Abstract
Bacterial phospholipase C has been reported to play a role in the pathogenesis of many bacteria. In order to gain a better understanding of the potential role of Mycobacterium tuberculosis phospholipase C in the pathogenesis of human tuberculosis, we investigated the genetic diversity of the four M. tuberculosis phospholipase C-encoding genes (plcA, plcB, plcC, and plcD) resulting from the IS6110 insertion and associated deletion, among 106 clinical isolates obtained from Turkey, by using PCR, Southern hybridization, and DNA sequencing. Two sequenced M. tuberculosis strains, H37Rv and CDC1551, were used as the references in the comparison. Sixty-six (62.3%) of the 106 isolates had an intact plcD gene, and 40 (37.7%) showed an interruption of the gene. Of the latter 40 isolates, 19 (47.5%) had an IS6110 insertion with no associated deletion in the plcD gene, 2 (5%) had an IS6110 insertion and an associated deletion within the plcD gene, 15 (37.5%) had an IS6110 insertion in the plcD gene that was associated with a partial deletion of the plcD gene and its right forward adjacent region, and 4 (10%) had a complete deletion of the plcD gene. The proportions of the isolates with an interrupted plcA, plcB, or plcC gene were 3.8, 1.9, and 3.8%, respectively. The data indicate that there is a much higher frequency of IS6110 insertion and deletion in the plcD gene than in the plcA, plcB, and plcC genes of M. tuberculosis.
Tuberculosis remains a leading infectious killer worldwide (14). In order to develop a better vaccine and more efficient therapeutic agents for tuberculosis prevention and control, it is essential to gain a better understanding of the pathogenicity and the virulence of Mycobacterium tuberculosis.
Bacterial phospholipase C (PLC) cleaves phospholipids and has been reported to play a role in the pathogenesis of many bacteria, including Clostridium perfringens, Listeria monocytogenes, and Pseudomonas aeruginosa (11). The activities of the two PLCs of L. monocytogenes, PLCA and PLCB, appear to overlap during the course of the intracellular infection (10). PLCA is thought to have a role in lysing the primary phagosome, and mutations in the gene encoding PLCA result in a decreased ability of L. monocytogenes to replicate in mouse peritoneal macrophages (1, 5, 10). The PLCs of P. aeruginosa, PLCH and PLCN, are thought to be particularly important in pathogenesis in the lungs because of their ability to degrade phosphatidylcholine in the lung surfactant, and a moderate reduction in alveolar epithelial injury was seen when rabbit lungs were infected with a PLC double mutant of P. aeruginosa (9, 12).
Two PLC-encoding genes, designated plcA and plcB, were identified in M. tuberculosis by Leão et al. before the completion of the M. tuberculosis strain H37Rv genome sequencing. The enzymes encoded by these two genes of M. tuberculosis, PLCA and PLCB, are similar to PLCN and PLCH of P. aeruginosa, respectively (7). The sequencing of the genome of M. tuberculosis H37Rv identified two additional M. tuberculosis plc genes, plcC and plcD (2). Three of the four genes, plcA, plcB, and plcC, are clustered together on the chromosome, while plcD is located in a different genomic region (2, 3). Previous studies using small sets of clinical isolates have demonstrated the variations in the plc genes of M. tuberculosis due to the IS6110 insertion and associated deletion (6, 13).
It was recently demonstrated that all four plc genes of M. tuberculosis are functional and that all are involved in PLC activity. The plcABC triple mutants and plcABCD quadruple mutants were found to be attenuated for growth in the lungs and spleens of mice, and the expression of the different plc genes is important for virulence at the different time points of the infection (8).
In order to gain a better understanding of the potential role of M. tuberculosis PLC in the pathogenesis of human tuberculosis, we investigated insertion- and deletion-associated genetic diversity in the four plc genes of M. tuberculosis among 106 clinical isolates obtained from Malatya, Turkey.
MATERIALS AND METHODS
M. tuberculosis isolates.
The study sample included a total of 106 M. tuberculosis clinical isolates obtained from 106 patients with active tuberculosis who were diagnosed at the Healthcare Centers of Malatya in eastern Turkey between 1 January and 31 December 2000. The isolates were identified as M. tuberculosis by conventional mycobacteriology speciation methods, including nitrate reduction, the niacin accumulation test, the BACTEC p-nitro-α-acetylamino-β-hydroxypropiophenone test (Becton Dickinson), and growth characteristics (4). DNA fingerprinting data of the study isolates were obtained from a molecular epidemiological database available at the Department of Clinical Microbiology, Inonu University, Malatya, Turkey. Of the 106 isolates, 75 (70.8%) had a unique IS6110 fingerprint, while the other 31 (29.2%) belonged to 12 different IS6110 fingerprint clusters, each of which included two to five isolates. Thus, the 106 isolates were considered to represent a total of 87 different strains.
PCR of plcD.
A PCR assay designated plcD-PCR1 was conducted to investigate the genetic diversity of the plcD gene due to the IS6110 insertion. The primers used in plcD-PCR1 were plcD1-F and plcD1-R (Table 1). M. tuberculosis CDC1551, which has an intact plcD gene (3), was used as a positive control, and M. tuberculosis H37Rv, which has a truncated plcD gene (2), was used as a negative control. A BD Advantage-GC 2 PCR kit (BD Biosciences Clontech, Palo Alto, Calif.) was used. Each standard 50-μl reaction mixture consisted of 10 μl of 5× reaction buffer, 5 μl of GC-Melt, 20 pmol of each primer in 2 μl, 1 μl of a 50× deoxyribonucleoside triphosphate mix, 1 μl of a 50× BD Advantage 2 polymerase mix, 2 μl of DNA solution containing 20 ng of DNA template, and 27 μl of PCR-grade water. The thermocycling program used for the plcD-PCR1 assay was 1 cycle at 94°C for 1 min; 26 cycles at 94°C for 30 s, 68°C for 30 s, and 72°C for 2.5 min; and a final cycle at 72°C for 10 min.
TABLE 1.
Oligonucleotide primers used in different PCR assays
| PCR assay | Primer | Sequence | Positiona |
|---|---|---|---|
| plcD-PCR1 | plcD1-F | 5′-TCG CCC GGA CAG GTC AAC AAG GTG-3′ | 107 bp to left |
| plcD1-R | 5′-CGC GCG CCG CCG CGC CGA AAT-3′ | 240 bp to right | |
| plcA-PCR | plcA-F | 5′-TCG AAC GCC GGG AGA TTA CC-3′ | 28 bp to left |
| plcA-R | 5′-GCA GGA AGG CAG GGC AAG TG-3′ | 17 bp to right | |
| plcB-PCR | plcB-F | 5′-TCC GGC GAA TGC ACC TTG GCT CAC-3′ | 82 bp to left |
| plcB-R | 5′-CGG CAG GCA GGC GGA ATC AGA ACA-3′ | 138 bp to right | |
| plcC-PCR | plcC-F | 5′-GGG CGG CAA AGG CGG ACC AAG AG-3′ | 224 bp to left |
| plcC-R | 5′-AAG CCG AAA TAC ACG AGG GAG AGC-3′ | 55 bp to right | |
| plcD-PCR2 | plcD2-F | 5′-GTC CAC TGT CGC CCG GAC AGG TCA ACA AGG TGT-3′ | 115 bp to left |
| plcD2-R | 5′-CGC TGG TAC ACC TGG GGA ATC TGG TGA CGT AGA-3′ | 18.2 kb to right |
Position in relation to the start site of the plcD gene sequence for the plcD-PCR1 and plcD-PCR2 assays and the start site of the plcA, plcB, and plcC gene sequences for the plcA-PCR, plcB-PCR, and plcC-PCR assays, respectively.
PCR of plcABC.
The sequence alterations in the plcA, plcB, and plcC genes resulting from the IS6110 insertion and the insertion-associated deletion were investigated using PCR assays designated plcA-PCR, plcB-PCR, and plcC-PCR, respectively. The primer pairs used in these three assays were plcA-F and plcA-R, plcB-F and plcB-R, and plcC-F and plcC-R, respectively (Table 1). The PCR reagents were from the BD Advantage 2 PCR kit (BD Biosciences Clontech). Each standard 50-μl reaction mix was the same as that used for plcD-PCR1, except that no GC-Melt was used and 5 μl of 10× reaction buffer was used. The thermocycling program used for the plcA-PCR, plcB-PCR, and plcC-PCR assays was the same as that used for the plcD-PCR1 assay.
Southern hybridization.
For the isolates that failed to produce a detectable product in plcD-PCR1, Southern hybridization using the plcD probe was performed to determine whether the plcD gene was present and whether the negative result was due to a disruption of the plcD-PCR1 priming sites. The genomic DNA of these isolates was restricted with the restriction endonuclease PvuII. The restriction fragments were separated by 1.0% (wt/vol) agarose gel electrophoresis and then transferred onto a Hybond-N+ nylon membrane (Amersham Pharmacia Biotech UK Limited, Little Chalfont, Buckinghamshire, United Kingdom), which was then hybridized with the plcD probe labeled using an enhanced-chemifluorescence random-priming labeling and amplification kit according to the manufacturer's instructions (Amersham Pharmacia Biotech UK Limited). Due to the sequence homology between the plcD and the plcA, -B, and -C genes, the plcD probe was also expected to hybridize with the plcA, -B, and -C genes. The plcD-specific bands were determined by excluding the bands attributable to plcD probe hybridization with the plcA, -B, and -C genes, based on the expected size of the plcABC-hybridizing bands and the results of the plcA-PCR, plcB-PCR, and plcC-PCR assays. A hypothetical map of the IS6110 insertions and associated deletions was constructed after analysis of the sizes of the plcD-specific bands.
PCR of the plcD gene-adjacent region.
Based on the results of the Southern hybridization, we hypothesized that the negative PCR results for many of the isolates were due to a large deletion of the region adjacent to the plcD gene, including the plcD1-R primer site. To test this hypothesis, we investigated the right forward adjacent region of the plcD gene in these isolates by using a PCR assay designated plcD-PCR2. The primers used in the plcD-PCR2 assay were plcD2-F and plcD2-R (Table 1). The MasterAmp Extra-Long PCR kit (Epicenter, Madison, Wis.) was used for this PCR assay. Each standard 50-μl reaction mixture consisted of 25 μl of 2× Premix 7 (containing PCR buffer and deoxyribonucleoside triphosphate) from the kit, 20 pmol of each primer in 2 μl, 2.5 U of MasterAmp Extra-Long DNA polymerase in 1 μl, 100 ng of DNA template in 10 μl, and 10 μl of PCR-grade water. The thermocycling program used for the plcD-PCR2 assay was 1 cycle of 94°C for 3 min; 31 cycles of 94°C for 1 min, 68°C for 1 min, and 72°C for 14.5 min; and 1 final cycle of 72°C for 10 min. All the PCR assays were performed using a 96-well Perkin-Elmer thermocycler, P-E 960 (Applied Biosystems, Foster City, Calif.). PCR products were examined by 0.8% (wt/vol) agarose gel electrophoresis performed in 1× Tris-borate-EDTA buffer.
Automated DNA sequencing.
PCR products of the plcD-PCR1, plcA-PCR, plcB-PCR, and plcC-PCR assays that were larger than that of the positive control and the PCR products of the plcD-PCR2 assay were sequenced to determine the site of IS6110 insertion and the length of any associated deletion. PCR products were purified using a QIAquick PCR purification kit according to the manufacturer's instructions (QIAGEN, Inc., Valencia, Calif.). Sequencing was performed in Applied Biosystems DNA sequencers (models 3700 and 3730) at the Sequencing Core of the University of Michigan. Sequence analysis was done using the National Center for Biotechnology Information BLAST program (www.ncbi.nlm.nih.gov/BLAST).
Definitions.
Isolates with an interruption in a given plc gene by IS6110 insertion and an associated deletion were identified as a mutant type of this particular gene; isolates for which the PCR results were identical to that of CDC1551 were designated a wild type.
RESULTS
Diversity of the plcD gene.
The 106 isolates fell into five different groups based on the sizes of their plcD-PCR1 products. Sixty-six (62.3%) of the 106 isolates had a PCR product of 1.9 kb, which is identical in size to the product of CDC1551. The other 40 (37.7%) of the 106 isolates either had a PCR product that was larger than that of CDC1551 or failed to produce a PCR product. Of these 40 isolates, 19 (47.5%) had a PCR product of 3.2 kb, a size equal to that of an intact plcD gene (1.9 kb) plus that of a copy of IS6110 (1.3 kb); 2 (5.0%) had a PCR product of a size (2.7 or 2.1 kb) that indicated a partial deletion of the plcD gene and an insertion of a copy of IS6110; and 19 (47.5%) were negative for the plcD-PCR1 assay (Table 2). Of the 12 clusters of isolates, 10 were found to have identical plcD genotypes for all the isolates in the same clusters, while two clusters showed different plcD genotypes for the different isolates belonging to the same clusters.
TABLE 2.
PCR results of four plc genes of 106 clinical isolates of M. tuberculosis from Malatya, Turkey
| Gene | PCR grouping | Size of PCR product (kb)c | No. (%) of isolates (n = 106) | No. (%) of strains (n = 86)b |
|---|---|---|---|---|
| plcA | plcA-I | 1.6a | 102 (96.2) | 84 (97.6) |
| plcA-II | 3.0 | 2 (1.9) | 1 (1.2) | |
| plcA-III | Negative | 2 (1.9) | 1 (1.2) | |
| plcB | plcB-I | 1.8a | 104 (98.1) | 85 (98.8) |
| plcB-II | Negative | 2 (1.9) | 1 (1.2) | |
| plcC | plcC-I | 1.8a | 102 (96.2) | 83 (96.5) |
| plcC-II | 3.0 | 3 (2.8) | 3 (3.5) | |
| plcC-III | Negative | 1 (0.9) | ||
| plcD | plcD-I | 1.9a | 66 (62.3) | 50 (58.8) |
| plcD-II | 3.2 | 19 (17.9) | 17 (20.0) | |
| plcD-III | 2.7 | 1 (0.9) | 1 (1.2) | |
| plcD-IV | 2.1 | 1 (0.9) | 1 (1.2) | |
| plcD-V | Negative | 19 (17.9) | 16 (18.8) |
Size identical to that of the PCR products of the positive control strain CDC1551.
The frequency of each plcABC genotype among the strains was determined based on a total of 86 strains, as two isolates of one strain were found to have different plcABC genotypes. The frequency of each plcD genotype among the strains was calculated based on the data for 85 of the 87 strains, as two strains showed a plcD genotype different from that of the clustered isolates.
Negative, no PCR product.
Diversity of plcABC.
Compared with the plcD gene, the plcA, plcB, and plcC genes were found to have much less IS6110 insertion-mediated diversity among the isolates studied. The proportions of the mutant types of the plcA, plcB, and plcC genes found in this set of isolates were 3.8% (four isolates), 1.9% (two isolates), and 3.8% (four isolates), respectively. These mutant types included those having PCR products larger than that of CDC1551 and those types that were PCR negative. The frequency of each genotype of the individual plc gene determined based on the number of isolates was very similar to that calculated based on the number of strains. Of the 12 clusters of isolates, 11 were found to have identical plcABC genotypes for all the isolates in the same clusters, while one cluster showed different plcABC genotypes for the two different isolates representing this strain (Table 2).
Southern hybridization.
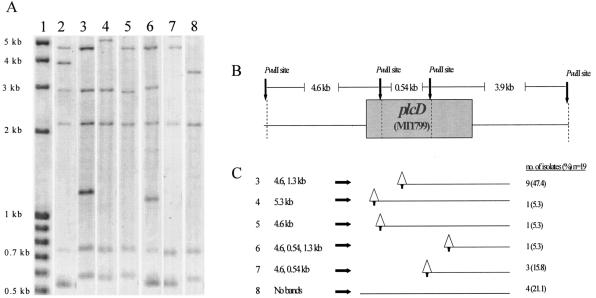
The 19 isolates that were negative for plcD-PCR1 fell into six different groups based on the results of Southern hybridization with the plcD probe. As shown by the hypothetical map of IS6110 insertions and associated deletions, 15 (78.9%) of the 19 isolates appeared to have a deletion of one of the plcD-PCR1 primer sites associated with the IS6110 insertion, and 4 (21.1%) of the 19 isolates did not have any plcD-specific bands, suggesting a deletion of the entire gene (Fig. 1).
FIG. 1.
Representative patterns of Southern hybridization of the 19 isolates that were negative by the plcD-PCR1 assay. (A) plcD Southern hybridization. Lane 1 contains the molecular size standard, lane 2 contains CDC1551, and lanes 3 through 7 indicate the five different banding patterns having bands that are plcD specific. The sizes of the plcD-specific bands in lanes 3 through 7 are shown in panel C. In lane 8, no plcD-specific band was detected. (B) PvuII restriction map of the plcD gene region and the expected restriction fragment size for CDC1551. (C) Hypothetical map of IS6110 insertion and associated deletion generated based on the analysis of the observed plcD band size. Triangles represent the positions of the IS6110 insertions predicted by Southern hybridization, and lines extending from the triangles represent the predicted deletion of unknown length. The arrows on the bottoms of the triangles represent the PvuII restriction site that is contained within the IS6110 sequence.
Diversity of the plcD-adjacent region.
Fifteen (78.9%) of the 19 isolates that failed to produce a product in plcD-PCR1 generated a detectable product in plcD-PCR2. Based on the results of plcD-PCR2, these 19 isolates were grouped into five groups. The size of the plcD-PCR2 products ranged from 3.5 to 5 kb, much smaller than the expected size of 20 kb. This observation confirmed the prediction of the Southern hybridization that a large region to the right of the plcD gene was deleted in these isolates. Of the 19 isolates, 9 (47.4%) had a 3.5-kb PCR product, 4 (21.1%) had a 4.0-kb PCR product, 1 (5.3%) had a 3.0-kb PCR product, and 1 (5.3%) had a 5.0-kb PCR product. The other four (21.1%) isolates were negative for plcD-PCR2. These four isolates were also found to lack the plcD gene by Southern hybridization. Sequence analysis of the 15 isolates that were positive by plcD-PCR2 confirmed the hypothetical map of IS6110 insertion and deletion generated based on the results of Southern hybridization, except for one isolate, for which the location of IS6110 as determined by DNA sequencing slightly differed from the prediction of Southern hybridization. This isolate was represented in lane 6 of Fig. 1, but upon sequencing, it was found to belong to the group represented in lane 7 of Fig. 1. Eight different plc gene profiles were revealed based on the combined results of all the PCR assays, the Southern hybridization, and the DNA sequencing (Table 3).
TABLE 3.
Grouping of 106 clinical isolates of M. tuberculosis according to genotypes based on their four plc genes
| Group | No. (%) of isolates (n = 106) | Genotype of isolate(s)a
|
|||
|---|---|---|---|---|---|
| plcD | plcA | plcB | plcC | ||
| PLC-I | 65 (61.3) | WT | WT | WT | WT |
| PLC-II | 1 (0.9) | WT | Mutant | WT | WT |
| PLC-III | 32 (30.2) | Mutant | WT | WT | WT |
| PLC-IV | 3 (2.8) | Mutant | WT | WT | Mutant |
| PLC-V | 1 (0.9) | Mutant | Mutant | WT | WT |
| PLC-VI | 2 (1.9) | Neg | WT | WT | WT |
| PLC-VII | 1 (0.9) | Neg | Neg | Neg | WT |
| PLC-VIII | 1 (0.9) | Neg | Neg | Neg | Neg |
WT, wild type; Neg, negative.
Sequence analysis of plc genes.
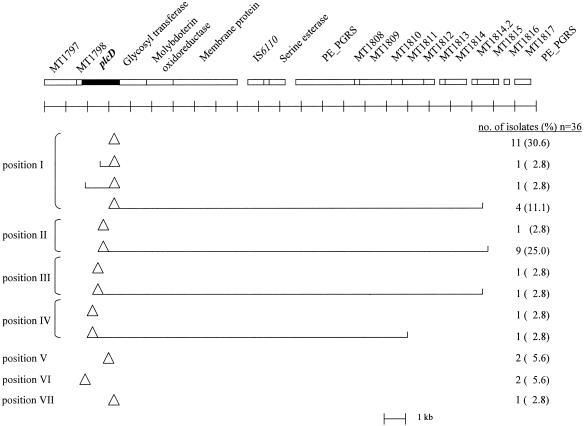
The PCR products of plcD-PCR1 and plcD-PCR2 that had altered sizes compared with those of the PCR products of CDC1551 were sequenced to confirm the insertion of IS6110 and to determine the location of the insertion and the size of any associated deletion. Of the 36 isolates sequenced, 19 (52.8%) had an IS6110 insertion within the plcD gene with no deletion, 2 (5.6%) had an IS6110 insertion and a partial deletion of the plcD gene, and 15 (41.7%) had an IS6110 insertion within the plcD gene followed by a partial deletion of the plcD gene and a large deletion of the plcD gene-adjacent region. Deletions of the region adjacent to the right side of the plcD gene ranged from 16.0 to 18.2 kb. Four (10%) of the 40 isolates with a mutant-type plcD gene were not sequenced because they did not contain the plcD gene, as suggested by the results of Southern hybridization. Characterization of the IS6110-mediated mutations for these four isolates was based only on Southern hybridization.
The insertion of IS6110 was found in seven different positions within the plcD gene, designated positions I through VII (Fig. 2). Seventeen (47.2%) of the 36 isolates had an IS6110 insertion at position I, 1,096 bp to the right of the start of the plcD gene; 10 (27.8%) had the insertion at position II, 603 bp to the right of the start of the plcD gene. Of the 36 isolates, two each (5.6%) had an IS6110 insertion at positions III, IV, V, or VI, 231, 154, 720, or 115 bp to the right of the start site of the plcD gene, respectively. One (2.8%) of the 36 isolates had an IS6110 insertion at position VII, 792 bp to the right of the start of the plcD gene (Fig. 2).
FIG. 2.
Gene map of the plcD gene region of CDC1551 and map of IS6110 insertion and deletion identified among the study isolates by DNA sequencing of PCR products. Triangles represent IS6110 insertions, and open brackets extending from the triangles represent deletions. The positions of the IS6110 insertion were labeled with Roman numerals from I to VII, indicating locations of 1,096, 603, 231, 154, 720, 115, and 792 bp to the right of the plcD gene start site, respectively.
Both of the plcA-PCR products that were larger than that of CDC1551 had an IS6110 insertion 137 bp to the right of the start of the plcA gene. Of the three plcC-PCR products that were larger than that of CDC1551, two had an IS6110 insertion 132 bp to the left of the plcC gene start site and one had an IS6110 insertion 102 bp to the right of the plcC gene start site. The IS6110 insertion in the former two isolates did not interrupt the plcC gene.
DISCUSSION
Because of the demonstrated role of PLC in the pathogenicity of an increasing number of pathogenic bacteria, including M. tuberculosis, and the highly conserved nature of the genomic sequence of M. tuberculosis, the diversity of the plc genes among clinical isolates is of interest. Our study investigated a collection of 106 clinical isolates containing predominantly unique strains and found their plcD genes to be highly varied due to the IS6110 insertion and associated deletion, especially in comparison with the plcA, -B, and -C genes. Although the genetic diversity of the plcABC gene region resulting from the IS6110 insertion is limited among clinical isolates, an increased level of genetic diversity was observed among the isolates when the plc gene profile of each individual isolate was examined based on the genotypes of all four of the plc genes. Since the protein product of each plc gene has been demonstrated to contribute to the overall PLC activity of M. tuberculosis, examining the genetic diversity of all four of the genes of each isolate allows for a better estimation of the potential diversity in PLC activity among clinical isolates (8). Further studies that consider all four plc genes of each isolate would be more informative of the contributions of each of the four PLCs.
Forty (37.7%) of the 106 isolates and 37 (42.5%) of the 87 strains investigated in our study were found to have an interrupted plcD gene, supporting the previous findings that the plcD gene region is a highly varied region and a hot spot for IS6110 insertion and associated deletion (6). Ho et al. investigated the genetic diversity of the 20-kb region of M. tuberculosis that contains the plcD gene and its right forward adjacent genes, and they found that 5 (22.7%) of 22 clinical isolates from the United Kingdom had an IS6110 insertion within the plcD gene (6).
The plcABC gene region was found to have very limited IS6110 insertion-mediated genetic diversity among clinical isolates, especially in comparison with the plcD gene. This finding contrasts with the findings of a recent study of the genetic diversity of all four of the plc genes of M. tuberculosis by Viana-Niero et al. (13), which found that 12 (48%), 10 (40%), 13 (52%), and 15 (60%) of the 25 isolates included in their study had polymorphisms in the plcA, plcB, plcC, and plcD genes, respectively, according to the PCR assays used. However, given the differences in the natures and sizes of the study samples between the two studies, it was not unexpected that different findings were obtained from the two different studies. In the earlier study, the isolates examined were chosen based on the results of PCR of the mtp40 sequence, which is located within the plcABC gene region and contains part of plcA and plcB. Twelve (48%) of the 25 isolates selected had a polymorphism in the mtp40 region, which may result in a much higher percentage of isolates with mutation of the plcABC genes than what might be found in the general population of M. tuberculosis (13). In contrast, the present study used a collection of clinical isolates that represented all the culture-proven tuberculosis cases diagnosed at the Healthcare Centers of Malatya in eastern Turkey over a 1-year time period. In addition, it is also possible that the genetic diversity of each of the genes was underestimated in the present study because the study was focused on describing and comparing insertion and deletion-related polymorphisms in the four plc genes and not on investigating small deletions or single-nucleotide polymorphisms in these genes.
Although the present study cannot lead to any conclusion about the pathogenicity of M. tuberculosis in relation to the interruption of the plc genes by IS6110 insertion or deletion, this study provides the first compelling evidence that there is a much higher rate of IS6110 insertion and deletion in the plcD gene than in the plcA, -B, and -C genes of M. tuberculosis. Understanding the genetic diversity of all four of these genes in the general population of M. tuberculosis is important because it is uncertain how much the activity of these four PLCs can complement each other during the course of the disease. Future studies will need to address how the variations of these genes among clinical isolates relate to the pathogenicity and virulence of M. tuberculosis by correlating genetic polymorphisms of the isolates with clinical and epidemiological data of the patients from whom the isolates were obtained. Identification of genes that show genetic polymorphisms having a strong association with the important clinical and epidemiological characteristics of the patients can provide information useful for a rational selection of genes for future in vitro and in vivo functional analysis and allow for a more focused search for virulence factors of M. tuberculosis, which will ultimately contribute to the development of better vaccines and therapeutic agents for tuberculosis prevention and control.
Acknowledgments
This study was supported by a grant (NIH-R01-AI151975) from the National Institutes of Health.
We are indebted to Ercument Evliyaoglu and Aydin Senoglu at Tuberculosis Control Dispensary in Malatya, Turkey, for their contributions to the collection of the study isolates and to Biolog Selami Gunal at the Department of Clinical Microbiology, Inonu University Medical Faculty, for his technical assistance in M. tuberculosis culture and DNA extraction. We also thank Dong Yang for her laboratory assistance and Carl Marrs, Betsy Foxman, and Lixin Zhang at the Center of Molecular and Clinical Epidemiology at the Epidemiology Department of the University of Michigan, School of Public Health, for their helpful discussion.
REFERENCES
- 1.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole, S., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann, R. D., D. Alland, J. A. Eisen, L. Carpenter, O. White, J. Peterson, R. DeBoy, R. Dodson, M. Gwinn, D. Haft, E. Hickey, J. F. Kolonay, W. C. Nelson, L. A. Umayam, M. Ermolaeva, S. L. Salzberg, A. Delcher, T. Utterback, J. Weidman, H. Khouri, J. Gill, A. Mikula, W. Bishai, W. R. Jacobs, Jr., J. C. Venter, and C. M. Fraser. 2002. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J. Bacteriol. 184:5479-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes, B. A., D. F. Sham, and A. S. Weissfeld. 2002. Bailey and Scott's diagnostic microbiology, 11th ed. Mosby, Inc., St. Louis, Mo.
- 5.Goldfine, H., C. Knob, D. Alford, and J. Bentz. 1995. Membrane permeabilization by Listeria monocytogenes phosphatidylinositol-specific phospholipase C is independent of phospholipid hydrolysis and cooperative with listeriolysin O. Proc. Natl. Acad. Sci. USA 92:2979-2983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Ho, T. B., B. D. Robertson, G. M. Taylor, R. J. Shaw, and D. B. Young. 2000. Comparison of Mycobacterium tuberculosis genomes reveals frequent deletions in a 20 kb variable region in clinical isolates. Yeast 17:272-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leão, S. C., C. L. Rocha, L. A. Murillo, C. A. Parra, and M. E. Patarroyo. 1995. A species-specific nucleotide sequence of Mycobacterium tuberculosis encodes a protein that exhibits hemolytic activity when expressed in Escherichia coli. Infect. Immun. 63:4301-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raynaud, C., C. Guilhot, J. Rauzier, Y. Bordat, V. Pelicic, R. Manganelli, I. Smith, B. Gicquel, and M. Jackson. 2002. Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 45:203-217. [DOI] [PubMed] [Google Scholar]
- 9.Saiman, L., G. Cacalano, D. Gruenert, and A. Prince. 1992. Comparison of adherence of Pseudomonas aeruginosa to respiratory epithelial cells from cystic fibrosis patients and healthy subjects. Infect. Immun. 60:2808-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith, G. A., H. Marquis, S. Jones, N. C. Johnston, D. A. Portnoy, and H. Goldfine. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63:4231-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Songer, J. G. 1997. Bacterial phospholipases and their role in virulence. Trends Microbiol. 5:156-161. [DOI] [PubMed] [Google Scholar]
- 12.Southern, P. M., Jr., B. B. Mays, A. K. Pierce, and J. P. Sanford. 1970. Pulmonary clearance of Pseudomonas aeruginosa. J. Lab. Clin. Med. 76:548-559. [PubMed] [Google Scholar]
- 13.Viana-Niero, C., P. E. de Haas, D. van Soolingen, and S. C. Leao. 2004. Analysis of genetic polymorphisms affecting the four phospholipase C (plc) genes in Mycobacterium tuberculosis complex clinical isolates. Microbiology 150:967-978. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. 1997. WHO report on the tuberculosis epidemic. World Health Organization, Geneva, Switzerland.