Abstract
Objectives
We sought to assess the effect of congenital heart disease requiring infant surgery with cardiopulmonary bypass on neurodevelopmental outcomes and growth at four years of age, while matching for gestational age, socioeconomic status, maternal gestational conditions, home environment, and parental intelligence by studying multiple gestation births.
Methods
We performed within-family comparison of 14 multiple gestation births in which one child had congenital heart disease requiring surgery with cardiopulmonary bypass at ≤ 6 months of age. Between four and five years of age, a comprehensive neurodevelopmental assessment was performed. Paired comparisons were conducted between siblings with and without heart defects using a series of non-parametric tests.
Results
On average, the children qualified as late preterm (mean gestational age 35.4 ± 2.6 weeks). At an average age of 4.8 ± 0.1 years, children with congenital heart disease weighed less than their siblings (median weight for age z-score −0.4 vs 0.1, p = 0.02) and had worse performance for cognition (median full scale IQ 99 vs. 109, p = 0.02) and fine motor skills (median Wide Range Assessment of Visual Motor Ability, Fine Motor score 94.5 vs. 107.5, p < 0.01).
Conclusions
After controlling for socioeconomic status, home environment, parental intelligence, and gestational factors by using multiple gestation births, congenital heart disease requiring surgery with cardiopulmonary bypass at ≤ 6 months of age is associated with lower weight, cognitive abilities and fine motor skills at 4 years of age.
Introduction
Neurodevelopmental (ND) deficits are common and significantly disabling complications of congenital heart disease (CHD) and its treatment.1,2 Previous studies have identified an increased incidence of mild cognitive impairment, impaired social interactions, deficits in fine motor skills and executive function, inattention and impulsivity compared to the general population. In studies of ND outcomes, it is difficult to control for potential confounders such as gestational age, maternal conditions during pregnancy, home environment, socioeconomic status (SES), and parental intelligence, all of which are likely to highly influence ND outcome.3–8 Multiple gestation births in which one child has CHD provide a unique opportunity to match for these factors. We have previously shown in such a cohort that at 1 year of age, the children with CHD had lower scores on the Bayley Scales of Infant Development than their siblings.9 However, ND measures assessed at 1 year of age may not predict long term outcomes.10,11 Therefore, the cohort was re-evaluated at 4 years of age.
Patients and Methods
Patient Population
Between September 1998 and April 2003, 550 infants with CHD undergoing surgery with cardiopulmonary bypass (CPB) at ≤ 188 days (6 months) of age were enrolled in a single-institution study of the association between apolipoprotein E (APOE) genotype and postoperative neurodevelopmental dysfunction.12 Exclusion criteria included (1) multiple congenital anomalies, (2) recognizable genetic or phenotypic syndrome other than chromosome 22q11 microdeletion, and (3) language other than English spoken in the home.
Among this cohort, children who were the product of a multiple gestation were identified; this group forms the study population that is the focus of this report. Sibling(s) of the same gestation were recruited to participate in the follow-up evaluation with the intent to perform a matched pairs evaluation. Children with identified genetic abnormalities (including microdeletion of chromosome 22q11) or marked dysmorphisms as determined by a geneticist (see Four-Year Follow-up Evaluation below for details) were excluded from this study. In addition, children were excluded from the study if the sibling was not available for evaluation or if the sibling also had CHD. Gestational age was recorded in completed weeks by best obstetric estimate.
As a measure of complexity of CHD, patients were categorized according to a previously described classification that has been shown to be predictive of postoperative mortality.13 Class I includes patients whose repair achieves a biventricular circulation and in whom there is no preoperative arch obstruction; Class II, biventricular circulation with arch obstruction; Class III, single ventricle circulation without arch obstruction; and Class IV, single ventricle circulation with arch obstruction. In general, patients with Class I or II CHD achieve normal physiology after a single operation, while those in Class III or IV require multiple palliative operations and may experience ongoing cyanosis and/or congestive heart failure.
The Institutional Review Board at The Children’s Hospital of Philadelphia approved the study. Written informed consent was obtained from the parent or guardian.
Intraoperative Management
Operations were performed by one of three cardiac surgeons with a dedicated team of cardiac anesthesiologists. Alpha-stat blood gas management was used. Deep hypothermic circulatory arrest (DHCA) was used at the surgeon’s discretion. Before DHCA, patients were cooled to a nasopharyngeal temperature of 18°C using a combination of core cooling on CPB and topical hypothermia. Modified ultrafiltration was performed in all patients.
Four-Year Follow-up Evaluation
A study follow-up visit was conducted between the 4th and 5th birthdays. Siblings were assessed on the same day. Growth parameters (weight, height, and head circumference) were measured. A comprehensive ND assessment was performed. This included investigator-administrated instruments to assess cognition (Wechsler Preschool and Primary Scale of Intelligence, Full Scale IQ (FSIQ)),14 language (Preschool Language Scale-IV),15 executive function (NEPSY executive function subdomain),16 visual-motor integration (Developmental Test of Visual Motor Integration),17 fine motor skills (Wide Range Assessment of Visual Motor Abilities pegboard),18 and academic skills (Woodcock-Johnson III reading and math clusters).19 All of these instruments are designed to have a mean score of 100 in the normal population with a standard deviation of 15. Throughout the project, study personnel were trained to use standardized assessment practices. Each protocol was reviewed for accuracy, with attention to all subsets, including those requiring interpretation of verbal responses. Inter-rater reliability and administration standards for every test were established and maintained. The assessors were not fully masked to the child’s CHD status; however, no information was directly provided to the assessor about the child’s history. Language testing was conducted by one of two speech language pathologists experienced in test administration and scoring. Audiologic evaluations were conducted using standard pediatric assessment methods based on developmental ability. For the fine motor assessment, hand dominance was established first, and testing was then performed only for the dominant hand. Parents completed questionnaires to report social competence,20 hyperactivity/impulsivity and attention,21 socioeconomic status (SES, Hollingshead index),22 interim medical history, and race/ethnicity. For details of the instruments used, see the Appendix. Each child with CHD was evaluated by a genetic dysmorphologist (DMM, EZ) at either the one year or four year visit. Based on this evaluation, further genetic testing, including single nucleotide polymorphism microarray to rule out copy number variants or single gene disorder studies (e.g. PTPN11 to rule out Noonan syndrome), was completed if clinically indicated. Any child found to have a clinically significant result was excluded from the analysis.
Statistical Analysis
Data analysis occurred in two phases, a descriptive phase and a comparative phase. In the descriptive phase of the study, measures of central tendency, variability, and association were computed for all study-related variables with an emphasis on ND outcomes. Parametric measures were used for variables which appeared normally distributed upon inspection; otherwise, non-parametric measures were selected. In the comparative phase, two sets of analyses were conducted. First, children with CHD born as a part of a multiple-gestation pregnancy were compared with singleton children with CHD in the larger APOE study cohort who completed the 4 year evaluation. The purpose of this analysis was to shed light on subsequent generalizability of our findings in the multiple gestation cohort. Comparisons were conducted for demographic, perinatal, and surgical outcomes using Fisher’s exact, one-sample sign-rank, and one-sample t-tests, depending upon the nature of the data. Second, children with CHD born as a part of a multiple-gestation birth were compared with their non-affected, same gestation siblings using traditional growth parameters (head circumference, weight, and length expressed as z-scores) and ten different neuropsychological measures using Wilcoxon sign-rank tests. The sign-rank test, a non-parametric test for evaluating matched pairs, was deemed appropriate given the dependent nature of the samples (multiple-gestation births) and the small, non-normative nature of the distributions. Handedness was compared using McNemar’s test. For triplet families, both siblings were evaluated and one was then chosen randomly to serve as the comparator in the analyses. The criterion for statistical significance was held constant at the nominal a α 0.05 level across all comparisons. All data were analyzed using STATA v12.1 (College Station, TX). As a means of further characterizing the outcomes with statistically significant differences between children with CHD and their siblings, we plotted the child level data for each family, ordered by gestational age.
Results
Description of the Study Population
Among 550 children enrolled in the larger cohort, 30 children (29 families) were the product of a multiple gestation. In 10 families, one or more children died before 1 year of age (in 8, the child with CHD expired; in 1 the sibling without CHD died; in 1 both members of a twin pair expired). No children died between 1 and 4 years of age. Three families were excluded due to microdeletion of chromosome 22q11 or diagnosis of another genetic abnormality in the child with CHD. One family was excluded due to presence of CHD in both members of the twin pair. In 14/15 remaining families (93%, 12 twin sets, 2 triplet sets), both the child with CHD and their sibling(s) were evaluated. Compared to our prior evaluation of this cohort at 1 year of age, which included 11 multiple gestation families, four additional families were evaluated and one family was lost to follow up. There were no significant differences between the included and not included multiple gestation children with respect to gender, race/ethnicity, gestational age, mode of delivery, birth weight, complexity of CHD or age at initial operation (data not shown).
The characteristics of the cohort of multiple gestation children with CHD (n = 14) described in this report are summarized in Table 1. In general, this was a predominantly white, preterm population delivered by C-section with a mean gestational age of 35.4 ± 2.6 weeks and a mean birthweight of 2.4 ± 0.65 kg. Children with CHD were predominantly male (10/14), while their non-affected siblings were predominantly female (9/14), although the difference was not statistically significant (p = 0.13 by Fisher’s exact test). All mothers had completed at least high school; 43% had college or graduate degrees. The children with CHD were relatively evenly split between two ventricle heart disease (8/14) and single ventricle heart disease (6/14). Accordingly, 8/14 underwent a single operation with cardiopulmonary bypass, while 6/14 had multiple operations. One twin pair was affected by twin-twin transfusion syndrome in utero. The ND evaluation was performed between 3/2005 and 9/2007 at an average age of 4.8 ± 0.1 years.
Table 1.
Comparison of multiple gestation children with CHD included in this study with singleton birth children with CHD who completed the 4 year follow-up evaluation.
| Parameter | Multiple gestation CHD cohort (n = 14) | Singleton gestation CHD cohort (n = 362) | P | |
|---|---|---|---|---|
|
| ||||
| Demographic | Female sex | 4 (28.6%) | 157 (43.4%) | 0.411 |
|
| ||||
| Race | ||||
| White | 12 (85.7%) | 245 (67.7%) | ||
| Black or African American | 2 (14.3%) | 78 (21.4%) | 0.471 | |
| Other | 39 (10.8%) [n = 362] |
|||
|
| ||||
| Maternal education | ||||
| Less than high school | 0 | 19 (5.3%) | ||
| High school/some college | 8 (57.1%) | 155 (42.8%) | ||
| College degree | 5 (35.7%) | 123 (34.5%) | 0.711 | |
| Graduate degree | 1 (7.1%) | 63 (17.4%) [n = 362] |
||
|
| ||||
| SES, Hollingshead Index | ||||
| 1 | 1 | 12 | ||
| 2 | 0 | 31 | ||
| 3 | 3 | 75 | 0.671 | |
| 4 | 4 | 114 | ||
| 5 | 6 | 128 [n = 360] | ||
|
| ||||
| Perinatal information | Mean gestational age, weeks (range) | 35.4 ± 2.6 (30–38) |
38.6 ± 1.9 (30–42) [n = 359] |
<0.012 |
|
| ||||
| Term (>37 weeks EGA) | 6 (42.9%) |
316 (88.0%) [n = 359] |
<0.011 | |
|
| ||||
| Type of delivery | ||||
| Vaginal C-section | 3 (21.4%) 11 (78.6%) |
262 (73.2%) 96 (26.8%) [n = 358] |
<0.011 | |
|
| ||||
| Mean birth weight, kg (range) | 2.40 ± 0.65 (1.26–3.23) |
3.16 ± 0.60 (1.41–5.14) [n = 360] |
<0.012 | |
|
| ||||
| CHD and surgery | CHD Class | |||
| I | 7 (50.0 %) | 193 (53.3%) | ||
| II | 1 (7.1%) | 44 (12.2%) | 0.871 | |
| III | 1 (7.1%) | 35 (9.7%) | ||
| IV | 5 (35.7%) | 90 (24.9%) | ||
|
| ||||
| Median age at first operation, days (range) | 14.5 (1–169) | 8 (1–188) | 1.03 | |
|
| ||||
| Operation at ≤ 30 days of age | 8 (57.1%) | 225 (62.2%) | 0.781 | |
|
| ||||
| Total no. operations with CPB Median (range) | 1 (1–3) | 1 (1–5) | ||
| One | 8 (57.1%) | 215 (59.4%) | ||
| Two | 2 (14.3%) | 43 (11.9%) | 0.901 | |
| Three | 4 (28.6%) | 94 (26.0%) | ||
| Four | 8 (2.2%) | |||
| Five | 2 (0.6%) | |||
|
| ||||
| DHCA employed during any operation | ||||
| Yes | 8 (57.1%) | 219 (60.5%) | 0.791 | |
| No | 6 (42.9%) | 143 (39.5%) | ||
|
| ||||
| Median cumulative DHCA time over all operations, minutes (range) | 19 (0–136) | 27 (0–226) | 0.793 | |
|
| ||||
| Median length of stay (first operation) | 11 (3–42) | 11 (2–109) | 1.03 | |
Fisher’s exact test
One sample t-test
One sample non-parametric sign test
SES, socioeconomic status; EGA, estimated gestational age; CHD, congenital heart disease; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest.
Comparison between Multiple-Gestation Children with CHD and Singletons
When compared with the singleton cohort, the multiple-gestation cohort evidenced significantly lower gestational age and birth weight as well as significantly higher percentages of infants delivered by Cesarean section (all p’s < 0.01). Demographic and surgical variables were not significantly different between the two groups (see Table 1).
Comparison between Multiple Gestation Children with CHD and Non-affected Siblings
Growth parameters at birth were available for both members of a multiple gestation in 10 families for weight, 9 families for length, and 8 families for head circumference. No significant differences were observed between children with CHD and their non-affected siblings (p = 0.88 for weight, p = 0.37 for length, and p = 0.53 for head circumference, data not shown).
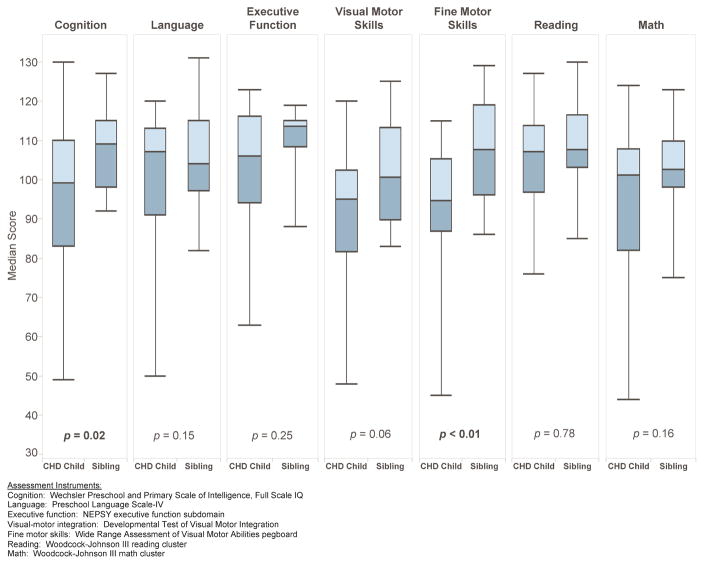
Summary results of the 4 year ND outcome measures comparing children with CHD to their non-affected siblings are shown in Table 2. Among the growth parameters, only weight z-score was observed to be significantly lower among children with CHD than their non-affected siblings (p = 0.02). When neurodevelopmental outcomes were examined, cognition score (p = 0.02) and fine-motor score (p < 0.01) were significantly lower among children with CHD. The analysis was repeated for these two outcome measures, excluding two families in which the child with CHD performed extremely poorly (cognition and fine-motor score both <50) to determine whether these outliers were driving the conclusions. Children with CHD still scored more poorly on these measures; the resulting p-values were p = 0.055 for cognition and p < 0.01 for fine-motor score, indicating the differences observed were not solely attributable to outliers. All remaining variables were not significantly different between the two groups, although visual motor integration (p = 0.06), and social competence (p = 0.06), two factors known to affect school performance and social interactions, approached the threshold of statistical significance. Handedness did not differ significantly between the two groups (p = 0.63, data not shown).
Table 2.
Comparison of outcome measures from the 4 year neurodevelopmental evaluation: multiple gestation children with CHD compared to their non-affected siblings.
| Domain Measure |
Families n | CHD Children Median (Range) | Non-Affected Siblings Median (Range) | p-value3 |
|---|---|---|---|---|
| Growth Parameters (WHO z-score)1 | ||||
| Head Circumference | 14 | 0.2 (−3.7, 2.2) | 0.7 (−3.3, 2.2) | 0.18 |
| Weight | 13 | −0.4 (−2.3, 0.4) | 0.1 (−1.2, 2.8) | 0.02 |
| Length | 13 | −0.6 (−3.9, 0.5) | −0.8 (−2.0, 1.0) | 0.07 |
| Investigator Administered2 | ||||
| Cognition | 13 | 99 (49, 130) | 109 (92, 127) | 0.02 |
| Language | 13 | 107 (50, 120) | 104 (82, 131) | 0.15 |
| Executive Function | 12 | 106 (63, 123) | 113.5 (88, 119) | 0.25 |
| VM Integration | 14 | 95 (48, 120) | 100.5 (83, 125) | 0.06 |
| Fine Motor Skills | 14 | 94.5 (45, 115) | 107.5 (86, 129) | <0.01 |
| Reading Achievement | 12 | 107 (76, 127) | 107.5 (85, 130) | 0.78 |
| Math Achievement | 14 | 101 (44, 124) | 102.5 (75, 123) | 0.16 |
| Parent Report | ||||
| Attention | 13 | 6 (0, 22) | 2 (0, 25) | 0.14 |
| Hyperactivity/Impulsivity | 13 | 6 (1, 17) | 4 (1, 24) | 0.78 |
| Social Competence | 13 | 115 (84, 123) | 116 (101, 123) | 0.06 |
Note. CHD, congenital heart disease; WHO, World Health Organization.
Population mean z-score = 0, 1 z-score = 1 standard deviation.
All scales in this category have a population norm of 100, standard deviation 15.
All p-values derived from non-parametric Wilcoxon sign-rank tests
With respect to other medical conditions which could influence performance on the ND evaluation, the two groups were statistically similar. Audiology evaluation demonstrated hearing loss in 4 children with CHD (1 bilateral sensorineural, 1 bilateral conductive, 2 indeterminate) and 2 siblings (1 bilateral conductive, 1 unilateral conductive), p = 0.65. Parents reported visual impairment (not quantified) in two children with CHD and no siblings (p = 0.48 by Fisher’s exact test). No children (either CHD or siblings) were reported to have chronic lung disease, although one child with CHD and his sibling were both reported to use medications for reactive airway disease. This sibling pair was born at 31 weeks gestation and both were reported to have had respiratory syncytial virus infection in the first year of life. One sibling was reported to have undergone major non-cardiac surgery, specifically a malrotation repair.
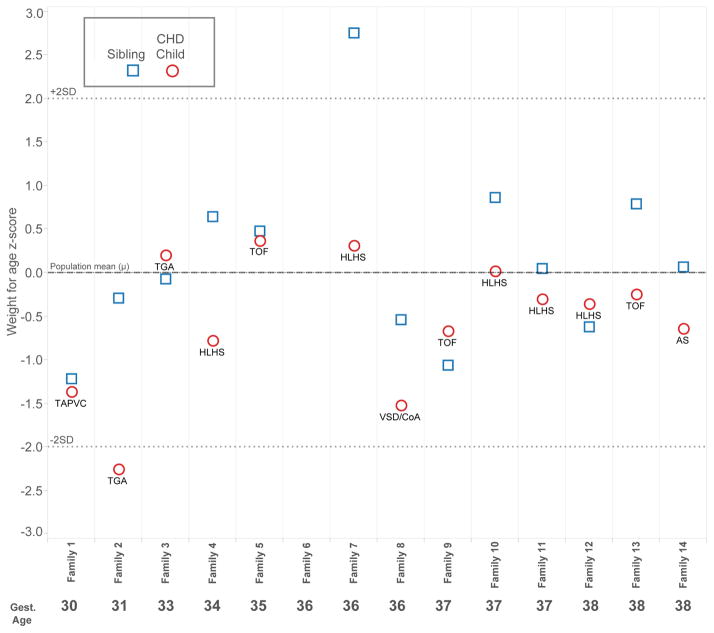
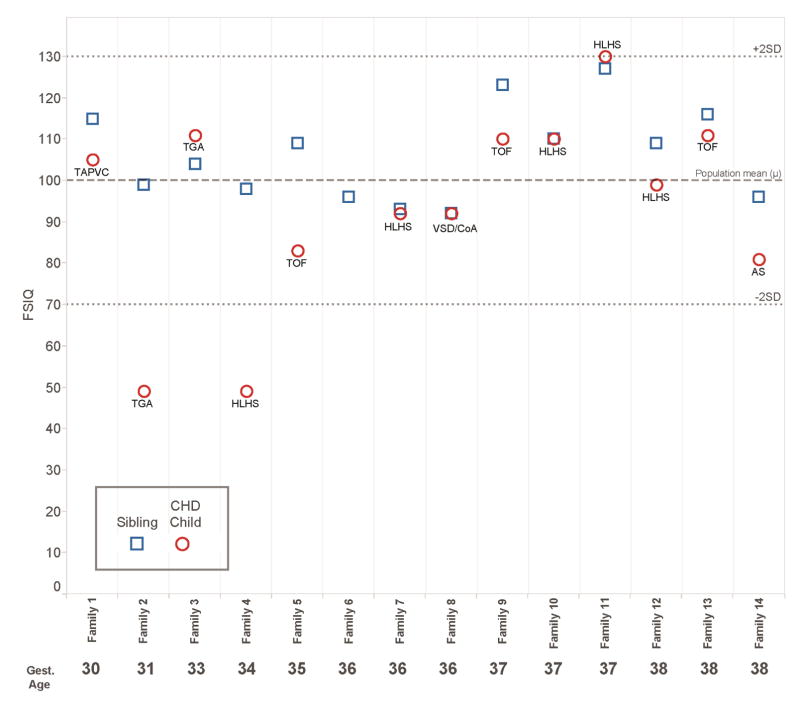
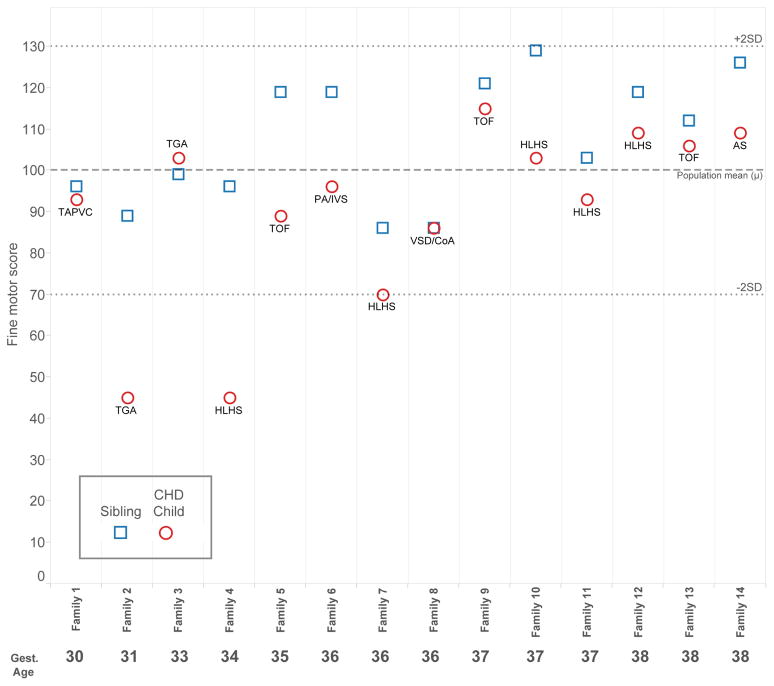
Figures 1, 2, and 3 present child level data for the outcome measures for which statistically different results were observed between children with CHD and their siblings. Note that most values fell within the respective normal ranges (± 2 standard deviations). For weight for age z-score, data were available for 13 families. Ten of 13 children with CHD weighed less than their sibling, although all but one child with CHD achieved a normal weight (±2 z-scores). For FSIQ, complete data were available for 13 families. In 9 of 13 families, the child with CHD performed worse than the sibling. The most notable discrepancies occurred in significantly preterm children (families 2 and 4, 31 and 34 weeks gestation, respectively). All 14 families had scores available for fine motor skills. The pattern observed for FSIQ was more pronounced in the area of fine motor skills; in 12 families, the child with CHD performed worse than the sibling, and overall, the discrepancies appear greater. The worst performances and greatest discrepancies within families occurred in the preterm families, specifically families 2 and 4. Notably, the siblings without CHD generally performed well, despite the fact that 8 of 14 families were preterm, and none achieved greater than 38 weeks gestational age.
Figure 1.
Child-level data for weight for age z-score at 4 years, grouped by family and ordered by gestational age. Cardiac diagnosis for the child with CHD is noted. Z-score = 0 is defined as the mean in the general population. The normal range falls between dotted lines at ± 2 z-scores.
Figure 2.
Child-level data for full scale IQ at 4 years, grouped by family and ordered by gestational age. Cardiac diagnosis for the child with CHD is noted. FSIQ = 100 represents the mean in the general population. The normal range falls between dotted lines at ± 2 standard deviations.
Figure 3.
Child-level data for Fine Motor Score at 4 years, grouped by family and ordered by gestational age. Cardiac diagnosis for the child with CHD is noted. Fine Motor Score = 100 represents the mean in the general population. The normal range falls between dotted lines at ± 2 standard deviations.
Discussion
This study demonstrates that among a cohort of multiple gestation births, the presence of CHD requiring surgery with CPB in the first 6 months of life is associated with persistent deficits in weight gain, cognition, and fine motor skills at 4 years of age. The observed deficits in cognition and fine motor skills are consistent with prior studies,1 but were obtained from a unique patient population, namely multiple gestation births in which one child has CHD. The design of this study allowed us to control for gestational age, maternal conditions during pregnancy, home environment, SES, and parental intelligence in a manner not possible in other studies. These potential confounders are important determinants of cognitive outcomes and have effects much greater in magnitude than many surgical, anesthetic, and hospital-related variables studied. For example, social class, which may affect access to care and educational achievement via multiple mechanisms, explained 23.7% of the variance in full scale IQ scores at eight years of age in the Boston Circulatory Arrest Trial, while assignment to intraoperative support strategy explained only 0.3%.3 A meta-analysis of twin studies has demonstrated that the heritability of IQ is approximately 50%, while in utero environment (20%) and shared post-natal environmental factors (17%) also contribute importantly to explaining variance in IQ.5,23 Mean cognitive scores of children born prematurely decline in a linear fashion with decreasing birth weight.24 Having controlled for these confounders, children with CHD had deficits in developmental achievement at four years of age when compared to their same-gestation siblings.
The neurodevelopmental deficits observed in children with CHD are likely multifactorial in etiology. In utero brain development, genetic factors (which may or may not be associated with clinical dysmorphisms), pre- and post-operative care strategies, the timing of cardiac surgery, intraoperative management strategies and maternal mental health have all been implicated with respect to their effects on ND.1,2,25 This study does not attempt to assign causality to any one of these multiple potential factors. Rather, this study confirms the association between CHD of sufficient severity to require infant surgery and adverse ND outcomes, while controlling for some important gestational and home environmental factors.
In contrast to many cohorts of children with CHD whose ND outcomes have been reported, the patient population studied here is more significantly affected by an additional factor—prematurity. Of note, 8/14 families included in the study were preterm and no family achieved a gestational age of greater than 38 completed weeks. Studies of neonates with critical CHD confirm increased morbidity and mortality in infants born before 39 weeks gestation.26, 27 An analysis of the larger cohort from which this study is drawn demonstrated worse neurodevelopmental outcomes at 4 years in children with CHD born before 39 weeks gestation.28 Our child level data indicate that the worst scores and greatest discrepancies between siblings on ND scales were observed in the families with lowest gestational age.
This cohort differs from the general population of infants undergoing surgery for CHD, and thus the generalizability of these findings to singleton, term infants must be considered. Aside from the issue of prematurity alluded to above, multiple gestation could be postulated to have an independent effect on neurodevelopmental outcome. Review of the current literature29,30 indicates that twin gestations are known to have a higher risk of cerebral palsy than singleton infants, when birth weight and gestational age are taken into account. Cognitive deficits independent of birth weight and gestation in twins have not been definitively shown; if present, the magnitude is likely small. Overall, however, our ND findings are consistent with patterns observed when cohorts of predominantly singleton, term infants with CHD are compared to population norms. Thus, we believe that the results from this cohort further the understanding of ND deficits in the larger population of children with CHD.
The data on somatic growth indicate evidence of decreased weight gain among the children with CHD. Median weight z-score was significantly lower among children with CHD than their non-affected siblings (p = 0.02). This finding is not surprising, given that many of the children with CHD underwent multiple operations, were palliated or potentially had residual hemodynamic lesions. Notably, we found no difference in head circumference, despite the overall concern about neurodevelopment in children with CHD and the known elevated incidence of microcephaly at birth in several forms of complex CHD.31,32
One limitation of this study is our sample size, which was constrained by the number of available multiple gestation children in a large cohort. While the sample size is admittedly small, we were sufficiently powered to detect differences between siblings in weight for age z-score, FSIQ and fine motor skills. It is possible that we did not have sufficient power to detect differences in scores in some domains examined. Given the small sample size, this cohort does not provide a precise point estimate of average performance for a congenital heart disease cohort on the instruments utilized. The strength of this study design lies in its ability to make paired comparisons that account for some common confounders of ND outcomes. In addition to the small sample size, we elected not to adjust for correlated endpoints, due in part to the relatively small number of measures used, the potential to overlook modest but emerging trends in the data, and the role of this study. In this study, we seek to add another dimension to the body of literature on ND in children with CHD by using a novel design.
Second, while this design achieves the best possible matching of maternal conditions during pregnancy and home environment between the groups with and without CHD, environmental differences may still remain. Parents may have treated their child with CHD differently than the non-affected sibling. Placental sufficiency may have differed for two fetuses carried in a single pregnancy.
Third, the issue of bias in the outcome evaluations is considered. The investigators performing the ND evaluation were not blinded to CHD status. However, as detailed in the Methods, administration and scoring of the tasks comprising the ND evaluation are highly standardized.
Finally, as alluded to above, this study highlights an association between CHD requiring infant surgery with CPB and ND deficits, while controlling for some important gestational and home environmental factors. It does not attempt to determine more specific causal factors leading to the observed deficits and the list of such possible factors remains long. As one example, there may be residual confounding by undetected genetic factors that lead to both CHD and ND deficits. In this study, a clinical evaluation by a genetic dysmorphologist, determined the need for further genetic testing. Genetic variants which did not cause clinical dysmorphisms or CHD characteristic of a particular known genetic condition could remain undetected.
Conclusion
Within multiple gestation births, children with CHD requiring surgery with CPB in the first 6 months of life weighed less and had worse performance than their siblings on measures of cognition and fine motor skills at 4 years of age. By using multiple gestation births, we controlled for gestational age, maternal conditions during pregnancy, home environment, SES, and parental intelligence. These findings suggest that CHD, its treatment, and other confounding factors such as genetic variants, have adverse effects on ND outcomes and weight gain. Our findings are consistent with other studies that have shown ND deficits among children with CHD compared to general population norms. These results underscore the need for strategies to promote optimal ND outcomes among children with CHD requiring surgery in infancy.
Figure 4. Central Picture.
Developmental outcomes: children with congenital heart disease vs same-gestation siblings.
Perspective.
Many studies find developmental deficits in children with congenital heart disease (CHD) compared to norms, but cannot adjust for gestational factors, home environment, or parental intelligence. Our unique cohort compares children with CHD requiring infant surgery to same-gestation siblings. Developmental deficits at 4 years were associated with CHD, controlling for important confounders.
Central Message.
Controlling for family- and pregnancy-related factors, congenital heart disease requiring infant surgery is associated with developmental deficits at 4 years.
Acknowledgments
Funding Sources: Supported by an American Heart Association National Grant-in-Aid (9950480N), HL071834 from the National Institutes of Health, the Pew Biomedical Scholar Program, the Daniel M. Tabas Endowed Chair in Pediatric Cardiothoracic Surgery, and the Fannie E. Rippel Foundation.
The authors express their appreciation to Nancy Burnham, CRNP, MSN, CCRC and the rest of the APOE study staff for their support in the writing of this manuscript.
Abbreviations
- ND
neurodevelopmental
- CHD
congenital heart disease
- CPB
cardiopulmonary bypass
- APOE
Apolipoprotein E
- SES
socioeconomic status
- DHCA
deep hypothermic circulatory arrest
- FSIQ
Full Scale IQ
- TOF
tetralogy of Fallot
- HLHS
hypoplastic left heart syndrome
- D-TGA
D-transposition of the great arteries
- IVS
intact ventricular septum
- VSD
ventricular septal defect
- AS
aortic stenosis
- TAPVR
total anomalous pulmonary venous return
- PA
pulmonary atresia, WHO, World Health Organization
Appendix
Note: portions of the text that follow have been previously published in studies reporting other outcomes from the APOE cohort.33,34
Cognition
The Wechsler Preschool and Primary Scale of Intelligence, Third Edition, is a standardized test of intelligence for children 3.5 to 7 years of age.14 It is commonly used in both clinical settings and research settings; it takes ~45 minutes to administer and yields 3 summary scores and 12 subtest scores. Scores include full-scale IQ (FSIQ), verbal IQ, and performance IQ, with means of 100 and SDs of 15. Only the FSIQ was utilized in the present study. The test covers a wide range of cognitive tasks. There is a large body of data explaining the meaning of test findings. The Wechsler Preschool and Primary Scale of Intelligence, Revised proved to have moderate to strong reliability (coefficients for FSIQ was 0.92) and validity (correlation with other cognitive tests in the positive and significant range of 0.74–0.90) in a variety of studies.
Language
The Preschool Language Scale-IV is a general test of early language skills.15 It provides a measure of language comprehension and expressive communication. Standard scores are derived on the basis of age and performance. The Total Language Scale (mean: 100; SD: 15), utilized in this study, is derived on the basis of performance on the receptive and expressive sections.
Executive Function
The NEPSY is a developmental neuropsychological assessment tool that was initially published in 1998 and then revised in 2007.16 It is a direct child measure that assesses key domains of neuropsychological functioning including attention/executive functioning, language, sensorimotor, visuospatial and memory. This study utilized the Attention and Executive Function subdomains of the 2nd edition of the NEPSY and used the visual attention and the statue subtests. The executive function subdomain yields a Core domain score with a mean of 100 and standard deviation of 15. The NEPSY is a norm-referenced and standardized assessment tool that has strong validity and high reliability (0.71) that was established through multiple studies.
Visual Motor Integration
Visual-motor integration was assessed with the developmental test of Visual Motor Integration, a simple copying task that assesses the child’s fine motor and visual motor coordination skills.17 It takes 10 minutes to complete and yields standard scores with a mean of 100 and standard deviation of 15. Handedness is also noted on the VMI. The interrater reliability had a median of 0.93. Generally, researchers have found the VMI to be a valuable predictor when used in combination with other measures. The positive correlation with other tests of visual skills and motor skills was documented in the manual as ranging from 0.72 to 0.76.
Fine Motor Skills
Fine motor skills were tested using the Wide Range Assessment of Visual Motor Abilities pegboard, a manipulative dexterity test.18 The child inserts as many pegs as possible within 90 seconds using a nearly square pegboard. The pegboard is “waffled” to add to its fine motor demands, as well as to increase its esthetic appeal. The test is typically completed first with the dominant hand and then with the non-dominant hand. For the current study, only results from the dominant hand were included. This test was chosen from the collection of pegboard tasks because it is the only one designed and standardized for a 4-year-old population. The scores are provided as standard scores and percentiles, which were published in 1995. The reliability and validity for the proposed group are strong.
Academic Skills (reading, math)
The Woodcock-Johnson III is a standardized achievement test for children 2 years to adulthood. It has recently been normed and revised.19 For the purpose of the present study, only the reading and math clusters were used. These subtests take about 10 minutes each and measure a preschooler’s skills in these areas. The norming procedures of the Woodcock-Johnson III were excellent and reflected the most recent census data. The data on reliability ranged from 0.80 to 0.87 for individual tests. This is one of the few achievements tests that have been normed for preschoolers.
Attention and Hyperactivity/Impulsivity
The ADHD Rating Scale-IV, Preschool Version, is an 18 item questionnaire that requires parents to rate the frequency of occurrence of ADHD symptoms as defined in the DSM-IV.21 The respondent rates each item on a Likert scale of 0 (not at all) to 3 (very often). This scale was developed specifically for children 3 to 6 years of age. Normative data were collected from a stratified sample of 907 children. Mean scores are provided for inattention and hyperactivity/impulsivity.
Social Competence
The Preschool and Kindergarten Behavior Scales was designed for evaluation of social skills and problem behavior patterns of children ages 3–6. It is a norm-referenced and standardized parent-report instrument. It yields two separate major scales: Social Skills which included 34 items and Problem Behaviors which include 42 items. 20 The Social Skills score was included for analysis here. Normative data were obtained from 2855 preschool-aged children (3–6 years of age). Reliability was reported to range from 0.81 to 0.97 in internal consistency and in the moderate to high range (0.58–0.87) in test-retest reliability. Predictive validity studies suggested that PKBS scores were able to predict need for special education services. Validity studies comparing the PKBS with other tests of social skills indicated strong correlations.
Footnotes
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Clinical Trial Registration: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–72. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 2.Tabbutt S, Gaynor JW, Newburger JW. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. 2012;27:82–91. doi: 10.1097/HCO.0b013e328350197b. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DC, Wypij D, du Plessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextrotransposition of the great arteriese: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 4.Devlin B, Daniels M, Roeder K. The heritability of IQ. Nature. 1997;388:468–71. doi: 10.1038/41319. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard TJ, Jr, McGue M. Genetic and environmental influences on human psychological differences. J Neurobiol. 2003;54:4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- 6.Petrill SA, Deater-Deckard K. Task orientation, parental warmth and SES account for a significant proportion of the shared environmental variance in general cognitive ability in early childhood: evidence from a twin study. Dev Sci. 2004;7:25–32. doi: 10.1111/j.1467-7687.2004.00319.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 8.Forbess JM, Visconti KJ, Hancock-Friesen C, Howe RC, Bellinger DC, Jonas RA. Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation. 2002;106:95–102. [PubMed] [Google Scholar]
- 9.Schultz AH, Jarvik GP, Wernovsky G, Bernbaum J, Clancy RR, D’Agostino JA, et al. Effect of congenital heart disease on neurodevelopmental outcomes within multiple gestation births. J Thorac Cardiovasc Surg. 2005;130:1511–16. doi: 10.1016/j.jtcvs.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116:333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 11.McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics. 2004;114:e572–e576. doi: 10.1542/peds.2003-0983-L. [DOI] [PubMed] [Google Scholar]
- 12.Gaynor JW, Gerdes M, Zackai EH, Bernbaum J, Wernovsky G, Clancy RR, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1736–45. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 13.Clancy RR, McGaurn SA, Wernovsky G, Spray TL, Norwood WI, Jacobs ML, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119:347–57. doi: 10.1016/S0022-5223(00)70191-7. [DOI] [PubMed] [Google Scholar]
- 14.Weschler D. Weschler preschool and primary scale of intelligence. 3. San Antonio (TX): Harcourt Assessment; 2002. [Google Scholar]
- 15.Zimmerman I, Steiner VP, Evatt R. Preschool language scale-IV. 4. San Antonio (TX): Harcourt Assessment; 2002. [Google Scholar]
- 16.Korkman M, Kirk U, Kemp S. NEPSY. 2. San Antonio (TX): Psychcorp/Harcourt Assessment; 2007. [Google Scholar]
- 17.Beery K. The VMI: developmental test of visual motor integration—administration, scoring, and testing manual. Cleveland (OH): Modern Circulation Press; 1989. [Google Scholar]
- 18.Adams D, Sheslow D. Wide range assessment of visual motor abilities. Wilmington (DE): Wide Range; 1995. [Google Scholar]
- 19.Mather N, Woodcock R. Woodcock Johnson Achievement Battery III Manual. 3. Rolling Meadows, IL: Riverside Publishing; 2001. [Google Scholar]
- 20.Merrell K. Preschool and Kindergarten Behavior Scales. Austin, TX: Psychological Corp; 1994. [Google Scholar]
- 21.DuPaul GJ, Powers TJ, Anastopoulos AD, Reid R. The ADHD rating scale IV manual. New York, NY: Guilford Press; 1999. [Google Scholar]
- 22.Hollingshead A. Four factor index of social status. New Haven (CT): Department of Sociology, Yale University; 1975. [Google Scholar]
- 23.Devlin B, Daniels M, Roeder K. The heritability of IQ. Nature. 1997;388:468–71. doi: 10.1038/41319. [DOI] [PubMed] [Google Scholar]
- 24.Bhutta AT, Cleves MA, Casey PH, Cardock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 25.McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A randomized controlled trial of interventions to promote adjustment in children with congenital heart disease entering school and their families. J Pediatric Psychology. 2012;37:1089–1103. doi: 10.1093/jpepsy/jss092. [DOI] [PubMed] [Google Scholar]
- 26.Costello JM, Polito A, Brown DW, McElrath TF, Graham DA, Thiagarajan RR, et al. Birth before 39 weeks gestation is associated with worse outcomes in neonates with heart disease. Pediatrics. 2010;126:e277–84. doi: 10.1542/peds.2009-3640. [DOI] [PubMed] [Google Scholar]
- 27.Costello JM, Pasquali SK, Jacobs JP, He X, Hill KD, Cooper DS, et al. Gestational age at birth and outcomes after neonatal cardiac surgery. Circulation. 2014;129(24):2511–2517. doi: 10.1161/CIRCULATIONAHA.113.005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goff DA, Luan X, Gerdes M, Bernbaum J, D’Agostino JA, Rychik J, et al. Younger gestational age is associated with worse neurodevelopmental outcomes after cardiac surgery in infancy. J Thorac Cardiovasc Surg. 2012;143:535–42. doi: 10.1016/j.jtcvs.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooke RWI. Does neonatal and infant neurodevelopmental morbidity of multiples and singletons differ? Sem Fetal Neonat Med. 2010;15:362–66. doi: 10.1016/j.siny.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz J. Neurodevelopmental outcome of twins. Sem Perinatol. 2012;36:201–12. doi: 10.1053/j.semperi.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Shillingford AJ, Ittenbach RF, Marino BS, Rychik J, Clancy RR, Spray TL, et al. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young. 2007;17(2):189–195. doi: 10.1017/S1047951107000248. [DOI] [PubMed] [Google Scholar]
- 32.Barbu D, Mert I, Kruger M, Bahado-Singh RO. Evidence of fetal central nervous system injury in isolated congenital heart defects: microcephaly at birth. Am J Obstet Gynecol. 2009;201(43):e1–7. doi: 10.1016/j.ajog.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Gaynor JW, Nord AS, Wernovsky G, Bernbaum J, Solot CV, Burnham N, et al. Apolipoprotein E genotype modifies the risk of behavior problems after infant cardiac surgery. Pediatrics. 2009;124:241–250. doi: 10.1542/peds.2008-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaynor JW, Gerdes M, Nord AS, Bernbaum J, Zackai E, Wernovsky G, et al. Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy? J Thorac Cardiovasc Surg. 2010;140:1230–1237. doi: 10.1016/j.jtcvs.2010.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]