Abstract
Recently published in vitro and in vivo findings strongly suggest that BBB impairment and increased risk for stroke by tobacco smoke (TS) closely resemble that of type-2 diabetes (2DM) and develop largely in response to common key modulators such oxidative stress (OS), inflammation and alterations of the endogenous antioxidative response system (ARE) regulated by the nuclear factor erythroid 2-related factor (Nrf2). Preclinical studies have also shown that nicotine (the principal e-liquid's ingredient used in e-cigarettes) can also cause OS, exacerbation of cerebral ischemia and secondary brain injury. Herein we provide evidence that likewise to TS, chronic e-Cigarette (e-Cig) vaping can be prodromal to the loss of blood-brain barrier (BBB) integrity and vascular inflammation as well as act as a promoting factor for the onset of stroke and worsening of post-ischemic brain injury. In addition, recent reports have shown that Metformin (MF) treatment before and after ischemic injury reduces stress and inhibits inflammatory responses. Recent published data by our group revealead that MF promotes the activation of counteractive mechanisms mediated by the activation of Nrf2 which drastically reduce TS toxicity at the brain and cerebrovascular levels and protect BBB integrity. In this study we provide additional in vivo evidence showing that MF can effectively reduce the oxidative and inflammatory risk for stroke and attenuate post-ischemic brain injury promoted by TS and e-Cig vaping. Our data also suggest that MF administration could be extended as prophylactic care during the time window required for the renormalization of the risk levels of stroke following smoking cessation thus further studies in that direction are warrated.
Abbreviations: ARE, Anti-Oxidant Response Element; BBB, Blood-Brain Barrier; CS, Cigarette Smoke; CSE, Cigarette Smoke Extract; 2DM, Type 2 Diabetes Mellitus; FJC, Fluoro-Jade C; FTC, Federal Trade Control; ISO, International Organization for Standardization; MF, Metformin; Nic, Nicotine; Cot, Cotinine; NQO-1, NAD(P)H: Quinone reductase I; Nrf2, Nuclear factor erythroid 2-related factor; PECAM-1, Platelet Endothelial Cell Adhesion Molecule-1; ROS, Reactive Oxygen Species; TJ, Tight Junction; tMCAO, ransient middle carotid artery occlusion; TS, Tobacco smoke; TTC, Triphenyl tetrazolium chloride; ZO-1, Zonulae occludentes-1
Keywords: Oxidative stress; Cigarette smoke; Vaping; Metformin; Ischemia, Blood Hemostasis; Blood-brain barrier; Inflammation; Nrf2
Graphical abstract
Highlights
-
•
Chronic cigarette and e-cigarette exposure downregulate throbomodulin and Nrf2.
-
•
Chronic CS and e-Cig exposure worsen stroke outcome in mice undergoing tMCAO.
-
•
Metformin ameliorate stroke outcomes in CS and e-Cig exposed mice undergoing tMCAO.
-
•
MF protective effect correlates with renormalization of Nrf2 levels.
1. Introduction
In the past decade a number of alternative vaping products have hit the market, rapidly gaining consumers among adults and, especially, adolescents [1]. Electronic nicotine delivery systems or e-cigarettes (e-Cigs) have become the sought-after product partly due to the belief that they are much safer than traditional cigarettes (e-Cig use has surpassed that of conventional cigarettes [2]). Moreover, tobacco smoking (TS) has been associated with vascular endothelial dysfunction [3], [4], [5], [6], [7], [8] in a causative and dose dependent manner [9] primarily related to the TS content of reactive oxygen species (ROS) [4], [10], nicotine [11], [12], [13], [14], [15], [16], and oxidative stress (OS) -driven inflammation [17]. Current scientific opinion considers OS-mediated pathways to play a major role in the pathogenesis of these disorders, especially stroke [18]. Preclinical studies (and data presented herein) have shown that nicotine (the principal ingredient of e-liquid) can cause OS, exacerbation of cerebral ischemia and secondary brain injury [19], [20], [21]. Likewise, chronic e-Cig vaping could be prodromal to cerebrovascular impairment and promote cerebrovascular conditions that favor the onset of stroke and post-ischemic brain injury [22]. The health impact of e-Cig vaping is currently unknown and the limited research and dearth of regulatory guidelines for the content of the vaping solution for e-Cigs (various harmful compounds including aldehydes, nitrosamines etc. have been detected in the e-Cig vapors [23], [24], [25], [26]) has become a critical public and regulatory concern. Further, we and others have found that TS promotes glucose intolerance and increases the risk of developing type-2 diabetes mellitus (2DM) [27], [28] with which it shares other pathogenic traits including the high risk of cerebrovascular and neurological disorders like stroke [29] via ROS generation, inflammation, and blood-brain barrier (BBB) impairment [30], [31], [32]. Recent findings [33], [34] from our group, supports an additive release pattern of angiogenic, oxidative and inflammatory factors by BBB endothelial cells in response to hyperglycemia (HG) and/or stroke conditions with comcomitant exposure to cigarette smoke extracts (CSE), thus suggesting the involvement of common pathogenic modulators of BBB impairment. To this end, Metformin (MF; a widely prescribed, firstline anti-diabetic drug) before and after stroke injury has been shown to reduce stress and inhibit the inflammatory responses [34], [35]. These data and recent published work by our group strongly suggests that MF activates counteractive mechanisms mediated by the activation of nuclear factor erythroid 2-related factor (Nrf2) [34], [36] which drastically reduce TS toxicity at the level of the BBB. Further, long term health impact and toxicity of e-Cigs are practically unknown.
TS alone is accountable for 434,000 deaths each year in United States (US) [37] and is a prodromal factor for the onset of stroke, Alzheimer's [9], [38], [39], depression [40] and vascular dementia [41]. OS, inflammation and the resulting BBB impairment [42], [43], [44] are the major prodromal factors for ischemic damage. Even upon smoking cessation, former chronic smokers remain at considerable risk for stroke for several years [45]. The only FDA approved drug treatment for ischemic stroke is tissue plasminogen activator (tPA) for which the therapeutic efficacy and safety is drastically decreased if not administered within 4.5 h from the onset of stroke [46]. Herein we provide alarming evidences (both in vitro and in vivo) demonstrating that e-Cigs are not safer than conventional tobacco products from a cerebrovascular perspective. Further we also show that MF can indeed provide partial protection against the negative impact of smoking and e-Cig vaping on stroke injury.
2. Methods
2.1. Materials and reagents
Sterile culture ware and molecular biology grade chemicals and reagents were obtained from standard commercial sources (Fisher Scientific, Pittsburgh, PA, USA; Sigma-Aldrich, St. Louis, MO, USA; and Bio-rad laboratories, Hercules, CA, USA). Fluorescein isothiocyanate (FITC; 3000–5000 MW; #FD4) and Rhodamine B isothiocyanate (RITC; 70,000 MW; #R9379) dextrans were purchased from Sigma-Aldrich, while Cascade Blue®-dextran (10,000 MW; #D-1976) was obtained from life technologies (Grand Island, NY, USA). Gel electrophoresis was carried out by using Mini-Protean® TGXTM gels 4–15% (#456–1084) from Bio-rad laboratories (Hercules, CA, USA). The antibodies were obtained from the following sources: Rabbit anti-ZO-1 (# sc-10804), mouse anti-ICAM-1 (#sc-18853), mouse anti-VCAM-1 (#sc-13160), mouse anti-PECAM-1 (#sc-376764), rabbit anti-Nrf2 (#sc-722), mouse anti-NQO1 (#sc-376023). Donkey anti-rabbit (#NA934) and sheep anti-mouse (#NA931) HRP-linked secondary antibodies were obtained from GE Healthcare (Piscataway, NJ, USA); goat anti-rabbit (#A11008, A21428) conjugated to Alexa Fluor® 488 and 555 respectively and anti-mouse (#A11001, A21422) conjugated to Alexa Fluor® 488 and 555 respectively from Invitrogen (Camarillo, CA, USA). Reference full flavor cigarettes (3R4F, 9.4 mg tar and 0.726 mg nicotine per cigarette), were obtained from the Center for Tobacco Reference Products (Kentucky Tobacco Research & Development Center, Lexington, KY) while e- cigarettes (Blu™, 24 mg/ml nicotine) were obtained from commercial sources.
2.2. Experimental design (In Vivo)
The animal protocol for this work was approved by the Institutional Animal Care and Use Committee, TTUHSC, Lubbock, Texas. C57BL/6 J male mice (age range 8–10 weeks old) were purchased from Jackson Laboratories. Mice where divided into 3 major groups including control, TS exposed and e-Cig exposed. Of these the TS and e-Cig exposed groups were divided into 2 subgroups including MF treated and Sham treated (saline). Mice were chronically exposed (via direct inhalation) to cigarette smoke (CS) of e-Cig vapor mixed with oxygenated air or oxygenated air alone, 6 times/day; 2 cigarettes/hour, 6–8 h/day, 7 days/week for 2 weeks following International Organization for Standardization/ Federal Trade Commission (ISO/FTC) standard smoking protocol (35 ml draw, 2 s puff duration, 1 puff per 60 s). CS and e-Cig vapor were separately and independently generated using a Single Cigarette Smoking Machines (SCSM, CH Technologies Inc., Westwood, NJ, USA) following previously published methods [47]. Mice were sacrificed and samples were collected for further analysis. MF (Sigma, St. Louis, MO, USA) was dissolved in sterile saline at a concentration of 30 mg/ml. MF was administered daily (via intraperitoneal Injections of doses of 200 mg/kg [34], [48], [49]) to mice either exposed to CS or e-Cig. At the end of the study, mice were sacrificed and samples (plasma and brain) were collected for further analysis. In a parallel study, a similar cohort of mice underwent identical CS or e-Cig exposures and MF treatments (200 mg/kg/day IP) for 10 days followed by tMCAO (see below for details).
2.3. Transient Middle Cerebral Artery Occlusion (tMCAO)
Brain ischemic injury by tMCAO in TS and e-Cig exposed C57BL/6 J male mice was performed as previously reported with slight modifications. Surgery was performed using a Zeiss OPMI pico I surgical microscope (Carl Zeiss GmbH, Jena, Germany). Temperature was maintained at 37 °C, controlled by the thermostatic blanket (TC-1000 Temperature Controller, CWE, USA). Mice were anesthetized with 4% and maintained at 1–1.5% isoflurane with a facemask. The cerebral blood flow was continuously monitored throughout the surgery to confirm the occlusion and reperfusion of the brain by using non-invasive, real-time microcirculation imaging, Pericam PSI system (Perimed Inc., Marble Falls, TX) placed over the exposed skull in the territory of the left middle cerebral artery (MCA) perfusion area. A midline incision was made at the neck about 1.5 cm long. The left carotid bifurcation, external carotid artery (ECA) and common carotid artery (CCA) were isolated from the adjacent tissue. After occlusion of CCA using a micro clip, the left ECA was ligated, coagulated, and cut distally to the cranial thyroid artery. 6–0 nylon monofilament with a silicone coated tip (0.20–0.23 mm; Doccol Corporation) was gently introduced up to ~8.5–9 mm to block the origin of the MCA. IR injury was produced by tMCAO (30 min) according to established procedures in Dr. Abbruscato's laboratory [50]. After 30 min occlusion, the suture was withdrawn up to the left carotid bifurcation to restore blood flow, i.e. reperfusion. Animals that failed to recover at least 80% of baseline within 10 min after reperfusion were excluded from the experimental group. After reperfusion, neurological evaluation using several sensory-motor tests was carried out. These include neurologic deficit score on a four-point scale [50]. Once the brain tissue was resected, coronal sections (10 µm thick) from the contralateral and ipsilateral hemispheres were also analyzed by fluorescent microscopy to assess neuronal degeneration by using fluoro-Jade C (FJC) which is an anionic dye that specifically stains soma and neurites of degenerating neurons [51].
2.4. Behavioral tests
Neurological deficits where assessed using a five-point scale as previously described [50]. In brief neurologic evaluation was carried out 96 h after reperfusion. The mouse was held gently by tail, suspended at about 50 cm above the bench and monitored for forelimb flexion. Extension of both forelimbs straight toward the floor was considered absence of a neurologic deficit and score 0 is assigned to the animal. Score of 1 (mild neurological deficit) was assigned to animals flexing the forelimb contralateral to the injured hemisphere. Mice were then placed on a large sheet of soft plastic coated paper to make sure that were able to grip firmly by their claws. While holding from the tail mice were pushed behind the shoulder with gentle lateral pressure until the forelimb slid several inches. Score of 2 (moderate neurological deficit) was assigned to animals showing decreased resistance to lateral push and forelimb flexion. A score of 3 (severe neurological deficit) was assigned to animals showing the same behavior as for score 2, plus a circling movement. A score of 4 was assigned to animal showing severe rotation progressing into barreling, loss of walking or righting reflex. A score of 5 is assigned to animals that are comatose or moribund. All behavioral assays were carried out between 5:00 p.m. and 10:00 p.m.
2.5. Cell culture
C57BL/6 mouse primary brain endothelial cells (mBMEC, P3, #C57-6023) were obtained from Cell Biologics (Illinois, USA). mBMEC (at passages 4) were seeded on gelatin coated cell culture flasks or glass chamber slides, cultured in recommended medium (M1168) and maintained at 37 °C with 5% CO2 exposure. The culture medium was changed every other day until the cells reached confluency. Phase contrast microscopy and the expression of characteristic phenotypic markers confirmed the monolayer integrity of both mBMEC at confluency. Established cultures were exposed to 5% CSE of e-CSE for 24 h (model of mainstream smoke exposure previously described by our group [52])
2.6. Transwell cell culture setup
Clear polyester transwell inserts (Costar® Transwell® polyester membrane cell culture inserts 0.4 µm pore membrane insert; #3470) were seeded with mBMEC cells (P4) on the luminal side and grown in M1168 medium. The wells were coated with matrigel prior to seeding. Phase contrast microscopy and Trans-endothelial electrical resistance (TEER) measurement were used to assess the confluence and integrity of the cell monolayers respectively.
2.7. Soluble cigarette smoke and e-Cig extract preparation
Soluble CS (CSE) and e-Cig (e-CSE) extracts was prepared according to the FTC standard smoking protocol (35 ml draw, 2 s puff duration, 1 puff per 60 s) using a Single Cigarette Smoking Machine (SCSM, CH Technologies Inc., Westwood, NJ, USA) according to previously published methods [53], [54]. Extracts were prepared fresh for each cycle and used in culture at a 5% dilution [34], [55].
2.8. Preparation of protein extracts
Cells were lysed using either RIPA lysis buffer or subcellular protein fractionation kit for cultured cells (Thermo scientific, # 78840) as per manufacturer's guidelines, such that total, nuclear, cytosolic and membrane fractions were collected. For brain tissue lysis, a weighed amount of minced brain was taken in a tube to which ice-cold RIPA lysis buffer (containing protease and Phosphatase inhibitors,15 µl/mg of brain tissue weight) was added. It was homogenized and allowed to stand on a shaker for 30 min. Total protein extract was collected by centrifugation at 14000 g for 30 mins. Samples were then aliquoted and stored at −80 °C until needed for protein expression analysis by western blotting.
2.9. Western blotting
Proteins expression was quantified by using Pierce BCA Protein Assay Kit (Thermo Scientific, # 23225). Samples (15–30 μg for cell lysates, 60–90 μg for tissue lysates) were then prepared following method as described in our previous lab report [52]. The denatured samples were run on SDS-PAGE (4–15% gradient gel) and transferred to PVDF membranes for further blotting. Band densities were analyzed by Image Studio Lite Ver 3.1 and calculated as percentage change over control protein expression.
2.10. Immunofluorescence
mBMEC cells were seeded in two-well chamber slides, grown and treated as mentioned earlier then fixed (using 16%, methanol free formaldehyde diluted 1 in 4 in 1X PBS; from Polysciences Inc. # 18814), washed and permeabilized (using 0.02% Triton 100X). Cells were then blocked with 5% goat serum in PBS (blocking buffer) at room temperature for one hour and incubated with primary antibodies prepared in blocking buffer for overnight at 4 °C. The following day, cells were washed, stained with Alexa Fluor® 488 or 555 conjugated goat anti-rabbit or anti-mouse antibodies or vice-versa at RT and mounted with DAPI in prolonged gold anti-fade mounting media (Invitrogen, OR, USA). Mounted slides upon overnight drying were observed under EVOS digital inverted fluorescence microscope. Cells stained only with secondary antibodies were used as negative controls [52].
2.11. ELISA
Quantitative determination of thrombomodulin in plasma samples collected from mice were analyzed by Quantikine ELISA kits (R&D systems, Minneapolis, MN, USA) according to the manufacturer's protocol.
2.12. BBB integrity
BBB integrity in transwell systems was assessed using 2 different methods: 1) Through TEER measurement (expressed as Ω cm2) using an EVOM 2 chamber (World Precision Instruments, Sarasota, FL, USA) as previously described [56]; 2) by permeability assessment (lumen to albumen) to a mixture of labeled dextrans in PBS (FITC ~4 kDa, 10 mg/ml; Cascade Blue ~10 kDa, and RITC ~70 kDa, 10 mg/ml) [57]. Dextrans were added to the luminal compartment of the transwells prior and then after termination of the treatment cycles. 50 µl of media sample were collected at time 0, 5, 15 and 30 min from the abluminal compartment and replaced with equal volumes of fresh media to maintain appropriate sink conditions. Media samples without dextran and that from abluminal compartments of cell free inserts with dextran added to the luminal compartment were taken into consideration during calculations. The permeability measurements were reported as percentage of controls.
2.13. Statistical analysis
Data from all experiments were expressed as standard deviation (SD) and analyzed by one-way ANOVA using GraphPad Prism 6 Software Inc. (La Jolla, CA, USA). Post hoc multiple comparison tests were performed with Tukey's or Dunnett's test. P values < 0.05 were considered statistically significant. Results are reported as mean ± SEM.
3. Results
3.1. Effect of e-Cig and TS extract on mouse primary brain microvascular endothelial cells
Comparative data from side by side experiments investigating the impact of e-Cig (Blu™; 24 mg/ml nicotine) vs. TS (3R4F research cigarettes containing 9.4 mg tar and 0.726 mg nicotine/cigarette and equivalent to full flavor brands; University of Kentucky) on mouse primary brain microvascular endothelial cells (mBMEC). Cellular oxidative stress following e-Cig and TS exposure for 24 h was assess using a fluorogenic probe (CellROX, absorption/emission maxima at ~644/665 nm). Our data revealed that OS promoted by the e-Cigs extract (e-CSE) was not dissimilar from that induced by cigarette smoke extract (CSE from 3R4F; Fig. 1A) and both were remarkably higher than control. Nrf2, one of the major transcription factors regulating the anti-oxidant defense response, was similarly strongly activated by e-Cig and TS as also demonstrated by the upregulation of its downstream signaling molecule NAD(P)H quinone dehydrogenase 1(NQO-1; Fig. 1B). In addition, nicotine (the main component of the e-liquids) released in the e-Cig vapors and rapidly distributed across the cerebrovascular system, effectively induces a dose and time dependent antioxidative stress response from BBB endothelial cells (overexpression and nuclear translocation of Nrf2 (Fig. 1C)).
Fig. 1.
Effect of e-Cig and TS extract on Mouse Primary Brain Microvascular Endothelial cells. A) CellRox staining demonstrating cellular oxidative stress on mBMEC promoted by TS and e-Cig exposure. B) Immunofluorescence & Western blot analysis emphasizing nuclear translocation and activation of Nrf2 and over expression of downstream detoxifying molecule NQO-1 in TS and e-Cig exposed BBB endothelial layers. C) Dose and time dependent effects of nicotine on the BBB endothelium. Note that cellular OS in response to nicotine exposure is both dose and time dependent. “*” =P<0.05; “**” P<0.01; compared to control. n = 4 biological replicates.
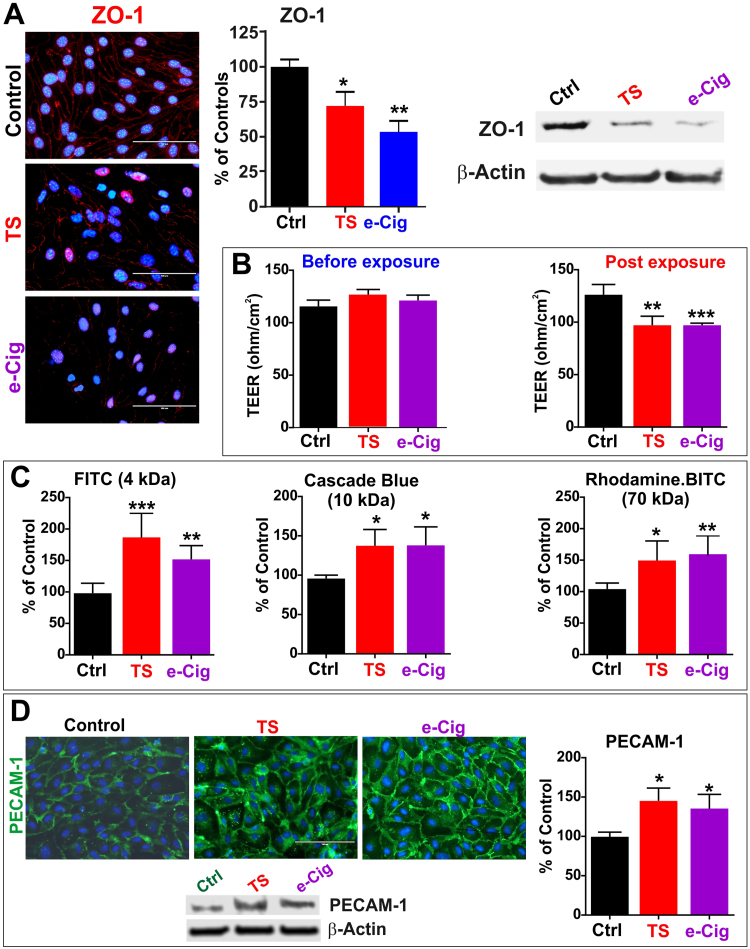
3.2. e-Cig and TS impact BBB integrity and promote inflammation
Likewise TS, e-Cig extracts downregulated the expression level of the tight junctional protein ZO-1 (Fig. 2A) as demonstrated by immunofluorescent analysis of the cell monolayers and confirmed by WB analysis (p <0.05 and p<0.001 for TS and e-Cig respectively vs controls). These data suggest an overall impairment of BBB integrity which was then confirmed in vitro using a transwell setup and TEER measurements (Fig. 2B). Chronic exposure to e-Cig and TS extracts (3 cycles of 24 h each) caused a significant decrease of TEER when compared against controls (p <0.001 and p<0.0001 for TS and e-Cig respectively vs controls). Loss of BBB integrity was further confirmed by permeability assays to fluorescent dextrans (ranging from 4 to 70 kDa; see Fig. 2C). In each case, BBB permeability of bMVEC monolayers on transwell support either exposed to TS or e-Cig were statistically significantly higher than controls and no meaningful differences were observed when comparing TS vs. e-Cig exposed cultures. Similarly, expression levels of the pro-inflammatory adhesion molecule PECAM-1 was upregulated in both TS and e-Cig exposed bMVEC (Fig. 2D) as demonstrated by immunofluorescent and WB analysis (p<0.05 for TS and e-Cig vs controls). These data suggest that from a functional point of view of the BBB, e-Cig exposure is as harmful as that of TS and both elicit a significant inflammatory response. In fact, similar correlative results were observed in vivo when we analyzed the expression levels of the vascular adhesion molecules PECAM-1, ICAM-1 and VCAM-1 in brain homogenates of mice chronically exposed to e-Cig or TS (via direct inhalation) against control animals (Fig. 3). Levels of these inflammatory markers were all significantly upregulated in TS and e-Cig exposed mice while no significant differences were observed when comparing TS against e-Cig exposed groups.
Fig. 2.
Effect of e-Cig and TS extract on BBB integrity. A) Immunofluorescence staining: demonstrating a comparable downregulation of ZO-1 in TS and e-Cig exposed BBB endothelial cell monolayers. B) TEER measurement prior and after treatments demonstrated a significant loss of BBB integrity in TS and e-Cig treated cultures vs. controls. The negative impact of the barrier integrity was further corroborated by in increased permeability to fluorescently labeled dextran molecules ranging from 4 to 70 kDa (C). D) Expression levels of the inflammatory marker PECAM-1 was also substantially increased by TS and e-Cig exposure as demonstrated by the immunofluorescence and WB analysis. “*” =P<0.05; “**” P<0.01; “***” P<0.001 compared to control. n = 4 biological replicates.
Fig. 3.
Vascular inflammatory response in vivo to TS and e-Cig exposure. Level of expression of PECAM-1, VCAM-1 and ICAM-1 were assessed in brain homogenates of mice chronically exposed to either TS or e-Cig. Note how these levels are comparatively higher compared to controls. “*” =P<0.05; “**” P<0.01 compared to control. N=6 mice per group.
3.3. Metformin provides partial protection against the harmful effects of smoking and e-Cig exposure on stroke injury
Animals were chronically exposed to TS or e-Cig vapors generated according to the FTC standard protocol and forced directly into two airtight smoking chambers housing the mice (4 mice/cage) by a dedicated CSM-SCSM cigarette smoking machine (CH Technologies, Westwood, NJ; see Fig. 4A). The smoking inlet is also dually connected to a feeding tube and a ventilator systems supplying O2 at atmospheric levels. In vivo results clearly show that TS exposure significantly downregulates circulating level of thrombomodulin (Fig. 4B), suggesting a pro-coagulant pre-disposition and increased risk of stroke in these animals. Furthermore, analyses of whole brain tissue homogenates also clearly show a parallel downregulation of Nrf2 expression. Surprisingly, e-Cig exposed mice also showed a downregulation of thrombomodulin and Nrf2 comparable (or slightly enhanced) to that elicited by 3R4F cigarettes. This was further confirmed by a similar trent of expression of its downstream target NQO-1.
Fig. 4.
Effect of smoking and e-Cig exposure on stroke injury. A) Direct inhalation of side stream smoke/e-Cig aerosol using a CSM-SCSM cigarette smoking machine. B) Reduced thrombomodulin plasma levels and total brain content of Nrf2 in TS and e-Cig exposed mice are paired by an increased expression of the vascular inflammatory marker PECAM-1 while the expression levels of Nrf2 and his downstream targets NQO-1 are both significantly downregulated. C) TTC-stained mouse brain slice after experimental stroke (tMCAO, 30 min occlusion, 24 h reperfusion) in mice chronically exposed to either TS (C1) or e-Cig (C2). Assessment of brain damage in either condition was matched by a correlative neurological evaluation 24 h-post reperfusion. Note that in both cases, MF treated mice had a statistically significant better outcome which correlated with renormalization of Nrf2. Panel C3 shows the comparative analysis of TNF-α levels in blood plasma including the baseline level (Ctrl BASE), stroke control (Ctrl NS) and mice chronically exposed to TS or e-Cig w/wo Metformin. D) TTC and FJC overlay in ipsilateral and contralateral brain slices. NS= non-smoke; TS=Tobacco smoker; e-Cig= e-cigarette vaper. N=6 mice per group; “*” =P<0.05; “**” P<0.01; “***” = P<0.001; “****” =P<0.0001 compare to control-NS mice; “#” =P<0.05, “##” =P<0.01 compare to MF treated mice. For figure C3, “#” =P<0.05, “##” =P<0.01 compare to stroke controls and “+” =P<0.05, “++” =P<0.01 versus mice exposed to either TS or e-Cig receiving MF.
Analysis of post stroke coronal brain sections revealed an increased brain infarct area (using 2, 3, 5-triphenyltetrazolium chloride-TTC staining) and worse neurological deficits in mice chronically exposed (2 weeks; direct inhalation) to TS (Fig. 4C1) and then subjected to 30 min tMCAO and 24 h of reperfusion compared to control animals. Our data also showed a similar trend (infarcted brain area and neurological deficits) In mice chronically exposed to e-Cig vapors (same protocol as for TS) (Fig. 4C2), thus validating our in vitro results. Of striking clinical interest, animals who received daily doses of MF along with either TS or e-Cig exposure, exhibited a significantly better stroke outcome when compared to their untreated counterparts (TS and e-Cig exposed animals not receiving MF). This is clearly demonstrated by the “reduced” infarct area (compared to the corresponding TS and e-Cig exposed animals) and better neurological scores (Fig. 4C1 and C2). Also of significant interest is the fact that brain levels of Nrf2 in TS and e-Cig exposed mice receiving MF were significantly higher (p<0.05) than those measured in their corresponding test group not receiving MF.
Inflammation is also a determinant of stroke outcome and has shown in Fig. 4C3, measurement of the pro-inflammatory cytokine TNF-α in blood plasma samples of our cohort of animals clearly revealed that MF treated mice exposed to TS or e-Cig exhibited a reduce upregulation of TNF-α versus their untreated counterparts when compared against the baseline level (Ctrl BASE - control mice not exposed to TS or e-Cig and not undergoing tMCAO; p<0.05 and p<0.001 respectively).
Current evaluation of infarct size with TTC staining was limited in discerning more subtle effects such as degenerating neurons, thus we confirmed TTC staining with Fluoro-Jade staining (FJC) which is represented in Fig. 4D. FJC stains effectively allowed us to observe degenerating (penumbral) neurons thus, providing a subtler evaluation of the changes in penumbral regions compared to TTC.
4. Discussion and Conclusion
Tobacco smoking is associated with vascular endothelial dysfunction [3], [4], [5], [6], [7], [8] in a causative and dose dependent way [9] primarily related to the TS content of reactive oxygen species (ROS) [4], [10], nicotine [11], [12], [13], [14], [15], [16], and inflammation [17]. Current scientific opinion considers ROS/oxidative stress (OS)-mediated pathways to play a major role in the pathogenesis of these disorders, especially stroke [18]. Recent published findings [33], [34] from our group support the idea of an additive release pattern of angiogenic and inflammatory factors (besides activation of common anti-oxidant mechanisms) by BBB endothelial cells in response to stroke conditions with comcomitant exposure to cigarette smoke extracts. Further, the Center for Tobacco Products (CTP) has revealed an alarming rise of e-cigarettes (e-Cigs) consumption among adolescents and adults [1]. The health impact of chronic e-Cigs vaping is currently unknown and despite the common belief that e-Cigs are free from the thousands of toxicants present in traditional tobacco-based products, various harmful compounds (aldehydes, nitrosamines etc.) have been detected in the e-Cig vapors [23], [24], [25], [26]. Further, preclinical studies (and data presented herein) have shown that nicotine (the principal e-liquid's ingredient) can cause OS, exacerbation of cerebral ischemia and secondary brain injury [19], [20], [21]. Likewise, chronic e-Cig vaping could be prodromal to cerebrovascular impairment and promote the onset of stroke and post-ischemic brain injury as also suggested by our findings. Our data suggest that e-Cig toxicity in respect to the BBB and the cerebrovascular system is clearly as dangerous as TS and while e-Cig may not contain/release the many toxic compounds and carcinogens identified in TS, their OS potential and possible harmful impact on the BBB is not dissimilar from that of full flavor conventional cigarette (see Fig. 1). In fact, in addition to cellular oxidative stress also indicated by the increased expression and nuclear translocation of Nrf2 (one of the major transcription factors regulating the anti-oxidant defense response [58]) and its downstream effector protein downstream signaling molecule NAD(P)H quinone dehydrogenase 1(NQO-1 which exerts acute detoxification and cytoprotective functions [34], [59]), e-Cig exposure also promoted loss of BBB integrity (see Fig. 2) and cellular inflammation (see Fig. 3). All the negative effects were comparable to those observed for TS. Nicotine can certainly play a major role in e-Cig toxicity since it can induce cellular oxidative stress (see Fig. 1C). It has been shown that 100 puffs of “first hand aerosol” from a single e-cigarette (4–5 sessions of vaping) can expose the vaper to up to 24 mg of nicotine [60] which rapidly distributed across the cerebrovascular system. To reach similar levels of nicotine exposure it would require smoking ≈ 15 traditional cigarettes [61]. While nicotine may be responsible for some of these pro-oxidative and inflammatory effects, we cannot rule out the possibility that other factors (such as the solvent contained in the e-liquid) may play an important role as well. In fact, in addition to propylene glycol and glycerin, e-liquids contain a number of aldehydes (including formaldehyde, benzaldehyde, acrolein, etc.) which form during the heating process necessary to vaporize the nicotine. Another recent but highly controversial report suggests that the reaction between the e-liquid vehicle (propylene glycol and glycerol) and formaldehyde (a known degradation byproduct of propylene glycol generated by heat) during vaping at high voltage (5.0 V) can lead to the formation of highly carcinogenic hemiacetal in significant concentration. Hemiacetals release formaldehyde and are commonly used as biocides [62]. Given these possibilities, it is conceivable that these substances could negatively impact the cerebrovascular system, thus requiring further and detailed future investigations. This is also important in view of the fact that a detailed side by side comparison between the cigarette smoke composition (which contains over 7000 different substances identified so far) versus e-Cig vapor as not be done yet although limited information on this topic are now becoming available [63], [64].
It is well know that TS exposure is a major prodromal factor for the onset of stroke [65], however the impact of e-Cig vaping on the risk/severity of stroke and neurological impairments has never been investigated before. Our in vitro data do suggest that, from a cerebrovascular perspective, e-Cig and TS have a similar negative impact, thus one of the major objective of this work was to assess whether this similarity extended also to ischemia and stroke outcome. Our in vivo data strongly suggest that indeed e-Cig vaping may act as a prodromal factor for stroke from a thrombolytic perspective. This is demonstrated by the decrease level of the anticoagulant factor thrombomodulin as well as increased expression level of the pro-inflammatory cytokine TNF-α (see Fig. 4B) in animals chronically exposed for 2 weeks to e-Cig vapors. Results were comparable to that observed in animals undergoing TS exposure and were substantially worse (lower thrombomodulin and higher TNF-α expression) than control animals. In addition e-Cig (and TS) worsened stroke injury (as demonstrated by the increased infarct size) and stroke outcome (assess through neurological evaluations; see also Fig. 4C1 and C2). Together, these data seems to confirm our central hypothesis that excessive OS caused by TS and e-Cigs dysregulation of the cellular antioxidant response system is a likely underling mechanism prodromal to cerebrovascular toxicity and highten risk and/or severity of stroke.
MF is an oral antidiabetic drug belonging to the biguanide class which works by inhibiting gluconeogenesis, thus suppressing hepatic glucose production [66]. It is currently the first-line drug of choice for the treatment of 2DM and has been investigated for other diseases where insulin resistance may be an important factor. Several experimental studies in vivo suggest that MF reduces cardiac ischemia/reperfusion injury, improves cardiac function, reduces the infarct size and reduces the percentage of apoptotic cardiomyocytes [67], [68], [69]. Although these studies clearly prove the protective effects of MF on cardiovascular risk/mortality, new evidence has emerged strongly supporting a possible therapeutic use of this drug in stroke prevention and protection [70]. To this end clinical reports have shown that MF treatment before and after ischemic injury reduces stress and inhibits inflammatory responses [35] and in a recent published study we revealed that MF activates counteractive mechanisms which drastically reduce TS toxicity at the cerebrovascular and BBB levels [34]. These beneficial effects appear to be mediated by MF activation of Nrf2 [36]. Given this premise, we wanted to assess the viability and effectiveness of MF treatment to prevent/reduce BBB damage and subsequent ischemic stroke injuries promoted by the chronic conditions of TS and possibly e-Cig vaping. Our results do support a protective role for MF in reducing the impact of TS and e-Cig on stroke including the risk of onset (as previously demonstrated [34]) as well stroke outcome (including reduced infarct area and better neurological evaluation). Our in vivo data also confirmed and strenghtened our previous in vitro observation and published results [71] that this protective effect is correlated with an enhancement of Nrf2. In fact, animals receiving MF along with TS or e-Cig exposure exibited a much lesser reduction of Nrf2 and a better stroke outcome. This is further confirmed by the fact that the corresponding post-ischemic plasma levels of TNF-α were significantly reduced in animal exposed to TS and e-Cig receiving MF versus those that did not receive MF (Fig. 4C3).
All togheter, these data well correlate with a number of experimental studies strongly supporting a possible therapeutic use of this drug in stroke prevention and protection [70]. For example, MF was shown to inhibit stroke-induced nitrosative signaling while promoting post-stroke reparative angiogenesis [72]. Recent in vivo studies have shown neuroprotective effects of MF associated with antioxidative defense in cerebral ischemia [73]. Furthermore, MF has been shown to attenuate BBB disruption in mice following middle cerebral artery occlusion and this neuroprotective effect was abolished in the presence of a selective AMPK inhibitor [74]. It is important to note that the dose, timing and route of MF administration plays a key role in its ability to be neuroprotective/restorative during stroke [75], [76] and this aspect should be further investigated in the near future. This is of outmost importance since although chronic smoking (and likely vaping) carry high risks for cardiovascular diseases (CVD) and stroke [77], [78], [79], care treatment(s) only begins upon clinical manifestation of the disease. Further, even upon smoking cessation, former chronic smokers remain at considerable risk for stroke for several years [45]. From this point of view, “repurposing” or extending the use of MF to counteract BBB damage and/or exacerbation of post-ischemic neuronal damage by TS/e-Cig could provide a shift and extension of practice paradigms and enable a rapid transition to prophylactic and/or therapeutic care for high risk stroke patients.
In addition, MF has been shown to protect against apoptotic cell death in primary cortical neurons, promote neurogenesis, and it is a potential therapy for injured or degenerating nervous system in cellular and animal models [80], [81], [82], [83]. The effect we observed however. seems to have a vascular component as well (e.g., thrombomodulin increase and reduce inflammation) or specific to the vascular endothelium (e.g. BBB integrity). MF has been shown to cross the BBB and differentially accumulate in various brain regions [84] however, as to the mode of passage MF as low passive lipoidal permeability and although passive diffusion may not completely excluded, MF has also been demonstrated to be a substrate for the Organic Cation Transporter 3 (OCT3) which is also expressed at the BBB [85]. This could explain how MF also attenuates CNS-based inflammation.
It is also important to note that while acute TS and e-Cig exposure initially upregulates Nrf2 (as a demonstration of oxidative stress and activation of the antioxidative response system), the effect is reversed in a condition of prolonged (chronic) exposure where the antioxidative response system becomes impaired [55], [71]. This phenomenon in also confirmed by our current results when we compare the in vitro (acute TS and e-Cig exposure; see also Fig. 1B) vs. In vivo (chronic exposure; see also Fig. 4B) data concerning Nrf2 expression.
Conflict of interest statement
The authors confirm that they have no competing interests.
Acknowledgements
Funding: This work was supported by the National Institutes of Health/National Institute on Drug Abuse R01-DA029121 to Drs. Luca Cucullo and Thomas Abbruscato.
Contributor Information
Mohammad A. Kaisar, Email: md.a.kaisar@ttuhsc.edu.
Heidi Villalba, Email: heidi.villalba@ttuhsc.edu.
Shikha Prasad, Email: prasad.shikha@ttuhsc.edu.
Taylor Liles, Email: taylor.liles@ttuhsc.edu.
Ali Ehsan Sifat, Email: ali.sifat@ttuhsc.edu.
Ravi K. Sajja, Email: ravi.sajja@ttuhsc.edu.
Thomas J. Abbruscato, Email: Thomas.Abbruscato@ttuhsc.edu.
Luca Cucullo, Email: luca.cucullo@ttuhsc.edu.
References
- 1.Youth Tobacco Use: Results from the 2014 National Youth Tobacco Survey. FDA. Ref Type: Report, 2016.
- 2.Hildick-Smith G.J., Pesko M.F., Shearer L., Hughes J.M., Chang J., Loughlin G.M. A practitioner's guide to electronic cigarettes in the adolescent population. J. Adolesc. Health. 2015;57:574–579. doi: 10.1016/j.jadohealth.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Hossain M., Sathe T., Fazio V., Mazzone P., Weksler B., Janigro D. Tobacco smoke: a critical etiological factor for vascular impairment at the blood-brain barrier. Brain Res. 2009;1287:192–205. doi: 10.1016/j.brainres.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naik P., Fofaria N., Prasad S., Sajja R.K., Weksler B., Couraud P.O. Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci. 2014;15:51. doi: 10.1186/1471-2202-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H.W., Chien M.L., Chaung Y.H., Lii C.K., Wang T.S. Extracts from cigarette smoke induce DNA damage and cell adhesion molecule expression through different pathways. Chem. Biol. Interact. 2004;150:233–241. doi: 10.1016/j.cbi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Nagy J., Demaster E.G., Wittmann I., Shultz P., Raij L. Induction of endothelial cell injury by cigarette smoke. Endothelium. 1997;5:251–263. doi: 10.3109/10623329709052590. [DOI] [PubMed] [Google Scholar]
- 7.Noronha-Dutra A.A., Epperlein M.M., Woolf N. Effect of cigarette smoking on cultured human endothelial cells. Cardiovasc. Res. 1993;27:774–778. doi: 10.1093/cvr/27.5.774. [DOI] [PubMed] [Google Scholar]
- 8.Raij L., Demaster E.G., Jaimes E.A. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J. Hypertens. 2001;19:891–897. doi: 10.1097/00004872-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Gill J.S., Shipley M.J., Tsementzis S.A., Hornby R., Gill S.K., Hitchcock E.R. Cigarette smoking. A risk factor for hemorrhagic and nonhemorrhagic stroke. Arch. Intern. Med. 1989;149:2053–2057. doi: 10.1001/archinte.149.9.2053. [DOI] [PubMed] [Google Scholar]
- 10.Panda K., Chattopadhyay R., Ghosh M.K., Chattopadhyay D.J., Chatterjee I.B. Vitamin C prevents cigarette smoke induced oxidative damage of proteins and increased proteolysis. Free Radic. Biol. Med. 1999;27:1064–1079. doi: 10.1016/s0891-5849(99)00154-9. [DOI] [PubMed] [Google Scholar]
- 11.Hanna S.T. Nicotine effect on cardiovascular system and ion channels. J. Cardiovasc. Pharmacol. 2006;47:348–358. doi: 10.1097/01.fjc.0000205984.13395.9e. [DOI] [PubMed] [Google Scholar]
- 12.Wang L., Kittaka M., Sun N., Schreiber S.S., Zlokovic B.V. Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J. Cereb. Blood Flow Metab. 1997;17:136–146. doi: 10.1097/00004647-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Paulson J.R., Yang T., Selvaraj P.K., Mdzinarishvili A., Van der Schyf C.J., Klein J. Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J. Pharmacol. Exp. Ther. 2010;332:371–379. doi: 10.1124/jpet.109.157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heeschen C., Weis M., Cooke J.P. Nicotine promotes arteriogenesis. J. Am. Coll. Cardiol. 2003;41:489–496. doi: 10.1016/s0735-1097(02)02818-8. [DOI] [PubMed] [Google Scholar]
- 15.Catanzaro D.F., Zhou Y., Chen R., Yu F., Catanzaro S.E., De Lorenzo M.S. Potentially reduced exposure cigarettes accelerate atherosclerosis: evidence for the role of nicotine. Cardiovasc. Toxicol. 2007;7:192–201. doi: 10.1007/s12012-007-0027-z. [DOI] [PubMed] [Google Scholar]
- 16.Das S., Gautam N., Dey S.K., Maiti T., Roy S. Oxidative stress in the brain of nicotine-induced toxicity: protective role of Andrographis paniculata Nees and vitamin E. Appl. Physiol. Nutr. Metab. 2009;34:124–135. doi: 10.1139/H08-147. [DOI] [PubMed] [Google Scholar]
- 17.Arnson Y., Shoenfeld Y., Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2009 doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Cojocaru I.M., Cojocaru M., Sapira V., Ionescu A. Evaluation of oxidative stress in patients with acute ischemic stroke. Rom. J. Intern. Med. 2013;51:97–106. [PubMed] [Google Scholar]
- 19.Li C., Sun H., Arrick D.M., Mayhan W.G. Chronic nicotine exposure exacerbates transient focal cerebral ischemia-induced brain injury. J. Appl. Physiol. 1985;120:328–333. doi: 10.1152/japplphysiol.00663.2015. [DOI] [PubMed] [Google Scholar]
- 20.Bradford S.T., Stamatovic S.M., Dondeti R.S., Keep R.F., Andjelkovic A.V. Nicotine aggravates the brain postischemic inflammatory response. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H1518–H1529. doi: 10.1152/ajpheart.00928.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulson J.R., Yang T., Selvaraj P.K., Mdzinarishvili A., Van der Schyf C.J., Klein J. Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J. Pharmacol. Exp. Ther. 2010;332:371–379. doi: 10.1124/jpet.109.157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah K.K., Boreddy P.R., Abbruscato T.J. Nicotine pre-exposure reduces stroke-induced glucose transporter-1 activity at the blood-brain barrier in mice. Fluids Barriers CNS. 2015;12:10. doi: 10.1186/s12987-015-0005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillman I.G., Kistler K.A., Stewart E.W., Paolantonio A.R. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul. Toxicol. Pharmacol. 2016;75:58–65. doi: 10.1016/j.yrtph.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Goniewicz M.L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchiyama S., Senoo Y., Hayashida H., Inaba Y., Nakagome H., Kunugita N. Determination of chemical compounds generated from Second-generation E-cigarettes using a Sorbent Cartridge Followed by a Two-step Elution Method. Anal Sci. 2016;32:549–555. doi: 10.2116/analsci.32.549. [DOI] [PubMed] [Google Scholar]
- 26.Varlet V., Farsalinos K., Augsburger M., Thomas A., Etter J.F. Toxicity assessment of refill liquids for electronic cigarettes. Int. J. Environ. Res. Public Health. 2015;12:4796–4815. doi: 10.3390/ijerph120504796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Will J.C., Galuska D.A., Ford E.S., Mokdad A., Calle E.E. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int. J. Epidemiol. 2001;30:540–546. doi: 10.1093/ije/30.3.540. [DOI] [PubMed] [Google Scholar]
- 28.Willi C., Bodenmann P., Ghali W.A., Faris P.D., Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 29.The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Ref Type: Report, 2014.
- 30.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman W.H., Stamatovic S.M., Andjelkovic A.V. Inflammatory mediators and blood brain barrier disruption in fatal brain edema of diabetic ketoacidosis. Brain Res. 2009;1254:138–148. doi: 10.1016/j.brainres.2008.11.100. [DOI] [PubMed] [Google Scholar]
- 32.Wellen K.E., Hotamisligil G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad S., Sajja R.K., Park J.H., Naik P., Kaisar M.A., Cucullo L. Impact of cigarette smoke extract and hyperglycemic conditions on blood-brain barrier endothelial cells. Fluids Barriers CNS. 2015;12:18. doi: 10.1186/s12987-015-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prasad S., Sajja R.K., Kaisar M.A., Park J.H., Villalba H., Liles T. Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol. 2017;12:58–69. doi: 10.1016/j.redox.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30:747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 36.Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2014 doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 37.The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. U.S. Department of Health and Human Services. Ref Type: Generic, 2006. [PubMed]
- 38.Howard G., Wagenknecht L.E., Burke G.L., Diez-Roux A., Evans G.W., McGovern P. Cigarette smoking and progression of atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279:119–124. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 39.Cataldo J.K., Prochaska J.J., Glantz S.A. Cigarette smoking is a risk factor for Alzheimer's Disease: an analysis controlling for tobacco industry affiliation. J. Alzheimers Dis. 2010;19:465–480. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haire-Joshu D., Glasgow R.E., Tibbs T.L. Smoking and diabetes. Diabetes Care. 1999;22:1887–1898. doi: 10.2337/diacare.22.11.1887. [DOI] [PubMed] [Google Scholar]
- 41.Anstey K.J., von S.C., Salim A., O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am. J. Epidemiol. 2007;166:367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 42.Arnson Y., Shoenfeld Y., Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010;34:J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg G.A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012;32:1139–1151. doi: 10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah R.S., Cole J.W. Smoking and stroke: the more you smoke the more you stroke. Expert Rev. Cardiovasc. Ther. 2010;8:917–932. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurewich V. Thrombolysis; a critical first-line therapy with an unfulfilled potential. Am. J. Med. 2015 doi: 10.1016/j.amjmed.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 47.Naik P., Fofaria N., Prasad S., Sajja R.K., Weksler B., Couraud P.O. Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci. 2014;15:51. doi: 10.1186/1471-2202-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y., Tang G., Li Y., Wang Y., Chen X., Gu X. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J. Neuroinflamm. 2014;11:177. doi: 10.1186/s12974-014-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin-Montalvo A., Mercken E.M., Mitchell S.J., Palacios H.H., Mote P.L., Scheibye-Knudsen M. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L., Wang H., Shah K., Karamyan V.T., Abbruscato T.J. Opioid receptor agonists reduce brain edema in stroke. Brain Res. 2011;1383:307–316. doi: 10.1016/j.brainres.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 51.Yang L., Shah K., Wang H., Karamyan V.T., Abbruscato T.J. Characterization of neuroprotective effects of biphalin, an opioid receptor agonist, in a model of focal brain ischemia. J. Pharmacol. Exp. Ther. 2011;339:499–508. doi: 10.1124/jpet.111.184127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasad S., Sajja R.K., Park J.H., Naik P., Kaisar M.A., Cucullo L. Impact of cigarette smoke extract and hyperglycemic conditions on blood-brain barrier endothelial cells. Fluids Barriers CNS. 2015;12:18. doi: 10.1186/s12987-015-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naik P., Fofaria N., Prasad S., Sajja R.K., Weksler B., Couraud P.O. Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci. 2014;15:51. doi: 10.1186/1471-2202-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naik P., Sajja R.K., Prasad S., Cucullo L. Effect of full flavor and denicotinized cigarettes exposure on the brain microvascular endothelium: a microarray-based gene expression study using a human immortalized BBB endothelial cell line. BMC Neurosci. 2015;16:38. doi: 10.1186/s12868-015-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naik P., Sajja R.K., Prasad S., Cucullo L. Effect of full flavor and denicotinized cigarettes exposure on the brain microvascular endothelium: a microarray-based gene expression study using a human immortalized BBB endothelial cell line. BMC Neurosci. 2015;16:38. doi: 10.1186/s12868-015-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sajja R.K., Prasad S., Cucullo L. Impact of altered glycaemia on blood-brain barrier endothelium: an in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS. 2014;11:8. doi: 10.1186/2045-8118-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sajja R.K., Green K.N., Cucullo L. Altered Nrf2 signaling mediates hypoglycemia-induced blood-brain barrier endothelial dysfunction in vitro. PLoS One. 2015;10:e0122358. doi: 10.1371/journal.pone.0122358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sajja R.K., Prasad S., Tang S., Kaisar M.A., Cucullo L. Blood-brain barrier disruption in diabetic mice is linked to Nrf2 signaling deficits: role of ABCB10? Neurosci. Lett. 2017 doi: 10.1016/j.neulet.2017.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dinkova-Kostova A.T., Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trehy Michael L., Ye Wei, Hadwiger Michael E., Moore Terry W., Allgire James F., Woodruff Jeffrey T. Analysis of electronic cigarette cartridges, refill solutions, AND smoke for nicotine and nicotine related impurities. J. Liq. Chromatogr. Relat. Technol. 2011;34:1442–1458. [Google Scholar]
- 61.Cheng T. Vol. 23. 2014. Chemical evaluation of electronic cigarettes; pp. ii11–ii17. (Tob. Control). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen R.P., Luo W., Pankow J.F., Strongin R.M., Peyton D.H. Hidden formaldehyde in e-cigarette aerosols. N. Engl. J. Med. 2015;372:392–394. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- 63.Kaisar M.A., Prasad S., Liles T., Cucullo L. A decade of e-Cigarettes: limited research & unresolved safety concerns. Toxicology. 2016 doi: 10.1016/j.tox.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naik P., Cucullo L. Pathobiology of tobacco smoking and neurovascular disorders: untied strings and alternative products. Fluids Barriers CNS. 2015;12:25. doi: 10.1186/s12987-015-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah R.S., Cole J.W. Smoking and stroke: the more you smoke the more you stroke. Expert Rev. Cardiovasc. Ther. 2010;8:917–932. doi: 10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Natali A., Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49:434–441. doi: 10.1007/s00125-006-0141-7. [DOI] [PubMed] [Google Scholar]
- 67.Kravchuk E., Grineva E., Bairamov A., Galagudza M., Vlasov T. The effect of metformin on the myocardial tolerance to ischemia-reperfusion injury in the rat model of diabetes mellitus type II. Exp. Diabetes Res. 2011;2011:907496. doi: 10.1155/2011/907496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin M., van der Horst I.C., van Melle J.P., Qian C., van Gilst W.H., Sillje H.H. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H459–H468. doi: 10.1152/ajpheart.00054.2011. [DOI] [PubMed] [Google Scholar]
- 69.Yeh C.H., Chen T.P., Wang Y.C., Lin Y.M., Fang S.W. AMP-activated protein kinase activation during cardioplegia-induced hypoxia/reoxygenation injury attenuates cardiomyocytic apoptosis via reduction of endoplasmic reticulum stress. Mediat. Inflamm. 2010;2010:130636. doi: 10.1155/2010/130636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng Y.Y., Leu H.B., Chen T.J., Chen C.L., Kuo C.H., Lee S.D. Metformin-inclusive therapy reduces the risk of stroke in patients with diabetes: a 4-year follow-up study. J. Stroke Cerebrovasc. Dis. 2014;23:e99–e105. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Prasad S., Sajja R.K., Kaisar M.A., Park J.H., Villalba H., Liles T. Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol. 2017;12:58–69. doi: 10.1016/j.redox.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdelsaid M., Prakash R., Li W., Coucha M., Hafez S., Johnson M.H. Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes. 2015;64:1804–1817. doi: 10.2337/db14-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abd-Elsameea A.A., Moustaf A.A., Mohamed A.M. Modulation of the oxidative stress by metformin in the cerebrum of rats exposed to global cerebral ischemia and ischemia/reperfusion. Eur. Rev. Med. Pharmacol. Sci. 2014;18:2387–2392. [PubMed] [Google Scholar]
- 74.Liu Y., Tang G., Li Y., Wang Y., Chen X., Gu X. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J. Neuroinflamm. 2014;11:177. doi: 10.1186/s12974-014-0177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J., Benashski S.E., Venna V.R., McCullough L.D. Effects of metformin in experimental stroke. Stroke. 2010;41:2645–2652. doi: 10.1161/STROKEAHA.110.589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tureyen K., Kapadia R., Bowen K.K., Satriotomo I., Liang J., Feinstein D.L. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J. Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- 77.Howard G., Wagenknecht L.E., Cai J., Cooper L., Kraut M.A., Toole J.F. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. Stroke. 1998;29:913–917. doi: 10.1161/01.str.29.5.913. [DOI] [PubMed] [Google Scholar]
- 78.Shinton R., Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ. 1989;298:789–794. doi: 10.1136/bmj.298.6676.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mannami T., Iso H., Baba S., Sasaki S., Okada K., Konishi M. Cigarette smoking and risk of stroke and its subtypes among middle-aged Japanese men and women: the JPHC Study Cohort I. Stroke. 2004;35:1248–1253. doi: 10.1161/01.STR.0000128794.30660.e8. [DOI] [PubMed] [Google Scholar]
- 80.Nath N., Khan M., Paintlia M.K., Singh I., Hoda M.N., Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J. Immunol. 2009;182:8005–8014. doi: 10.4049/jimmunol.0803563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma T.C., Buescher J.L., Oatis B., Funk J.A., Nash A.J., Carrier R.L. Metformin therapy in a transgenic mouse model of Huntington's disease. Neurosci. Lett. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 82.Wang J., Gallagher D., DeVito L.M., Cancino G.I., Tsui D., He L. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 83.El-Mir M.Y., Detaille D., Villanueva G., Delgado-Esteban M., Guigas B., Attia S. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J. Mol. Neurosci. 2008;34:77–87. doi: 10.1007/s12031-007-9002-1. [DOI] [PubMed] [Google Scholar]
- 84.Labuzek K., Suchy D., Gabryel B., Bielecka A., Liber S., Okopien B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010;62:956–965. doi: 10.1016/s1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- 85.Geier E.G., Chen E.C., Webb A., Papp A.C., Yee S.W., Sadee W. Profiling solute carrier transporters in the human blood-brain barrier. Clin. Pharmacol. Ther. 2013;94:636–639. doi: 10.1038/clpt.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]