Abstract
Taiwan has witnessed an emerging syndrome of liver abscess caused by Klebsiella pneumoniae carrying the magA gene required for exopolysaccharide web biosynthesis. We report a patient transferred from Alaska to Washington State with a magA+ K. pneumoniae liver abscess and describe a simple approach for recognition of these hypervirulent strains.
CASE REPORT
A 62-year-old man presented to a physician in Alaska with complaints of weight loss, anorexia, malaise, shortness of breath, and polydipsia of approximately 5 weeks' duration. His past medical history was notable only for tobacco use. The patient works at an Alaska cannery each summer and resides in the Philippines during the remainder of the year. He had returned from the Philippines three and a half weeks prior to admission. A physical examination was notable only for a liver edge palpable 3 cm below the right costal margin. However, laboratory evaluation demonstrated diabetic ketoacidosis with a serum glucose concentration exceeding 1,100 mg/deciliter. Highly elevated serum amylase (1,600 IU/liter) and lipase (12,000 IU/liter) levels prompted an abdominal ultrasound, followed by computerized tomography scanning, which revealed an 8.4- by 7.9-cm abscess with an air-fluid level in the right lobe of the liver. The patient was referred to a tertiary care medical center in Washington State. Echinococcus and Entamoeba histolytica serologies were negative, but cultures of blood and fluid from the liver abscess subsequently grew Klebsiella pneumoniae. The patient was treated with a fluoroquinolone antibiotic and percutaneous drainage with a good clinical response.
Klebsiella pneumoniae is a gram-negative lactose-fermenting enteric bacillus that forms large mucoid colonies. Although K. pneumoniae can cause pneumonia (e.g., Friedländer's pneumonia), it is more frequently encountered in the clinical laboratory as a cause of urinary tract infections, bacteremia, and wound and miscellaneous other nosocomial infections. Little is known about Klebsiella virulence factors beyond some iron acquisition systems and the antiphagocytic and anticomplement role of capsular polysaccharide (1, 5, 6, 8).
Since 1981, a distinctive syndrome of community-acquired K. pneumoniae septicemia with liver abscess has been reported in Taiwan (2, 7, 10, 12). This syndrome is notable for high mortality (10 to 40%), and some cases have been complicated by meningitis or endophthalmitis (2, 10, 11). Nearly 1,000 cases have been reported to date, and K. pneumoniae is now by far the most common cause of pyogenic liver abscess in Taiwan. A few cases in Korea, Singapore, Japan, India, and Thailand have also been described previously (7). Diabetes mellitus is an important predisposing factor, present in approximately half of afflicted patients. Unusual reported complications have included lung abscess, brain abscess, prostate abscess, osteomyelitis, septic arthritis, and psoas abscess.
Molecular typing of Taiwanese strains by pulsed-field gel electrophoresis has indicated that the liver isolates do not represent a single clonal population, although a predominant cluster of related strains has been identified in some series (9). Multiple capsular types have also been noted, with a predominance of K1 sometimes observed (4). Recently, a group of Taiwanese researchers identified a novel gene designated magA (for “mucoviscosity associated gene A”) that was present in 52 of 53 liver isolates of K. pneumoniae compared with only 9 of 52 strains not associated with liver abscess (3). The magA gene was found to be located within a 35-kbp locus containing 24 open reading frames with homology to genes involved in exopolysaccharide biosynthesis or export and glycosylation. This locus is not found within the sequenced genome of ATCC strain K. pneumoniae MGH78578.
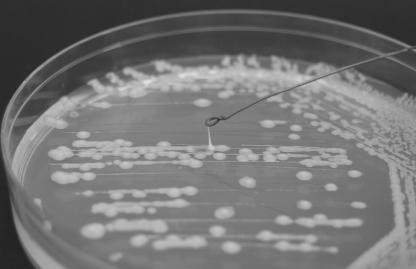
K. pneumoniae strains carrying magA were found to have a characteristic hyperviscous phenotype, characterized by the formation of elongated (>5 mm) mucoviscous strings when a loop is passed through a colony. In addition, magA+ K. pneumoniae was found to form a capsule-associated mucopolysaccharide web and exhibit increased serum resistance, resistance to phagocytosis, and virulence in mice compared with an isogenic magA mutant strain. Infected mice were found to have liver and brain abscesses similar to those seen in humans (3).
These observations suggest that the genetic locus containing magA represents a novel pathogenicity island responsible for the emergence of increased virulence in certain Taiwanese K. pneumoniae strains. Due to the similarity of our patient's presentation to those reported in Taiwan, we examined our K. pneumoniae isolate for the presence of hypermucoviscosity and found that colonies readily formed long mucoviscous strings (Fig. 1) that were not formed by other nonliver K. pneumoniae isolates in our laboratory. Genomic DNA was extracted from liquid cultures of the liver abscess isolate and two unrelated K. pneumoniae strains by use of a MasterPureTM kit (Epicentre, Madison, Wis.). After resuspension in Tris-EDTA buffer, PCR was employed with Taq polymerase to amplify 80 bp of 16S ribosomal DNA (primers 5′-GCGGTAATACGGAGGGTGC and 5′-CAC ATCCGACTTGACAGACC; GenBank AF453251) and 540-bp (primers 5′-CGCCG CAAATACGAGAAGTG and 5′-GCAATCGAAGTGAAGAGTGC) or 1,282-bp (primers 5′-GGTGCTCTTTACATCATTGC and 5′-GCAATGGCCATTTGCGTTAG) magA fragments that were detected by electrophoresis through 1.5% agarose. PCRs were performed with a 100-μl final volume at 95°C for 5 min followed by 40 cycles of 95°C for 0.5 min, 55°C for 0.5 min, and 72°C for 2 min and then 72°C for 10 min before holding at 10°C. All K. pneumoniae strains contained the 16S ribosomal DNA fragment, but only the hyperviscous liver isolate was found to contain magA (data not shown), which was confirmed by sequencing of the amplified fragment and comparison to the published sequence (GenBank AB085741).
FIG. 1.
Hypermucoviscous phenotype of magA+ Klebsiella pneumoniae.
This represents the first reported case of magA+ Klebsiella pneumoniae associated with liver abscess and bacteremia in North America. The infection appears to have been acquired in the Philippines. These hypervirulent K. pneumoniae strains can be readily detected by the presence of a hypermucoviscous colonial phenotype, and confirmation by PCR amplification of magA is straightforward. Further geographic dissemination of magA+ K. pneumoniae is likely, and our report underscores the importance of the clinical laboratory in the surveillance of emerging infections.
Acknowledgments
We are grateful to the staff of the Harborview Medical Center Clinical Microbiology Laboratory for their skillful technical assistance.
REFERENCES
- 1.Alvarez, D., S. Merino, J. M. Tomas, V. J. Benedi, and S. Alberti. 2000. Capsular polysaccharide is a major complement resistance factor in lipopolysaccharide O side chain-deficient Klebsiella pneumoniae clinical isolates. Infect. Immun. 68:953-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng, D. L., Y. C. Liu, M. Y. Yen, C. Y. Liu, and R. S. Wang. 1991. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch. Intern. Med. 151:1557-1559. [PubMed] [Google Scholar]
- 3.Fang, C. T., Y. P. Chuang, C. T. Shun, S. C. Chang, and J. T. Wang. 2004. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med. 199:697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung, C. P., F. Y. Chang, S. C. Lee, B. S. Hu, B. I. Kuo, C. Y. Liu, M. Ho, and L. K. Siu. 2002. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Highsmith, A. K., and W. R. Jarvis. 1985. Klebsiella pneumoniae: selected virulence factors that contribute to pathogenicity. Infect. Control 6:75-77. [DOI] [PubMed] [Google Scholar]
- 6.Kabha, K., L. Nissimov, A. Athamna, Y. Keisari, H. Parolis, L. A. Parolis, R. M. Grue, J. Schlepper-Schafer, A. R. Ezekowitz, D. E. Ohman, et al. 1995. Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect. Immun. 63:847-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko, W. C., D. L. Paterson, A. J. Sagnimeni, D. S. Hansen, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, J. G. McCormack, and V. L. Yu. 2002. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg. Infect. Dis. 8:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koczura, R., and A. Kaznowski. 2003. Occurrence of the Yersinia high-pathogenicity island and iron uptake systems in clinical isolates of Klebsiella pneumoniae. Microb. Pathog. 35:197-202. [DOI] [PubMed] [Google Scholar]
- 9.Lau, Y. J., B. S. Hu, W. L. Wu, Y. H. Lin, H. Y. Chang, and Z. Y. Shi. 2000. Identification of a major cluster of Klebsiella pneumoniae isolates from patients with liver abscess in Taiwan. J. Clin. Microbiol. 38:412-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, Y. C., D. L. Cheng, and C. L. Lin. 1986. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch. Intern. Med. 146:1913-1916. [PubMed] [Google Scholar]
- 11.Tang, L. M., and S. T. Chen. 1994. Klebsiella pneumoniae meningitis: prognostic factors. Scand. J. Infect. Dis. 26:95-102. [DOI] [PubMed] [Google Scholar]
- 12.Wang, J. H., Y. C. Liu, S. S. Lee, M. Y. Yen, Y. S. Chen, S. R. Wann, and H. H. Lin. 1998. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 26:1434-1438. [DOI] [PubMed] [Google Scholar]