Abstract
The experimental metastatic potential of 13762NF mammary adenocarcinoma clone MTLn3 was tested after pretreatment in serum-free medium containing transforming growth factor (TGF) beta 1 at 0-5000 pg/ml. Lung colonies were measured 2 weeks after inoculation in syngeneic F344 rats, and a bell-shaped dose-response curve with 2- to 3-fold increase in number of surface lung metastases was seen. Maximal enhancement occurred at the 50 pg/ml dose level. The effect was specific because addition of neutralizing anti-TGF-beta antibody blocked the stimulatory activity at all levels of TGF-beta 1 pretreatment, but when antibody was given alone, neutralizing anti-TGF-beta antibody had no effect on untreated cells. Increased metastatic potential appears to be from an increased propensity of cells to extravasate as tested in the membrane invasion culture system. MTLn3 cells penetrated reconstituted basement-membrane barriers 2- to 3.5-fold more than did untreated control cells, depending upon length of TGF-beta 1 exposure. Increased invasive potential is apparently due, in part, to a 2- to 6-fold increase in type IV collagenolytic (gelatinolytic) and a 2.4-fold increase in heparanase activity. TGF-beta 1 treatment of MTLn3 cells did not alter their growth rate or morphology in the presence of serum; however, growth was inhibited in serum-free medium. Likewise, adhesion to human umbilical vein endothelial cell monolayers or to immobilized reconstituted basement membrane or fibronectin matrices was unchanged. These results suggest that TGF-beta 1 may modulate metastatic potential of mammary tumor cells by controlling their ability to break down and penetrate basement-membrane barriers.
Full text
PDF

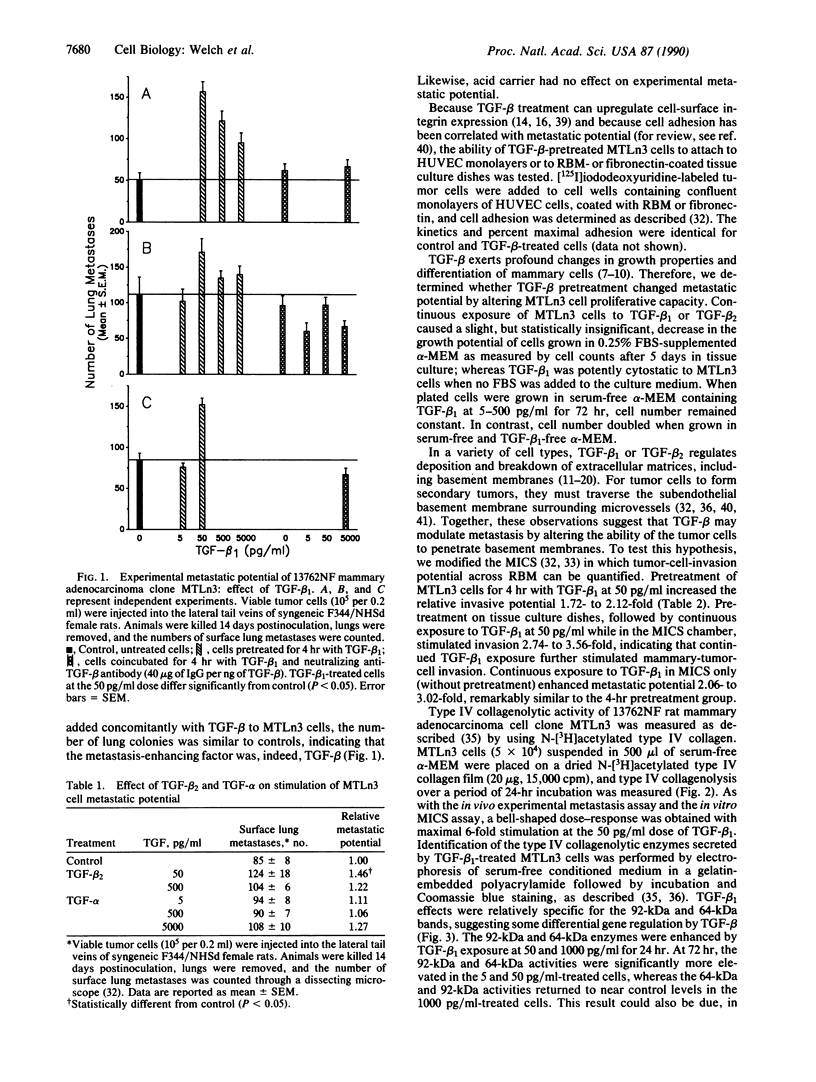
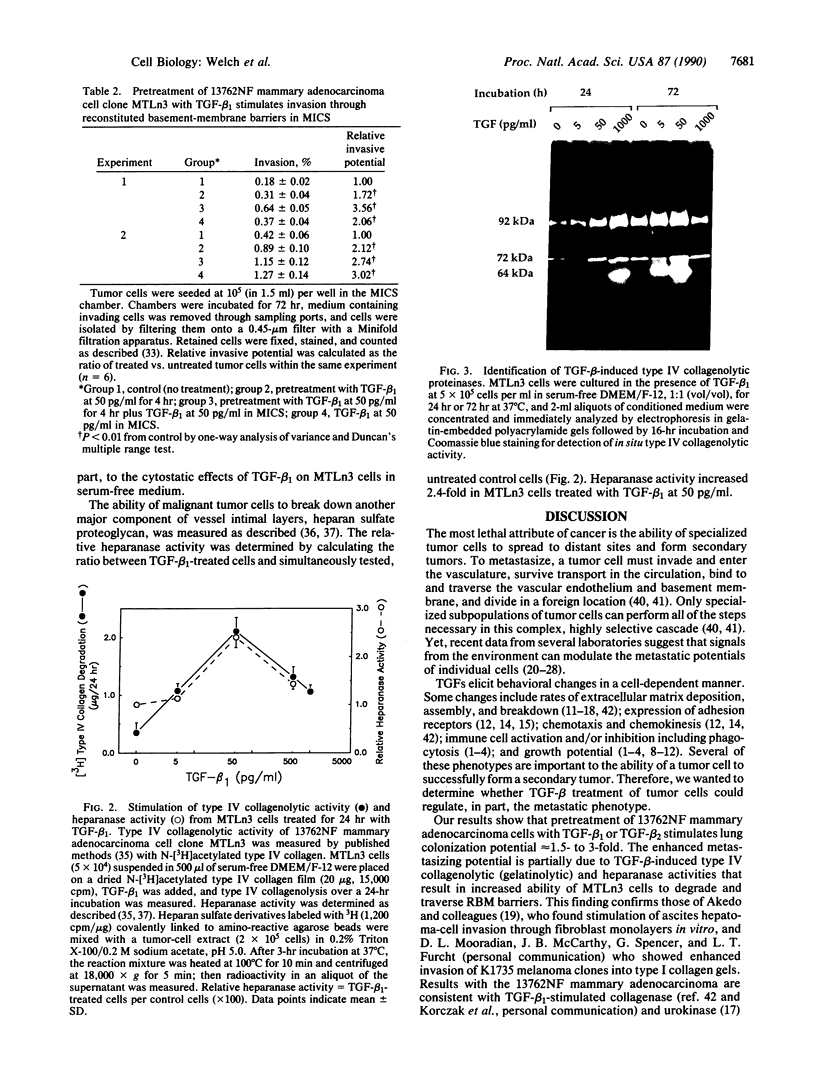

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeed P. A., Nakajima M., Welch D. R. The role of polymorphonuclear leukocytes (PMN) on the growth and metastatic potential of 13762NF mammary adenocarcinoma cells. Int J Cancer. 1988 Nov 15;42(5):748–759. doi: 10.1002/ijc.2910420521. [DOI] [PubMed] [Google Scholar]
- Akedo H., Shinkai K., Mukai M., Komatsu K. Potentiation and inhibition of tumor cell invasion by host cells and mediators. Invasion Metastasis. 1989;9(2):134–148. [PubMed] [Google Scholar]
- Arrick B. A., Korc M., Derynck R. Differential regulation of expression of three transforming growth factor beta species in human breast cancer cell lines by estradiol. Cancer Res. 1990 Jan 15;50(2):299–303. [PubMed] [Google Scholar]
- Chua C. C., Geiman D. E., Keller G. H., Ladda R. L. Induction of collagenase secretion in human fibroblast cultures by growth promoting factors. J Biol Chem. 1985 May 10;260(9):5213–5216. [PubMed] [Google Scholar]
- Dabbous M. K., Haney L., Carter L. M., Paul A. K., Reger J. Heterogeneity of fibroblast response in host-tumor cell-cell interactions in metastatic tumors. J Cell Biochem. 1987 Dec;35(4):333–344. doi: 10.1002/jcb.240350408. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Budmen M. B. Characterization in vivo and in vitro of tumor cells selected for resistance to syngeneic lymphocyte-mediated cytotoxicity. Cancer Res. 1976 Sep;36(9 PT1):3160–3165. [PubMed] [Google Scholar]
- Freter C. E., Lippman M. E., Cheville A., Zinn S., Gelmann E. P. Alterations in phosphoinositide metabolism associated with 17 beta-estradiol and growth factor treatment of MCF-7 breast cancer cells. Mol Endocrinol. 1988 Feb;2(2):159–166. doi: 10.1210/mend-2-2-159. [DOI] [PubMed] [Google Scholar]
- Gorelik E., Wiltrout R. H., Copeland D., Herberman R. B. Modulation of formation of tumor metastases by peritoneal macrophages elicited by various agents. Cancer Immunol Immunother. 1985;19(1):35–42. doi: 10.1007/BF00199309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Seftor R. E., Fidler I. J. A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett. 1987 Dec;38(1-2):137–147. doi: 10.1016/0304-3835(87)90209-6. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Karey K. P., Sirbasku D. A. Differential responsiveness of human breast cancer cell lines MCF-7 and T47D to growth factors and 17 beta-estradiol. Cancer Res. 1988 Jul 15;48(14):4083–4092. [PubMed] [Google Scholar]
- Keski-Oja J., Blasi F., Leof E. B., Moses H. L. Regulation of the synthesis and activity of urokinase plasminogen activator in A549 human lung carcinoma cells by transforming growth factor-beta. J Cell Biol. 1988 Feb;106(2):451–459. doi: 10.1083/jcb.106.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Postlethwaite A. E., Moses H. L. Transforming growth factors in the regulation of malignant cell growth and invasion. Cancer Invest. 1988;6(6):705–724. doi: 10.3109/07357908809078038. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Miller F. R., McEachern D., Miller B. E. Growth regulation of mouse mammary tumor cells in collagen gel cultures by diffusible factors produced by normal mammary gland epithelium and stromal fibroblasts. Cancer Res. 1989 Nov 1;49(21):6091–6097. [PubMed] [Google Scholar]
- Miller F. R. Tumor subpopulation interactions in metastasis. Invasion Metastasis. 1983;3(4):234–242. [PubMed] [Google Scholar]
- Müller G., Behrens J., Nussbaumer U., Böhlen P., Birchmeier W. Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5600–5604. doi: 10.1073/pnas.84.16.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Irimura T., Nicolson G. L. A solid-phase substrate of heparanase: its application to assay of human melanoma for heparan sulfate degradative activity. Anal Biochem. 1986 Aug 15;157(1):162–171. doi: 10.1016/0003-2697(86)90209-5. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Irimura T., Nicolson G. L. Heparanases and tumor metastasis. J Cell Biochem. 1988 Feb;36(2):157–167. doi: 10.1002/jcb.240360207. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Lotan D., Baig M. M., Carralero R. M., Wood W. R., Hendrix M. J., Lotan R. Inhibition by retinoic acid of type IV collagenolysis and invasion through reconstituted basement membrane by metastatic rat mammary adenocarcinoma cells. Cancer Res. 1989 Apr 1;49(7):1698–1706. [PubMed] [Google Scholar]
- Nakajima M., Welch D. R., Belloni P. N., Nicolson G. L. Degradation of basement membrane type IV collagen and lung subendothelial matrix by rat mammary adenocarcinoma cell clones of differing metastatic potentials. Cancer Res. 1987 Sep 15;47(18):4869–4876. [PubMed] [Google Scholar]
- Neri A., Welch D., Kawaguchi T., Nicolson G. L. Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J Natl Cancer Inst. 1982 Mar;68(3):507–517. [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis: tumor cell and host organ properties important in metastasis to specific secondary sites. Biochim Biophys Acta. 1988 Nov 15;948(2):175–224. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Pignatelli M., Bodmer W. F. Integrin-receptor-mediated differentiation and growth inhibition are enhanced by transforming growth factor-beta in colorectal tumour cells grown in collagen gel. Int J Cancer. 1989 Sep 15;44(3):518–523. doi: 10.1002/ijc.2910440324. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. Transforming growth factor (type beta) promotes the addition of chondroitin sulfate chains to the cell surface proteoglycan (syndecan) of mouse mammary epithelia. J Cell Biol. 1989 Nov;109(5):2509–2518. doi: 10.1083/jcb.109.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz L. C., Gingras M. C., Goldberg G., Greenberg A. H., Wright J. A. Loss of growth factor dependence and conversion of transforming growth factor-beta 1 inhibition to stimulation in metastatic H-ras-transformed murine fibroblasts. Cancer Res. 1988 Dec 15;48(24 Pt 1):6999–7003. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers M. T., Barrett-Lee P. J., Berger U., Luqmani Y. A., Gazet J. C., Powles T. J., Coombes R. C. Growth factor expression in normal, benign, and malignant breast tissue. Br Med J (Clin Res Ed) 1988 Jun 11;296(6637):1621–1624. doi: 10.1136/bmj.296.6637.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverius E. M., Walker-Jones D., Bates S. E., Stampfer M. R., Clark R., McCormick F., Dickson R. B., Lippman M. E. Production of and responsiveness to transforming growth factor-beta in normal and oncogene-transformed human mammary epithelial cells. Cancer Res. 1989 Nov 15;49(22):6269–6274. [PubMed] [Google Scholar]
- Weiss L. Dynamic aspects of cancer cell populations in metastasis. Am J Pathol. 1979 Dec;97(3):601–608. [PMC free article] [PubMed] [Google Scholar]
- Welch D. R., Lobl T. J., Seftor E. A., Wack P. J., Aeed P. A., Yohem K. H., Seftor R. E., Hendrix M. J. Use of the Membrane Invasion Culture System (MICS) as a screen for anti-invasive agents. Int J Cancer. 1989 Mar 15;43(3):449–457. doi: 10.1002/ijc.2910430318. [DOI] [PubMed] [Google Scholar]
- Welch D. R., Neri A., Nicolson G. L. Comparison of 'spontaneous' and 'experimental' metastasis using rat 13762 mammary adenocarcinoma metastatic cell clones. Invasion Metastasis. 1983;3(2):65–80. [PubMed] [Google Scholar]
- Welch D. R., Schissel D. J., Howrey R. P., Aeed P. A. Tumor-elicited polymorphonuclear cells, in contrast to "normal" circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5859–5863. doi: 10.1073/pnas.86.15.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman D. M., Polverini P. J., Kamp D. W., Leibovich S. J. Transforming growth factor-beta (TGF beta) is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem Biophys Res Commun. 1988 Dec 15;157(2):793–800. doi: 10.1016/s0006-291x(88)80319-x. [DOI] [PubMed] [Google Scholar]




