Abstract
Responders to acute-phase cognitive therapy (A-CT) for major depressive disorder (MDD) often relapse or recur, but continuation-phase cognitive therapy (C-CT) or fluoxetine reduces risks for some patients. We tested composite moderators of C-CT versus fluoxetine’s preventive effects to inform continuation treatment selection. Responders to A-CT for MDD judged to be at higher risk for relapse due to unstable or partial remission (N=172) were randomized to 8 months of C-CT or fluoxetine with clinical management and assessed, free from protocol treatment, for 24 additional months. Pre-continuation-treatment characteristics that in survival analyses moderated treatments’ effects on relapse over 8 months of continuation-phase treatment (residual symptoms and negative temperament) and on relapse/recurrence over the full observation period’s 32 months (residual symptoms and age) were combined to estimate the potential advantage of C-CT versus fluoxetine for individual patients. Assigning patients to optimal continuation treatment (i.e., to C-CT or fluoxetine, depending on patients’ pre-continuation-treatment characteristics) resulted in absolute reduction of relapse or recurrence risk by 16–21% compared to the other non-optimal treatment. Although these novel results require replication before clinical application, selecting optimal continuation treatment (i.e., personalizing treatment) for higher risk A-CT responders may decrease risks of MDD relapse and recurrence substantively.
Keywords: major depressive disorder, cognitive therapy, fluoxetine, relapse, recurrence, personalized advantage index, personalized medicine
1. Introduction
The global health burden of major depressive disorder (MDD) is amplified by the disorder’s frequently chronic or recurrent course (Kessler et al., 2014). Acute-phase treatments, including cognitive therapy (A-CT) and pharmacotherapy, reduce depressive symptoms substantially, but major depressive relapses or recurrences are common (Cuijpers et al., 2013a, 2013b; Vittengl et al., 2007). After acute-phase treatment response, continuation-phase treatments, including cognitive therapy (C-CT) and pharmacotherapy, reduce patients’ absolute risk of relapse by an average of about 19–29% compared to inactive control groups (Biesheuvel-Leliefeld et al., 2015; Hansen et al., 2008; Vittengl et al., 2007). Which continuation-phase treatment is best for individual patients responding to A-CT is unclear, however, and requires knowledge of treatment moderators to realize the aim of personalized medicine. The current analyses aimed to develop and test an index of pre-continuation-phase treatment characteristics that moderated the effects of C-CT versus continuation-phase fluoxetine (FLX), drawing data from a randomized clinical trial (Jarrett et al., 2013).
Previous clinical trials comparing cognitive therapies to pharmacotherapies among patients in remission or recovery from MDD have revealed few moderators of the treatments’ effects on relapse or recurrence (e.g., Blackburn et al., 1986; Blackburn and Moore, 1997; Jarrett et al., 2000; Kuyken et al., 2008, 2015; Segal et al., 2010). However, detecting and using moderators to guide treatment selection is often difficult because many moderator effects are small (Kraemer, 2013). Consequently, researchers have proposed aggregating variables with weaker moderator effects into stronger composite moderators (DeRubeis et al., 2014; Kraemer, 2013).
For example, Wallace et al. (2013) analyzed data from a randomized clinical trial comparing acute-phase interpersonal psychotherapy versus pharmacotherapy for MDD. There was no main effect of treatment on a global outcome measure, but a composite of eight variables (empirically selected from among 32 candidate variables) with weak individual moderator effects on the outcome variable (|median| = 0.08 SD) showed a stronger combined moderator effect (0.31 SD). The aggregate moderator identified patients with better outcomes in acute-phase psychotherapy versus pharmacotherapy. Similarly, Smagula et al. (2016) applied this technique to inform medication augmentation among older adults with MDD.
In the same spirit, DeRubeis et al. (2014) introduced the “personalized advantage index” to forecast outcomes in acute-phase cognitive therapy versus pharmacotherapy for MDD. Among numerous candidate variables, nine variables that statistically significantly predicted or moderated treatment response in the full sample were combined via linear multiple regression models to estimate the advantage if individual patients had been assigned to the optimal versus non-optimal treatment. Optimal treatment was defined as the treatment forecast to yield the lowest depressive symptom score, and the other treatment was considered non-optimal. The optimal treatment varied based on patients’ pre-treatment characteristics that moderated treatment outcome. Optimal versus non-optimal treatment yielded a mean difference in depressive symptoms of 0.58 SD. Similarly, Huibers et al. (2015) applied the personalized advantage index to inform selection of acute-phase CT versus interpersonal psychotherapy for MDD, focusing on post-treatment symptom severity.
Here, we extended the personalized advantage index technology to continuation-phase CT (C-CT) versus FLX. Our purpose was to identify the better continuation-phase treatment for specific patients. Data were drawn from a two-phase clinical trial. In the acute phase, adults with recurrent MDD all received A-CT. Acute-phase responders judged to be at higher risk for relapse due to partial or unstable remission were randomized to 8 months of C-CT (n = 86), FLX (n = 86), or pill placebo with clinical management (n = 69) and followed 24 additional months (Jarrett et al., 2013). In survival analyses, relapse in the 8-month continuation phase was more frequent in pill placebo (32.7%) than in C-CT (18.3%) and FLX (18.0%), for which relapse did not differ significantly. Across the continuation-plus-follow-up phases (32 months), relapse/recurrence in C-CT (45.2%), FLX (41.1%), and pill placebo (56.3%) did not differ significantly (Jarrett et al., 2013). The pill placebo arm was not analyzed here because pill placebo is rarely used in routine clinical practice and yielded more relapse than C-CT or FLX.
The goals of the current analyses were (1) to identify sets of pre-continuation-treatment patient characteristics that significantly moderated the continuation treatments’ effects in multi-moderator models; and (2) to compute and test a personalized advantage index using these moderator variables, focusing on risk reductions in optimal versus non-optimal continuation-phase treatments. Outcome variables were major depressive relapse during the 8-month period of active continuation-phase treatment, plus relapse or recurrence over the full 32 months of observation. Optimal treatment was defined as the continuation-phase treatment (either C-CT or FLX) that yielded the lower expected probability of relapse or recurrence for individual patients. Thus, we aimed to support personalized treatment by providing empirical guidance for selection of continuation-phase treatment for adults with recurrent MDD who responded to A-CT with higher risk for relapse.
2. Method
Jarrett and Thase (2010) and Jarrett et al. (2013) described in detail the randomized clinical trial that provided the data analyzed here. The trial was reviewed by a Data Management and Safety Board as well as the Institutional Review Boards at each site annually. Here we summarize methods relevant to the current analyses.
2.1. Participants
Outpatients were recruited through clinical referrals and newspaper, bulletin board, and Internet announcements. Participants (a) provided written informed consent; (b) met criteria for recurrent MDD (American Psychiatric Association, 2000) on the Structured Clinical Interview for DSM-IV (First et al., 1996); (c) had a history of remission between depressive episodes, ≥ 1 depressive episode with complete inter-episode recovery, or antecedent dysthymic disorder; and (d) scored ≥ 14 on the 17-item Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960).1 Exclusion criteria were (a) severe or poorly controlled concurrent medical disorders that could cause depression, (b) psychotic or organic mental disorders, bipolar disorder, active substance dependence, or primary obsessive-compulsive or eating disorders, (c) inability to complete questionnaires in English, (d) active suicide risk, (e) <18 or >70 years old, (f) history of non-response to ≥ 8 weeks of CT or 6 weeks of fluoxetine, and (g) pregnancy, current or planned within 11 months post-intake.
Among 523 patients enrolled in A-CT, 292 responded (no major depressive episode and final HRSD ≤ 12). Among the responders, 241 had higher risk for relapse based on a priori criteria for unstable or partial remission (at least 1 of the last 7 acute-phase HRSD scores ≥ 7; Jarrett and Thase, 2010) and consented to randomization to C-CT (n = 86), FLX (n = 86), or pill placebo with clinical management (n = 69).2 Patients assigned to pill placebo are not analyzed here. Analyzed patients (n = 172) were M = 42.4 (SD = 11.6) years old with M = 15.8 (SD = 2.8) years of education; 69.8% women; 83.1% white, 9.3% black, and 7.6% other races/ethnicities. Participants’ mean age of MDD onset was 20.4 (SD = 10.1) years and, at intake to the acute phase, their current depressive episode had lasted M = 24.6 (median = 8.0; SD = 49.1) months. Including the episode present at study intake, participants had experienced a median of 4 (minimum 2) major depressive episodes. Over their lives, 49.4% of patients had been treated previously for depression with antidepressant medication and 40.7% with psychotherapy.
2.2. Acute phase
Patients were withdrawn from any psychotropic medications (including antidepressants and anxiolytics) before–and were not prescribed medications during–the acute-phase protocol. Acute-phase CT (Beck et al., 1979) aims to increase patients’ engagement with sources of reinforcement and to improve functioning; to identify and change patients’ maladaptive automatic thoughts; and to assess and restructure patients’ broader negative assumptions (i.e., schema) about the self, world, and future. Acute-phase CT lasted 12 weeks (with 2 additional weeks allowed for rescheduling) and included 16 or 20 sessions: Patients received 2 CT sessions per week for 4 weeks. Patients with ≥ 40% reduction in HRSD scores then received 8 weekly sessions (16 total), whereas patients with < 40% reduction in HRSD scores then received 8 twice-weekly plus 4 weekly sessions (20 total).3 Patients with less early symptom reduction received more sessions to increase their chances of response. The 16 cognitive therapists had completed at least 1 year of supervised training and maintained Cognitive Therapy Scale (Young and Beck, 1980) mean scores ≥ 40, demonstrating competence. Therapists submitted session videotapes for review, participated in weekly group supervision, and received feedback on their sessions’ strengths and weaknesses.
2.3. Continuation and follow-up phases
Building on A-CT, C-CT patients learn to apply compensatory skills to residual and emergent depressive symptoms, to restructure depressive assumptions, and to generalize CT skills across problems, time, and situations (Jarrett, 1989; Jarrett et al., 2008). The C-CT protocol included 4 biweekly followed by 6 monthly (10 total) sessions of approximately 60 minutes each. Patients’ acute- and continuation-phase therapists were the same, with a few exceptions (e.g., due to a therapist’s maternity leave).
Patients randomized to FLX or matching pill placebo received double-blinded clinical management (Fawcett et al., 1987) from experienced pharmacotherapists. Clinical management included supportive contact addressing signs and symptoms of depression, effects of medication, and information about depression. Pharmacotherapists were prohibited from using specific C-CT methods. The clinical-management protocol included one 45-minute and then nine 30-minute sessions on the same schedule as C-CT. Research pharmacies dispensed identical capsules with active fluoxetine or pill placebo. Patients received 10 mg/day for 2 weeks, then 20 mg/day for 2 weeks, and 40 mg/day thereafter. Pharmacotherapists were allowed to decrease doses to lessen patients’ side effects, but patients who could not tolerate at least 10mg/day were removed from medication and received only clinical management. Most patients (73%) achieved the target dose of 40mg/day of fluoxetine or pill placebo (Jarrett et al., 2013). All patients randomized to C-CT or FLX are included in the current analyses.
After the continuation phase, patients entered a 24-month follow-up without protocol treatment. In the continuation and follow-up phases, independent evaluators blinded to treatment-group membership assessed patients every 4 months. Patients were encouraged to contact study staff for interim evaluation if they experienced depressive symptoms. If they experienced a major depressive relapse or recurrence, patients were referred out for treatment.
2.4. Measures
2.4.1. Relapse and recurrence
Independent evaluators completed the Longitudinal Interval Follow-Up Evaluation (LIFE; Keller et al., 1987) every 4 months after A-CT, at study exit, and when patients, therapists, or follow-up evaluators suspected major depressive relapse or recurrence. This semi-structured retrospective interview yielded weekly psychiatric status ratings of DSM-IV MDD on a scale of 1 = no symptoms, 2 = one or two mild symptoms, 3 = obvious symptoms with moderate impairment, 4 = major symptoms and impairment but does not meet full MDD criteria, 5 = meets full MDD criteria, 6 = meets MDD criteria with severe impairment and/or psychosis. Ratings of 1–2 for ≥ 35 continuous weeks defined recovery. Ratings of 5–6 for ≥ 2 continuous weeks defined relapse and recurrence before and after, respectively, meeting criteria for recovery (Jarrett and Thase, 2010). Reliability for the LIFE’s many indices is generally > 0.70 (Keller et al. 1987), and MDD psychiatric status ratings showed high retest reliability in the current study (median lag-1 correlation = 0.87).
2.4.2. Residual symptoms
Patients completed the 21-item Beck Depression Inventory (BDI; Beck et al., 1961) and 30-item Inventory for Depressive Symptomatology self-report (IDS-SR; Rush et al., 1996), plus independent evaluators administered the 17-item HRSD after A-CT. Because these measures reflect the same depressive symptom construct during CT for MDD (Vittengl et al., 2013), we standardized the measures’ total scores based on their distributions (M and SD) at acute-phase intake and averaged them to form a robust residual symptom composite. Higher composite scores indicated more severe depressive symptomatology. Treating the three scales as items, the residual symptom composite showed high internal consistency (alpha = 0.95).
2.4.3. Personality
Patients completed the Schedule for Nonadaptive and Adaptive Personality-2nd Edition (SNAP-2; Clark et al., 2014) late in A-CT (week 12). The SNAP-2 is a factor analytically derived self-report inventory measuring 15 trait dimensions relevant to personality disorder with 390 true-false items. Scales analyzed here were negative temperament (high scorers experience a wide range of negative emotions and overreact to daily stresses and hassles; low scorers experience little distress and recover quickly from negative experiences), mistrust (high scorers have pervasively suspicious and cynical attitudes toward others; low scorers have trustful, even naïvely positive, attitudes toward others), and propriety (high scorers express preferences for traditional, conservative morality and “proper” conduct; low scorers reject social rules and conventions, with little concern for traditional “right” and “wrong”). The negative temperament (0.90), mistrust (0.87), and propriety (0.83) scales had high alpha internal consistency reliability in the current study.
2.5. Prior single-variable analyses of moderators
The current analyses focused on developing multi-moderator models supporting computation of the personalized advantage index. Candidates for the multi-moderator models were individual variables that demonstrated statistically significant moderator effects with at least modest effect sizes in prior single-variable analyses (Vittengl et al., 2015; see their online supplements for individual variables’ moderator effects). During the 8-month experimental phase, residual symptoms and lower propriety predicted relapse in C-CT but not in FLX. In contrast, greater negative temperament and mistrust predicted relapse in FLX but not in C-CT. Over 32 months, younger age predicted relapse/recurrence in FLX but not in C-CT. Finally, greater residual symptoms predicted relapse/recurrence in C-CT but not in FLX. Appendix 1 shows that these variables had moderator effect sizes (differences in standardized simple slopes between C-CT and FLX) stronger than 0.20, whereas other variables tested in prior analyses had weaker moderator effects.
2.6. Statistical analyses
In the current analyses, residual symptoms, propriety, negative temperament, and mistrust were tested in a multi-variable Cox regression survival (time-to-event) analysis of relapse over 8 months (the duration of active continuation treatment); and residual symptoms and age were tested in a multi-variable Cox regression analysis of relapse/recurrence over 32 months (active continuation treatment plus follow-up). The test variables were entered as both main effects and interactions with treatment. Variables with independent moderator effects significant at p < 0.05, two-tailed, were retained in the multi-variable models for use in computing the personalized advantage index (see Table 1). Treatment was coded C-CT = +0.5 and FLX = −0.5, and continuous variables were standardized (M = 0, SD = 1), before computing treatment interaction (moderator) terms to minimize collinearity between main effects and interactions.
Table 1.
Cox Regression Models of Relapse over 8 Months and Relapse/Recurrence over 32 Months
| Parameter | Estimate | SE | p |
|---|---|---|---|
| Relapse over 8 Months | |||
| Treatment | −0.071 | 0.509 | 0.889 |
| Residual symptoms | 0.232 | 0.213 | 0.276 |
| Negative temperament | 0.150 | 0.267 | 0.574 |
| Treatment × residual symptoms | 1.483 | 0.426 | 0.001 |
| Treatment × negative temperament | −1.345 | 0.515 | 0.009 |
| Relapse/recurrence over 32 Months | |||
| Treatment | 0.035 | 0.309 | 0.909 |
| Residual symptoms | 0.162 | 0.144 | 0.261 |
| Age | −0.201 | 0.143 | 0.161 |
| Treatment × residual symptoms | 0.834 | 0.292 | 0.004 |
| Treatment × age | 0.764 | 0.285 | 0.007 |
Note. N = 172. Treatment: Continuation cognitive therapy = +0.5, continuation fluoxetine plus clinical management = −0.5. Residual symptoms, negative temperament, and age variables have been standardized.
These intent-to-treat analyses used complete or multiply imputed data. Data for patient age, residual symptoms, relapse, and recurrence were complete. For personality, 12.8% of data were missing, likely due to patient assessment fatigue. We generated 10 data sets with missing values imputed via the Markov chain Monte Carlo method in PROC MI, computed standard analyses on each dataset, and pooled the results via PROC MIANALYZE in SAS software version 9.3 (SAS Institute, Inc., Cary, NC).
The final models shown in Table 1 were then rerun in a series of leave-one-out (i.e., jackknife) analyses, as suggested by DeRubeis et al. (2014). In each jackknife analysis, a different patient’s data were excluded. This process was repeated over 172 patients × 10 imputed datasets. The resulting jackknife regression parameters were then used to forecast whether each patient had been randomized to the “optimal” or “non-optimal” continuation treatment (C-CT or FLX). Thus, the risks predicted for each participant avoided potential bias attributable to including the same patient’s data during computation of the regression parameters. The treatment predicted to yield the lower relapse or recurrence hazard rate was “optimal” and the other treatment was “non-optimal.” Comparison of these predictions defined the “personalized advantage index” for each patient. The observed difference in relapse and recurrence between optimal and non-optimal assignment clarified the potential value of matching patients to continuation treatments and validated the personalized advantage index.
3. Results
3.1. Relapse during 8 months of continuation treatment
We selected moderators of the continuation-phase treatments’ effects on relapse over 8 months using Cox regression. This 8-month period defined the duration of the continuation-phase treatments. We entered residual symptoms plus trait propriety, negative temperament, and mistrust into the multi-variable model as main effects and interactions with treatment. Backward and forward selection procedures both identified residual depressive symptoms and negative temperament as variables with independent moderator effects significant at p < 0.05, two-tailed, whereas trait propriety and mistrust were excluded from the final model based on this criterion. The regression coefficients in the top portion of Table 1 indicate that patients with lower residual symptoms or higher negative temperament relapsed less in C-CT, whereas patients with higher residual symptoms or lower negative temperament relapsed less in FLX.
Using the variables in the top portion of Table 1, we next entered patients’ residual symptoms and negative temperament scores into a series of jackknife regression analyses to produce estimates of relapse risk in optimal versus non-optimal continuation treatment (i.e., either C-CT or FLX depending on each patient’s combination of residual symptoms and negative temperament scores). By randomization after higher risk response to A-CT, 90 patients received their optimal treatment and 82 received their non-optimal treatment. The reduction in relapse risk with optimal versus non-optimal treatment was significant, beta = −1.051, SE = 0.464, p = 0.024, hazard ratio = 0.349, in Cox regression analysis.4 Based on this analysis, 10.2% of patients relapsed during 8 months of optimal continuation treatment whereas 26.4% relapsed in non-optimal treatment, risk difference = 16.2%, number needed to treat = 6.2. Table 2 shows descriptive statistics for residual symptoms and negative temperament among patients optimally assigned to continuation treatments.
Table 2.
Descriptive Statistics for Patients Optimally Assigned to C-CT or FLX
| Assigned to C-CT | Assigned to FLX | |||
|---|---|---|---|---|
| Outcome: Moderator variables | M | SD | M | SD |
| Relapse over 8 months: | ||||
| Residual symptoms | −0.521 | 0.743 | 0.444 | 0.889 |
| Negative temperament | 0.420 | 0.999 | -0.466 | 0.936 |
| Relapse/recurrence over 32 months: | ||||
| Residual symptoms | −0.597 | 0.695 | 0.461 | 0.886 |
| Age | −0.521 | 0.759 | 0.679 | 0.708 |
Note. Continuation cognitive therapy (C-CT) and fluoxetine with clinical management (FLX) were provided for 8 months after higher-risk response to acute-phase CT. After continuation treatment, patients were followed 24 months, yielding 32 months of post-acute assessment. Residual symptoms, negative temperament, and age variables were standardized in the full sample.
3.2. Relapse/recurrence by 32 months post-randomization
Analyses of relapse/recurrence over 32 months paralleled analyses of relapse. The 32-month period included 8 months of active continuation treatment plus 24 months of follow-up. We entered residual symptoms and patient age into a Cox regression model of relapse/recurrence. Backward and forward selection procedures both identified residual symptoms and age as significant moderators in the multi-variable model. The regression coefficients in the bottom portion of Table 1 indicate that patients with lower residual symptoms and younger age relapsed/recurred less in C-CT, whereas patients with higher residual symptoms and older age relapsed/recurred less in FLX.
Using the variables in the bottom portion of Table 1, jackknife regression analyses produced estimates of relapse/recurrence risk in optimal versus non-optimal continuation treatment based on patients’ age and residual symptoms. By randomization after higher risk response to A-CT, 82 patients received their optimal treatment and 90 received their non-optimal treatment. The reduction in relapse/recurrence risk with optimal versus non-optimal treatment was significant, beta = −0.686, SE = 0.286, p = 0.016, hazard ratio = 0.503, in Cox regression analysis.5 Based on this analysis, 31.8% of patients relapsed/recurred during 8 months of optimal continuation treatment plus 24 months follow-up whereas 53.2% relapsed/recurred in non-optimal treatment and follow-up, risk difference = 21.4%, number needed to treat = 4.7. Figure 1 displays curves for time to relapse/recurrence with optimal and non-optimal treatment. Table 2 shows descriptive statistics for residual symptoms and age among patients optimally assigned to continuation treatments.
Figure 1.
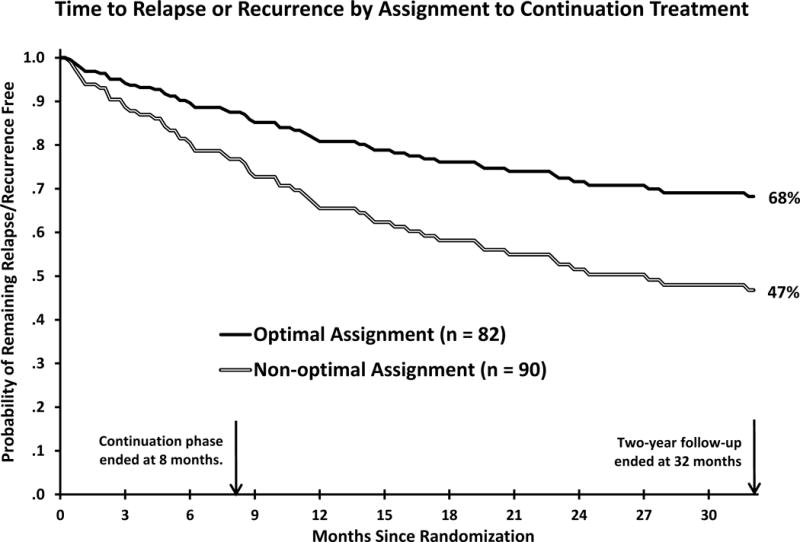
Among higher risk responders to acute phase cognitive therapy (N= 172), relapse or recurrence of major depressive disorder was less frequent with optimal versus non-optimal assignment to 8 months of continuation phase cognitive therapy or fluoxetine plus 24 months of follow-up.
3.3. Example: Computing a Personalized Advantage Index
Although these novel results require replication, their potential application can be illustrated. For example, the regression coefficients in the bottom portion of Table 1 could be used to estimate risk of relapse/recurrence within 32 months if a higher risk A-CT responder were choosing between C-CT or FLX. In particular, the composite moderator score (i.e., personality advantage index) would be computed as
where S and A are a patient’s standardized residual symptom intensity and age, respectively. (Standardized values were computed by subtracting the patient’s score from the sample mean and then dividing the difference by the sample SD; see Appendix 1 for sample means and SDs.) Resulting composite values > 0 favor FLX, whereas values < 0 values favor C-CT.
For example, suppose that a patient with recurrent MDD responded to A-CT with residual symptoms 1.0 SD below the current mean (−1.0; e.g., a score of about 4 on the HRSD) and age 0.2 SD above the current mean (+0.2; about 45 years old). If the sum of the treatment coefficient (0.035; see Table 1), the treatment × residual symptoms coefficient (0.834) multiplied by the individual patient’s standardized residual symptom score (-1.0), and the treatment × age coefficient (0.764) multiplied by the individual patient’s standardized age (0.2) is positive, then the patient would be better treated with FLX. But if the sum is negative, then the patient would be better treated with C-CT. In the current example,
so this patient would be better treated with C-CT. The extent to which treatment with C-CT (versus FLX) would be important for this particular patient can be expressed as the hazard ratio = e(−0.646) = 0 524 Thus, patients with this specific profile of residual symptoms and age would have an expected rate of relapse/recurrence with C-CT roughly one-half (0.524) that with FLX.
4. Discussion
“Personalized” or “precision” treatment for depression requires knowledge of reliable and replicable treatment moderators (Simon and Perlis, 2010; Wallace et al., 2013). In the current analyses, we computed a personalized advantage index (DeRubeis et al., 2014) comparing two continuation-phase treatments, C-CT versus FLX, provided to patients with recurrent MDD with higher risk (i.e., partial or unstable) remission during A-CT (Jarrett et al., 2013). The personalized advantage index combined individual moderator variables to estimate risk reduction with optimal selection of C-CT or FLX.
After A-CT, the personalized advantage index was computed from each patient’s residual symptoms plus either negative temperament (focusing on reducing relapse during active continuation treatment) or age (focusing on reducing relapse or recurrence over continuation treatment plus follow-up). Randomization to C-CT or FLX created a “natural experiment” by which some patients received their optimal treatment but others received their non-optimal treatment. Optimal versus non-optimal treatment reduced relapse by 16% over 8 months (the duration of continuation-phase treatment) and relapse/recurrence by 21% over 32 months (continuation-phase treatment plus 24 months of follow-up), with this larger risk reduction perhaps having greater importance for public health. These risk reductions are similar in size to the mean advantage of continuation treatments versus inactive control conditions for MDD (19–29%; Biesheuvel-Leliefeld et al., 2015; Hansen et al., 2008; Vittengl et al., 2007). Thus, optimally selecting among continuation treatments may be as important as the initial decision to continue or discontinue treatment after the acute phase.
The current clinical trial comparing C-CT with FLX among higher risk A-CT responders is unique to date, so our moderator findings are novel. However, they are broadly consistent with past research. For example, our finding that higher residual symptoms among A-CT responders predicted more relapse and recurrence among C-CT versus FLX patients might be interpreted in light of sequential-treatment models. Meta-analysis supports the value of acute-phase pharmacotherapy followed by C-CT for prevention of MDD relapse/recurrence (Guidi et al., 2016). A hypothesized strength of C-CT (compared to continuation-phase medication) is that C-CT can be tailored to individual patients’ specific residual symptoms, whereas a particular antidepressant medication (dose notwithstanding) is the same for each patient. Nonetheless, differences between C-CT and FLX following A-CT were nonsignificant in the current dataset (Jarrett et al., 2013). However, in the moderator analyses herein and among the responders stratified to higher risk with the most residual symptoms at randomization, patients treated with FLX had less relapse and recurrence over 32 months compared to those treated with C-CT. Thus, another hypothesis is that patients who show substantial residual symptoms after acute treatment in one modality might be better switched to treatment in a second modality. These interesting possibilities require empirical clarification to improve understanding of sequential treatments and their possible moderators.
Our findings of better outcomes (i.e., less relapse or recurrence) in C-CT for younger adults and for patients with higher negative temperament fit the theory and goals of C-CT (Jarrett, 1989; Jarrett et al., 2008). Younger patients (who often have an earlier age of MDD onset) and patients with higher negative temperament (neuroticism, and related trait dimensions such as mistrust) may have greater depressive illness liabilities (e.g., Hardeveld et al., 2013; Jarrett et al., 2001; Vittengl et al., 2010). Moreover, depression can be viewed as a part or consequence of a “general neurotic syndrome” that predicts poorer long-term functional and emotional outcomes (Tyrer et al., 2016). Continuation-phase CT aims to “neutralize” such liabilities by teaching and rehearsing the use of compensatory skills and preemptive coping with behavioral and cognitive risks for relapse. In contrast, FLX may dampen depressive symptoms via bottom-up neurochemical processes (Ma, 2015) and without the continued reinforcement of behavioral skills and cognitive shifts found in A-CT. Frequent assessment of A-CT responders’ use of CT skills during a trial of C-CT versus FLX would inform tests of the processes by which the continuation treatments may prevent relapse (Jarrett et al., 2011).
Several study design features limit the generalization and scope of the current findings. First, the extent to which our results generalize to different patient populations, treatment settings, and treatment protocols is unknown. For example, our results contrasting C-CT with FLX among adults with recurrent MDD who responded to A-CT with residual symptoms may not fit other patients and treatments. Second, generalization of our results to other measures is unclear. For example, the current longitudinal assessment of relapse and recurrence likely captures more depressive episodes than do cross-sectional methods (Patten, 2009), and so may relate differently to patient characteristics. Third, withdrawal from antidepressant medications, such as fluoxetine, can produce rebound or new depressive symptoms (Chouinard and Chouinard, 2015). Although there was no obvious change in the rate of relapse/recurrence soon after the 8-month continuation treatments ended in the current study (see Figure 1 and Jarrett et al., 2013), the impact of fluoxetine withdrawal, if any, for individual patients in the current sample is unknown. Finally, our moderator analyses identified for whom C-CT and FLX offered stronger relapse/recurrence prevention, but moderators are different from mediators or mechanisms that clarify why patients fare better in particular treatments (Kazdin, 2009).
Moderator effects often fail to replicate across typically powered studies of treatments for patients with MDD. Consequently, our results require replication and clarification before they are applied in routine clinical practice and thus are best thought of as “initial steps.” Replication might take place in an independent clinical trial, or perhaps more expediently and powerfully in meta-analyses of existing trials’ pooled individual patient data (cf. Cuijpers et al., 2014). If residual symptoms, negative temperament/neuroticism, and patient age generally moderate the preventive effects of C-CT versus pharmacotherapy for MDD, then work on clinical translation and mechanisms will be important next steps. For example, clinicians likely will require practical tools for choosing among continuation-phase treatments, including widely available assessment instruments and algorithms to forecast relapse and recurrence risk in each treatment. Long assessment instruments may require shortening and simplification to make them practical in busy health systems, and selection algorithms likely would become more usable if accompanied by publically available software. To compute and use a validated personalized advantage index, after independent replication, we recommend that clinicians input current data (e.g., residual symptoms measured after acute-phase treatment) and obtain patient consent for continuation-phase treatment at the time that this treatment decision needs to be made. Further, mechanisms might be clarified by testing relations of moderators with targets for change in continuation treatment (e.g., CT skills). In the meantime, the current results demonstrate the potential for personalizing selection of C-CT versus FLX for individual patients, as well as the importance of continued investigation of sequential treatments.
Highlights.
Responders to cognitive therapy for depression often relapse or recur.
Continuation cognitive therapy or fluoxetine reduce risk for some patients.
Using patient characteristics to select a continuation treatment is demonstrated.
Patient characteristics included residual symptoms, age, and negative temperament.
Selecting the optimal continuation treatment may reduce absolute risk by 16–21%.
Acknowledgments
We appreciate the careful review by members of the trial’s Data Safety and Monitoring Board. We are indebted to our research teams and our colleagues at The University of Texas Southwestern Medical Center, the University of Pittsburgh (where Dr. Thase was located during patient accrual), and the University of Pennsylvania (Dr. Thase’s current affiliation). We appreciate the participation of colleagues, previously named, and study participants without whom such research could not have been completed.
Funding
This report was supported by Grants Number K24 MH001571, R01 MH58397, R01 MH69619 (to Robin B. Jarrett, Ph.D.) and R01 MH58356 and R01 MH69618 (to Michael E. Thase, M.D.) from the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health.
Appendix 1. Descriptive Statistics and Individual-variable Moderator Effect Sizes of Treatment Outcomes
| Moderator Effect Size | |||||
|---|---|---|---|---|---|
| Variable | N | Mean/% | SD | Relapse | Relapse/Recurrence |
| Assessed at Intake to Acute-phase Cognitive Therapy | |||||
| Age in years | 172 | 42.37 | 11.61 | 0.12 | 0.26 |
| Female gender | 172 | 70% | −0.02 | 0.06 | |
| White ethnicity | 172 | 83% | −0.08 | −0.01 | |
| Education years | 172 | 15.85 | 2.82 | −0.06 | −0.11 |
| Age of MDD onset | 172 | 20.39 | 10.12 | 0.11 | 0.19 |
| Length (months) of current depressive episode | 172 | 24.58 | 49.09 | 0.09 | 0.04 |
| Number of depressive episodes | 172 | 4.13 | 1.68 | 0.09 | 0.09 |
| Family history of depressive illness | 172 | 68% | 0.03 | 0.08 | |
| Comorbid Axis I disorders | 172 | 42.37 | 0.80 | −0.05 | −0.13 |
| Assessed at Randomization to Continuation Phase Treatments | |||||
| Residual depressive symptoms | 172 | 17.63 | 7.38 | 0.27 | 0.29 |
| Social Adjustment Scale–Self-report | 155 | 1.90 | 0.37 | 0.12 | 0.09 |
| Inventory of Interpersonal Problems | 157 | 1.08 | 0.50 | −0.02 | 0.07 |
| Employed | 156 | 65% | 0.04 | 0.10 | |
| Dysfunctional Attitudes Scale | 171 | 112.40 | 30.84 | 0.03 | −0.02 |
| Beck Hopelessness Scale | 156 | 4.65 | 4.05 | 0.04 | 0.17 |
| Attributional Style Questionnaire–Global Failure | 156 | 0.12 | 1.11 | 0.08 | 0.05 |
| Attributional Style Questionnaire–Stable Failure | 156 | 0.17 | 0.94 | 0.02 | 0.04 |
| Self-control Scale | 157 | 22.52 | 23.33 | −0.08 | −0.16 |
| Work Alliance Inventory–Therapist report | 163 | 6.01 | 0.63 | 0.01 | 0.16 |
| Work Alliance Inventory–Patient report | 154 | 6.28 | 0.54 | −0.05 | 0.02 |
| Skills of Cognitive Therapy–Therapist report | 167 | 3.50 | 0.79 | −0.02 | 0.04 |
| Skills of Cognitive Therapy–Patient report | 159 | 3.68 | 0.68 | 0.02 | −0.02 |
| Schedule for Nonadaptive and Adaptive Personality | |||||
| Negative Temperament | 150 | 55.26 | 8.85 | −0.22 | −0.05 |
| Mistrust | 150 | 54.16 | 10.99 | −0.22 | −0.07 |
| Manipulativeness | 150 | 49.96 | 8.20 | −0.03 | −0.07 |
| Aggression | 150 | 50.93 | 9.52 | −0.20 | 0.03 |
| Self-harm | 150 | 59.56 | 12.30 | −0.18 | −0.11 |
| Low Self-esteem | 150 | 63.33 | 15.27 | −0.14 | −0.08 |
| Suicide Potential | 150 | 53.91 | 9.93 | −0.17 | −0.12 |
| Eccentric Perceptions | 150 | 46.02 | 7.19 | −0.07 | −0.05 |
| Dependency | 150 | 52.86 | 11.20 | −0.04 | −0.03 |
| Positive Temperament | 150 | 39.07 | 11.27 | 0.02 | −0.13 |
| Exhibitionism | 150 | 45.30 | 9.60 | −0.05 | −0.07 |
| Entitlement | 150 | 46.82 | 10.98 | −0.06 | −0.13 |
| Detachment | 150 | 55.66 | 10.81 | −0.12 | −0.11 |
| Disinhibition | 150 | 48.64 | 8.06 | −0.02 | 0.05 |
| Impulsivity | 150 | 49.47 | 9.69 | 0.13 | 0.13 |
| Propriety | 150 | 49.26 | 9.47 | −0.26 | −0.17 |
| Workaholism | 150 | 49.28 | 10.30 | −0.05 | −0.07 |
Note. Personality scale means are T-scores based on community norms. Residual depressive symptoms is a composite of the 17-item Hamilton Rating Scale for depression (M = 7.48, SD = 3.50, in the tabled sample; Hamilton, 1960), 30-item Beck Depression Inventory (M = 7.06, SD = 5.20, in the tabled sample; Beck et al., 1961), and 28-item Inventory for Depressive Symptomatology–Self-report (M = 11.51, SD = 7.04, in the tabled sample; Rush et al., 1996), based on the measures’ distributions in the full sample at acute-phase intake (Vittengl et al., 2013). Moderator effect size is the difference in the standardized simple slopes reported by Vittengl et al. (2015b) for continuation-phase cognitive therapy minus fluoxetine; effect size absolute values > 0.20 are bolded.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Due to a scoring error, 2 patients erroneously entered A-CT with HRSD = 13 at one of two diagnostic visits. During A-CT, one of these patients responded and one dropped out. As recommended by the Data Safety and Monitoring Board, the two patients are analyzed here as they were treated during data collection.
Three non-responders were randomized in error. As recommended by the Data Safety and Monitoring Board, they are analyzed here as they were treated during data collection.
Four (1 early and 3 late) responders were misclassified as late and early responders, respectively. As recommended by the Data Safety and Monitoring Board, they are analyzed here as they were treated during data collection.
Analysis of relapse over 8 months used multiply imputed values for 22 patients missing negative temperament data; no patients were missing residual symptoms or relapse data. Results without imputed data (N = 150) were very similar. Parallel to the results in Table 1, regression estimates were: treatment = −0.399 (SE = 0.600), residual symptoms = 0.331 (SE = 0.237), negative temperament = 0.145 (SE = 0.298), treatment × residual symptoms = 1.493 (SE = 0.475), and treatment × negative temperament = −1.860 (SE = 0.597). The comparison of relapse with optimal versus non-optimal treatment yielded beta = −1.000, SE = 0.483, p = 0.039, hazard ratio = 0.368, in Cox regression analysis.
Analysis of relapse/recurrence over 32 months did not use imputed data because no patients were missing residual symptoms, age, or relapse/recurrence data.
Declaration of Interest
Dr. Vittengl is a paid reviewer for UpToDate. Dr. Clark is author and copyright owner of the Schedule for Adaptive and Nonadaptive Personality, 2nd Edition (SNAP-2). Fees for commercial and funded, non-commercial usage licenses support her students’ research (unfunded, non-commercial research and clinical usage licenses are free of charge). Dr. Thase has no conflicts of interest pertaining to this paper, although he does report the following relationships with companies that develop treatment for depression or provide education pertaining to those treatments: During the past three years Dr. Thase has consulted with and/or served on advisory boards for Advir, Alkermes, Allergan, AstraZeneca, Avenir, Bristol-Myers Squibb Company, Cerecor, Cerenex, Eli Lilly and Company, Forest Laboratories, Janssen Pharmaceutica, Johnson & Johnson, Lundbeck, MedAvante, Merck, Moksha8, Naurex, Neuronetics, Novartis, Otsuka, Nestlé (formerly Pamlab), Pfizer Pharmaceuticals, Roche, Shire, Sunovion, Takeda, and Teva. During this time, he has received grant support from Alkermes, Assurerx, AstraZeneca, Avenir, Eli Lilly and Company, Forest Laboratories, Janssen/Johnson & Johnson, Otsuka, and Roche, as well as funding from the Agency for Healthcare Research and Quality and the NIMH. He has equity holdings for MedAvante, Inc. and has received royalties from American Psychiatric Publishing, Inc. (APPI), Guilford Publications, Herald House, and W.W. Norton & Company, Inc. Two books currently promoted by the APPI specifically pertain to cognitive therapy. Dr. Thase also discloses that his spouse is an employee of Peloton Advantage, which does business with several pharmaceutical companies that market medications used to treat depression. Dr. Jarrett is a paid consultant to the NIH, NIMH, and UpToDate. Her medical center charges fees for the cognitive therapy she provides to patients.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. fourth. American Psychiatric Association; Washington, DC: 2000. text rev. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. Guilford Press; New York: 1979. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Biesheuvel-Leliefeld KM, Kok GD, Bockting CH, Cuijpers P, Hollon SD, van Marwijk HJ, Smit F. Effectiveness of psychological interventions in preventing recurrence of depressive disorder: Meta-analysis and meta-regression. J Affect Disord. 2015;174:400–410. doi: 10.1016/j.jad.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Blackburn IM, Eunson KM, Bishop S. A two-year naturalistic follow-up of depressed patients treated with cognitive therapy, pharmacotherapy and a combination of both. J Affect Disord. 1986;10:67–75. doi: 10.1016/0165-0327(86)90050-9. [DOI] [PubMed] [Google Scholar]
- Blackburn I, Moore RG. Controlled acute and follow-up trial of cognitive therapy and pharmacotherapy in out-patients with recurrent depression. Br J Psychiatry. 1997;171:328–334. doi: 10.1192/bjp.171.4.328. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Chouinard V. New classification of selective serotonin reuptake inhibitor withdrawal. Psychother Psychosom. 2015;84:63–71. doi: 10.1159/000371865. [DOI] [PubMed] [Google Scholar]
- Clark LA, Simms LJ, Wu KD, Casillas A. Schedule for Nonadapative and Adaptive Personality–2nd Edition (SNAP-2): Manual for Administration, Scoring, and Interpretation. University of Notre Dame; Notre Dame, IN: 2014. [Google Scholar]
- Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry. 2013a;58:376–385. doi: 10.1177/070674371305800702. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, Andersson G. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013b;3(4) doi: 10.1136/bmjopen-2012-002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Weitz E, Twisk J, Kuehner C, Cristea I, David D, DeRubeis RJ, Dimidjian S, Dunlop BW, Faramarzi M, Hegerl U, Jarrett RB, Kennedy SH, Kheirkhah F, Mergl R, Miranda J, Mohr DC, Segal ZV, Siddique J, Simons AD, Vittengl JR, Hollon SD. Gender as predictor and moderator of outcome in cognitive behavior therapy and pharmacotherapy for adult depression: An ‘individual patient data’ meta-analysis. Depress Anxiety. 2014;31:941–951. doi: 10.1002/da.22328. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Cohen ZD, Forand NR, Fournier JC, Gelfand LA, Lorenzo-Luaces L. The Personalized Advantage Index: Translating research on prediction into individualized treatment recommendations. A demonstration PLoS One. 2014;9(1):e83875. doi: 10.1371/journal.pone.0083875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester S, Elkin I, Autry J. Clinical management–imipramine/placebo administration manual. NIMH Treatment of Depression Collaborative Research Program. Psychopharmacol Bull. 1987;23:309–324. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York State Psychiatric Institute, Biometrics Research Department; New York: 1996. [Google Scholar]
- Guidi J, Tomba E, Fava GA. The sequential integration of pharmacotherapy and psychotherapy in the treatment of major depressive disorder: A meta-analysis of the sequential model and a critical review of the literature. Am J Psychiatry. 2016;173:128–137. doi: 10.1176/appi.ajp.2015.15040476. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R, Gaynes B, Thieda P, Gartlehner G, Deveaugh-Geiss A, Krebs E, Lohr K. Meta-analysis of major depressive disorder relapse and recurrence with second-generation antidepressants. Psychiatr Serv. 2008;59:1121–1130. doi: 10.1176/appi.ps.59.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeveld F, Spijker J, De Graaf R, Nolen WA, Beekman AF. Recurrence of major depressive disorder and its predictors in the general population: Results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS) Psychol, Med. 2013;43:39–48. doi: 10.1017/S0033291712002395. [DOI] [PubMed] [Google Scholar]
- Huibers MH, Cohen ZD, Lemmens LM, Arntz A, Peeters FL, Cuijpers P, DeRubeis RJ. Predicting optimal outcomes in cognitive therapy or interpersonal psychotherapy for depressed Individuals using the personalized advantage index approach. Plos One. 2015;10(11):e0140771. doi: 10.1371/journal.pone.0140771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB. Cognitive therapy for recurrent unipolar major depressive disorder: The continuation/maintenance phase. 1989 Unpublished treatment manual. [Google Scholar]
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase. Arch Gen Psychiatry. 2001;58:381–388. doi: 10.1001/archpsyc.58.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Kraft D, Schaffer M, Witt-Browder A, Risser R, Atkins DH, Doyle J. Reducing relapse in depressed outpatients with atypical features: A pilot study. Psychother Psychosom. 2000;69:232–239. doi: 10.1159/000012401. [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, Thase ME. Preventing depressive relapse and recurrence in higher risk cognitive therapy responders: A randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatry. 2013;70:1152–1160. doi: 10.1001/jamapsychiatry.2013.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Vittengl JR, Clark LA, Thase ME. Quantifying and qualifying the preventive effects of acute-phase cognitive therapy: Pathways to personalizing care. J Consult Clin Psychol. 2015;84:365–376. doi: 10.1037/ccp0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl JR, Clark LA. Preventing recurrent depression. In: Whisman MA, editor. Adapting Cognitive Therapy for Depression: Managing Complexity and Comorbidity. Guilford Press; New York: 2008. pp. 132–156. [Google Scholar]
- Jarrett RB, Vittengl JR, Clark LA, Thase ME. Skills of Cognitive Therapy (SoCT): A new measure of patients’ comprehension and use. Psychol Assess. 2011;23:578–586. doi: 10.1037/a0022485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Thase ME. Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: design of a double-blinded, fluoxetine- and pill placebo-controlled, randomized trial with 2-year follow-up. Contemp Clin Trials. 2010;31:355–377. doi: 10.1016/j.cct.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE. Understanding how and why psychotherapy leads to change. Psychother Res. 2009;19:418–428. doi: 10.1080/10503300802448899. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kessler RC, de Jonge P, Shahly V, van Loo HM, Wang PS, Wilcox MA. Epidemiology of depression. In: Gotlib IH, Hammen CL, Gotlib IH, Hammen CL, editors. Handbook of Depression. third. Guilford; New York: 2014. pp. 7–24. [Google Scholar]
- Kraemer H. Discovering, comparing, and combining moderators of treatment on outcome after randomized clinical trials: a parametric approach. Stat Med. 2013;32:1964–1973. doi: 10.1002/sim.5734. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, Barrett B, Byng R, Evans A, Mullan E, Teasdale JD. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol. 2008;76:966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J, Causley A, Cowderoy S, Evans A, Gradinger F, Kaur S, Lanham P, Morant N, Richards J, Shah P, Sutton H, Vicary R, Weaver A, Wilks J, Williams M, Taylor RS, Byford S. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): A randomised controlled trial. Lancet. 2015;386:63–73. doi: 10.1016/S0140-6736(14)62222-4. [DOI] [PubMed] [Google Scholar]
- Ma Y. Neuropsychological mechanism underlying antidepressant effect: A systematic meta-analysis. Mol Psychiatry. 2015;20:311–319. doi: 10.1038/mp.2014.24. [DOI] [PubMed] [Google Scholar]
- Patten SB. Accumulation of major depressive episodes over time in a prospective study indicates that retrospectively assessed lifetime prevalence estimates are too low. BMC Psychiatry. 2009;9:19. doi: 10.1186/1471-244X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, Bloch R, Levitan RD. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67:1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Perlis RH. Personalized medicine for depression: Can we match patients with treatments? Am J Psychiatry. 2010;167:1445–1455. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Wallace ML, Anderson SJ, Karp JF, Lenze EJ, Mulsant BH, Butters MA, Blumberger DM, Diniz BS, Lotrich FE, Dew MA, Reynolds CI. Combining moderators to identify clinical profiles of patients who will, and will not, benefit from aripiprazole augmentation for treatment resistant late-life major depressive disorder. J Psychiatr Res. 2016;8:1112–118. doi: 10.1016/j.jpsychires.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrer P, Tyrer H, Guo B. The general neurotic syndrome: A re-evaluation. Psychother Psychosom. 2016;85:193–197. doi: 10.1159/000444196. [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: A comparative meta-analysis of cognitive-behavioral therapy’s effects. J Consult Clin Psychol. 2007;75:475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Jarrett RB. Moderators of continuation phase cognitive therapy’s effects on relapse, recurrence, remission, and recovery from depression. Behav Res Ther. 2010;48:449–458. doi: 10.1016/j.brat.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Nomothetic and idiographic symptom change trajectories in acute-phase cognitive therapy for recurrent depression. J Consult Clin Psychol. 2013;81:615–626. doi: 10.1037/a0032879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Stable remission and recovery after acute-phase cognitive therapy for recurrent major depressive disorder. J Consult Clin Psychol. 2014;82:1049–1059. doi: 10.1037/a0037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, Jarrett RB. Predictors of longitudinal outcomes after unstable response to acute-phase cognitive therapy for major depressive disorder. Psychotherapy. 2015;52:268–277. doi: 10.1037/pst0000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Frank E, Kraemer H. A novel approach for developing and interpreting treatment moderator profiles in randomized clinical trials. JAMA Psychiatry. 2013;70:1241–1247. doi: 10.1001/jamapsychiatry.2013.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Beck AT. Cognitive Therapy Scale: Rating Manual. Center for Cognitive Therapy; Philadelphia, PA: 1980. [Google Scholar]


