Abstract
The aim of the present study is to elucidate the neuronal pathways associated to NSAIDs causing a reduction of the risk and progression of Alzheimer's disease. The research was developed administering the active enantiomer of ibuprofen, dexibuprofen (DXI), in order to reduce associated gastric toxicity. DXI was administered from three to six-month-old female APPswe/PS1dE9 mice as a model of familial Alzheimer's disease. DXI treatment reduced the activation of glial cells and the cytokine release involved in the neurodegenerative process, especially TNFα. Moreover, DXI reduced soluble β-amyloid (Aβ1-42) plaque deposition by decreasing APP, BACE1 and facilitating Aβ degradation by enhancing insulin-degrading enzyme. DXI also decreased TAU hyperphosphorylation inhibiting c-Abl/CABLES/p-CDK5 activation signal pathway and prevented spatial learning and memory impairment in transgenic mice. Therefore, chronic DXI treatment could constitute a potential AD-modifying drug, both restoring cognitive functions and reversing multiple brain neuropathological hallmarks.
Abbreviations: AD, Alzheimer's disease; Aβ, Amyloid beta; APP, Amyloid precursor protein; NFTs, Neurofibrillary tangles; BACE1, β-secretase 1; TNF-α, Tumor necrosis factor; iNOS, Inducible nitric oxide synthase; NSAIDs, Non-steroidal anti-inflammatory drugs; PPARγ, Peroxisome proliferator-activated receptor-γ; COX-1, Cyclooxygenase-1; COX-2, Cyclooxygenase-2; NFĸβ, Nuclear factor kappa beta; IBU, Ibuprofen; pTAU, Phospho-TAU; DXI, Dexibuprofen; APP/PS1,, APPswe/PS1dE9; WT, Wild type; MWM, Morris water maze; NORT, Novel object recognition test; DI, Discrimination index; PFA, Paraformaldehide; nNOS, Neuronal Nitric Oxide Synthase; GFAP, Glial Fibrillary Acidic Protein; ADAM10, Disintegrin and metalloproteinase domain-containing protein 10; IDE, Insulin-degrading enzyme; CDK5, Cyclin-dependent kinase 5; GSK3β, Glycogen synthase kinase 3β; c-ABL, Abelson non-receptor tyrosine kinase; MAPK, Mitogen-activated protein kinase; PKA, Protein kinase A; SYP, Synaptophysin
Keywords: APPSwe/PS1dE9, Dexibuprofen, Insulin receptor, Mitochondria, Hippocampus, TAU, Memory impairment, Alzheimer's disease
Graphical abstract
Highlights
-
•
DXI treatment improves cognitive impairment in APPswe/PS1dE9 mice through multiple targets.
-
•
Aβ reduction occurs with DXI inhibiting APP, BACE1 and increasing IDE1 hippocampal protein levels.
-
•
DXI contributes to TAU hyperphosphorylation decreasing but does not alter total TAU expression.
-
•
CDK5 pathway is altered by DXI inhibiting CDK5 phosphorylation via TNFα/c-ABLl/CABLES.
-
•
DXI decreases pAKT/pGSK3β protein levels which are involved in insulin signaling pathway.
1. Introduction
Alzheimer's disease (AD) is the most common form of dementia affecting elderly people [1]. AD is clinically characterized by loss of memory and cognitive functions. Moreover, histopathological hallmarks include extracellular amyloid peptide (Aβ) deposition in neuritic plaques, and intracellular deposits of hyperphosphorylated TAU (pTAU), causing the formation of neurofibrillary tangles (NFTs) and, finally, neuronal loss [2].
Despite its high prevalence and mortality, the molecular mechanisms associated with neuronal loss are still unknown. However, Aβ peptides, especially Aβ(1-42), are currently considered the main neurotoxins responsible for AD neuronal loss. According to the amyloidogenic cascade hypothesis, Aβ peptides are generated via the amyloid precursor protein (APP) through two proteolytic enzymes, the β-secretase 1 (BACE1) and the γ-secretase [3], [4].
Since there are no effective treatments to prevent or cure AD, great efforts are currently directed towards identifying disease-modifying therapies, involving several compounds in different phases of development [5], [6], [7]. However, due to the complex nature of AD, one of the main concerns in drug development relies on the ability to obtain compounds capable of acting in more than a single specific disease target. Thereby, some researchers propose that the popular amyloid cascade hypothesis should be slightly modified to incorporate additional cellular and physiological components contributing to the neurodegenerative process, such as neuroinflammatory glial cells [5], [7]. This hypothesis suggests that glial activation, mainly microglia, would lead to an upregulation of brain cytokines and chemokines such as IL-1β, IL-12, tumor necrosis factor (TNFα), and inducible nitric oxide synthase activation (iNOS), thus causing neuronal loss in different brain areas, especially the hippocampus [6], [8].
Nevertheless, the dual effects of the inflammatory response add complexity to the process. At the beginning of the disease, inflammation constitutes a neuroprotective response intended to reduce Aβ accumulation. However, as the disease progresses, the excessive or chronic neuroinflammatory response contributes to AD development [7]. For this reason, non-steroidal anti-inflammatory drugs (NSAIDs) are being on the research focus towards AD treatment over the last years [9]. These drugs might influence the inflammatory response by activating the peroxisome proliferator-activated receptor-γ (PPARγ) and inhibiting cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). In addition, some studies have revealed other interesting NSAIDs targets, such as nuclear factor kappa beta (NFĸβ) and β and γ-secretases [8]. In addition, some authors reported that these benefits may only be observed in early phases of the disease [6]. Although there are still some discrepancies between epidemiological results, some studies indicate that patients under long-term treatment with NSAIDs for other conditions such as rheumatoid arthritis, present a reduced incidence of AD, suggesting that these drugs could restore some pathways altered in the disease [10], [11].
Ibuprofen (IBU) is one of the most commonly used NSAIDs. Preclinical studies with IBU showed a reduction in the levels of pTAU and Aβ deposition, as well as a decrease in COX-2 expression and activated microglia [12]. Moreover, a reduction in oxidative stress through a decrease in NOX2 and 4HNE expression was also reported [12]. Bearing in mind these promising findings, in the present study we investigated the effects of dexibuprofen (DXI), the active enantiomer of IBU [13]. DXI provides an improvement for the treatment of several inflammatory diseases, since lower doses than its racemic counterpart are necessary to obtain identical therapeutic efficacy [14]. Furthermore, the higher solubility in water and increased absorption of DXI allow to reduce undesirable ulcerogenic side effects associated with IBU or other NSAIDs [14], [15], [16]. Additionally, preclinical data have demonstrated that DXI have a more powerful anti-inflammatory and analgesic effect than IBU [17]. Taking into account the advantages of this compound, the aim of the present study was to investigate the effects of DXI on AD-associated neuropathology markers, as well as the underlying mechanisms related to memory loss, in female APPswe/PS1dE9 mice, a model of familial AD [18], [19].
2. Materials and methods
2.1. Animals and treatment
In this study, six-month-old female APPswe/PS1dE9 (APP/PS1) and C57BL/6 wild-type (WT) mice were used. This animal model was chosen according to previous studies reporting that female mice develop higher progressive memory impairment and AD-like neuropathology compared to male mice [20]. These transgenic mice express a Swedish (K594M/N595L) mutation of a chimeric mouse/human APP (mo/huAPP695swe), together with the human exon-9-deleted variant of PS1 (PS1-dE9).
Animals were divided in three groups (WT, APP/PS1 and APP/PS1 DXI) and, at least, 10 animals per group were used. APP/PS1 transgenic mice were non-treated (APP/PS1) or treated with water supplemented with DXI (APP/PS1 DXI), containing 50 mgDXI kg−1 day−1 for 3 months before sacrifice. WT mice did not receive any pharmacological treatment. All animals were given access to food or water ad libitum and kept under controlled temperature, humidity and light conditions. Every effort was made to reduce the number of animals and minimize animal suffering. Mice were treated in accordance with the European Community Council Directive 86/609/EEC and the procedures established by the Department d′Agricultura, Ramaderia i Pesca of the Generalitat de Catalunya.
2.2. Cognitive function test: novel object recognition
Before sacrifice, the hippocampal-dependent recognition memory of treated and non-treated mice was assessed by a novel object recognition test (NORT). The first three days, each mouse was left to get used to the open field box, without any objects (10 min/session). On the fourth day, mice were left for 10 min to explore two identical objects (A+A). On the fifth day, each mouse was exposed for 10 min to a familiar object A and a novel object, namely B. After this, the objects and the open field box were cleaned with soap and water in order to avoid the presence of olfactory signs. Recorded videos were analyzed and the discrimination index (DI) was calculated dividing the exploration time of the novel object by the total exploration time [21] Exploration was defined as sniffing or touching an object. Mice with a total exploration time of < 5 s for an object were removed from analyses [22].
2.3. Immunofluorescence
Mice were sacrificed at 6 months and, at least, 5 animals of each group were perfused before brain extraction using 4% paraformaldehyde (PFA). Tissues were processed and stained as previously described by our group [23], [24]. The antibodies used in this study are supplied as Table 1 of Supplementary material.
2.4. Measurement of β-amyloid peptides in cortical tissues by ELISA
Soluble and insoluble Aβ (1−42) were measured in cortical extracts using commercially available human ELISA kits (Cat # KHB3441; Invitrogen, Camarillo, CA, USA) according to manufacturer's guidelines. Data obtained from the cortical homogenates was expressed as picograms of Aβ content per milligrams of total protein (pg/mg) [25].
2.5. Sample preparation for Western blotting and Real-Time PCR
At 6 months, 5–8 animals of each group were sacrificed by cervical dislocation prior to brain dissection. For protein extraction, hippocampi were homogenized in lysis buffer (50 mM TrisHCl pH: 7.4, 150 mM NaCl, 5 mM EDTA, 1%Triton X-100) and protease inhibitor mixture (Complete, Roche Diagnostics, Barcelona, Spain). Samples were stored at −80 °C until use. Protein concentration was determined using Pierce BCA Protein Assay kit (Pierce Company, Rockford, MI, USA) [26].
2.6. Western blot analysis
Aliquots of samples containing 10 µg of protein were analyzed by Western Blotting. The samples were placed in sample buffer (0.5 M Tris-HCl, 10% glycerol, 2% SDS, 5% 2-mercaptoethanol, 0.05% bromophenol blue, pH 6.8) and denatured by boiling at 95 °C for 5 min. Samples were separated by electrophoresis on 10% acrylamide gels, transferred to PVDF (Polyvinylidene difluoride) membranes using transblot apparatus and blocked in 5% skim milk powder in TBS-T buffer (50 mM Tris, 1.5% NaCl, 0.05% Tween 20, pH 7.5) for 1 h at room temperature. They were incubated overnight at 4 °C with primary antibodies (Supplementary material, Table 2). After that, membranes were washed in TBS-T buffer and incubated with IgG secondary antibody for 1 h at room temperature. Bands were detected by chemiluminescence detection kit and using Chemi doc XRS + Molecular Imager detection system (Bio-Rad). The quantification was performed by Image Lab image analysis software. All results were normalized to GAPDH [26].
2.7. Real-Time PCR
For RNA extraction, hippocampi were homogenized by Trizol reagent (Life Technologies Corporation) and samples were stored at −80 °C until use. Equal concentrations of cDNA of each animal were used for q-PCR and each sample was analyzed by triplicate. The assays were performed on a StepOnePlus Real-Time PCR system (Applied Biosystems). The PCR reaction contained 2 µg of reverse-transcribed RNA, 2SYBRGreen qPCR Master Mix (K0253, Pierce, Thermo Fisher Scientific), and 100 mM of each primer. Analyzed genes are detailed in Table 3 of Supplementary material. Results were normalized to actin [26].
2.8. Gastric damage
After treatment, mice were sacrificed and stomachs were removed, cut and rinsed with ice-cold distilled water. The ulcer index (UI) was determined by calculating the severity of the lesions as previously reported by other authors [27].
2.9. Statistical analysis
Statistical analyses were performed using GraphPad Prism 6. Data were expressed as mean ± standard deviation. Significant differences were determined by one-way analysis of variance (ANOVA), followed by Tukey's post hoc test for multigroup comparisons to compare data from the experimental groups. The level of significance for the acceptance was p < 0.05.
3. Results
3.1. Dexibuprofen reduces neuroinflammation in APPswe/PS1dE9 mice brain
It is well known that activated astrocytes and microglia are usually localized in the brain area where Aβ deposition occurs in APP/PS1 mice [28]. We found a significant increase in NFκβ, TNFα, iNOS and neuronal Nitric Oxide Synthase (nNOS) protein level in APP/PS1 mice compared to WT (p < 0.05; Fig. 1A). In addition, DXI treatment significantly decreased the levels of all these proteins, with the exception of nNOS (no significant differences were observed).
Fig. 1.
A) Representative GAPDH-normalized immunoblotting images and quantification (n = 4–6 independent samples per group) of proteins related in neuroinflammation processes in the hippocampal extracts of WT, APP/PS1 and APP/PS1-DXI treated 6-month-old mice. B) Immunohistochemistry of ThS of Aβ plaque and GFAP in the brains of 6-month-old APP/PS1 and APP/PS1-DXI treated mice (n = 4–6 independent samples per group, with at least 5 slices analyzed per sample). C) Concentrations of soluble and insoluble human Aβ(1−42) peptides in the cortical extracts in untreated and DXI-treated APP/PS1 mice, expressed as pg/mg of total protein as determined by ELISA; n = 4–6 independent samples per group, with 3 technical replicates per sample (* denotes p < 0.05).
In order to study the astrocytes activation, brain sections were immunostained with an anti-GFAP (Glial Fibrillary Acidic Protein) antibody. The staining against GFAP was markedly higher in the brain of untreated APP/PS1 mice compared to DXI treated mice (Fig. 1B).
Additionally, potential gastric damage of DXI was compared using the same dose of it's racemic counterpart, IBU, in WT mice for three months. In agreement with other authors, DXI induced significantly less gastric damage than IBU (Supplementary material Fig. 1).
3.2. Dexibuprofen reduces plaque burden and inhibits APP processing toward the amyloidogenic pathway in APPswe/PS1dE9 mice
APP/PS1 mice exhibit a time-dependent Aβ production and deposition in the brain with increasing age [29]. To determine the effect of DXI treatment on this process, Thioflavin-S (ThS) was used for detection of fibrillary plaques (Fig. 1B). The analysis revealed that long-term administration of DXI significantly reduced the area occupied by fibrillary plaques in the cerebral cortex of APP/PS1 mice. Additionally, as shown in Fig. 1C, DXI treatment caused a significant decrease in the levels of soluble and insoluble Aβ (1-42) (p<0.05).
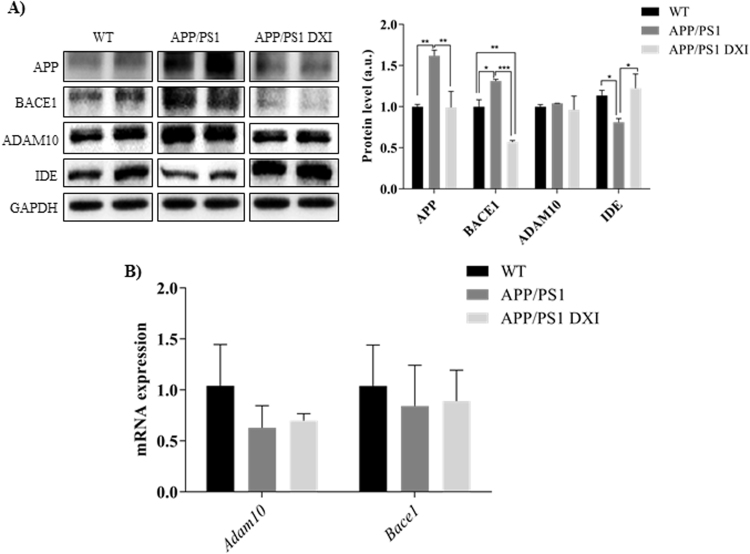
Western Blot analyses were carried out to investigate the mechanisms involved in APP processing that contribute to Aβ soluble production. Quantitative analysis (Fig. 2A) indicated that the levels of full-length APP were significantly increased in the hippocampus of non-treated transgenic group (p < 0.01), whereas a significantly decrease in the levels of this protein was found in DXI-treated mice (p < 0.01). In addition, DXI significantly decreased protein levels of BACE1 in the hippocampus of transgenic mice compared to non-treated APP/PS1 mice (p < 0.001). Moreover, DXI treatment significantly increased IDE protein levels in APP/PS1 mice hippocampus (p < 0.05). However, changes in ADAM10 in APP/PS1 mice after DXI treatment were not observed. As it is shown in Fig. 2B, no significant differences between mRNA expression of ADAM10 nor BACE1 were observed after DXI treatment. Therefore, these results indicate that DXI inhibits APP processing toward amyloidogenic pathway, thus suggesting that DXI lowers the plaque burden by promoting Aβ degradation through the inhibition of BACE1 and the synthesis of APP in the brain hippocampus of transgenic mice.
Fig. 2.
A) Representative GAPDH-normalized immunoblotting images and quantification (n = 4–6 independent samples per group) of key molecules involved in APP processing APP, BACE, ADAM10 and IDE in the hippocampal extracts of WT, APP/PS1 and APP/PS1-DXI treated 6-month-old mice (* denotes p < 0.05, ** denotes p < 0.01). B) Quantitative analysis of mRNA expression of ADAM10 and BACE1 determined by RT-PCR.
3.3. Dexibuprofen attenuates TAU phosphorylation in APPswe/ PS1dE9 mice
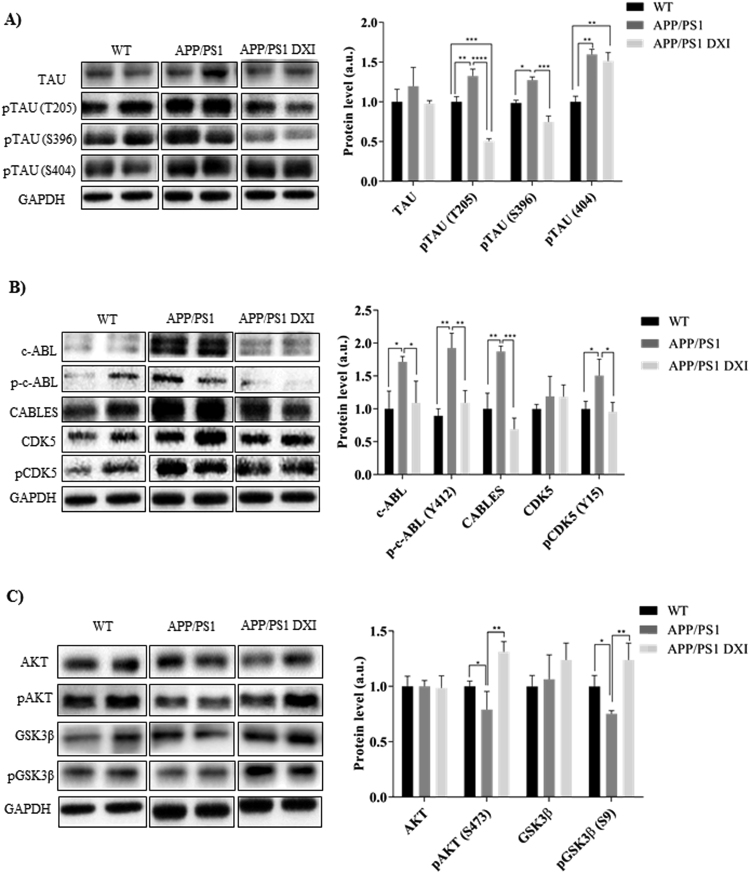
The effects of DXI on TAU hyperphosphorylation were assessed by Western blot. Data analysis showed that the levels of pTAU at Thr205 and Ser 396 were significantly decreased in the hippocampus of DXI APP/PS1 mice compared with non-treated transgenic mice (p < 0.0001; p < 0.001 respectively; Fig. 3A).
Fig. 3.
Representative GAPDH-normalized immunoblotting images and quantification (n = 4–6 independent samples per group) of key molecules involved in TAU hyperphosphorilation A) Protein levels of TAU, pTAU (T205), pTAU(S396) and pTAU (S404) of WT, APP/PS1 and APP/PS1 treated with DXI, B) Protein levels of c-ABL, p-c-ABL (Y412), CABLES, CDK5 and pCDK5(Y15) of WT, APP/PS1 and APP/PS1 treated with DXI and C) Protein levels of AKT, pAKT, GSK3β AND PGSK3β of WT, APP/PS1 and APP/PS1 treated with DXI (* denotes p < 0.05, ** denotes p < 0.01).
The process of TAU hyperphosphorylation is mediated mainly by two kinases: the cyclin-dependent kinase 5 (CDK5) and the glycogen synthase kinase 3β (GSK3β). In addition, upstream regulation of both kinases is mediated by c-ABL. Thus, analysis of the c-ABL signaling pathway in the hippocampus at 6 months revealed an increase in the protein levels of total and phosphorylated c-ABL on Tyr412 in APP/PS1 compared with WT mice (p < 0.05; p < 0.01; Fig. 3B). Moreover, consequently with the c-ABL-dependent CDK5 activation, CDK5Tyr15 phosphorylation was also markedly elevated (p < 0.05). Furthermore, in this signaling pathway, CABLES mediates the interaction between c-ABL and CDK5, and regulates CDK5 tyrosine phosphorylation by c-ABL. Western blot analyses showed a significant increase in CABLES protein levels in APP/PS1 mice compared with WT mice (p < 0.01). DXI-treated transgenic group showed a significant decrease in the expression of activated proteins involved in c-ABL pathway (p˂0.05). Likewise, DXI prevented the inhibition of brain insulin signaling pathway measured by p-AKT and p-GSK3β analyses, which showed a significant increased phosphorylation compared with non-treated APP/PS1 mice (p < 0.05; p < 0.01; Fig. 3C).
3.4. Dexibuprofen improves the cognitive process in APPswe/PS1dE9 mice
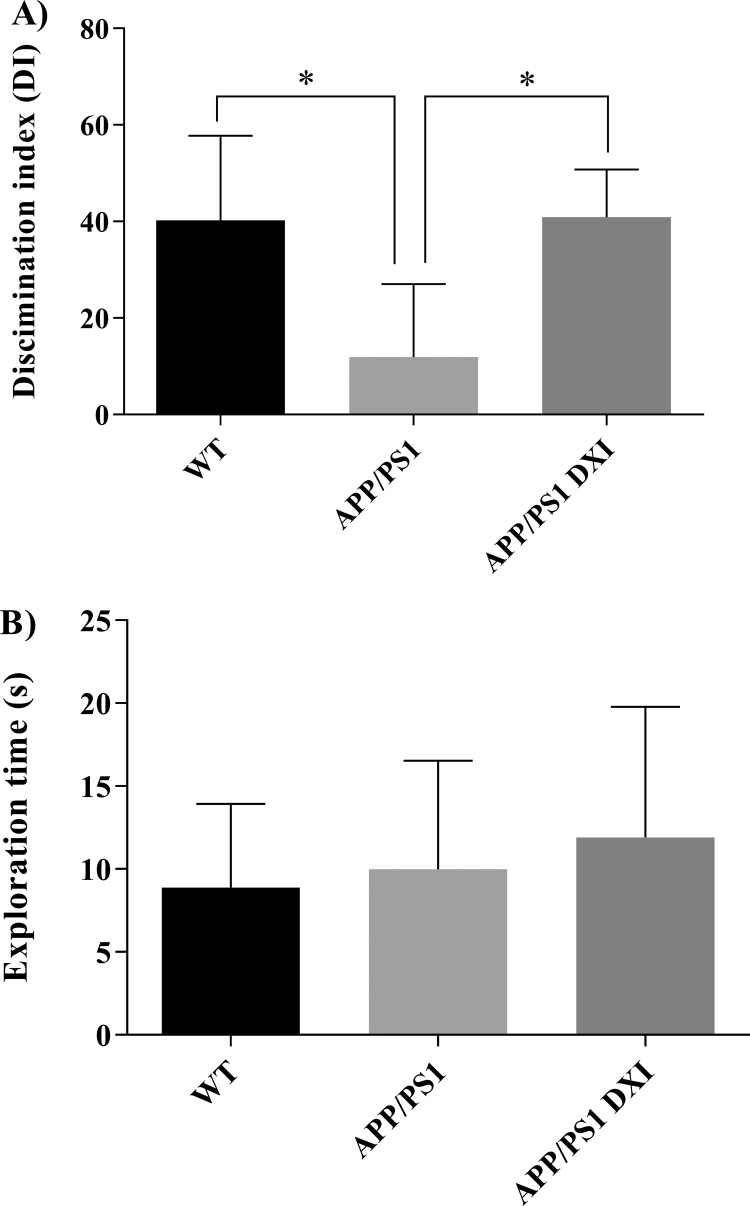
In the NOR test, APP/PS1 DXI mice presented a significant increase of DI compared to non-treated transgenic mice, thus indicating drug enhanced memory effects (p < 0.05; Fig. 4A). In addition, no differences in total exploration time were observed among the experimental groups (Fig. 4B).
Fig. 4.
Novel object recognition test. A) Discrimination index (DI) expressed as a percentage comparison between WT, APP/PS1 and APP/PS1 DXI. B) No significant effect on total exploration time during the test was found between the assessed groups (* denotes p < 0.05).
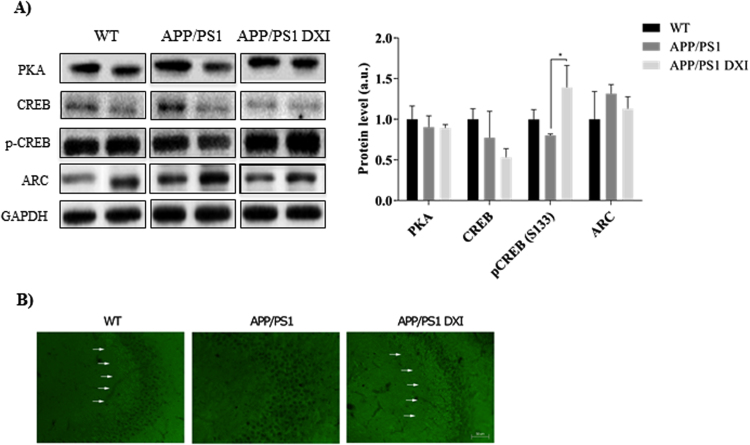
At the molecular level, APP/PS1 treated with DXI for three months showed a significant increase in CREB phosphorylation at serine 133 (Ser 133) in the hippocampus compared to non-treated APP/PS1 mice (p < 0.05). This phosphorylation leads to the transcription of memory associated genes. Moreover, no significant hippocampal alterations in the levels of protein kinase A (PKA) nor ARC were observed in APP/PS1 mice compared to WT groups (Fig. 5A).
Fig. 5.
A) Representative GAPDH-normalized immunoblotting images and quantification (n = 4–6 independent samples per group) of proteins related to memory processes in the hippocampal extracts of WT, APP/PS1 and APP/PS1 treated with DXI in 6-month-old mice. (* denotes p < 0.05). B) Immunohistochemistry of synaptophysin and Hoestch in the hippocampal CA3 region.
Finally, in order to evaluate synaptic integrity, we investigated whether the protein levels of the synaptic marker synaptophysin (SYP) in transgenic mice after a chronic DXI-treatment were modified. DXI treatment induced a consistent increase in SYP protein levels of DXI APP/PS1 mice hippocampus compared with vehicle-treated APP/PS1 mice (Fig. 5B). Thus, these data indicate that DXI treatment could improve the memory process through the p-CREB.
4. Discussion
Several epidemiological studies have reported that chronic use of NSAIDs reduces the risk for AD [10]. In the same line, the present research demonstrates that a chronic DXI treatment ameliorates learning and memory deficits in APP/PS1 mice, thus suggesting that DXI could be an AD modifying drug. DXI exerts significant effects on the reduction of Aβ production, both enhancing Aβ clearance and inhibiting aggregation of Aβ into amyloid plaques, as well as inhibiting TAU phosphorylation.
Brain gliosis activation is an important pathological feature of all neurodegenerative disorders, also AD [30]. Previous preclinical research has reported that activation of microglia and astrocytes is markedly enhanced and probably correlates with cognitive deficits and amyloid plaques in the brain of APP/PS1 [31], [32]. The results of the present investigation show that DXI treatment markedly reduces the activated astrocytes as well as the cytokine expression -mainly TNFα- in the hippocampus of APP/PS1 mice. Microglia activation and cytokine increase in brain are important in the onset of AD, since it has been demonstrated that drugs against TNFα improve AD neuropathology [17], [33]. Therefore, the significant reduction of inflammatory mediators observed in DXI treated APP/PS1 mice could partially explain the neuroprotective properties of this NSAID.
The DXI effect on decreasing brain Aβ accumulation might be attributed to two main factors: 1) the direct APP inhibition and 2) the inhibition of hippocampal BACE1 expression, thus regulating Aβ processing towards amyloidogenic pathways [34]. Furthermore, our results show that DXI treatment markedly increased IDE expression in the hippocampus, suggesting that the effect of DXI on lowering Aβ deposition might also be attributable to its role in promoting Aβ degradation [35].
Besides Aβ pathology, the abnormal increase of the hyperphosphorylated TAU protein, the major component of intracellular neurofibrillary tangles (NFTs), contributes to the development of AD [36]. The results of the current study show that DXI treatment significantly reduced pTAU at multiple sites in the hippocampus of APP/PS1 mice, a matter of importance given that hyperphosphorylated TAU is directly implicated in memory dysfunction in AD [23], [37]. However, Kitazawa and colleagues demonstrated that lipopolysaccharide treatment, a compound which activates brain microglia, significantly exacerbated the TAU pathology in the 3xTg-AD mice without affecting APP processing [37], [38], [39]. Thus, microglia activation could directly affect TAU phosphorylation, independently of Aβ modulation.
To further elucidate the mechanism for the DXI-dependent reduction in TAU phosphorylation, we assessed the changes in activation of CDK5 and GSK3β -the two major kinases that phosphorylate TAU- by using specific antibodies [40]. It has been demonstrated that CDK5 activity is regulated by its phosphorylation at Tyr15 site, exhibiting an increased CDK5 activation in APP/PS1 mice [40], [41]. Moreover, in vitro and in vivo studies have shown that active c-ABL, a kinase triggered by Aβ, promotes CDK5 phosphorylation at Tyr15 site and increases its activity leading to TAU phosphorylation [41]. We found that DXI treatment reduced the elevated levels of pCDK5 at Tyr15 site in the brain of APP/PS1 mice, suggesting that DXI attenuates TAU hyperphosphorylation through its inhibitory effect on CDK5 signal pathway. Moreover, it is known that c-ABL phosphorylates CDK5 on Y15 through the adaptor protein CABLES, thus stimulating the CDK5 kinase activity [41]. Furthermore, it has been reported that c-ABL can be activated in response to oxidative stress, DNA damage and inflammation. Our results showing a significant reduction of c-ABL, CABLES, pCDK5 (Y15) and some TAU phosphoepitopes in the DXI treated group are in accordance to this data. These findings might be explained by the reduction in the levels of cytokines and interleukins -especifically TNFα- involved in the activation of c-ABL. Moreover, since our results indicate that DXI could alter the CDK5 pathway by decreasing the inflammatory response, CDK5 could be the link between glial activation and TAU phosphorylation. Therefore, c-ABL might be upstream of CDK5 activation and DXI might attenuate TAU phosphorylation through inhibiting the CDK5 signal pathway TNFα/c-ABL/CABLES/CDK5.
The role of impaired brain insulin signaling in the pathogenesis of AD is also supported by several recent preclinical studies showing improvements in cognition, memory and TAU phosphorylation by treatment with intranasal insulin. Our results have shown that DXI treatment increased both pGSK3β and pAKT activity in APP/PS1.
Our results also demonstrate that DXI reverses cognitive deficits in the APP/PS1 mice, since the performance of the NOR test was improved in the DXI treated group against the non-treated APP/PS1. In previous studies, Trinchese and colleagues reported that synaptic plasticity and memory loss in APP/PS1 mice occur as early as 3–4 months of age and it is independent of plaque formation [42], [43]. Thus, targeting synapses and molecular mechanisms of memory, mainly CREB phosphorylation, could be a suitable target for AD improvement, since synaptic dysfunction occurs independently of plaque formation.
Previous studies reported that loss of synapses is one of the main neuropathological hallmarks of AD, which strongly correlates with the cognitive decline [14]. The beneficial effects of DXI on cognitive improvement in the APP/PS1 mice are likely attributable to the combined effects of the reduction in pro-inflammatory molecules and BACE1, as well as the increase in p-CREB expression and synaptic integrity [44].
PKA is the main kinase involved in CREB phosphorylation and contributes to the process of memory formation by regulating the classical cAMP/PKA/CREB pathway [44]. However, no-changes on PKA expression were observed in the hippocampus of APP/PS1 mice. Since BACE 1 is involved in the regulation of CREB phosphorylation, we suggest that DXI treatment could increase pCREB levels by targeting BACE1 [45].
We considered APP/PS1 transgenic mice the best model to study DXI effects and mechanisms involved in AD, since these animals show amyloid plaques deposits and TAU hyperphosphorylation. Notwithsantding, no animal model reproduces all the features involved in the human AD. Moreover, only female mice were used in this study, since it has been demonstrated that they present higher vulnerability to AD in comparison with age-matched males.
5. Conclusions
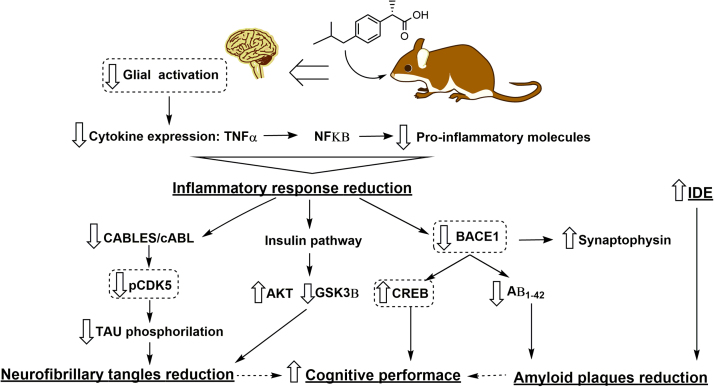
In conclusion, this study shows that DXI could exert multiple beneficial effects against AD, both decreasing the inflammatory response and the levels of amyloid plaques and neurofibrillary tangles, while enhancing the memory. Thus, our results provide more evidence showing that NSAIDs could reduce AD risk through multiple mechanisms. In addition, this is the first study reporting new targets that could clarify the mechanisms involved in DXI enhancement of memory and cognitive performance in a familial AD animal model. These mechanisms are TNFα/c-ABL/CDK5/TAU inhibition, insulin signaling activation and BACE1/SYP/p-CREB modulation (Fig. 6).
Fig. 6.
A proposed mechanism where Dexibuprofen could constitute a potential treatment for AD. DXI causes a reduction in neuroinflammatory process which decreases three key signaling pathways, CABLES/c-ABL, hippocampal insulin pathway and the β-secretase enzyme, which are directly involved in the production and metabolism of Aβ, TAU phosphorylation and memory improvement. These signaling pathways constitute an early target for the treatment of AD with DXI, probably in a presymptomatic stage which can explain the potential use of this drug in neurodegenerative diseases.
Competing interests
None declared.
Acknowledgements
This work was supported by Grants from the Spanish Ministry of Science and Innovation: MAT 2014-59134-R project, SAF-2016-33307, PI2016/01, CB06/05/0024 (CIBERNED) and the European Regional Development Founds. Research team from UB and URV belongs to 2014SGR-525 from Generalitat de Catalunya. MLG, and ESL belong to 2014SGR-1023. The author, ESL, acknowledges the support of the Spanish Ministry for the PhD scholarship FPI-MICINN (BES-2012-056083). The authors are grateful to Roxanne Rowles for the English revision of the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.06.003.
Appendix A. Supplementary material
Supplementary material
References
- 1.Bedse G., Di Domenico F., Serviddio G. Aberrant insulin signaling in Alzheimer's disease: current knowledge. Front. Neurosci. 2015;9:1–13. doi: 10.3389/fnins.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winblad B., Amouyel P., Andrieu S. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T., Kimura A., Hata S. Alternative cleavage mechanism of human APP by BACE1. Alzheimer's Dement. 2016:12. [Google Scholar]
- 4.Anand R., Gill K.D., Mahdi A.A. Therapeutics of Alzheimer's disease: past, present and future. Neuropharmacology. 2014;76:27–50. doi: 10.1016/j.neuropharm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 2016;8:1–14. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calsolaro V., Edison P. Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimers Dement. 2016;12:719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Viola K.L., Klein W.L. Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129:183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGeer P.L., Rogers J., McGeer E.G. Inflammation, antiinflammatory agents, and Alzheimer's disease: the last 22 years. J. Alzheimer's Dis. 2016;54:853–857. doi: 10.3233/JAD-160488. [DOI] [PubMed] [Google Scholar]
- 9.Weggen S., Eriksen J.L., Das P. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 10.Côté S., Carmichael P.-H., Verreault R. Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer's disease. Alzheimer's Dement. 2012;8:219–226. doi: 10.1016/j.jalz.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 11.McGeer P.L., Schulzer M., Edith G.M. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 12.Eriksen J.L., Sagi S. a., Smith T.E. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J. Clin. Investig. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sánchez-López E., Egea M.A., Cano A. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen– in vitro, ex vivo and in vivo characterization. Colloids Surf. B. 2016;145:241–250. doi: 10.1016/j.colsurfb.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 14.Ankarcrona M., Winblad B., Monteiro C. Current and future treatment of amyloid diseases. J. Intern. Med. 2016;280:177–202. doi: 10.1111/joim.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Liu X., Gong T. In vitro and in vivo investigation of dexibuprofen derivatives for CNS delivery. Acta Pharmacol. Sin. 2012;33:279–288. doi: 10.1038/aps.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin D.Q., Sung J.Y., Hwang Y.K. Dexibuprofen (S(+)-isomer ibuprofen) reduces microglial activation and impairments of spatial working memory induced by chronic lipopolysaccharide infusion. Pharmacol. Biochem. Behav. 2008;89:404–411. doi: 10.1016/j.pbb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira S.T., Clarke J.R., Bomfim T.R. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer's disease. Alzheimer's Dement. 2014;10:S76–S83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Puzzo D., Gulisano W., Palmeri A. Rodent models for Alzheimer's disease drug discovery. Expert Opin. Drug Discov. 2015;10:703–711. doi: 10.1517/17460441.2015.1041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trushina E., Zhang L., Zhang S. Restoration of axonal trafficking of mitochondria averts cognitive decline in mouse models of familial Alzheimer's disease. Alzheimer's Dement. 2014;10(4):238. [Google Scholar]
- 20.Jiao S.S., Bu X. Le, Liu Y.H. Sex dimorphism profile of Alzheimer's disease-type pathologies in an APP/PS1 mouse model. Neurotox. Res. 2016;29:256–266. doi: 10.1007/s12640-015-9589-x. [DOI] [PubMed] [Google Scholar]
- 21.Abad S., Camarasa J., Pubill D. Adaptive plasticity in the hippocampus of young mice intermittently exposed to MDMA could be the origin of memory deficits. Mol Neurobiol. 2016;53:7271–7283. doi: 10.1007/s12035-015-9618-z. [DOI] [PubMed] [Google Scholar]
- 22.Ettcheto M., Petrov D., Pedros I. Evaluation of neuropathological effects of a high-fat diet in a presymptomatic Alzheimer's disease stage in APP/PS1 mice. J. Alzheimer's Dis. 2016;54:233–251. doi: 10.3233/JAD-160150. [DOI] [PubMed] [Google Scholar]
- 23.Porquet D., Andrés-Benito P., Griñán-Ferré C. Amyloid and tau pathology of familial Alzheimer's disease APP/PS1 mouse model in a senescence phenotype background (SAMP8) Age. 2015;37:1–17. doi: 10.1007/s11357-015-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busquets O., Ettcheto M., Pallàs M. Long-term exposition to a high fat diet favors the appearance of β-amyloid depositions in the brain of C57BL/6J mice. A potential model of sporadic Alzheimer's disease. Mech. Ageing Dev. 2016 doi: 10.1016/j.mad.2016.11.002. in press. [DOI] [PubMed] [Google Scholar]
- 25.Pedrós I., Petrov D., Allgaier M. Early alterations in energy metabolism in the hippocampus of APPswe/PS1dE9 mouse model of Alzheimer's disease. Biochim. Biophys. Acta – Mol. Basis Dis. 2014;1842:1556–1566. doi: 10.1016/j.bbadis.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Ettcheto M., Petrov D., Pedrós I. Hypercholesterolemia and neurodegeneration. comparison of hippocampal phenotypes in LDLr knockout and APPswe/PS1dE9 mice. Exp. Gerontol. 2015;65:69–78. doi: 10.1016/j.exger.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.H., Li J., Huang Y. Gastroprotective effect and mechanism of amtolmetin guacyl in mice. World J. Gastroenterol. 2004;10:3616–3620. doi: 10.3748/wjg.v10.i24.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eikelenboom P., van Exel E., Hoozemans J.J. Neuroinflammation – an early event in both the history and pathogenesis of Alzheimer's disease. Neurodegener. Dis. 2010;7:38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Alloza M., Robbins E.M., Zhang-Nunes S.X. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Rojo L.E., Fernández J.A., Maccioni A.A. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer's Disease. Arch. Med. Res. 2008;39:1–16. doi: 10.1016/j.arcmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-López E., Ettcheto M., Egea M.A., Espina M., Calpena A.C., Folch J., Camins A., García M.L. New potential strategies for Alzheimer's disease prevention: pegylated biodegradable dexibuprofen nanospheres administration to APPswe/PS1dE9. Nanomedicine. 2017;13(3):1171–1182. doi: 10.1016/j.nano.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Ruan L., Kang Z., Pei G. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer's disease. Curr. Alzheimer Res. 2009;6:534–540. doi: 10.2174/156720509790147070. [DOI] [PubMed] [Google Scholar]
- 33.Habbas S., Santello M., Brecker D. Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell. 2015;163:1730–1741. doi: 10.1016/j.cell.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Laird F.M. BACE1, a major determinant of selective vulnerability of the brain to amyloid- amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baranello J., Robert L., Bharani K. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer's disease. Curr. Alzheimer Res. 2015;12:32–46. doi: 10.2174/1567205012666141218140953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J., Tan L., Wang H.F. Anti-Inflammatory drugs and risk of Alzheimer's Disease: an updated systematic review and meta-analysis. J. Alzheimers Dis. 2015;44:385–396. doi: 10.3233/JAD-141506. [DOI] [PubMed] [Google Scholar]
- 37.Iqbal K., Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: significance, etiopathogenesis, therapeutics and prevention: Alzheimer review series. J. Cell Mol. Med. 2008;12:38–55. doi: 10.1111/j.1582-4934.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitazawa M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J. Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carreras I., McKee A.C., Choi J.K. R-flurbiprofen improves tau, but not Aß pathology in a triple transgenic model of Alzheimer's disease. Brain Res. 2013;1541:115–127. doi: 10.1016/j.brainres.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patzke H., Maddineni U., Ayala R. Partial rescue of the p35-/- brain phenotype by low expression of a neuronal-specific enolase p25 transgene. J. Neurosci. 2003;23:2769–2778. doi: 10.1523/JNEUROSCI.23-07-02769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hantschel O., Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2004;5:33–44. doi: 10.1038/nrm1280. [DOI] [PubMed] [Google Scholar]
- 42.Trinchese F., Liu S., Battaglia F. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann. Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W., Bai M., Xi Y. Early memory deficits precede plaque deposition in APPswe/PS1dE9 mice: involvement of oxidative stress and cholinergic dysfunction. Free Radic. Biol. Med. 2012;52:1443–1452. doi: 10.1016/j.freeradbiomed.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 44.Teich A.F., Nicholls R.E., Puzzo D. Synaptic therapy in Alzheimer's Disease: a CREB-centric approach. Neurotherapeutics. 2015;12:29–41. doi: 10.1007/s13311-014-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y., Huang X., Zhang Y. Alzheimer's β-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of β-amyloid. J. Neurosci. 2012;32:11390–11395. doi: 10.1523/JNEUROSCI.0757-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material