closed squares) and A. polyphaga (open circles) to the following over 96 h: (a) octadecyl-PC (IC50s were between 3.906 and 7.813 μM for A. castellanii and between 31.25 and 62.5 μM for A. polyphaga); (b) hexadecyl-PC (IC50s were between 3.906 and 7.813 μM for A. castellanii and between 15.63 and 31.25 μM for A. polyphaga); and (c) eldefosine (IC50s were between 250 and 500 μM for A. castellanii and in excess of 500 μM for A. polyphaga). A. castellanii was significantly more susceptible than A. polyphaga to each of the phospholipid analogues tested (P < 0.05). Acanthamoeba cell numbers were assessed by measuring the percent alamarBlue reduction relative to that for the untreated control cultures over 96 h. The results are expressed as the means for triplicate cultures ± SEs.
Both A. castellanii and A. polyphaga were significantly (P < 0.05) more resistant to edelfosine than either hexadecyl-PC or octadecyl-PC at all concentrations used, with little effect observed below 250 μM (Fig. 4c). A. castellanii was significantly (P < 0.05) more susceptible than A. polyphaga to edelfosine at a concentration of 500 μM.
DISCUSSION
Species of the free-living protozoon Acanthamoeba are the causative agents of both Acanthamoeba keratitis and GAE in humans (7, 19, 20). At present, there is an urgent need for new, effective antimicrobials (18). To facilitate this challenge we have developed a colorimetric microtiter plate assay that uses the reduction of alamarBlue to assess Acanthamoeba numbers and thus determine the efficacies of potential antimicrobial agents. This assay is sensitive, the results can be read in a nonsubjective manner, and the assay is less labor-intensive than existing assays. This assay system has been optimized for A. castellanii and A. polyphaga, the two most common species isolated from patients with Acanthamoeba keratitis.
The results of initial studies with a standard 200-μl culture volume were disappointing, as alamarBlue reduction reached a plateau at approximately 60%. This prompted studies to determine if an increase in the surface area-to-volume ratio by reduction of the culture volume would increase metabolic activity and result in greater alamarBlue reduction. These studies established that the culture volume did influence the metabolic activities of both species of Acanthmoeba, as measured by their abilities to reduce alamarBlue. Thus, by culturing Acanthamoeba in a volume of 100 μl and not one of 200 μl, alamarBlue reduction can reach essentially 100%, effectively increasing the assay resolution by allowing the detection of small differences in cell numbers. The larger surface area-to-volume ratio presumably serves to allow better oxygenation of the culture medium, which increases the metabolic activity of the Acanthamoeba cells and which results in increased production of the factors responsible for alamarBlue reduction (NADH, reduced flavin adenine dinucleotide, reduced flavin mononucleotide). An additional benefit of smaller volumes of culture medium is the economy of reagents. By using a titration of each Acanthamoeba species, the optimal seeding densities, i.e., those that gave maximum alamarBlue reduction during the exponential phase, were easily determined for a number of assay lengths ranging from 1 to 4 days.
By using the established parameters, the assay system was validated by comparing the relative susceptibilities of A. castellanii and A. polyphaga to chlorhexidine over a 4-day period and comparing the results obtained with those obtained by the widely used manual counting method. The results obtained by both methods not only were in agreement with each other but also demonstrated that A. polyphaga is more resistant to chlorhexidine than A. castellanii, irrespective of the assay used. Chlorhexidine is known to be a membrane-active agent and to target acidic phospholipids, although other mechanisms, such as inhibition of ATP synthesis, have been proposed to occur in the presence of high chlorhexidine concentrations (15).
To further evaluate the utility of the assay that was developed, the susceptibilities of A. castellanii and A. polyphaga to APC and AGPC, phospholipid analogues, were assessed. A previous study (22) examined the effects of a number of APCs on Acanthamoeba species by a manual counting assay, but until now, AGPCs had yet to be examined. In agreement with the previous study (22), using the alamarBlue assay we found that hexadecyl-PC (16-carbon chain length) was completely effective (IC100) at similar concentrations against both A. castellanii and A. polyphaga. Similarly, we found octadecyl-PC (18-carbon chain length) to be less effective, an effect that was exemplified with A. polyphaga. Longer chain lengths with various degrees of saturation were tested and, with the exception of (Z,Z)-6,12-eicosadienylphosphocholine, were found to be largely ineffective at a concentration of 80 μM (22). We therefore examined the effect of edelfosine, an AGPC with an 18-carbon chain length. Our results indicate that this compound is poorly effective. Previous studies (10) have demonstrated that edelfosine is effective against Leishmania donovani and a number of other trypanosomatids at low-micromolar levels (<5 μM for L. donovani). This would indicate that Acanthamoeba species are susceptible to a more limited range of the available phospholipid analogues than the trypanosomatids. Furthermore, the relative differences in the susceptibilities of A. castellanii and A. polyphaga to chlorhexidine, octadecyl-PC, hexadecyl-PC, and edelfosine observed in this study suggest differences in the phospholipid compositions of their membranes.
A challenge in the field of Acanthamoeba research will be to develop antimicrobial agents better able to destroy the cyst stages. Our unpublished results suggest that the cyst stages are not highly metabolically active and that the alamarBlue assay is unlikely to be useful in enumerating these stages directly. However, an adaptation of the assay could be envisaged for this purpose. For this, cyst stages would be exposed to potential inhibitors for a set period of time, after which the growth medium would be replaced by fresh medium that lacked inhibitors. The number of viable cysts able to transform into trophozoites could then be assessed by their ability to reduce alamarBlue.
In conclusion, we have developed a colorimetric, microtiter plate assay based on the commercially available alamarBlue reagent to test the activities of antimicrobial compounds against Acanthamoeba. This method offers a number of advantages over many of the systems used at present. It is rapid, the results can be read in a nonsubjective manner, the assay measures only live cells, and minimal hazards are associated with the reagents used. The low seeding requirements of a 96-h study means that a single 75-cm2 flask containing 107 cells provides sufficient numbers of Acanthamoeba cells to assay more than 3,000 samples in triplicate. As it is based on a 96-well microtiter plate, it could easily be automated and is amenable to scaling for high throughput.
Acknowledgments
This work was funded by the William Ross Foundation and the University of Strathclyde Research and Development Fund.
REFERENCES
- 1.Ahmed, S. A., R. M. Gogal, Jr., and J. E. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. [DOI] [PubMed] [Google Scholar]
- 2.Auran, J. D., M. B. Starr, and F. A. Jakobiec. 1987. Acanthamoeba keratitis. A review of the literature. Cornea 6:2-26. [PubMed] [Google Scholar]
- 3.Baker, C. N., and F. C. Tenover. 1996. Evaluation of Alamar colorimetric broth microdilution susceptibility testing method for staphylococci and enterococci. J. Clin. Microbiol. 34:2654-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beattie, T. K., D. V. Seal, A. Tomlinson, A. K. McFadyen, and A. M. Grimason. 2003. Determination of amoebicidal activities of multipurpose contact lens solutions by using a most probable number enumeration technique. J. Clin. Microbiol. 41:2992-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borazjani, R. N., L. L. May, J. A. Noble, S. V. Avery, and D. G. Ahearn. 2000. Flow cytometry for determination of the efficacy of contact lens disinfecting solutions against Acanthamoeba spp. Appl. Environ. Microbiol. 66:1057-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck, S. L., and R. A. Rosenthal. 1996. A quantitative method to evaluate neutralizer toxicity against Acanthamoeba castellanii. Appl. Environ. Microbiol. 62:3521-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, E. J., H. W. Buchanan, P. A. Laughrea, C. P. Adams, P. G. Galentine, G. S. Visvesvara, R. Folberg, J. J. Arentsen, and P. R. Laibson. 1985. Diagnosis and management of Acanthamoeba keratitis. Am. J. Ophthalmol. 100:389-395. [DOI] [PubMed] [Google Scholar]
- 8.Collins, L., and S. G. Franzblau. 1997. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor, C. G., S. L. Hopkins, and R. D. Salisbury. 1991. Effectivity of contact lens disinfection systems against Acanthamoeba culbertsoni. Optom. Vis. Sci. 68:138-141. [DOI] [PubMed] [Google Scholar]
- 10.Croft, S. L., K. Seifert, and M. Duchene. 2003. Antiprotozoal activities of phospholipid analogues. Mol. Biochem. Parasitol. 126:165-172. [DOI] [PubMed] [Google Scholar]
- 11.Culbertson, C. G., P. W. Ensminger, and W. M. Overton. 1966. Hartmannella (Acanthamoeba). Experimental chronic, granulomatous brain infections produced by new isolates of low virulence. J. Clin. Pathol. 46:305-314. [DOI] [PubMed] [Google Scholar]
- 12.Franzblau, S. G., R. S. Witzig, J. C. McLaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B. Cook, V. K. Quenzer, R. M. Ferguson, and R. H. Gilman. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 36:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hugo, E. R., W. R. McLaughlin, K. H. Oh, and O. H. Tuovinen. 1991. Quantitative enumeration of Acanthamoeba for evaluation of cyst inactivation in contact lens care solutions. Investig. Ophthalmol. Vis. Sci. 32:655-657. [PubMed] [Google Scholar]
- 14.Khunkitti, W., S. V. Avery, D. Lloyd, J. R. Furr, and A. D. Russell. 1997. Effects of biocides on Acanthamoeba castellanii as measured by flow cytometry and plaque assay. J. Antimicrob. Chemother. 40:227-233. [DOI] [PubMed] [Google Scholar]
- 15.Maillard, J.-Y. 2002. Bacterial target sites for biocide action. J. Appl. Microbiol. Symp. Suppl. 92:16S-27S. [PubMed] [Google Scholar]
- 16.Marciano-Cabral, F., R. Puffenbarger, and G. A. Cabral. 2000. The increasing importance of Acanthamoeba infections. J. Eukaryot. Microbiol. 47:29-36. [DOI] [PubMed] [Google Scholar]
- 17.Moore, M. B., J. P. McCulley, M. Luckenbach, H. Gelender, C. Newton, M. B. McDonald, and G. S. Visvesvara. 1985. Acanthamoeba keratitis associated with soft contact lenses. Am. J. Ophthalmol. 100:396-403. [DOI] [PubMed] [Google Scholar]
- 18.Seal, D. V. 2003. Acanthamoeba keratitis update—incidence, molecular epidemiology and new drugs for treatment. Eye 17:893-905. [DOI] [PubMed] [Google Scholar]
- 19.Seijo Martinez, M., G. Gonzalez-Mediero, P. Santiago, A. Rodriguez De Lope, J. Diz, C. Conde, and G. S. Visvesvara. 2000. Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J. Clin. Microbiol. 38:3892-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stehr-Green, J. K., T. M. Bailey, and G. S. Visvesvara. 1989. The epidemiology of Acanthamoeba keratitis in the United States. Am. J. Ophthalmol. 107:331-336. [DOI] [PubMed] [Google Scholar]
- 21.Tiballi, R. N., X. He, L. T. Zarins, S. G. Revankar, and C. A. Kauffman. 1995. Use of a colorimetric system for yeast susceptibility testing. J. Clin. Microbiol. 33:915-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walochnik, J., M. Duchene, K. Seifert, A. Obwaller, T. Hottkowitz, G. Wiedermann, H. Eibl, and H. Aspock. 2002. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 46:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi, H., K. Uchida, K, Nagino, and T. Matsunaga. 2002. Usefulness of a colorimetric method for testing antifungal drug susceptibilities of Aspergillus species to voriconazole. J. Infect. Chemother. 8:374-377. [DOI] [PubMed] [Google Scholar]
- 24.Yu, H. G., H. Chung, Y. S. Yu, J. M. Seo, and J. W. Heo. 2003. A new rapid and non-radioactive assay for monitoring and determining the proliferation of retinal pigment epithelial cells. Korean J. Ophthalmol. 17:29-34. [DOI] [PubMed] [Google Scholar]