Abstract
Strains JW1T and JW3, isolated from surface seawater of the Arabian Sea, were subjected to polyphasic taxonomic analysis. Cells of both strains were Gram-stain-negative, aerobic, and rod-shaped. They formed violet pigment and produced violacein. On the basis of 16S rRNA gene sequence analysis, strains JW1T and JW3 showed high 16S rRNA gene sequence similarity with Pseudoalteromonas byunsanensis JCM12483T (98.2%), P. shioyasakiensis SE3T (97.8%), P. arabiensis JCM 17292T (97.3%), and P. gelatinilytica NH153T (97.1%). The 16S rRNA gene sequence similarity between JW1T and JW3 was 100%. Phylogenetic analyses revealed that both strains fell within the cluster of the genus Pseudoalteromonas and represented an independent lineage. The average nucleotide identity and in silico DNA-DNA hybridization values between JW1T and type strains of the closely related Pseudoalteromonas species were 70.9–83.3% and 20.0–26.4%, respectively. The sole respiratory quinone in both strains is ubiquinone 8 (Q-8). The principal fatty acids are summed feature 3 (C16:1ω7c and/or iso-C15:0 2OH), C18:1ω7c, and C16:0. The major polar lipids are phosphatidylethanolamine, phosphatidylglycerol, one unidentified glycolipid, one unidentified aminolipid, and one unidentified phospholipid. The DNA G+C content was 43.3 mol%. Differential phylogenetic distinctiveness, chemotaxonomic differences, and phenotypic properties indicated that strains JW1T and JW3 could be differentiated from the Pseudoalteromonas species with validly published names. Therefore, it is proposed that strains JW1T and JW3 represent a novel species of the genus Pseudoalteromonas, for which the name Pseudoalteromonas amylolytica sp. nov. (type strain, JW1T = CGMCC 1.15681T = KCTC 52406T = MCCC 1K02162T) is proposed.
Introduction
The genus Pseudoalteromonas, the type genus of the family Pseudoalteromonadaceae [1], was proposed by Gauthier et al. (1995) [2]. Initially, the genus Pseudoalteromonas was differentiated from the genus Alteromonas based on the phylogenetic analysis of 16S rRNA gene sequences [2]. Currently, the genus Pseudoalteromonas consists of 43 species with validly published names (http://www.bacterio.net/p/pseudoalteromonas.html). Members of the genus Pseudoalteromonas are widespread in nature and have a great adaptability to marine environments, such as coastal, open, and deep seawaters, sediments, marine invertebrates, fish, and algae [3]. The genus Pseudoalteromonas is Gram-negative, aerobic or facultatively anaerobic, and rod-shaped, it requires Na+ ions for growth, usually does not denitrify, and possesses ubiquinone-8 (Q8) as major respiratory quinone [3].
Some Pseudoalteromonas species produce a variety of primary and secondary metabolites, including antibiotics [2], exopolymers [4, 5], hydrolytic enzymes [6, 7], and pigments [2, 8]. Violacein is a natural indolocarbazole compound formed by condensation of two molecules of tryptophan [9]. It is a potential pharmaceutical agent owing to its extensive biological properties, such as antibacterial, antiviral, antioxidant, and antitumor activities [10]. Pseudoalteromonas luteoviolacea has been reported to produce violacein [11]. Here, we present a polyphasic study describing two novel violacein-producing strains, both of which were isolated from surface water of the Arabian Sea.
Materials and methods
Organisms and culture conditions
Strains JW1T and JW3 were isolated from the surface seawater collected from the Arabian Sea (E67° N24°). The seawater samples were stored at 4°C until use. Natural seawater agar (pH 7.2–7.4) supplemented with 0.05% peptone (w/v; BD, Sparks, MD, USA) and 0.01% yeast extract (w/v; BD) was used for isolation. The seawater samples were diluted using the standard ten-fold dilution plating technique and spread on natural seawater agar. After ten days of aerobic incubation at 30°C, two violet colonies, designated as JW1T and JW3, were picked from different samples and purified by repeated restreaking. The purity was confirmed by the uniformity of cell morphology. The reference strains P. byunsanensis JCM 12483T, P. shioyasakiensis JCM 18891T, and P. arabiensis JCM 17292T were obtained from the JCM (Japan Collection of Microorganisms). The reference strain P. gelatinilytica NH153T was available in our lab [12]. Unless otherwise stated, the two strains were routinely cultured in marine broth 2216 (MB; BD) or on marine agar 2216 (MA; BD) at 30°C and stored at –80°C with 30% (v/v) glycerol.
16S rRNA gene and genome sequence determination
The 16S rRNA gene was amplified and analyzed as described previously [13]. PCR products were cloned into the vector pMD 19-T (TaKaRa, Dalian, China) and then sequenced to determine the almost-complete sequence of the 16S rRNA gene. High-quality genomic DNA was extracted with the AxyPrep™ Bacterial Genomic DNA Miniprep Kit (Axygen Scientific, Inc., Union City, CA, USA). The genomes of strains JW1T, JW3, and P. byunsanensis JCM 12483T were sequenced using the Solexa paired-end sequencing technology with the Illumina HiSeq 2000 platform (Anoroad Gene Technology Co. Ltd, Beijing, China). One paired-end library was constructed with 500-bp insert size. The sequencing generated approx. 1 Gb of clean data (approx. 500-fold genome coverage). De novo assembly of the reads was carried out using SOAPdenovo (version 2.0.1) [14]. Assembly k-mer was tested from 57 to 64 for seeking the optimal value, using the abyss-pe script. Assembly quality was estimated using MUMmer [15]. Completeness of the genome sequence was addressed using the bioinformatics tool CheckM (http://ecogenomics.github.io/CheckM/) [16]. The complete sequence of the 16S rRNA gene was annotated via the RNAmmer 1.2 Server [17] and was compared with related sequences of reference organisms using the EzTaxon-e service [18].
Phylogenetic status
Phylogenetic analysis was carried out using ARB (release 6.0.2) [19] and the All-Species Living Tree Project database (LTPs123, September 2015) [20]. The 16S rRNA gene sequences of strains JW1T and JW3 were aligned with SILVA Incremental Aligner (SINA, version 1.2.11) (http://www.arb-silva.de) [21]. The alignment sequences were imported into the LTPs database and implemented in ARB. On the basis of the obtained All-Species Living Tree and the EzTaxon-e results, 24 species were selected and sequence data were aligned with ClustalW [22]. Phylogenetic trees were reconstructed using the MEGA 5 program package [23], using Algicola sagamiensis B-10-31T as the outgroup, by neighbor-joining [24], maximum-parsimony [25], and maximum-likelihood methods [26]. Tree topology was evaluated by bootstrap analysis using 1000 resample datasets. Kimura two-parameter model [27] was used to calculate evolutionary distances and reconstruct phylogeny (neighbor-joining and maximum-likelihood methods).
The genomes of 20 type strains of Pseudoalteromonas species were retrieved from the GenBank database (S1 Fig). Six housekeeping genes, atpD (beta subunit for ATP synthase), gyrB (DNA gyrase beta subunit), mreB (rod shape-determining protein), recA (RNA recombinase alpha subunit), rpoD (RNA polymerase), and topA (DNA topoisomerase I) were used for multilocus sequence analysis (MLSA). The concatenated sequence of six single genes was obtained from the genome and subjected to maximum-likelihood phylogenetic analyses [26] with MEGA 5.
Phenotypic characteristics
Cell morphology, size, and motility were examined using confocal laser scanning microscopy (TCS SP5; Leica) and transmission electron microscopy (JEM-1230; JEOL). The hanging-drop method was used for motility testing. Cell morphology and ultrastructure were observed using transmission electron micrographs.
The growth at various temperatures (4, 15, 20, 28, 30, 37, 45, and 50°C) was tested in MB. The pH range for growth was determined in the range of 5.0–10.5 with interval of 0.5 in MB by adding MES (pH 5.0–6.0), PIPES (pH 6.5–7.0), Tricine (pH 7.5–8.5), and CAPSO (pH 9.0–10.5) at a final concentration of 50 mM. pH values changed only minimally after autoclaving. Growth at different concentrations of NaCl (0, 0.5, 1.0, 3.0, 5.0, 7.5, 10.0, and 15.0%, w/v) was investigated using NaCl-free MB (prepared according to the MB formula, but without NaCl). Sea-salt requirement for growth was measured in the PY medium (peptone 5.0 g, yeast extract 1.0 g and distilled water 1 L, pH 7.6) supplemented with sea salts (Sigma) at various concentrations (0, 0.5, 1.0, 2.0, 3.0, 4.0, 4.5, and 5.0%, w/v). Growth was measured at 590 nm (OD590) with a UV/visible spectrophotometer (Ultrospec 6300 pro; Amersham Biosciences). Upper and lower limits for growth were confirmed when no growth was observed after cultivation for one month. For anaerobic growth, strains were incubated in the AnaeroPack-MicroAero anaerobic system (Mitsubishi). Sodium nitrate (20 mM) or sodium nitrite (20 mM) was used as a potential electron acceptor.
Gram reaction, oxidase and catalase activities, and hydrolysis of starch and Tween-20, -40, and -80 were tested according to [28]. Violacein was extracted according to [29] and the absorbance of the violet pigment was monitored from 350 nm to 1000 nm using a UV/visible spectrophotometer (DU800, Beckman Coulter). The molecular weight of violacein was deduced by LC-MS analysis according to [29]. Chromatography was carried out on a Agilent 1200. Compound separation was achieved on an analytical column (Extend-C18, 3.5 μm, 2.1 × 100 mm; Agilent). Analysis of the pigment by eletrospray ionization mass spectrometry was conducted with Finnigan LCQ DECA XP MAX mass spectrometer (Thermo Electron Corp., USA). The flow rate of the pigment solution was 15 μL/min. The utilization of carbon substrates as sole carbon and energy sources was tested in BM [30] supplemented with filter-sterilized complex nutrients (yeast extract, peptone and tryptone, 0.2%, w/v), sugars (0.2%, w/v), alcohols (0.2%, w/v), organic acids (0.1%, w/v), or amino acids (0.1%, w/v). Yeast extract (0.01%, w/v) was added as a growth factor. Acid production was evaluated using marine oxidation-fermentation medium supplemented with 1% filter-sterilized sugars [31]. API 20NE and API 20E tests (bioMérieux) were used according to the manufacturer’s instructions to determine additional physiological and biochemical characteristics. Strips were inoculated with a heavy bacterial suspension (MacFarland 5 standard) in AUX medium supplemented with 2% (w/v) sea salts (Sigma) [32]. API 20 NE and API 20 E strips were read after 48 h. Sensitivity to antimicrobial agents was determined with a two-layer plate method according to Wu et al. (2015)[33]. Four reference strains, P. byunsanensis JCM 12483T, P. shioyasakiensis JCM 18891T, P. arabiensis JCM 17292T, and P. gelatinilytica NH153T were used as controls in the above tests.
Chemotaxonomic characteristics
The cellular fatty acids of strains JW1T, JW3, and the reference strains were determined under identical conditions in parallel. The quadrant streak method was used for inoculation and cellular fatty-acid methyl esters were obtained from cells grown on MA at 30°C for 16 h from quadrant 3 (late exponential phase). Whole cell fatty acids were analyzed using the Microbial Identification System (MIDI Inc.) according to the manufacturer’s instructions. Isoprenoid quinones were extracted and purified by two-dimensional thin-layer chromatography (TLC) and then analyzed by LC-MS (Agilent 1200 and Thermo Finnigan LCQ DECA XP MAX mass spectrometer) [34]. Total lipids were extracted and separated by two-dimensional TLC [35] on silica gel 60 F254 plates (Merck). Four types of spray reagent were used to detect the corresponding lipids, including molybdophosphoric acid for total lipids, α-naphthol reagent for glycolipids, ninhydrin reagent for lipids containing free aminolipids, and molybdenum blue for phosphorus-containing lipids [36].
Average nucleotide identities and genome analysis
The average nucleotide identity (ANI) was calculated using the OrthoANIu algorithm by ChunLab's online Average Nucleotide Identity calculator [37]. In silico DNA-DNA hybridization (DDH) values were calculated by GGDC [38].
rRNA genes were identified using the RNAmmer 1.2 Server [17] and tRNA genes were searched with the tRNAscan-SE 2.0 online server [39]. Gene prediction and functional annotation were carried out using the Rapid Annotation using Subsystem Technology (RAST) server online [40]. Selected predicted genes were classified using RPSBLAST against the COG database [41]. Translated genes were assigned to KEGG pathway using KEGG Automatic Annotation Server (KAAS) with the BBH method [42, 43]. Orthologous cluster analyses were carried out using OrthoMCL [44]. Shared and unique orthologous clusters were established with in-house shell scripts. CRISPR structures in the genomes were predicted with the CRISPRFinder program online (http://crispr.i2bc.paris-saclay.fr/Server/).
Results and discussion
Phenotypic features
Strains JW1T and JW3 were Gram-stain-negative, aerobic and rod-shaped (0.7–1.2 μm in width and 1.8–3.0 μm in length) (S2 Fig). Colonies were violet, circular, convex, smooth, and 1–2 mm in diameter after one day of incubation at 30°C on MA. Optimal growth occurred at 30°C and pH 7.5. UV-visible absorption spectra and LC-MS analysis of the pigment isolated from strains JW1T, JW3, and P. byunsanensis JCM 12483T showed the presence of violacein. Maximal absorption of UV-visible light was at 575 nm (S3A Fig). Mass spectrometry of the pigment revealed a parent ion [M-H]−at m/z 342.1, which was identical to that of violacein (S3B Fig) [45]. Both strains were positive for catalase, oxidase, tryptophan deaminase, and Voges–Proskauer reaction, able to hydrolyze esculin, gelatin, starch, Tween-20, -40 and -80, susceptible to (μg per disc unless otherwise stated) chloramphenicol (30), erythromycin (10), gentamicin (10), kanamycin (30), mefoxin (30), neomycin (30), nitrofurantoin (300), norfloxacin (10), polymyxin B (300 IU), rifampicin (5), streptomycin (10), tetracycline (30), and vancomycin (30), and resistant to ampicillin (10), cefalexin (30), nystatin (100), and penicillin G (10 IU). Detailed phenotypic characteristics are given in the species description and Table 1.
Table 1. Differential phenotypic characteristics of strains JW1T, JW3, and closely related members of the genus Pseudoalteromonas.
Strains/species: 1, strain JW1T; 2, strain JW3; 3, P. byunsanensis JCM 12483T; 4, P. shioyasakiensis JCM 18891T; 5, P. arabiensis JCM 17292T; 6, P. gelatinilytica NH153T. All data were obtained in this study and under identical conditions, unless indicated otherwise.
| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Color | violet | violet | violet* | non-pigmented† | non-pigmented‡ | non-pigmented§ |
| Violacein production | + | + | + | – | – | – |
| Growth in NaCl (%): | ||||||
| Range | 0.5–10 | 0.5–10 | 0.5–5* | 0.5–12† | 0.5–10‡ | 0–10§ |
| Optimum | 1–3 | 1–3 | 1.5–2* | 1–3† | 2–3‡ | 3–5§ |
| Growth pH: | ||||||
| Range | 6–10.5 | 6–10.5 | 5–10* | 5.5–9.5† | 5.0–10.0‡ | 5.5–9.5§ |
| Optimum | 7.5 | 7.5 | 8.0* | 6.5–8.0† | 7.0–8.0‡ | 7.5–8.5§ |
| Growth temperature (ºC): | ||||||
| Range | 20–40 | 20–40 | 10–40* | 5–40† | 6–35‡ | 15–45§ |
| Optimum | 30 | 30 | 25–30* | 28–30† | 25‡ | 37§ |
| Arginine dihydrolase | – | – | – | + | + | – |
| Citrate utilization | – | – | – | + | + | + |
| Nitrate reduction | – | – | – | – | + | – |
| Tryptophan deaminase | + | + | + | – | – | – |
| Urease | – | – | – | – | + | + |
| Voges–Proskauer | + | + | + | + | – | + |
| Hydrolysis of: | ||||||
| Starch | + | + | + | – | – | – |
| Tween-40 | + | + | + | – | + | – |
| Utilization of: | ||||||
| L-Arabinose | – | – | – | + | + | + |
| Cellobiose | – | + | + | – | + | + |
| Ethanol | – | – | – | + | + | + |
| D-Fructose | – | – | – | + | – | + |
| D-Mannitol | – | – | – | – | – | + |
| D-Mannose | – | – | – | + | + | + |
| Sucrose | + | + | – | + | + | + |
| D-Trehalose | + | + | + | + | – | + |
| Acid production: | ||||||
| Cellobiose | – | + | + | – | + | – |
| Ethanol | – | – | – | – | + | – |
| D-fructose | – | – | – | + | – | – |
| D-Galactose | – | + | – | – | – | – |
| D-Glucose | + | + | + | – | – | + |
| D-Mannitol | – | – | – | – | – | + |
| D-Mannose | – | – | – | + | + | + |
| L-Rhamnose | – | + | – | – | – | – |
| Sucrose | + | + | – | + | + | – |
| D-Trehalose | + | + | + | + | – | + |
| Susceptibility to: | ||||||
| Gentamicin (10 μg) | + | + | + | + | – | + |
| Nitrofurantoin (300 μg) | + | + | + | – | – | – |
| Streptomycin (10 μg) | + | + | + | + | + | – |
| Tetracycline (30 μg) | + | + | + | – | – | + |
| ANI value (%) | ||||||
| With JW1T | 100 | 99.9 | 83.3 | 71.0 | 70.9 | 70.9 |
| in silico DDH values | ||||||
| With JW1T | 100 | 99.9 | 26.4 | 20.0 | 20.1 | 20.0 |
16S rRNA gene sequence similarities and phylogenetic analysis
The 16S rRNA gene sequences of strains JW1T and JW3 (1527 nt) were obtained. According to EzTaxon as well as ClustalW results of 16S rRNA gene sequence comparison to representative bacteria with validly published names, strains JW1T and JW3 showed high 16S rRNA gene sequence similarity to P. byunsanensis JCM12483T (98.2%), P. shioyasakiensis SE3T (97.8%), P. arabiensis JCM 17292T (97.3%), and P. gelatinilytica NH153T (97.1%), and exhibited less than 97.0% 16S rRNA gene sequence similarity with the type strains of other Pseudoalteromonas species. The 16S rRNA gene sequence similarity between strains JW1T and JW3 was 100%.
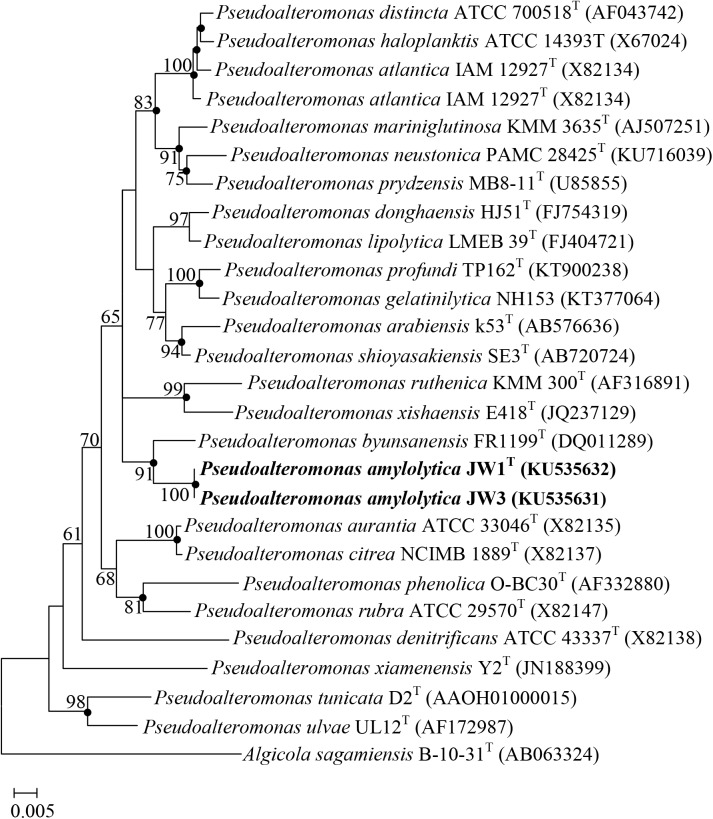
The All-Species Living Tree indicated that the genus Pseudoalteromonas forms a monophyletic clade and strains JW1T and JW3 fall within the cluster comprising the Pseudoalteromonas species. The topologies of neighbor-joining, maximum-likelihood, and maximum-parsimony phylogenetic trees based on the 16S rRNA gene also supported the notion that strains JW1T and JW3 formed a stable lineage, with a high bootstrap value of 100%, and a distinct lineage from P. byunsanensis (bootstrap value 91%) (Fig 1). Phylogenetic trees based on concatenated sequences of the six housekeeping genes atpD, gyrB, mreB, recA, rpoD, and topA confirmed that the two strains formed a clade with P. byunsanensis as well as P. citrea and could not be associated with any of the recognized species in the genus Pseudoalteromonas (S1 Fig). Phylogenetic analysis indicated JW1T and JW3 form another, independent lineage and might represent a novel member of the genus Pseudoalteromonas.
Fig 1. Neighbor-joining phylogenetic tree based on 16S rRNA gene sequences showing the phylogenetic relationships of JW1T, JW3, and related taxa.
Bootstrap values (>60%) based on 1,000 replications are shown at branch nodes. Filled circles indicate that the corresponding nodes were also recovered in the trees generated with the maximum-likelihood and maximum-parsimony algorithms. Bar, 0.005 substitutions per nucleotide position.
Chemotaxonomic analysis
Chemotaxonomic data supported the results of the phylogenetic analysis. The sole respiratory quinone found in strains JW1T and JW3 was Q8, in line with all members of the genus Pseudoalteromonas [3]. Fatty-acid analysis revealed that summed feature 3, C18:1ω7c, and C16:0 were the major fatty acids in JW1T and JW3 and the references strains (Table 2). JW1T and JW3 possessed phosphatidylethanolamine and phosphatidylglycerol as the major polar lipids, similar to the reference strains. In addition, JW1T and JW3 possessed three unidentified glycolipids (GL2–GL4), one unidentified aminolipid (AL2), and one unidentified phospholipid (PL1) as moderate-to-minor polar lipids, which were similar to those of P. byunsanensis JCM 12483T (S4 Fig). Moreover, JW1T and JW3 possessed aminolipid, glycolipid, and phospholipid, all of which were detected in the four reference strains (S4 Fig) [12].
Table 2. Fatty acid compositions (%) of JW1T, JW3, and related Pseudoalteromonas species.
Strains/species: 1, isolate JW1T; 2, isolate JW3; 3, P. byunsanensis JCM 12483T; 4, P. shioyasakiensis JCM 18891T; 5, P. arabiensis JCM 17292T; 6, P. gelatinilytica NH153T. All data were taken from this study. Fatty acids representing less than 0.1% in all strains were omitted.–, Not detected; tr, traces (<1.0%).
| Fatty acid | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Straight-chain | ||||||
| C10:0 | 1.7 | 1.7 | tr | – | – | – |
| C12:0 | 1.1 | 1.4 | 1.2 | 2.4 | 3.5 | 1.9 |
| C14:0 | tr | 1.1 | tr | 1.1 | 1.6 | 1.0 |
| C16:0 | 18.4 | 16.5 | 21.0 | 22.7 | 27.1 | 23.3 |
| C18:0 | 1.6 | 1.0 | 1.8 | 5.1 | 3.0 | 3.7 |
| Unsaturated | ||||||
| C15:1ω8c | – | tr | 1.0 | – | tr | tr |
| C17:1ω8c | tr | tr | tr | tr | tr | 1.2 |
| C18:1ω6c | 4.1 | 4.6 | – | – | – | – |
| C18:1ω7c | 18.4 | 13.9 | 21.2 | 20.4 | 14.6 | 22.6 |
| C18:1ω9c | tr | tr | tr | tr | tr | 1.2 |
| Hydroxy | ||||||
| C10:0 3OH | 6.3 | 7.9 | 7.3 | 1.0 | tr | 1.0 |
| C12:0 3OH | 7.2 | 9.3 | 6.2 | 10.4 | 9.8 | 7.6 |
| Summed feature* | ||||||
| 3 | 29.9 | 31.1 | 29.3 | 27.5 | 30.9 | 27.4 |
| Unknown | ||||||
| 11.799 | 5.5 | 6.2 | 5.7 | 1.9 | tr | 1.8 |
*Summed features represent groups of two fatty acids that could not be separated by GLC with the MIDI system. Summed feature 2 contained C14:0 3OH and/or iso-C16:1 I; Summed feature 3 contained C16:1ω7c and/or iso-C15:0 2OH.
Chemotaxonomic data of JW1T, JW3, and their relatives also showed some clear differences in fatty acid composition and polar lipid profile. The percentage of C16:0 of strains JW1T and JW3 (18.4% and 16.5%, respectively) was lower than that of the reference strains (21.0–27.1%). JW1T and JW3 contained C18:1ω6c (4.1% and 4.6%, respectively), which were not detected in the reference strains (Table 2). One unidentified aminolipid (AL1) was present in both strain JW1T and JW3, but was not detected in the P. byunsanensis JCM 12483T. P. byunsanensis JCM 1248 possessed three unidentified aminolipids (AL3–AL5) and three unidentified lipids (L3–L5), while strains JW1T and JW3 did not. Diphosphatidylglycerol was present in P. shioyasakiensis JCM 18891T, P. arabiensis JCM 17292T, and P. gelatinilytica NH153T [12], but not in JW1T and JW3 (S4 Fig).
In silico DNA-DNA relatedness
The DNA G+C content of strains JW1T and JW3 calculated from the genome sequence was 43.3 mol%, a value in the range reported for members of the genus Pseudoalteromonas, i.e. 38–48 mol% [46]. JW1T and the reference strains exhibited ANI values of 70.9–83.3% (Table 1). These ANI values were far below the threshold of species boundary (94–96%) [47], indicating low taxonomic relatedness between JW1T and the reference strains. The recommended results (formula 2) of the in silico DDH analysis revealed that JW1T and the reference strains shared 20.0–26.4% DNA relatedness (Table 1). The values were below 70%, indicating that the strains should be assigned to different genomic species [48]. In addition, the ANI and in silico DDH values between JW1T and JW3 were 99.9%. These values were above the species boundary (94–96% for ANI values and 70% for in silico DDH values), suggesting that JW1T and JW3 represent an identical genospecies.
Genomic features
General features of JW1T and JW3 are displayed in Table 3 and S1 Table. The bioinformatics tool CheckM indicated that the genome completeness was 100% for both JW1T and JW3, with a contamination percentage of 0.4% and 0.5%, respectively. Genome sequence completeness ≥95%, with ≤5% contamination, is considered to indicate an excellent reference genome for deeper analyses [16]. The genome size of the two strains and their related Pseudoalteromonas species varied from 4.5 Mb to 4.8 Mb. This variation can be attributed partially to the draft nature of the sequence. COG assignments were similar for all genomes. Twenty-two COG classes were annotated in the genomes of JW1T, JW3, P. byunsanensis JCM 12483T, and P. arabiensis JCM 17292T, and 23 COG classes (with an extra Cytoskeleton class) were detected in the genomes of P. shioyasakiensis JCM 18891T and P. gelatinilytica NH153T (S1 Table). Furthermore, 13 and 6 unique orthologous clusters were found in the genomes of JW1T and JW3, respectively (Table 3). The two strains shared 640 unique orthologous clusters.
Table 3. Genome statistics of JW1T, JW3, and related Pseudoalteromonas species.
Strains/species: 1, strain JW1T (MKJU00000000); 2, strain JW3 (MKJT00000000); 3, P. byunsanensis JCM 12483T (MNAN00000000); 4, P. shioyasakiensis JCM 18891T (LRUE00000000); 5, P. arabiensis JCM 17292T (LRUF00000000); 6, P. gelatinilytica NH153T (LRRU00000000). +, Detected;–, not detected.
| Genome characteristics | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Size (Mbp) | 4.86 | 4.85 | 4.74 | 4.81 | 4.46 | 4.8 |
| G+C content (mol%) | 43.3 | 43.3 | 42.5 | 41.3 | 40.9 | 41.4 |
| Total number of genes | 4212 | 4204 | 4096 | 4350 | 4302 | 4302 |
| Protein coding sequences (CDS) | 4035 | 4026 | 3919 | 4230 | 4184 | 4184 |
| Pseudogene | 61 | 58 | 54 | 25 | 16 | 16 |
| rRNA genes | 19 | 18 | 17 | 13 | 7 | 7 |
| tRNA genes | 95 | 98 | 97 | 78 | 94 | 91 |
| CRISPRs structure | 2 | 2 | 2 | 3 | 2 | 4 |
| vioABCDE operon | + | + | + | – | – | – |
| Unique orthologous clusters | 13 | 6 | 646 | 426 | 628 | 337 |
Genome analysis of JW1T, JW3, and the reference strains indicated the presence of genes encoding clustered regularly interspaced short palindromic repeats (CRISPRs; Table 3). CRISPRs, in association with CRISPR-associated (Cas) proteins, make up the immune system that confers resistance to foreign genetic elements, such as plasmids and phages [49]. The CRISPRs/Cas system is being used for gene editing. Colonies of JW1T and JW3 were violet and the two strains produced violacein. The biosynthesis of violacein begins with L-tryptophan and is successively catalyzed by enzymes VioA, B, E, D, and C, all of which are encoded by the vioABCDE operon [50]. Strains JW1T, JW3, and P. byunsanensis JCM 12483T produced violacein and possessed vioABCDE operon, while P. arabiensis JCM 17292T, P. shioyasakiensis JCM 18891T and P. gelatinilytica NH153T did not (Table 3 and S3 Fig).
The genomes of strains JW1T and JW3 were annotated and analyzed to identify the major metabolic pathways of carbon, nitrogen, sulfur, and phosphorus based on key genes. JW1T and JW3 can use organic carbon sources (refer to the species description). They harbor key genes of the Entener–Doudoroff pathway, glycolysis pathway, pentose phosphate pathway, and tricarboxylic acid cycle. The genomes of JW1T and JW3 possess an ammonium transporter gene, but they lack genes involved in nitrate reduction, nitrite reduction, nitrogen fixation, nitrification, or anaerobic ammonium oxidation. Thus, both strains can utilize only reduced nitrogen. Genes encoding urease and urea transporter were not detected, suggesting that JW1T and JW3 are incapable of utilizing urea as a C or N source. Both genomes possess a variety of sulfate permease genes involved in assimilatory SO42+ reduction. Sulfate can be reduced to sulfide and is subsequently incorporated into amino acids (e.g. cysteine). The genomes of JW1T and JW3 harbor genes encoding low-affinity inorganic phosphate transporter and sodium-dependent phosphate transporter. The presence of alkaline phosphatase genes in both genomes indicates that JW1T and JW3 are capable of utilizing both inorganic and organic forms of phosphorus.
Conclusion
Strains JW1T and JW3 possess some properties, particularly chemotaxonomic characteristics, that species of the genus Pseudoalteromonas all share. On the other hand, JW1T and JW3 could be distinguished from the type strains of their closely related species on the basis of phenotypic differences (e.g., color, NaCl, pH and temperature ranges and optima, arginine dihydrolase, citrate utilization, nitrate reduction, tryptophan deaminase, urease, Voges–Proskauer reaction, carbohydrate utilization, and acid production, Table 1). In addition, strains JW1T and JW3 produce violacein, a potential pharmaceutical agent. On the basis of phylogenetic, genome, and chemotaxonomic data, as well as phenotypic characteristics, strains JW1T and JW3 represent a novel species of the genus Pseudoalteromonas, for which the name Pseudoalteromonas amylolytica sp. nov. is proposed.
Description of Pseudoalteromonas amylolytica sp. nov
Pseudoalteromonas amylolytica (a.my.lo.ly'ti.ca. Gr. n. amylon, starch; N.L. adj. lyticus -a -um (from Gr. adj. lytikos -ê -on), able to loosen, able to dissolve; N.L. fem. adj. amylolytica, starch dissolving).
Cells are Gram-stain-negative, rod-shaped, 0.7–1.2 μm in width and 1.8–3.0 μm in length. Colonies are violet, circular, convex, smooth and 1–2 mm in diameter after one day of incubation at 30°C on MA. Grow on NaCl-free MB supplemented with 0.5–10% (w/v) NaCl (optimum 1.0–3.0%). pH and temperature ranges for growth are pH 6–10.5 and 20–40°C (optimum at pH 7.5 and 30°C). Require sea salts for growth. No anaerobic growth occurs on MA supplemented with sodium nitrate or sodium nitrite. Produce violacein. Positive for catalase, oxidase, tryptophan deaminase, and Voges–Proskauer reaction. Negative for arginine dihydrolase, citrate utilization, β-galactosidase, glucose fermentation, nitrate reduction, lysine and ornithine decarboxylases, indole formation, H2S production, and urease. Esculin, gelatin, starch, Tween-20, -40, and -80 are hydrolyzed. The following compounds are utilized as sole carbon and energy sources: N-acetyl-glucosamine, l -alanine, l-arginine, d-glucose, l -histidine, l -isoleucine, d-maltose, sodium acetate, sodium propionate, sodium pyruvate, sucrose and d-trehalose. Acid is produced from d-glucose, d-maltose, sucrose, and d-trehalose. Principal fatty acids (> 10%) are summed feature 3 (C16:1ω7c and/or iso-C15:0 2OH), C18:1ω7c, and C16:0. Sole respiratory quinone is Q-8. Major polar lipids are phosphatidylethanolamine, phosphatidylglycerol, one unidentified glycolipid, one unidentified aminolipid, and one unidentified phospholipid. In addition, moderate to minor amounts of three unidentified glycolipids, one unidentified aminolipid, one unidentified phospholipid, and two unidentified lipids are present. DNA G+C content is 43.3 mol%.
The type strain, JW1T (= CGMCC 1.15681T = KCTC 52406T = MCCC 1K02162T), and additional strain JW3 were isolated from surface seawater.
Supporting information
(DOCX)
The gene sequences were obtained from the genomes, the accession numbers of which are indicated in parentheses. Bootstrap values (>90%) based on 1,000 replications are shown at branch nodes. Bar, 0.05 substitutions per nucleotide position.
(TIF)
Transmission electron micrographs showing the cell ultrastructure of strains JW1T (a) and JW3 (b). Bar, 0.5 μm.
(TIF)
Absorption profile (a) and mass spectrum (b) of violacein.
(TIF)
Thin-layer chromatograms after staining with molybdatophosphoric acid, α-naphthol reagent, ninhydrin reagent, and molybdenum blue showing the total polar lipid profiles of strains JW1T (a1-a4), JW3 (b1-b4), and P. byunsanensis JCM 12483T (c1-c4). PE, Phosphatidylethanolamine; PG, phosphatidylglycerol; AL, aminolipid; GL, glycolipid; PL, phospholipid; L, other lipid.
(TIF)
Acknowledgments
We would like to thank Prof. Aharon Oren for help with etymology.
Data Availability
The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequence of strains JW1T and JW3 are KU535632 and KU535631. The GenBank accession numbers for the whole genome sequences of strains JW1T, JW3 and P. byunsanensis JCM12483T are MKJU00000000, MKJT00000000 and MNAN00000000, respectively. Other relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China granted to YW (no. 41406174, http://www.nsfc.gov.cn/publish/portal1/); the National Key Basic Research Program of China granted to XX (2014CB441503, http://most.gov.cn/); the Natural Science Foundation of Zhejiang Province granted to XX (LR17D060001, http://www.zjnsf.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ivanova EP, Flavier S, Christen R. Phylogenetic relationships among marine Alteromonas-like proteobacteria: emended description of the family Alteromonadaceae and proposal of Pseudoalteromonadaceae fam. nov., Colwelliaceae fam. nov., Shewanellaceae fam. nov., Moritellaceae fam. nov., Ferrimonadaceae fam. nov., Idiomarinaceae fam. nov. and Psychromonadaceae fam. nov. Int J Syst Evol Microbiol. 2004;54(5): 1773–1788. [DOI] [PubMed] [Google Scholar]
- 2.Gauthier G, Gauthier M, Christen R. Phylogenetic analysis of the genera Alteromonas, Shewanella, and Moritella using genes coding for small-subunit rRNA sequences and division of the genus Alteromonas into two genera, Alteromonas (emended) and Pseudoalteromonas gen. nov., and proposal of twelve new species combinations. Int J Syst Bacteriol. 1995;45(4): 755–61. doi: 10.1099/00207713-45-4-755 [DOI] [PubMed] [Google Scholar]
- 3.Ivanova EP, Jun Ng H, Webb HK. The Family Pseudoalteromonadaceae In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes. 4nd edn, Gammaproteobacteria New York: Springer; 2014. p. 575–582. [Google Scholar]
- 4.Matsuyama H, Sawazaki K, Minami H, Kasahara H, Horikawa K, Yumoto I. Pseudoalteromonas shioyasakiensis sp. nov., a marine polysaccharide-producing bacterium. Int J Syst Evol Microbiol. 2014;64(1): 101–106. [DOI] [PubMed] [Google Scholar]
- 5.Matsuyama H, Minami H, Kasahara H, Kato Y, Murayama M, Yumoto I. Pseudoalteromonas arabiensis sp. nov., a marine polysaccharide-producing bacterium. Int J Syst Evol Microbiol. 2013;63(5): 1805–1809. [DOI] [PubMed] [Google Scholar]
- 6.Xu XW, Wu YH, Wang CS, Gao XH, Wang XG, Wu M. Pseudoalteromonas lipolytica sp. nov., isolated from the Yangtze River estuary. Int J Syst Evol Microbiol. 2010;60(9): 2176–2181. [DOI] [PubMed] [Google Scholar]
- 7.Romanenko LA, Zhukova NV, Rohde M, Lysenko AM, Mikhailov VV, Stackebrandt E. Pseudoalteromonas agarivorans sp. nov., a novel marine agarolytic bacterium. Int J Syst Evol Microbiol. 2003;53(1): 125–131. [DOI] [PubMed] [Google Scholar]
- 8.Park YD, Baik KS, Yi H, Bae KS, Chun J. Pseudoalteromonas byunsanensis sp. nov., isolated from tidal flat sediment in Korea. Int J Syst Evol Microbiol. 2005;55(6): 2519–2523. [DOI] [PubMed] [Google Scholar]
- 9.Balibar CJ, Walsh CT. In vitro biosynthesis of violacein from L-tryptophan by the enzymes VioA-E from Chromobacterium violaceum. Biochemistry. 2006;45(51): 15444–15457. doi: 10.1021/bi061998z [DOI] [PubMed] [Google Scholar]
- 10.Sun H, Zhao D, Xiong B, Zhang C, Bi C. Engineering Corynebacterium glutamicum for violacein hyper production. Microb Cell Fact. 2016;15(1): 148 doi: 10.1186/s12934-016-0545-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durán M, Ponezi AN, Faljoni-Alario A, Teixeira MFS, Justo GZ, Durán N. Potential applications of violacein: a microbial pigment. Med Chem Res. 2012;21(7): 1524–1532. [Google Scholar]
- 12.Yan J, Wu Y-H, Meng F-X, Wang C-S, Xiong S-L, Zhang X-Y, et al. Pseudoalteromonas gelatinilytica sp. nov., isolated from surface seawater. Int J Syst Evol Microbiol. 2016;66(9): 3538–3545. doi: 10.1099/ijsem.0.001224 [DOI] [PubMed] [Google Scholar]
- 13.Xu XW, Wu YH, Zhou Z, Wang CS, Zhou YG, Zhang HB, et al. Halomonas saccharevitans sp. nov., Halomonas arcis sp. nov. and Halomonas subterranea sp. nov., halophilic bacteria isolated from hypersaline environments of China. Int J Syst Evol Microbiol. 2007;57(7): 1619–1624. [DOI] [PubMed] [Google Scholar]
- 14.Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1(1): 18 doi: 10.1186/2047-217X-1-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5(2): R12 doi: 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome res. 2015;25(7): 1043–1055. doi: 10.1101/gr.186072.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35(9): 3100–3108. doi: 10.1093/nar/gkm160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim O-S, Cho Y-J, Lee K, Yoon S-H, Kim M, Na H, et al. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62(3): 716–721. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, et al. ARB: a software environment for sequence data. Nucleic. Nucleic Acids Res. 2004;32(4): 1363–1371. doi: 10.1093/nar/gkh293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarza P, Richter M, Peplies J, Euzeby J, Amann R, Schleifer K-H, et al. The All-Species Living Tree project: A 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol. 2008;31(4): 241–250. doi: 10.1016/j.syapm.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 21.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35(21): 7188–7196. doi: 10.1093/nar/gkm864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22): 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10): 2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4): 406–425. [DOI] [PubMed] [Google Scholar]
- 25.Fitch WM. Toward defining the course of evolution: minimum change for a specific tree topology. Syst Zool. 1971;20(4): 406–416. [Google Scholar]
- 26.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6): 368–376. [DOI] [PubMed] [Google Scholar]
- 27.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16(2): 111–120. [DOI] [PubMed] [Google Scholar]
- 28.Dong X, Cai M. Determinative manual for routine bacteriology. Beijing: Scientific Press; (English translation); 2001. [Google Scholar]
- 29.Mireille Ayé A, Bonnin-Jusserand M, Brian-Jaisson F, Ortalo-Magne A, Culioli G, Koffi Nevry R, et al. Modulation of violacein production and phenotypes associated with biofilm by exogenous quorum sensing N-acylhomoserine lactones in the marine bacterium Pseudoalteromonas ulvae TC14. Microbiology. 2015;161(10): 2039–2051. doi: 10.1099/mic.0.000147 [DOI] [PubMed] [Google Scholar]
- 30.Farmer III JJ, Janda JM, Brenner FW, Cameron DN, Birkhead KM. Genus I. Vibrio Pacini 1854, 411AL In: Garrity GM, Brenner DJ, Krieg NR, Staley JT. editors. Bergey's Manual of Systematic Bacteriology, 2nd edn, vol. 2, The Proteobacteria, Part B, The Gammaproteobacteria. New York: Springer; 2005. p. 494–546. [Google Scholar]
- 31.Leifson E. Determination of carbohydrate metabolism of marine bacteria. J Bacteriol. 1963;85(5): 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YH, Xu L, Meng FX, Zhang DS, Wang CS, Oren A, et al. Altererythrobacter atlanticus sp. nov., isolated from deep-sea sediment. Int J Syst Evol Microbiol. 2014;64(1): 116–121. [DOI] [PubMed] [Google Scholar]
- 33.Wu YH, Xu L, Zhou P, Wang CS, Oren A, Xu XW. Brevirhabdus pacifica gen. nov., sp. nov., isolated from deep-sea sediment in a hydrothermal vent field. Int J Syst Evol Microbiol. 2015;65(10): 3645–3651. doi: 10.1099/ijsem.0.000469 [DOI] [PubMed] [Google Scholar]
- 34.Komagata K, Suzuki K-I. Lipid and cell-wall analysis in bacterial systematics. Methods Microbiol. 1987;19(16): 161–207. [Google Scholar]
- 35.Tindall BJ, Sikorski J, Smibert RA, Krieg NR. Phenotypic characterization and the principles of comparative systematics In: Reddy CA, Beveridge TJ, Breznak JA, Marzluf G, Schmidt TM, Snyder LR, editors. Methods for General and Molecular Microbiology. 3rd edn Washington DC, USA: ASM Press; 2007. p. 330–393. [Google Scholar]
- 36.Chen C, Su Y, Tao T, Fu G, Zhang C, Sun C, et al. Maripseudobacter aurantiacus gen. nov., sp. nov., a novel member of the family Flavobacteriaceae isolated from a sedimentation basin. Int J Syst Evol Microbiol. 2017;67(4): 778–783. doi: 10.1099/ijsem.0.001580 [DOI] [PubMed] [Google Scholar]
- 37.Lee I, Kim YO, Park SC, Chun J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 2016;66(2): 1100–1103. [DOI] [PubMed] [Google Scholar]
- 38.Meier-Kolthoff JP, Auch AF, Klenk H-P, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics. 2013;14(1): 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowe TM, Chan PP. tRNAscan-SE on-line: integrating search and contextual analysis of transfer RNA genes. Nucleic Acids Res. 2016;44(W1): W54–57. doi: 10.1093/nar/gkw413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9(1): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S, Zhu Z, Fu L, Niu B, Li W. WebMGA: a customizable web server for fast metagenomic sequence analysis. BMC Genomics. 2011;12(1): 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36 Suppl 1: D480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M. KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007;35 Suppl 2: W182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L, Stoeckert CJ Jr., Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13(9): 2178–2189. doi: 10.1101/gr.1224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yada S, Wang Y, Zou Y, Nagasaki K, Hosokawa K, Osaka I, Arakawa R, Enomoto K. Isolation and characterization of two groups of novel marine bacteria producing violacein. Mar Biotechnol. 2008;10(2): 128–132. doi: 10.1007/s10126-007-9046-9 [DOI] [PubMed] [Google Scholar]
- 46.Bowman JP, McMeekin TA. Genus XI. Pseudoalteromonas Gauthier, Gauthier and Christen 1995a, 759VP In Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey's Manual of Systematic Bacteriology. 2nd edn. New York: Springer; 2005. p. 467–478. [Google Scholar]
- 47.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. U. S. A. 2009;106(45): 19126–19131. doi: 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wayne LG, Brenner DJ, Colwell RR, Grimont AD, Kandler O, Krichevsky MI, et al. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol. 1987;37(10): 463–464. [Google Scholar]
- 49.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315(5819): 1709–1712. doi: 10.1126/science.1138140 [DOI] [PubMed] [Google Scholar]
- 50.Hoshino T. Violacein and related tryptophan metabolites produced by Chromobacterium violaceum: biosynthetic mechanism and pathway for construction of violacein core. Appl Microbiol Biotechnol. 2011;91(6): 1463–1475. doi: 10.1007/s00253-011-3468-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
The gene sequences were obtained from the genomes, the accession numbers of which are indicated in parentheses. Bootstrap values (>90%) based on 1,000 replications are shown at branch nodes. Bar, 0.05 substitutions per nucleotide position.
(TIF)
Transmission electron micrographs showing the cell ultrastructure of strains JW1T (a) and JW3 (b). Bar, 0.5 μm.
(TIF)
Absorption profile (a) and mass spectrum (b) of violacein.
(TIF)
Thin-layer chromatograms after staining with molybdatophosphoric acid, α-naphthol reagent, ninhydrin reagent, and molybdenum blue showing the total polar lipid profiles of strains JW1T (a1-a4), JW3 (b1-b4), and P. byunsanensis JCM 12483T (c1-c4). PE, Phosphatidylethanolamine; PG, phosphatidylglycerol; AL, aminolipid; GL, glycolipid; PL, phospholipid; L, other lipid.
(TIF)
Data Availability Statement
The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequence of strains JW1T and JW3 are KU535632 and KU535631. The GenBank accession numbers for the whole genome sequences of strains JW1T, JW3 and P. byunsanensis JCM12483T are MKJU00000000, MKJT00000000 and MNAN00000000, respectively. Other relevant data are within the paper and its Supporting Information files.