Abstract
The disparities in stroke mortality between blacks and whites, as well as the increased stroke mortality in the “stroke belt” have long been noted. The reasons for these disparities have yet to be fully explained. The association between trace element status and cardiovascular diseases, including stroke, has been suggested as a possible contributor to the disparities in stroke mortality but has not been fully explored. The purpose of this study is to investigate distributions of four trace elements (arsenic, mercury, magnesium, and selenium) in the environment in relation to stroke risk. The study population (N=27,770) is drawn from the Reasons for Geographic and Racial Disparities in Stroke (REGARDS) cohort. Environmental distribution of each trace element was determined using data from the United States Geological Survey (USGS) and was categorized in quartiles. A proportional hazards model, adjusted for demographic data and stroke risk factors, was used to examine the association of interest. The results showed that higher selenium levels in the environment were associated with increased stroke risk, and the hazard ratio for the 4th quartile compared to the 1st quartile was 1.33 (95% CI: 1.09, 1.62). However, there was no statistically significant relationship between environmental arsenic, mercury or magnesium and the risk of stroke. Because of dietary and non-dietary exposure as well as bioavailability, further research using biomarkers is warranted to examine the association between these trace elements and the risk of stroke.
Keywords: Stroke, Trace Elements, REGARDS, USGS
Introduction
The presence of geographic differences in stroke mortality has been known since early in the twentieth century, with the southeastern region of the United States experiencing higher levels of stroke mortality than the rest of the country[1]. Much work has been done in order to understand the underlying causes of the geographic disparities in stroke mortality, and several sources of the disparities have been hypothesized such as geographic disparities in stroke risk factors, including environmental risk factors. However, despite the research toward explaining the disparities in stroke mortality, the underlying causes are still not fully understood. Regional disparities in environmental exposure to trace elements have been hypothesized to play a role in the geographic disparities in stroke mortality. However, epidemiological data are limited on the relationship between the environmental distribution of trace elements and stroke risk.
Studies have linked serum levels of trace elements to cardiovascular diseases such as myocardial infarction, hypertension, and atherosclerosis [2–6]. The relationship between some trace elements and stroke have also been explored, but most studies investigating the link have been either have been focused on relatively small geographic areas and homogenous populations or have used only medical records to collect outcomes [7–11]. Thus, although some studies have shown a link between certain trace elements and stroke (or cerebrovascular diseases in general) [8, 9, 11], the relationship remains unclear. This study is concerned with the relationship between stroke and four different elements measured in the environment: arsenic, mercury, magnesium, and selenium. Arsenic is a naturally occurring trace element that is present in both soil and water, while mercury is a heavy metal whose presence in the environment can be attributed to both natural and artificial sources. Magnesium is a nutrient that most commonly enters the human body through diet and drinking water[12]. Selenium occurs naturally in the environment, and generally enters the human body through ingestion of selenium containing foods[13].
Because of the large geographic variation in the data, the Reasons for Geographical and Racial Differences in Stroke (REGARDS) cohort provides an excellent opportunity to further examine the relationship between the occurrence of these elements in the environment and stroke risk. In this study, we will use data from the REGARDS cohort, together with USGS National Geochemical Survey (NGS) data, to investigate the distribution of arsenic, mercury, magnesium, and selenium in the environment in relation to the risk of stroke.
Materials and Methods
Study Participants
The REGARDS study is a longitudinal cohort study designed to identify the underlying causes for the increased levels of stroke mortality in the Stroke Belt and among the black population. The REGARDS population is comprised of 30,239 individuals from across the contiguous 48 US states, aged 45 and up, either black or white. Participants in the REGARDS study were selected from a commercially available list and contacted through mail and telephone to be invited into the study. Once a participant provided consent by telephone and was enrolled in the study, demographic and self-reported medical data were collected. Following the phone interview, an in-home visit was performed in order to collect baseline anthropometric measurements and blood pressure, as well as to collect blood and urine samples and an ECG, and to obtain written informed consent. Following the collection of baseline data, phone interviews are performed every six months to collect follow-up data, including investigation of possible stroke events[14]. Stroke events in the REGARDS cohort were defined using the World Health Organization definition[15]. Medical records of REGARDS participants experiencing a possible stroke were reviewed by at least two physicians from a committee of stroke experts. In cases where the physicians disagreed, the possible stroke was adjudicated by the full committee. Study procedures were approved by the institutional review boards at all participating institutions.
Geochemical Information
The NGS database includes samples that were collected from a variety of sources, mostly stream and soil samples. Arsenic and selenium was determined using the hydride atomic absorption method and mercury was determined using the cold vapor atomic absorption method, and the results for these elements are expressed in parts per million (ppm). Magnesium concentration was determined using inductively coupled plasma spectrometry-mass spectrometry (ICP) after peroxide fusion or by ICP after acid dissolution, and the median value per county was computed. Magnesium was expressed in percentage by weight (wt%). More detailed information on the collection and testing of samples in the NGS database can be found on the NGS website: https://mrdata.usgs.gov/geochem/doc/analysis.htm.
REGARDS participants were geocoded using baseline home addresses and were classified by location into Federal Information Processing Standard (FIPS) codes, which provides a unique five digit number for each county in the United States. The NGS county level data were then used to determine the level of environmental trace elements for each REGARDS participant. When NGS data provided multiple measurements for a county, the median of the results was assigned to that county. Furthermore, the NGS data provide information on the level of contamination in a sample measurement. Participants residing in counties with measurements reported to have “moderate” or “heavy” degrees of contamination were excluded from the data. Assigned environmental levels of trace elements for individuals are categorized into quartiles, which are used as the primary exposure variables in the statistical models. More details about the use of the NGS data in REGARDS can be found in Rembert et al [16].
Covariates
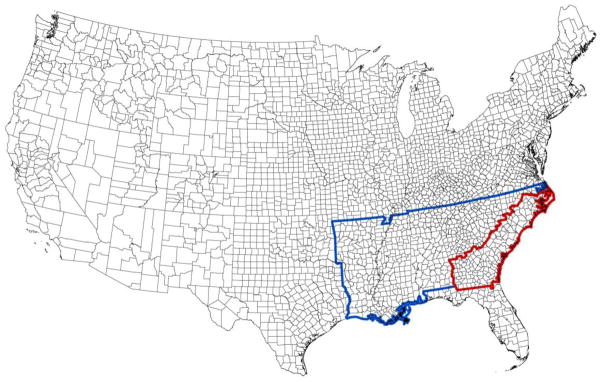
Gender, race, region, education level, age, smoking status, and diabetes were self-reported by REGARDS participants during the initial phone interview. Race was categorized as either black or white. People that identified as neither black nor white were excluded from the REGARDS study. The region of participant residence was categorized into the stroke buckle, stroke belt, and non-belt. The stroke buckle (Figure 1, highlighted in red) was defined as the coastal plain of North Carolina, South Carolina, and Georgia. The stroke belt (Figure 1, highlighted in blue) was defined as the states of Alabama, Mississippi, Tennessee, Arkansas, Louisiana, North Carolina, South Carolina, and Georgia excluding the portion in the stroke buckle. The non-belt region was defined as the remaining contiguous United States. Education level was categorized into less than high school, high school graduate, some college, and college graduate and above. Smoking status was categorized into current smoker, past smoker, and never smoked. Participant hypertension was determined from either measured systolic blood pressure (SBP) greater than or equal to 140 mmHg or diastolic blood pressure (DBP) greater than or equal to 90 mmHg during in-home visit or participant self-report of current use of medication to control blood pressure. Heart disease was determined from a self-report of myocardial infarction (MI), coronary artery bypass graft surgery, bypass, angioplasty, or stenting; or evidence of MI from electrocardiogram (ECG). Atrial fibrillation was determined by either participant self-report or ECG evidence. Left ventricular hypertrophy (LVH) was determined using the Sokolow-Lyon limb lead criteria from ECG, with RAVL greater than 1100 determining LVH.
Figure 1.
The stroke belt (highlighted in blue) and buckle (highlighted in red) regions
Statistical Analysis
For each element of interest, the association of the element with stroke risk was estimated using Cox proportional hazards models with the outcome of interest time to first stroke from the date of their in-home visit. Participants without recorded strokes were censored on the date of death or the most recent follow-up. For each element, univariate analysis of the association of the element with time to stroke was performed, followed by a second model with participant demographics as covariates. Demographic variables included in this model were gender, race, region, education level, and age. An age-race interaction term was also included in this model, due to the known result that the difference in stroke risk by race varies as a function of age. A third model was then fitted by including participants’ co-morbidities with the factors included in the second model. This included all covariates in the second model as well as hypertension, diabetes, smoking status, heart disease, atrial fibrillation, and LVH.
Results
Of the 30,239 REGARDS participants, 67 resided in counties with “moderate” or “heavy” degrees of contamination in the NGS data and were thus excluded from the analysis. 1,930 participants were excluded from this analysis because they reported having had a stroke at baseline. 468 participants were excluded due to missing dates of the in-home visit or censoring date and another 4 participants were excluded due to having an event or censoring date before the date of the in-home visit, leaving a final analytic sample of 27,770. Among the participants included in the analysis, 938 incident strokes were recorded during the course of the study. Table 1 describes the demographic and medical characteristics of the study population by whether a stroke event occurred during follow-up, supplemental Table 1 presents the same by quartiles of selenium. The average age among participants experiencing a stroke during the course of the study is five and a half years higher than that of stroke-free participants. Just over half of the stroke group was men compared to only 44% in the stroke-free group. About 72% of the stroke group was hypertensive, markedly higher than the proportion of hypertensive participants in the stroke-free group. The proportion of participants that had other stroke risk factors (such as diabetes, heart disease, atrial fibrillation, and LVH) was also notably higher in the stroke group. The most notable difference between the stroke group and the stroke free group in regards to the environmental element levels is the higher proportion of stroke group participants in the 4th quartile of environmental selenium.
Table 1.
Demographic and medical characteristics of the study population by stroke event during follow-up
| Variable (n missing) | Stroke event (n=938) | No stroke event (n=26832) | Full Population (n=27770) | |
|---|---|---|---|---|
| Arsenic Quartile (ppm) (1776) | 1–[0.75, 2.15) | 217 (24.7%) | 6837 (27.2%) | 7054 (27.1%) |
| 2–[2.15, 3.60) | 209 (23.8%) | 5794 (23.1%) | 6003 (23.1%) | |
| 3–[3.60, 6.42) | 224 (25.5%) | 6303 (25.1%) | 6527 (25.1%) | |
| 4–[6.42, 49.55] | 228 (26.0%) | 6182 (24.6%) | 6410 (24.7%) | |
| Mercury Quartile (ppm) (2614) | 1–[0.010, 0.035) | 276 (32.1%) | 7953 (32.7%) | 8229 (32.7%) |
| 2–[0.035, 0.045) | 190 (22.1%) | 5537 (22.8%) | 5727 (22.8%) | |
| 3–[0.045, 0.055) | 217 (25.2%) | 5798 (23.9%) | 6015 (23.9%) | |
| 4–[0.055, 3.540) | 178 (20.7%) | 5007 (20.6%) | 5185 (20.6%) | |
| Magnesium Quartile (wt%) (1694) | 1–[0.006, 0.058) | 212 (24.1%) | 6543 (26.0%) | 6755 (25.9%) |
| 2–[0.058, 0.201) | 228 (25.9%) | 6430 (25.5%) | 6658 (25.5%) | |
| 3–[0.201, 0.591) | 224 (25.4%) | 5946 (23.6%) | 6170 (23.7%) | |
| 4–[0.591, 4.316] | 217 (24.6%) | 6276 (24.9%) | 6493 (24.9%) | |
| Selenium Quartile (ppm) (2765) | 1–[0.10, 0.30) | 199 (23.5%) | 6460 (26.7%) | 6659 (26.6%) |
| 2–0.30 | 202 (23.8%) | 6273 (26.0%) | 6475 (25.9%) | |
| 3–(0.30, 0.45) | 206 (24.3%) | 5480 (22.7%) | 5686 (22.7%) | |
| 4–[0.45, 2.20] | 241 (28.4%) | 5944 (24.6%) | 6185 (24.7%) | |
| Age (0) | 70.0 ± 8.8 | 64.5 ± 9.4 | 64.7 ± 9.4 | |
| Gender (0) | Male | 482 (51.4%) | 11897 (44.3%) | 12379 (44.6%) |
| Race (0) | Black | 394 (42.0%) | 10832 (40.4%) | 11226 (40.4%) |
| Region (0) | Belt | 319 (34.0%) | 9304 (34.7%) | 9623 (34.7%) |
| Buckle | 199 (21.2%) | 5643 (21.0%) | 5842 (21.0%) | |
| Nonbelt | 420 (44.8%) | 11885 (44.3%) | 12305 (44.3%) | |
| Education Level (15) | Less than High School | 146 (15.6%) | 3117 (11.6%) | 3263 (11.8%) |
| High School Graduate | 286 (30.5%) | 6854 (25.6%) | 7140 (25.7%) | |
| Some College | 238 (25.4%) | 7215 (26.9%) | 7453 (26.9%) | |
| College Graduate and Above | 267 (28.5%) | 9632 (35.9%) | 9899 (35.7%) | |
| SBP, mmHg (65) | 133 ± 18 | 127 ± 16 | 127 ± 17 | |
| Hypertension (61) | Yes | 671 (71.8%) | 15350 (57.3%) | 16021 (57.8%) |
| Diabetes (98) | Yes | 277 (29.6%) | 5706 (21.3%) | 5983 (21.6%) |
| Smoking Status (109) | Current | 162 (17.3%) | 3757 (14.1%) | 3919 (14.2%) |
| Past | 393 (42.1%) | 10679 (40.0%) | 11072 (40.0%) | |
| Never | 379 (40.6%) | 12291 (46.0%) | 12670 (45.8%) | |
| Heart Disease (494) | Yes | 263 (28.6%) | 4325 (16.4%) | 4588 (16.8%) |
| Atrial Fibrillation (608) | Yes | 134 (14.6%) | 2138 (8.2%) | 2272 (8.4%) |
| LVH (442) | Yes | 138 (14.9%) | 2488 (9.4%) | 2626 (9.6%) |
For categorical measures, counts and percentages are reported. For continuous measures, mean and standard deviation are reported.
Higher levels of environmental selenium were associated with higher risk of stroke (Tables 2). and there was an apparent monotonic relationship between environmental selenium exposure and stroke. For those in the 4th quartile of selenium exposure, the risk of stroke was 33% higher than for those in the 1st quartile of exposure (HR: 1.33, 95% CI: 1.09, 1.62). Those in the 2nd and 3rd quartiles of exposure to selenium were at increased risk of stroke relative to those in the 1st, but these differences were not statistically significant in multivariable models (2nd quartile HR: 1.07, 95% CI: 0.87, 1.32; 3rd quartiles HR: 1.20, 95% CI: 0.97, 1.47). There was no significant association between environmental exposure to arsenic, mercury or magnesium and incident stroke (Table 2).
Table 2.
Associations between environmental distribution of trace elements by quartiles and incidence of stroke
| Model I | Model II | Model III | ||
|---|---|---|---|---|
| Arsenic | Wald Test p-value* | 0.451 | 0.285 | 0.291 |
| 1 | Ref | Ref | Ref | |
| 2 | 1.14 (0.94,1.38) | 1.14 (0.94,1.38) | 1.12 (0.91,1.37) | |
| 3 | 1.11 (0.92,1.34) | 1.17 (0.96,1.42) | 1.19 (0.98,1.45) | |
| 4 | 1.15 (0.95,1.38) | 1.20 (0.98,1.46) | 1.19 (0.97,1.46) | |
| Mercury | Wald Test p-value* | 0.706 | 0.710 | 0.741 |
| 1 | Ref | Ref | Ref | |
| 2 | 0.98 (0.82,1.18) | 0.99 (0.82,1.19) | 1.03 (0.85,1.25) | |
| 3 | 1.09 (0.91,1.30) | 1.09 (0.91,1.31) | 1.11 (0.92,1.33) | |
| 4 | 1.00 (0.83,1.21) | 1.05 (0.86,1.27) | 1.07 (0.88,1.30) | |
| Magnesium | Wald Test p-value* | 0.698 | 0.279 | 0.216 |
| 1 | Ref | Ref | Ref | |
| 2 | 1.08 (0.89,1.30) | 1.13 (0.93,1.39) | 1.18 (0.96,1.46) | |
| 3 | 1.12 (0.92,1.35) | 1.26 (1.00,1.58) | 1.28 (1.01,1.62) | |
| 4 | 1.04 (0.86,1.26) | 1.19 (0.93,1.54) | 1.22 (0.95,1.58) | |
| Selenium | Wald Test p-value* | 0.016 | 0.010 | 0.019 |
| 1 | Ref | Ref | Ref | |
| 2 | 1.05 (0.86,1.27) | 1.07 (0.87,1.31) | 1.08 (0.87,1.33) | |
| 3 | 1.22 (1.00,1.48) | 1.23 (1.01,1.50) | 1.21 (0.98,1.48) | |
| 4 | 1.31 (1.09,1.58) | 1.35 (1.11,1.64) | 1.34 (1.10,1.64) |
Three degree of freedom test
Model I–unadjusted model, Model II–Model I + age, gender, race, region, education, and age-race interaction Model III–Model II + SBP, hypertension, diabetes, smoking status, heart disease, atrial fibrillation, and LVH
Discussion and Conclusion
In this study, we found that environmental distribution of arsenic, mercury or magnesium was not associated with increased risk of stroke, whereas high levels of environmental selenium was, even after multivariable adjustment. While arsenic is a naturally occurring trace element, pollutants such as mining run-off and insecticides can increase the levels of arsenic in the environment as well. High levels of arsenic are known to be toxic to the human body and are linked to several cancers[17], and high levels of arsenic have been linked to hypertension and ischemic heart disease as well as cardiovascular diseases in general[18–20]. However, there is no complete consensus on the relationship between high arsenic levels and risk of many cardiovascular diseases including stroke. An environmental study suggested a possible link between arsenic in groundwater and higher rates of stroke and other cerebrovascular diseases [8, 9]. Our results indicated no statistically significant effect of environmental arsenic levels on stroke risk.
The primary pathway that environmental mercury can be introduced to the human body is through contaminated seafood, while other possible pathways for environmental mercury are not well known[21]. The known toxic effects of mercury once introduced into the human body, such as increased blood pressure, should theoretically increase the risk of cardiovascular diseases including stroke. However while the association between mercury toxicity and hypertension is clear, research into the relationship with other cardiovascular diseases has provided mixed results [22–24]. The results here indicate that environmental mercury levels are not related to stroke risk.
Magnesium exposure is most associated with the hardness of water [7]. It has been suggested that there is an inverse relationship between water hardness and risk of cardiovascular diseases, including stroke [25], and the protective effect is usually ascribed to the magnesium content in the water; however, studies supporting the relationship between environmental magnesium levels on cardiovascular diseases have used data on the community level [10], while case-control and cohort studies have provided mixed results [7, 11]. Our results indicate no relationship between the environmental magnesium levels where the REGARDS participants reside and elevated risk of stroke.
Human sources of environmental selenium include effluents from sewage treatment plants and oil refineries [13]. Like magnesium, it has been hypothesized that there is an inverse relationship between selenium levels in the body and risk of cardiovascular diseases [26]. Investigation into this hypothesis has provided mixed results. While some studies have found a connection between low selenium levels and heart disease, there is little evidence of a relationship between selenium levels and other cardiovascular diseases [27–29]. The results of this study did indicate that environmental selenium levels are linked to increased stroke risk. However, the direction of the hazards actually indicated that higher levels of selenium are associated with higher stroke risk. This unexpected result may be echoing other research that indicates that there is an optimal range for selenium levels for which moving above it will increase cardiovascular risk [30, 31]. These results prompted exploration into the possible differences in the demographic and medical characteristics between selenium quartile groups. The results of this post-hoc investigation are presented in Supplemental Table 1.
The research herein utilized the geographically broad REGARDS cohort, comprised of a large sample of both whites and blacks, in order to provide a more complete picture of the effects of environmental trace elements on stroke risk than has generally been provided in the literature, which has generally been focused on smaller or more homogenous populations. However, this study used environmental data to assess exposure to the trace elements of interest, which provides some inherent risk of residual confounding. For example, increased selenium levels are commonly associated with oil and metal refineries, and sewage treatment, which is then associated with lower socioeconomic status. We also considered participants’ baseline residence for merging with the USGS data and did not account for changes in residence since that time. This could result in misclassification bias. Additionally, levels of environmental exposure are not surrogates for levels of ingestion/absorption. Thus, future research must make use of blood or urinary levels of the trace elements among the REGARDS cohort, in order to provide a more direct exposure level of participants when assessing trace elements as a risk factor for stroke.
Supplementary Material
Acknowledgments
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.2 The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Additional funding was provided by NIH/NIEHS R01 ES021735-05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lanska DJ, Kuller LH. The geography of stroke mortality in the United States and the concept of a stroke belt. Stroke; a journal of cerebral circulation. 1995;26(7):1145–9. doi: 10.1161/01.str.26.7.1145. [DOI] [PubMed] [Google Scholar]
- 2.D'Alonzo CA, Pell S. A study of trace metals in myocardial infarction. Archives of environmental health. 1963;6:381–5. doi: 10.1080/00039896.1963.10663409. [DOI] [PubMed] [Google Scholar]
- 3.Hegde B, Griffith GC, Butt EM. Tissue and serum manganese levels in evaluation of heart muscle damage. A comparison with SGOT. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 1961;107:734–7. doi: 10.3181/00379727-107-26738. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder HA. Cadmium as a factor in hypertension. Journal of Chronic Diseases. 1965;18(7):647–656. [Google Scholar]
- 5.Skoczynska A, Poreba R, Steinmentz-Beck A, Martynowicz H, Affelska-Jercha A, Turczyn B, Wojakowska A, Jedrychowska I. The dependence between urinary mercury concentration and carotid arterial intima-media thickness in workers occupationally exposed to mercury vapour. International journal of occupational medicine and environmental health. 2009;22(2):135–42. doi: 10.2478/v10001-009-0017-4. [DOI] [PubMed] [Google Scholar]
- 6.Cheng TJ, Chuu JJ, Chang CY, Tsai WC, Chen KJ, Guo HR. Atherosclerosis induced by arsenic in drinking water in rats through altering lipid metabolism. Toxicology and applied pharmacology. 2011;256(2):146–53. doi: 10.1016/j.taap.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Leurs LJ, Schouten LJ, Mons MN, Goldbohm RA, van den Brandt PA. Relationship between tap water hardness, magnesium, and calcium concentration and mortality due to ischemic heart disease or stroke in The Netherlands. Environmental health perspectives. 2010;118(3):414–20. doi: 10.1289/ehp.0900782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisabeth LD, Ahn HJ, Chen JJ, Sealy-Jefferson S, Burke JF, Meliker JR. Arsenic in drinking water and stroke hospitalizations in Michigan. Stroke; a journal of cerebral circulation. 2010;41(11):2499–504. doi: 10.1161/STROKEAHA.110.585281. [DOI] [PubMed] [Google Scholar]
- 9.Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environmental health : a global access science source. 2007;6:4. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rylander R, Bonevik H, Rubenowitz E. Magnesium and calcium in drinking water and cardiovascular mortality. Scandinavian journal of work, environment & health. 1991;17(2):91–4. doi: 10.5271/sjweh.1722. [DOI] [PubMed] [Google Scholar]
- 11.Yang CY. Calcium and magnesium in drinking water and risk of death from cerebrovascular disease. Stroke; a journal of cerebral circulation. 1998;29(2):411–4. doi: 10.1161/01.str.29.2.411. [DOI] [PubMed] [Google Scholar]
- 12.Bo S, Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Current opinion in lipidology. 2008;19(1):50–6. doi: 10.1097/MOL.0b013e3282f33ccc. [DOI] [PubMed] [Google Scholar]
- 13.U.D.o.H.a.H. Services, editor. Toxicological Profile for Selenium. 2003. [PubMed] [Google Scholar]
- 14.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 15.Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke; a journal of cerebral circulation. 1989;20(10):1407–31. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 16.Rembert N, He K, Judd SE, McClure LA. The geographic distribution of trace elements in the environment: the REGARDS study. Environmental monitoring and assessment. 2017;189(2):84. doi: 10.1007/s10661-016-5733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.D.o.H.a.H. Services, editor. Toxicological Profile for Arsenic. 2007. [Google Scholar]
- 18.Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environmental health perspectives. 2012;120(4):494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CJ, Hsueh YM, Lai MS, Shyu MP, Chen SY, Wu MM, Kuo TL, Tai TY. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension. 1995;25(1):53–60. [PubMed] [Google Scholar]
- 20.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arteriosclerosis, thrombosis, and vascular biology. 1996;16(4):504–10. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- 21.U.D.o.H.a.H. Services, editor. Toxicological Profile for Mercury. 1999. [Google Scholar]
- 22.Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. Journal of clinical hypertension. 2011;13(8):621–7. doi: 10.1111/j.1751-7176.2011.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salonen JT, Seppanen K, Nyyssonen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91(3):645–55. doi: 10.1161/01.cir.91.3.645. [DOI] [PubMed] [Google Scholar]
- 24.Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, Valkonen VP, Seppanen K, Laukkanen JA, Salonen JT. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(1):228–33. doi: 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- 25.Larsson SC, Orsini N, Wolk A. Dietary magnesium intake and risk of stroke: a meta-analysis of prospective studies. The American journal of clinical nutrition. 2012;95(2):362–6. doi: 10.3945/ajcn.111.022376. [DOI] [PubMed] [Google Scholar]
- 26.Neve J. Selenium as a risk factor for cardiovascular diseases. Journal of cardiovascular risk. 1996;3(1):42–7. [PubMed] [Google Scholar]
- 27.Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue RP, Combs GF, Farinaro E, Clark LC, Reid ME. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. American journal of epidemiology. 2006;163(8):694–9. doi: 10.1093/aje/kwj097. [DOI] [PubMed] [Google Scholar]
- 28.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. The American journal of clinical nutrition. 2006;84(4):762–73. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Sun XD, Fan JH, Gunter EW, Taylor PR, Mark SD. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. The American journal of clinical nutrition. 2004;79(1):80–5. doi: 10.1093/ajcn/79.1.80. [DOI] [PubMed] [Google Scholar]
- 30.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Archives of internal medicine. 2008;168(4):404–10. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 31.Bleys J, Navas-Acien A, Laclaustra M, Pastor-Barriuso R, Menke A, Ordovas J, Stranges S, Guallar E. Serum selenium and peripheral arterial disease: results from the national health and nutrition examination survey, 2003–2004. American journal of epidemiology. 2009;169(8):996–1003. doi: 10.1093/aje/kwn414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.