Summary
Rectal gonorrhea and chlamydia increase the risk of a new diagnosis of HIV independent of rectal sexual behavior among men who have sex with men.
Background
Rectal sexually transmitted infections (STI) have been associated with HIV diagnosis, but inferring a causal association requires disentangling them from receptive anal intercourse (RAI).
Methods
We conducted a stratified case-control study by frequency matching 4 controls to each case within year using clinical data from men who have sex with men (MSM) attending the Seattle STD Clinic 2001–2014. Cases were MSM with a new HIV diagnosis and negative HIV test ≤12 months. Controls were HIV-negative MSM. All included men had rectal STI testing, tested negative for syphilis, and had complete sexual behavior data. We categorized men by RAI: (1) none; (2) condoms for all RAI; (3) condomless RAI (CRAI) only with HIV-negative partners; and (4) CRAI with HIV-positive or unknown-status partners. We created three logistic regression models: (1) three univariate models of concurrent rectal gonorrhea, rectal chlamydia, and rectal STI in ≤12 months with new HIV diagnosis; (2) those three infections, plus age, race, year, number of sexual partners ≤2 months, and methamphetamine use; and (3) model 2 with RAI categories. We calculated the population attributable risk of rectal STI on HIV diagnoses.
Results
Among 176 cases and 704 controls, rectal gonorrhea, chlamydia and rectal STI ≤12 months were associated with HIV diagnosis. The magnitude of these associations attenuated in the second model, but persisted in model 3 (gonorrhea aOR 2.3 95%CI 1.3 – 3.8; chlamydia aOR 2.5 95%CI 1.5 – 4.3; prior STI aOR 3.0 95%CI 1.5 – 6.2). One in 7 HIV diagnoses can be attributed to rectal STI.
Conclusion
Rectal STI are independently associated with HIV acquisition. These findings support the hypothesis that rectal STI play a biologically-mediated causal role in HIV acquisition and support screening/treatment of STI for HIV prevention.
Keywords: sexually transmitted diseases, gonorrhea, chlamydia, HIV, sexual behavior
Introduction
Numerous studies have documented a consistent epidemiologic association between rectal gonorrhea and chlamydial infection and new HIV diagnoses in men who have sex with men (MSM). Among MSM, rectal gonorrhea has been associated with a 2 to 17-fold increase in the risk of HIV acquisiton,(1-4) rectal chlamydia with 3.9-fold increase in risk(3) and either infection with 1.6 to 8.8-fold increase in risk.(5, 6) The causal nature of this association is supported by biologic plausibility – inflammatory sexually transmitted infections (STI) increase target leukocytes at sites of HIV exposure and cause mucosal disruption.(7, 8) However, most epidemiologic studies suffer from important limitations: STIs and HIV share a common causal pathway (i.e. sexual activity). Disentangling the potential biological role that rectal STIs may play in promoting HIV acquisition requires adjusting for sexual activity and partner HIV status in regression models that estimate the association between rectal STI and HIV. To date, only two studies have controlled for these covariates.(4, 9) Those studies suggest that STIs do independently increase the risk of HIV acquisition, but they are limited due to their small numbers of HIV seroconversions, 53 and 26 respectively. Moreover, only one looked at receptive anal intercourse by partner HIV status.(4) Using over 10 years of clinical data, we sought to determine whether the association between rectal gonococcal and chlamydial infections and HIV diagnosis is independent of receptive anal sexual behavior, and to estimate the population attributable risk percent (PAR%) of rectal bacterial STI on HIV diagnoses.
Methods
Using clinical data from Public Health– Seattle & King County (PHSKC) STD Clinic from January 1, 2001 through December 31, 2014, we created a retrospective stratified case-control study by frequency matching a ratio of controls to cases of 4:1 within year. The PHSKC STD Clinic is the only categorical STD clinic in the state of Washington and is located in downtown Seattle. It serves approximately 10,000 person-visits per year, about half of which are MSM visits. Cases were defined as MSM who were diagnosed with HIV at the STD Clinic during the study period and reported testing HIV negative in the prior 12 months; we excluded persons who had not tested HIV negative in the prior 12 months since our sexual history data was limited to that period and we wanted to limit HIV diagnoses to recent diagnoses. Controls were randomly selected MSM who tested HIV-negative at the STD clinic during the study period; we randomly sampled four controls for every one case based on year of case HIV diagnosis. All included men had rectal STI testing at the time of their HIV test visit, tested negative for syphilis at that visit, and had complete sexual behavior data. We excluded men with concurrent syphilis as it is known to be associated with HIV diagnosis.(1) We only used data gathered at a new problem visit. All data were collected as part of routine medical care, recorded in the clinic's electronic database, and de-identified for analysis. The University of Washington Human Subjects Division approved this study.
Clinical and Laboratory Procedures and Variable Definitions
During all new problem visits throughout the study period, MSM answered questions about sexual behavior by type of intercourse (receptive anal, insertive anal, receptive oral, insertive oral), and condom use (always, usually, sometimes, never) by sex partner HIV status (positive, negative, unknown). Sexual behaviour questions asked about sexual partners in aggregate over the prior 12 months. Clinicians collected this data through face-to-face interviews until 2010. In 2010, the clinic instituted a clinical computer assisted self-interview (CASI) for the collection of sexual behavior and other historical data. (Previous analyses have shown the differences in ascertainment of sexual behavior data between these two methods to be negligible.)(10, 11) We used this data to create four mutually exclusive categories of sexual behavior in the prior 12 months: (1) no receptive anal intercourse (RAI); (2) condoms for all RAI regardless of partner HIV status; (3) condomless RAI (CRAI) only with HIV-negative partners; and (4) CRAI with HIV-positive or unknown status partners.
Throughout the study period, clinicians tested for gonorrhea and chlamydia by reported anatomic sites of exposures. Until 2011, we used culture to diagnose rectal and pharyngeal gonorrhea and rectal chlamydia; thereafter, extra-genital gonorrhea and chlamydia were diagnosed by nucleic acid amplification tests (NAAT) with the AptimaCombo2 (Hologic, Inc; Bedford, MA). Either a positive culture or positive NAAT constituted a rectal infection. For gonorrhea and chlamydia infections in the prior 12 months, we only included those infections tested for and diagnosed in our clinic which may represent an underestimate.
At present, we diagnose syphilis in our clinic by a combination of clinical findings, darkfield microscopy, rapid-plasma reagin (RPR) and Treponema pallidum particle agglutination assay (TPPA). Since 2006, a single experienced disease intervention specialist has defined the final diagnosis and stage of infection for all syphilis cases based on a combination of clinical, historical and laboratory findings. We used this final syphilis diagnosis in our study. For data collected prior to 2006, we used the following composite laboratory and clinical findings to define early syphilis (primary, secondary, or early latent): (1) a clinical diagnosis of early syphilis with a positive RPR test and positive Treponema pallidum particle agglutination assay (TPPA), or (2) no clinical diagnosis and no history of syphilis with an RPR titer >1:32 and a positive TPPA, or (3) no clinical diagnosis and no history of syphilis with a VDRL >1:8 and a positive TPPA. We used these criteria to exclude men with syphilis.
Clinicians recommend HIV testing at routine clinical visits for all MSM not previously diagnosed with HIV. Until 2010, the PHSKC STD Clinic employed a second-generation HIV EIA (Vironostika HIV-1 Microelisa System; bioMerieux, Durham, NC or rLAV Genetic System; Bio-Rad Laboratories, Hercules, CA). After 2010, we used a third-generation EIA (Genetic Systems HIV1/2 Plus O EIA, Biorad Laboratories, Redmond, WA). High-risk MSM are offered a rapid HIV antibody tests (OraQuick, OraSure Technologies Inc., Bethlehem, PA until 2013; and INSTI, bioLytical Laboratories, Richmond, British Columbia, after 2013). Additionally, in order to diagnose acute HIV infection, we have conducted pooled HIV RNA testing since 2003.(12) We considered a positive result on any of these tests combined with self-report of previous negative HIV status to be a new HIV diagnosis.
Statistical Analyses
In order to examine rectal infections' independent association with HIV diagnosis, we conducted three analyses that utilized a stepwise addition of covariates to logistic regression models with robust standard errors to estimate the odds ratio (OR) of the association between rectal gonorrhea and chlamydial infection and new HIV diagnoses. In the first analysis, we conducted three separate univariate analyses stratified by year of the associations of HIV, 1) with concurrent rectal gonorrhea, 2) concurrent rectal chlamydia and 3) a history of rectal infection with gonorrhea, chlamydia or both in the prior 12 months. (We use the term ‘concurrent’ to indicate rectal infection diagnosed at the HIV testing visit as opposed to a previous rectal infection). In the next analysis, we examined these associations in a single model that included each STI separately controlling for age, race, number of sexual partners in the last 2 months, methamphetamine use in the last 12 months and calendar year. We included these factors as they have been previously found to be associated with either or both rectal STI and HIV. In the final analysis, we added the four sexual behavior categories as dummy variables (no RAI (referent group), condoms with all RAI, CRAI with only HIV negative partners, and CRAI with HIV positive or unknown status partners) to the multivariate model in the previous analysis. The results from this final analysis describe the risk of a new HIV diagnosis for men with rectal infection compared to those without a rectal STI among MSM who reported the same sexual behaviors.
We used the ORs from model three to determine the contribution (i.e. population attributable risk percent, PAR%) of each rectal STI and rectal sexual behaviors to HIV acquisition. Because the population prevalence of our outcome (HIV diagnosis) is less than 10%, we assumed that the OR approximated the RR and used these estimates to calculate the PAR% using the following formula:
where Pc is the proportion of cases exposed (i.e. the prevalence of the sexual behavior among the cases).(13) In order to compare cases and controls for descriptive statistics, we used t-test for parametric continuous variables (i.e. age), Mann-Whitney U test for non-parametric ordinal variables (i.e. number of sexual partners) and chi-square test for categorical variables. We used Stata v12.1 (StataCorp, College Station, TX) to conduct analyses and an alpha of 0.05.
Results
Study population
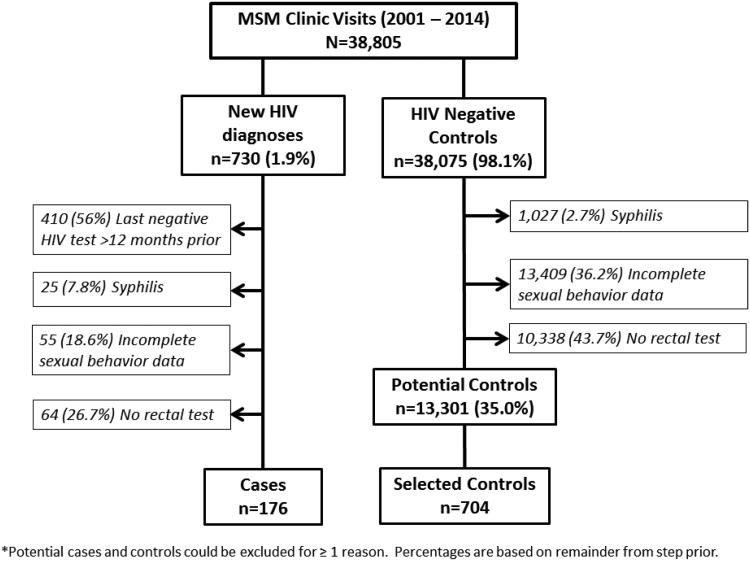
Between January 1, 2001 and December 31, 2014, there were 38,805 MSM new problem visits at the PHSKC STD Clinic and 730 (1.9%) new HIV diagnoses. Of the 730 MSM diagnosed with HIV at the PHSKC STD Clinic, after exclusions, our final analysis included 176 cases (Figure 1). Of the 38,075 potential MSM control visits, we excluded 24,774 visits leaving 13,301 potential control subjects from whom we randomly selected 704 controls. We excluded 10,338 MSM visits without rectal STI testing; at these visits, 3,487 (34%) reported no receptive anal sex in the last 12 months. Rectal STI testing increased over the study period from a low of 25% of MSM visits in 2001, to a high of 47% of MSM visits in 2014 (p<0.001).
Figure 1. Study Design.
*Potential cases and controls could be excluded for ≥ 1 reason. Percentages are based on reminder from step prior.
Due to the large number of excluded potential cases and controls, we compared these groups to assess for bias. Included cases were younger (31.4 versus 34.5 years), had a median of 1 more sexual partner in the prior 12 months, were more likely to report anal symptoms (18.2% vs 9.4%) and have pharyngeal gonorrhea (13.5% versus 7.9%) than excluded cases. Although only 335 of the excluded cases had full sexual behavior data, there was a difference in the distribution of sexual behaviors with excluded cases being more likely to deny RAI in the past 12 months (13.7% versus 4.0%) or more likely use condoms with all sexual partners (22.1% versus 17.6%). Cases were more likely to report CRAI with HIV negative partners only (35.8% versus 20.9%). When we compared all potential controls to selected controls, we found that selected controls were younger (33.0 v. 35.3 years), less likely to be black (4.3% versus 11.9%), have a urethral infection (gonorrhea 4.7% versus 6.8%; chlamydia 3.8% versus 5.9%), and to have not had RAI in the past 12 months (5.5% versus 17.7%). Selected controls were more likely to have been Asian/Pacific Islander (9.4% versus 5.1%), report anal symptoms (10.5% versus 7.4%), to report CRAI with HIV negative partners only (34.8% versus 28.7%) and to report CRAI with HIV-positive or unknown status partners (28.8% versus 24.2%).
Selected cases and controls were similar only in age (Table 1). Cases were more likely to report more sexual partners in the past 2 months (median 3 versus 2, p=0.015), use methamphetamine in the prior 12 months (29% vs. 13.6%, p<0.001) and be diagnosed with any STI (47.2% vs. 22.6%, p<0.001). Including both cases and controls, there were 97 cases of rectal gonorrhea and 98 cases of rectal chlamydia diagnosed in the study population, and >80% of these infections were asymptomatic. Thirty men with rectal infection were co-infected with both gonorrhea and chlamydia: 16 (2.2%) of controls and 14 (7.9%) of cases.
Table 1. Study population, key demographics, and sexual behaviors.
| Controls N=704 | Cases N=176 | P-value | |
|---|---|---|---|
| Age (mean, SD) | 33.0 (10.2) | 31.4 (9.3) | 0.061 |
|
| |||
| Race | 0.002 | ||
| White (non-Hispanic) | 488 (69.3) | 111 (63.1) | |
| Black (non-Hispanic) | 30 (4.3) | 15 (8.5) | |
| Asian/Pacific Islander | 66 (9.4) | 5 (2.8) | |
| Latino/Hispanic | 43 (6.1) | 14 (8.0) | |
| Native American/Alaskan Native | 5 (0.7) | 2 (1.1) | |
| Unknown/other* | 72 (10.2) | 29 (16.5) | |
|
| |||
| Number of sexual partners in <2 months (median, IQR) | 2 (1,4) | 3 (1,5) | 0.015 |
|
| |||
| Methamphetamine use in the prior 12 months | 96 (13.6) | 51 (29.0) | <0.001 |
|
| |||
| Anal symptoms | 74 (10.5) | 32 (18.2) | 0.005 |
|
| |||
| Prior Rectal Infection with Gonorrhea and/or Chlamydia | |||
|
| |||
| In the past 3 months | 10 (2.6) | 3 (3.0) | 0.824 |
|
| |||
| In the past 6 months | 24 (6.2) | 9 (8.9) | 0.327 |
|
| |||
| In the past 12 months | 31 (8.0) | 21 (20.8) | <0.001 |
|
| |||
| Concurrent Infections | |||
| Gonorrhea and/or Chlamydia at Any Site | 143 (22.6) | 76 (47.2) | <0.001 |
|
| |||
| Gonorrhea | |||
| Rectal | 56 (8.0) | 41 (23.3) | <0.001 |
| Pharyngeal | 38 (5.5) | 23 (13.5) | <0.001 |
| Urethral | 29 (4.7) | 14 (9.1) | 0.035 |
|
| |||
| Chlamydia | |||
| Rectal | 59 (8.5) | 39 (23.2) | <0.001 |
| Pharyngeal | 2 (0.9) | 2 (3.5) | 0.134 |
| Urethral | 24 (3.8) | 4 (2.6) | 0.451 |
|
| |||
| Receptive Anal Intercourse Sexual Behaviors | <0.001 | ||
| No receptive anal intercourse × 12 months | 39 (5.5) | 7 (4.0) | |
| Condoms for RAI** with ALL partners | 217 (30.8) | 31 (17.6) | |
| CRAI^ with Only Negative Partners | 245 (34.8) | 63 (35.8) | |
| CRAI with HIV positive or Unknown Status | 203 (28.8) | 75 (42.6) | |
Multiple races or did not respond.
RAI - receptive anal intercourse
CRAI - condomless receptive anal intercourse
Risk of new HIV Diagnosis Associated with Rectal Gonorrhea and Chlamydia
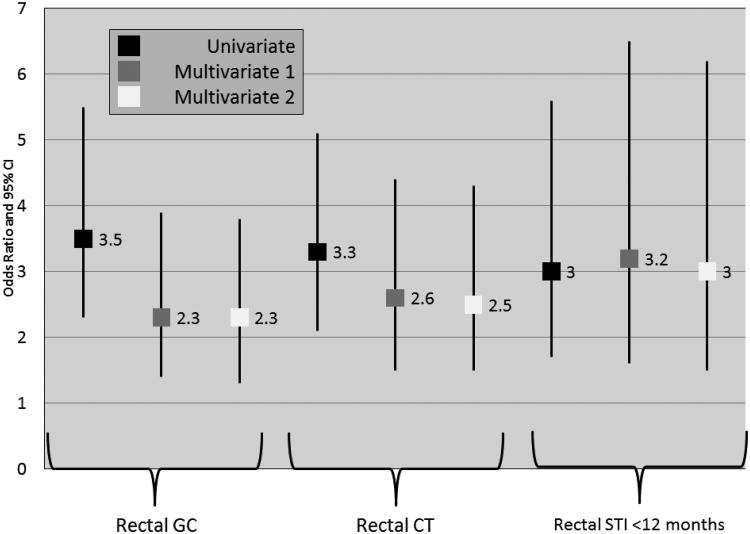
In univariate analyses, the risk of new HIV diagnosis with concurrent rectal gonorrhea was 3.5 (95% CI 2.3 – 5.5), concurrent rectal chlamydia 3.3 (95% CI 2.1 – 5.1) and with either infection in the past 12 month 3.0 (95% CI 1.7 – 5.6) (Figure 2). The addition to the model of age, race, year, number of sexual partners in the last 2 months and methamphetamine use in the past year attenuated this risk for concurrent rectal gonorrhea (OR 2.3 95% CI 1.4 – 3.9) and concurrent rectal chlamydia (OR 2.6 95% CI 1.5 – 4.4), and slightly increased the strength of association for rectal infection in the last 12 months (OR 3.2 95% CI 1.6 – 6.5). These associations persisted with the addition of sexual behavior to the model (Figure 2). Additionally, and as expected, condomless receptive anal intercourse with HIV- negative (OR 3.4 95% CI 1.2 – 10.2) or HIV-positive or unknown status partners (OR 4.2 95% CI 1.4 – 12.4) were also significantly associated with new HIV diagnosis.
Figure 2. Risk of New HIV Diagnosis with Concurrent Rectal Gonorrhea, Concurrent Rectal Chlamydia and History of Rectal Infection with Either Gonorrhea or Chlamydia in the Past Year: Results of a Stepwise Analysis (N = 176 cases and 704 controls).
GC – gonorrhea; CT – chlamydia; STI – either gonorrhea or chlamydia; CRAI – condomless receptive anal intercourse
Multivariate 1 – Adjusted for age, race, year, number of sexual partners in the past 2 months, methamphetamine use. Rectal GC and rectal CT included as separate variables.
Multivariate 2 – In addition to the covariates listed in Multivariate 1, receptive anal intercourse status – no RAI (referent), condoms with all RAI, CRAI with HIV- negative partners, CRAI with HIV-positive or unknown status partners.
Given the elevated risks of either current or prior rectal STI, we conducted an exploratory analysis to determine the association between individuals with both a current rectal STI and a prior rectal STI in the last 12 months. This exposure increased the risk of new HIV diagnosis by 3.8 (p=0.013) even after controlling for age, race, year, number of sexual partners in the last 2 months, methamphetamine use in the past year and rectal sexual behavior data.
The population attributable risk percentages are presented in Table 2. Approximately 14% of new HIV diagnoses were attributable to rectal STIs diagnosed concurrently with HIV or in the 12 months prior to HIV diagnosis.
Table 2. Population attributable risk percent (PAR%) of rectal sexually transmitted infections and sexual behaviors on new HIV diagnoses among MSM at the Public Health - Seattle & King County STD Clinic.
| Rectal Sexually Transmitted Infections and Sexual Behaviors | aOR* | 95% CI | PAR% | 95% CI |
|---|---|---|---|---|
| Rectal Gonorrhea | 2.3 | 1.3 – 3.8 | 13.2% | 5.7% – 19.2% |
| Rectal Chlamydia | 2.5 | 1.5 – 4.3 | 13.9% | 7.0%– 19.9% |
| Either Rectal STI <12 months | 3.0 | 1.5 – 6.2 | 13.9% | 5.8% – 20.9% |
| Condoms Always | 1.9 | 0.6 – 5.9 | 8.3% | --** – 16.2% |
| CRAI with HIV-negative partners | 3.4 | 1.2 – 10.2 | 25.3% | 5.1% – 34.7% |
| CRAI with HIV-positive or unknown status partners | 4.2 | 1.4 – 12.4 | 32.5% | 12.4% – 42.0% |
From model 3; adjusted for age, race, year, number of sexual partners in the past 2 months, methamphetamine use, other rectal GC/CT infections and sexual behaviors (no receptive anal intercourse (RAI) (referent), condoms with all RAI, condomless RAI (CRAI) with HIV- negative partners, CRAI with HIV-positive or unknown status partners.
Undefined.
Discussion
In this study, we found that rectal gonorrhea and chlamydial infection were independently associated with HIV acquisition, even after controlling for selected seroadaptive behaviors (i.e. selective condom use based on partners' HIV status); both infections were associated with a two and a half fold increase in the risk of HIV acquisition. Men who tested negative for both infections but who were diagnosed with rectal gonococcal and/or chlamydial infection in the prior year experienced a 3-fold risk of new HIV diagnoses, regardless of reported sexual behaviors, and those with current and prior infections had an even more elevated risk of HIV acquisition. Our study is one of only a few that have controlled for rectal sexual behaviors, and of those it is the largest with most HIV seroconversions. These findings strengthen the evidence that bacterial STIs independently increase the risk for HIV acquisition, most likely through biologic mechanisms.
Among the prior studies that have examined the association between rectal bacterial STI and HIV acquisition in MSM, some did not adjust for any sexual behaviors(1), while others adjusted only for number of sexual partners or a limited amount of condom use data.(5, 6) Several adjusted for condom use based on anal sexual role,(2, 4, 9, 14) but only one, The Health in Men [HIM] study,(4) like ours, adjusted for unprotected anal intercourse by partner HIV status and anal sexual role. The HIM study was a prospective longitudinal study that collected very detailed, partner specific data from 1381 Australian MSM, 47 (3.4%) of whom seroconverted,(4) and found that rectal gonorrhea was associated with incident HIV (HR 7.12, 95% CI 2.05 – 24.75) after adjusting for sexual behavior. However, because HIM started before the widespread availability of NAATs for extragenital testing, that finding was based on the occurrence of only 3 cases of rectal gonorrhea. While our study did not have nearly as detailed behavioral data as HIM, because we had many more HIV and rectal STI diagnoses, our findings offer greater precision than the estimate from HIM and reinforces the same conclusion: bacterial STI increase the risk of HIV acquisition independent of receptive anal intercourse, most likely through a biologic mechanism.
Interestingly, our primary outcome, the risk of new HIV diagnosis with concurrent rectal infection, is remarkably similar to results from other contemporary studies. In both papers from the involveMENt trial, the only other study to control for a measure of anal intercourse, the aHR for HIV seroconversion with rectal STI was 2.7.(9, 14) The adjusted odds of HIV infection given rectal gonococcal infection in an observational study from the pre-antiretroviral era (1982 – 1990) was 3.2.(2) In a retrospective analysis of New York City HIV/STD surveillance data, the RR of HIV diagnosis with a prior rectal gonorrhea or chlamydia was 2.6 (unadjusted).(6) Despite some statistical outliers, the consistency in the magnitude of association between either concurrent or prior rectal gonorrhea and chlamydia and new HIV diagnosis is about a two-and-a-half fold increase, similar to what we found. Also, our finding that 14% of HIV diagnoses can be attributed to rectal STI is consistent with the one other study on the subject. Kelley et al reported on a population attributable fraction of combined rectal gonorrhea and chlamydia infection of 14.6% in a cohort of MSM in Atlanta.(9)
Our finding that a history of rectal STI in the prior 12 months is associated with HIV acquisition merits comment. In most instances, persons diagnosed with a rectal STI in King County are tested concurrently for HIV infection.(15) As a result, it seems unlikely that most of these infections were present at the time of HIV acquisition. It is possible that rectal STI leads to a prolonged change in the rectal mucosa that increases one's susceptibility to HIV even after treatment. Alternatively, the finding that history of STI is associated with HIV acquisition highlights the extent to which such a history reflects unmeasured behavioral or sexual network factors that promote HIV acquisition, factors that may also confound the association between prevalent rectal STIs and HIV. Other studies that have also found an increased risk with prior rectal STI,(1, 5, 6) promoted these infections as a herald event which could, and arguably should, be used to indicate which patients deserved enhance HIV prevention services, such as pre-exposure prophylaxis (PrEP) and enhanced risk-reduction counselling.
Although our study has many strengths, it is also subject to many limitations. First and foremost, this is a retrospective study and subject to the limitations inherent to that study design. In particular, with respect to the concurrent rectal infections, we cannot be certain that the bacterial STI preceded HIV acquisition, which would help establish temporality and suggest causality. Secondly, our choice of exclusion criteria created differences in some factors between included and excluded cases and controls. Although there were not differences in the main exposure variables (i.e. rectal STI), there were differences in the distribution of reported sexual behaviors among both the cases and controls. Additionally, there were differences in report anal symptoms: included cases were more likely than excluded cases to report anal symptoms, which might suggest a more inflammatory rectal infection and increased risk of HIV, thus biasing our results. However, selected controls also reported more anal symptoms than potential controls, which should negate this potential bias. For the most part, the differences in excluded and included cases and controls occurred simultaneously, and in the same direction, but we cannot be certain that this did not affect our results. Thirdly, sexual behavior data was self-reported and subject to recall and social desirability bias. It is possible that men inaccurately recorded their condom use and sex partner HIV status data. However, CRAI with HIV-positive and unknown status partners was strongly associated with new HIV diagnosis, suggesting that this data is at least partially valid. We also used two different methods for obtaining sexual behavior data over the study period – clinician collected and CASI. Although previous analyses have found negligible differences in these methods,(10, 11) it is possible that some bias exists by time period. However, this bias would have affected both cases and controls evenly and thus not affect the results (i.e. non-differential misclassification). Fourth, although our study adjusted for many aspects of sexual behavior, as indicated above, the risk of HIV acquisition reflects an array of biologic, behavioral and network factors, and our findings may be affected by residual confounding. In particular, we did not exclude or adjust for infection with Herpes Simplex Virus which is known to increase one's risk of HIV acquisition. Fifth, we did not control for insertive anal intercourse by condom status, a known risk factor for HIV, because it is not on the causal pathway with rectal STI and HIV, nor did we control for urethral or pharyngeal gonorrhea or chlamydia infections, because we believe that the magnitude of risk associated with insertive anal intercourse, and pharyngeal and urethral STI, is much smaller than that of RAI and rectal infections. However, it is possible that by only controlling for receptive anal intercourse by condom use and sex partner HIV status that we overlooked other important modes of sexual transmission such as partial penetration or rimming (oral-anal intercourse). Since, to the best of our knowledge, only RAI is associated with both rectal gonorrhea and chlamydia and HIV acquisition, we believe this to be an accurate method to control for this confounder. Sixth, during the majority of the study period, we diagnosed rectal STI by culture, which is a less sensitive method than NAAT, thus we likely underestimated our exposure. However, since this method was used consistently across both cases and controls, it likely did not affect the association. Lastly, this study took place in a single STD Clinic in a city with a high HIV testing rate among MSM. The generalizability outside of this population is unknown.
In conclusion, we found that rectal gonorrhea and chlamydia infection are independent risk factors for HIV acquisition. While the biological role of rectal infections in promoting HIV acquisition can probably never be proven definitively, the consistency of this finding, as well as a plausible biological mechanism through which these STIs could increase the risk of HIV acquisition, support a causal role for bacterial STIs – particularly rectal infections - in promoting HIV acquisition and the associated need to continue and expand STI control efforts as an HIV prevention control strategy. Importantly, ensuring that men are screened for gonorrhea and chlamydia at all exposed sites, particularly the rectum, should remain a priority, and HIV-negative men with rectal STI should be offered PrEP.
Acknowledgments
We appreciate the biostatistical assistance provided by Dr. Jim Hughes. We are grateful to the Public Health Seattle & King County patients and staff without whom this work would be impossible.
Funding: This work was funded by the National Institutes of Health (K23 - AI113185 to L.A.B., and T32 AI07140 trainee support to C.M.K) and the University of Washington Center for AIDS Research, an NIH-funded program [grant P30 AI027757] which is supported by the following NIH Institutes and Centers: National Institute of Allergy and Infectious Diseases, National Career Institute, National Institutes of Mental Health, National Institute on Drug Abuse, National Institute of Child Health and Human Development, National Heart, Lung, and Blood Institute, National Institute on Aging.
Footnotes
Conflicts of Interests: L.A.B. has received research support from Hologic.
References
- 1.Zetola NM, Bernstein KT, Wong E, et al. Exploring the relationship between sexually transmitted diseases and HIV acquisition by using different study designs. J Acquir Immune Defic Syndr. 2009;50(5):546–51. doi: 10.1097/qai.0b013e318195bd2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craib KJ, Meddings DR, Strathdee SA, et al. Rectal gonorrhoea as an independent risk factor for HIV infection in a cohort of homosexual men. Genitourin Med. 1995;71(3):150–4. doi: 10.1136/sti.71.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott KC, Philip S, Ahrens K, et al. High prevalence of gonococcal and chlamydial infection in men who have sex with men with newly diagnosed HIV infection: an opportunity for same-day presumptive treatment. J Acquir Immune Defic Syndr (1999) 2008;48(1):109–12. doi: 10.1097/QAI.0b013e318165dc0b. [DOI] [PubMed] [Google Scholar]
- 4.Jin F, Prestage GP, Imrie J, et al. Anal sexually transmitted infections and risk of HIV infection in homosexual men. J Acquir Immune Defic Syndr (1999) 2010;53(1):144–9. doi: 10.1097/QAI.0b013e3181b48f33. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr (1999) 2010;53(4):537–43. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 6.Pathela P, Braunstein SL, Blank S, et al. HIV incidence among men with and those without sexually transmitted rectal infections: estimates from matching against an HIV case registry. Clin Infect Dis. 2013;57(8):1203–9. doi: 10.1093/cid/cit437. [DOI] [PubMed] [Google Scholar]
- 7.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35(11):946–59. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 8.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley CF, Vaughan AS, Luisi N, et al. The Effect of High Rates of Bacterial Sexually Transmitted Infections on HIV Incidence in a Cohort of Black and White Men Who Have Sex with Men in Atlanta, Georgia. AIDS Res Hum Retroviruses. 2015;31(6):587–92. doi: 10.1089/aid.2015.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dombrowski J, Kerani R, Golden M. Routine CASI Increased Sexual History Completeness among MSM STD Clinic Patients. CDC STD Prevention Conference; Minneapolis, MN: 2012. [Google Scholar]
- 11.Dombrowski J, Golden M. STD Clinic Triage Based on Computer-Assisted Self Interview: The King County Experience. International Society for STD Research World Congress; Quebec, Canada: 2011. [Google Scholar]
- 12.Stekler J, Swenson PD, Wood RW, et al. Targeted screening for primary HIV infection through pooled HIV-RNA testing in men who have sex with men. AIDS. 2005;19(12):1323–5. doi: 10.1097/01.aids.0000180105.73264.81. [DOI] [PubMed] [Google Scholar]
- 13.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–9. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughan AS, Kelley CF, Luisi N, et al. An application of propensity score weighting to quantify the causal effect of rectal sexually transmitted infections on incident HIV among men who have sex with men. BMC Med Res Methodol. 2015;15:25. doi: 10.1186/s12874-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz DA, Dombrowski JC, Bell TR, et al. HIV Incidence Among Men Who Have Sex With Men After Diagnosis With Sexually Transmitted Infections. Sex Transm Dis. 2016;43(4):249–54. doi: 10.1097/OLQ.0000000000000423. [DOI] [PMC free article] [PubMed] [Google Scholar]