Abstract
Quantitative microanalysis of brains from patients with Alzheimer's disease (AD) find neuronal loss and neuroinflammation in structures that control cognitive function. Though historically difficult to recapitulate in experimental models, several groups have recently reported that by middle-age, transgenic mice that co-express high levels of two AD-associated mutations, amyloid-β protein precursor (AβPPswe) and presenilin 1 (PS1ΔE9), undergo significant AD-type neuron loss in sub-cortical nuclei with heavy catecholaminergic projections to the hippocampal formation. Here we report that by 13 months of age these dtg AβPPswe/PS1ΔE9 mice also show significant loss of pyramidal neuron in a critical region for learning and memory, the CA1 subregion of hippocampus, as a direct function of amyloid-β (Aβ) aggregation. We used these mice to test whether 17α-estradiol (17αE2), a less feminizing and non-carcinogenic enantiomer of 17β-estradiol, protects against this CA1 neuron loss. Female dtg AβPPswe/PS1ΔE9 mice were ovariectomized at 8–9 months of age and treated for 60 days with either 17αE2 or placebo via subcutaneous pellets. Computerized stereology revealed that 17αE2 ameliorated the loss of neurons in CA1 and reduced microglial activation in the hippocampus. These findings support the view that 17αE2, which may act through non-genomic mechanisms independent of traditional estrogen receptors, could prevent or delay the progression of AD in older men and women.
Keywords: Alzheimer's disease, amyloid-β, stereology, transgenic mice
INTRODUCTION
Alzheimer's disease (AD) is diagnosed at autopsy by the presence of gross and microscopic brain changes, including high densities of amyloid-β (Aβ)-containing plaques and neurofibrillary tangles in cortical tissue [1, 2], widespread neurogliosis [3, 4], marked cortical atrophy [5, 6], and severe neuron loss. Brain regions that show particularly severe neuron loss include pyramidal neurons in the CA1 subregion of hippocampus and noradrenergic neurons in the locus coeruleus (LC), two neuronal populations that mediate attention, learning, memory and other neural processes associated with cognitive function [1, 7–10]. The mechanisms that underlie this neuron loss are not understood, and there are no approved methods to slow this neurodegeneration.
Both estrogen and testosterone have been established as endogenous neuroprotective factors against AD-related neuropathology (for review, see [11]). The gonadal steroid 17β-estradiol (17βE2) mediates sexual differentiation of the developing brain [12, 13], improves spatial memory performance in middle-aged and aged female rodents [14–16], and provides neuroprotection against a range of age-related and experimental damage in adult brains [17–27]. Both receptor-dependent and receptor-independent mechanisms appear to mediate these effects [28–30]; however, in the past decade concerns about the safety of long-term 17βE2 treatment have raised serious questions about its future therapeutic potential [31–33].
A number of studies indicate that 17αE2, an enantiomer of 17βE2 with markedly lower feminizing and carcinogenic effects, could provide similar though potentially safer neuroprotective treatment against AD for aging men and women [22, 26, 27, 34–38]. Because of its low binding affinity to classical estrogen receptors, ERα and ERβ, and its inability to activate canonical genomic pathways at clinical doses, 17αE2 was long considered biologically inactive. Recent reports, however, indicate that 17αE2 stimulates rapid effects on a number of intracellular signaling cascades [22, 39] and exerts important neuroprotective effects in vitro [40–42]. The potential for protection against AD-type neuropathology, together with low feminization and carcinogenic side effects, suggests that 17αE2 offers a novel therapeutic option for prevention and management of AD, and possibly other neurodegenerative conditions. To date, few studies have investigated the potential of 17αE2 to protect against AD-type neuropathology using in vivo experimental models.
Several groups have reported middle-aged and older double transgenic (dtg) mice that overexpress two mutations related to familial AD, amyloid-β protein precursor (AβPPswe) and presenilin 1 (PS1ΔE9), undergo age-related neuron loss similar to that in AD. Using design-based stereological approaches, different groups have confirmed loss of tyrosine hydroxylase (TH) positive neurons in the LC, a region that provides heavy catecholarninergic projections to the hippocampal formation [43, 44], and alterations in synaptic numbers and size [45]. In the present study we report that at about 11 months of age (range 9–13 months), the brains of male and female dtg AβPPswe/PS1ΔE9 mice contain significantly fewer numbers of pyramidal neurons in the CA1 subregion of hippocampus. Furthermore, we report that treatment of female dtg AβPPswe/PS1ΔE9 mice with 60 days of 17αE2 via slow-release subcutaneous pellets prevents this loss of pyramidal CA1 neurons in hippocampus.
MATERIALS AND METHODS
Mice
Male and female dtg AβPPswe/PS1ΔE9 and non-transgenic littermate control (nou-Tg) mice were raised from birth in the vivarium at the Laboratory of Experimental Gerontology at the Gerontology Research Center (GRC, NIA/NIH, Baltimore, MD). These mice were raised from founders donated by Drs. David Borchelt and Michael Lee at the Johns Hopkins University School of Medicine that over-express AβPPswe and PS1ΔE9 mutations. Mice were group housed (n = 2–5) in plastic cages with corncob bedding with ad libitum access to food (NIH formula 07) and filtered water. Conditions within the vivarium were maintained at a 12:12 hour light:dark cycle and temperature of 22 ± 2°C. Animal husbandry and maintenance were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Study design
Effects of gender and transgene expression on CA1 neuron number
The initial phase of this study was designed to assess gender and transgene effects on total number of CA1 neurons in hippocampus. These experiments used a total of 24 mice, including 12 dtg AβPPswe/PS1ΔE9 mice (6 per gender, average age 13.5 ± 0.9 months) and 12 non-tg mice (6 per gender, average age 12.6 ± 0.9 months). All mice were sacrificed by anesthesia overdose and intracardial perfusion with aldehydes, with no significant age differences between groups, and brains were removed for histological processing and stereological quantification of the total number of pyramidal neurons in CA1, as detailed below. We have previously reported age-related increases and no gender differences in amyloid load or regional volume in hippocampus of both male and female dtg AβPPswe/PS1ΔE9 mice compared to age- and gender-matched non-tg controls [46–48]. Rather than repeat this analysis, in the present study amyloid load was analyzed only in one gender of dtg AβPPswe/PS1ΔE9 mice for the purpose of correlation analyses with loss of CA1 pyramidal neurons in these mice. The decision was made to analyze this correlation in female dtg AβPPswe/PS1ΔE9 mice because these mice were used to test the neuroprotective effects of 17αE2 on CA1 neuron loss, as detailed in the following section.
Neuroprotective effects of 17αE2 on CA1 neuron number
In the second phase of the study, female dtg AβPPswe/PS1ΔE9 mice were used to test whether 17αE2 protects against neuron loss in the CA1 sub-region of hippocampus. A total of 21 female mice aged 8–9 months were used in these studies, including 1) 16 female dtg AβPPswe/PS1ΔE9 mice; and 2) 5 age-matched female non-tg mice. Under anesthesia from a 100 mg/kg ketamine/10 mg/kg xylazine mixture, 16 female dtg AβPPswe/PS1ΔE9 mice underwent either bilateral ovariectomy (OVX) surgery (n = 10) or sham surgery (n = 6). The non-transgenic control mice did not receive any surgery. While under anesthesia, the 10 mice that received OVX were randomly assigned into two groups for implantation with dissolvable subcutaneous pellets (Innovative Research, Sarasota, F1) that deliver sustained release of either 1.5 mg 17αE2 (n = 5), or placebo (cholesterol, n = 5) over a 60-day period; the third group of dtg AβPPswe/PS1ΔE9 mice that received sham OVX surgery also underwent sham implantation surgery (see [21] for details of OVX, pellet implantation, and sham surgeries). All mice maintained normal consumption of food and water, with no weight differences or fluctuations during the 60-day treatment period. At 10–11 months of age, mice were sacrificed by anesthesia overdose and intracardial perfusion with 4% paraformaldehyde, and brains were removed for histological processing as detailed below. At the time of necropsy, the uterus from each mouse was removed and weighed to assess exposure to exogenous 17αE2. In addition, a group of 6 age-matched female dtg AβPPswe/PS1ΔE9 mice that underwent sham surgery for OVX+pellet implantation were included in the analysis of uterine weight. Because the uterine weights of the 17αE2-treated dtg AβPPswe/PS1ΔE9 mice were the same as those of the sham-treated dtg AβPPswe/PS1ΔE9 group, no further analysis, i.e., stereological studies, were carried on the brains from the sham surgery group.
Tissue preparation
On the day of sacrifice, mice were anesthetized with ketamine and killed by intracardial perfusion with 0.9% saline followed by 4% paraformaldehyde. Brains were removed, postfixed in 4% paraformaldehyde, and cryoprotected in a 30% sucrose solution before being stored at −80°C until sectioning. For studies using computerized stereology analysis, each brain was serially sectioned in the coronal plane from the frontal pole through the brainstem at a microtome setting of 40 μm. Serial sections were sub-sampled in a systematic-random manner to generate 8–10 sections through each entire reference space (CA1, CA2/3). That is, the first section through each reference space was selected at random, and each successive section selected in a systematic manner. For example, for a total of 80 sections through CA1, taking every 10th section, with a random start in the interval 1 to 10, would generate a systematic-random sample of 8 sections.
Histology
To visualize pyramidal neurons in CA regions, sampled sections were stained in a 0.1% solution of cresyl violet, rinsed, dehydrated through an ascending graded series of alcohol, cleared in xylene and coverslipped. Furthermore, two adjacent sets of systematic-random sampled sections through the hippocampus were processed to reveal microglia cells and Aβ. The first set was immunostained with antibodies to ionized calcium binding adaptor molecule 1 (Iba1). The Iba1 antibody is specifically expressed in macrophages/microglia and is upregulated during the activation of these cells, with no cross-reactivity to astrocytes or neurons (Chemicon International, Temecula, CA). The second set of systematic-random sections through the hippocampal formation was stained with Congo red to visualize amyloid deposits for determination of total amyloid volume (amyloid load), as detailed previously [43, 46]. The average section thickness after all tissue processing ranged from 14 to 17 μm.
Quantification of amyloid load and pyramidal cell numbers
With assistance from the Stereo Investigator system (MicroBrightField Inc., Williston, VT), design-based stereology was used to estimate total volume of amyloid plaques on Congo red sections (amyloid load) and mean total number of pyramidal cells in CA subregions. Estimates of amyloid load in molecular layers of the CA1 and CA2/3 regions of hippocampus were quantified using volume fraction and the Cavalieri-point counting method on Congo red-stained sections [43, 48]. Because previous studies have reported increased amyloid load in male dtg AβPPswe/PS1ΔE9 mice, studies of amyloid load in the present study were limited to the molecular layer in hippocampus of female dtg AβPPswe/PS1ΔE9 mice. The mean total numbers of pyramidal neurons in CA1 and CA2/3 regions of hippocampus were quantified using the optical fractionator method [49, 50], as previously reported [7, 21, 43, 44, 47, 51–54]. Briefly, the reference spaces on each sampled section were outlined under low power magnification (4X), and volume of Aβ and total number of cells quantified using a high resolution, oil immersion objective (60X, 1.4 numerical aperture). Deposits of Aβ appeared as pinkish red, while neurons were identified on the basis of a Nissl-stained neuronal phenotype, i.e., a dark violet blue nucleolus and a well-formed nuclear membrane, in CA subregions. Th avoid artifacts at the sectioning surface, e.g., lost caps, a guard volume was observed 2–3 μm above and below the disector where no cells were counted. Sampling of all parameters was continued to a mean coefficient of error (CE) of 0.05 to 0.10, according to Gundersen et al. [55]. All stereological parameters were quantified with assistance from a computerized stereology system by an operator blind to treatment and according to established principles, as detailed previously [21, 43, 47, 48, 51].
Statistical analysis
One-way ANOVA analyses and the Bonferroni/Dunn post hoc test were carried out to assess the effects of gender on total number of neurons in the CA1 region in male and female dtg AβPP/PS1 mice. Analysis of covariance (ANCOVA) was carried out with assistance from SAS statistical software (JMP, Cary, NC) to assess the influence of age at sacrifice (Age) on total number of CA1 neurons (Total n) in female mice for two independent variables: Transgene (wt vs. dtg AβPPswe/PS1ΔE9 mice + placebo); and Treatment (dtg AβPPswe/PS1ΔE9 mice + placebo vs. dtg AβPPswe/PS1ΔE9 mice +17αE2).
RESULTS
We investigated gender and transgene effects on neuron loss in hippocampal CA regions of 9 to 13 month-old male and female dtg AβPPswe/PS1ΔE9 mice and age- and gender-matched non-tg mice. Based on the finding of significantly fewer CA1 pyramidal neurons in female dtg AβPPswe/PS1ΔE9 mice compared to female non-tg controls, in a follow-up study we tested the hypothesis that this neuron loss in the CA regions of hippocampus could be mitigated by long-term (60 days) subcutaneous 17αE2 treatment of female dtg AβPPswe/PS1ΔE9 mice with bilateral OVX.
Gender and transgene effects on neuron number in hippocampal CA1 region
Loss of CA1 neurons and amyloid load
No gender differences were observed in total number of CA1 neurons for non-tg mice [male (n = 6) mean 80,781 (SEM 7758); female (n = 6) mean 84,173 (SEM 7651); p = 0.72]; or dtg AβPPswe/PS1ΔE9 mice [male mean 45,525 (SEM 4326), female mean 54,231 (SEM 4780) p = 0.20]. Comparison of data from non-tg and dtg AβPPswe/PS1ΔE9 mice revealed a significant 37% reduction in mean total number of CA1 neurons (F = 13.01; p < 0.01), as shown in Fig. 1.
Fig 1.
The total number of CA1 neurons in hippocampus of 9–13-month-old dtg AβPPswe/PS1ΔE9 mice (n = 12) was significantly lower than that in age- and gender-matched non-transgenic littermate controls (n = 12); * p < 0.01.
ANCOVA confirmed that the effect of Transgene and Age on Total n was due to a significant effect of Transgene (F = 9.22; p < 0.03) with no effect of Age (0.05; p < 0.83). Furthermore, there was a significant correlation between increasing amyloid load in hippocampal molecular layers of female dtg AβPPswe/PS1ΔE9 and the loss of CA1 pyramidal neurons (n = 6; R2 = 0.914, p < 0.01).
Stereological analysis showed no differences in the total numbers of pyramidal cells in the CA2/3 hippocampal subregion of dtg AβPPswe/PS1ΔE9 mice [mean 62,000 (SEM 3650) cells] compared to non-tg mice [76,500 (SEM 4650) cells; p = 0.14]. There was no correlation between CA2/3 pyramidal neuron number and Aβ load in molecular layers of the hippocampus (p = 0.88; data not shown).
Neuroprotective effect of 17αE2
Female dtg AβPPswe/PS1ΔE9 mice maintained normal weight and food/water consumption during the 60 days of treatment with 17αE2 and placebo. Uteri were removed and weighed blind to treatment history. As shown in Fig. 2, the two groups that did not receive OVX, i.e., non-tag or sham dtg AβPPswe/PS1ΔE9 mice, showed no significant differences in uterine weight; in contrast, uterine weights for placebo-treated dtg AβPPswe/PS1ΔE9 mice were significantly lower than that for the three other groups.
Fig. 2.
Weight values of uteri from OVX female dtg AβPPswe/PS1ΔE9 mice treated with 17αE2 or placebo, non-OVX dtg AβPPswe/PS1ΔE9 mice (sham), and non-tg mice. The weight of dtg uteri was significantly different among the treatment groups; n = 5–6/group; *p < 0.005.
Neuroprotective effects of 17αE2
There was a significant effect of 17αE2 on neuron number in the CA1 subregion of hippocampus of female dtg AβPPswe/PS1ΔE9 mice (F = 7.66;p < 0.02), as shown in Figs 3 and 4.
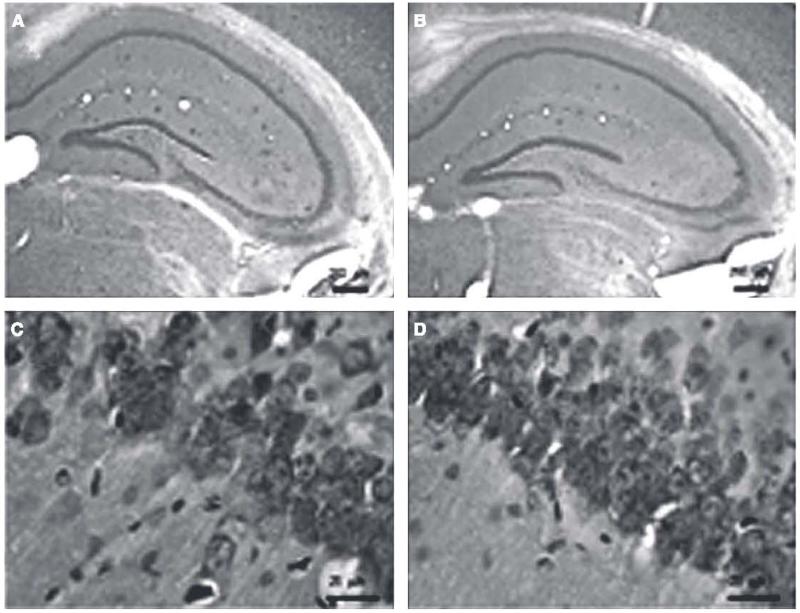
Fig. 3.
Low-magnification micrograph images of Congo red staining of Aβ plaques in the hippocampus region of dtg AβPPswe/PS1ΔE9 mice treated with either placebo (A) or 17αE2 (B). High-magnification images of pyramidal neurons stained with violet in the CA1 region of the hippocampus in placebo-treated (C) and 17αE2-treated (D) dtg AβPPswe/PS1ΔE9 mice.
Fig. 4.
Total number of pyramidal cells in the CA1 hippocampal layer in brains from non-tg mice and ovariectomied (OVX) female dtg AβPPswe/PS1ΔE9 mice sacrificed at about 11 months of age (9 to 13 months) following treatment with either 17αE2 or placebo. The number of CA1 pyramidal cells in dtg AβPPswe/PS1ΔE9 mice treated with placebo was significantly decreased compared to non-tg mice. The number of CA1 neurons in 17αE2-treated mice was significantly elevated (39%) compared to placebo treated animals, and not statistically diiferent from non-tg mice. n = 5/group; * p < 0.05.
The significant effect of Treatment (placebo or 17αE2) and Age on Total N in CA1 was due to a significant effect of Treatment (F = 13.8; p < 0.0075) and not Age (F = 0.126; p < 0.73), as shown by ANCOVA. The finding of no significant differences in the mean total number of CA1 neurons in the hippocampus of non-tg female mice compared to that in the 17αE2-treated female mice indicates that 17αE2 treatment prevented the loss of CA1 neurons observed in the placebo-treated female AβPPswe/PS1ΔE9 mice.
As shown in Fig. 3A and B, amyloid load in dtg AβPPswe/PS1ΔE9 mice treated with 17αE2 was reduced by over 25% compared to that in placebo-treated dtg AβPPswe/PS1ΔE9 mice [6965 (SEM 720) mm3 vs. 9770 (SEM 1525) mm3]; however, this effect did not reach statistical significance (p = 0.17; data not shown).
Figure 5 shows low and high magnification views of Iba1-positive microglia and presents results for the mean total number of Iba1-immunopositive microglia in hippocampus. The images at high magnification in the inset shows examples of a less activated phenotype, with more numerous immunoreactive processes on microglia following treatment with 17αE2 for 60 days. The mean total number of Iba1-positive microglia was significantly higher in the placebo-treated dtg AβPPswe/PS1ΔE9 mice compared to both non-tg and 17αE2-treated mice (p < 0.0001). There was no significant difference in number of Iba1-positive microglia between the non-tg and the 17αE2-treated mice. Thus, 60 days of 17αE2 treatment appeared to have attenuated microglial activation in the molecular layers of hippocampus of female dtg AβPPswe/PS1ΔE9 mice.
Fig. 5.
Images of Iba1-positive microglia in the hippocampus of non-tg mice (A), OVX female dtg AβPPswe/PS1ΔE9 mice treated with placebo (B) or 17αE2 (C). Upper panel shows lower magnification (4X) views of immunostained microglia in (60X). D) Mean total numbers of Iba1-positive microglia in hippocampal molecular layers of non-tg mice and OVX: female dtg AβPPswe/PS1ΔE9 mice sacrificed about 11 months of age following 60 days of treatment with 17αE2 or placebo. The mean total number of Iba1-positive microglia in placebo-treated mice was significantly elevated compared to both non-tg and 17αE2-treated mice. There was no statistical difference between non-tg and 17αE2-treated mice. n = 5/group; * p < 0.05.
DISCUSSION
Previous studies in single tg AβPP mice [52, 53, 60] and double transgenic APP670-671/PS1M146L mice that express relatively low levels of mutant Aβ, and especially low levels of the more fibrillar Aβ1–42 peptide, report relatively minor or no AD-type neuron loss in brain regions associated with cognitive function [61, 68]. Transgenic mice that express higher levels of the more fibrillar Aβ species following co-expression of two AβPP mutations (APP751 and APP670-671) and a PS1 knock-in reportedly show loss of pyramidal cells in CA1/2 but not in CA3 or dentate gyrus [69]. Other studies in mice that express five AD-associated mutations report qualitative evidence of reduced numbers of large pyramidal neurons in layer V of neocortex and subiculum at 9 months of age [70]. Mice expressing multiple AβPP mutations (APPK670N, M671L, APPV717I, under the mouse Thy1 promoter) and mutant PS1 (PS-1M146L under the pHMG promoter) show qualitative evidence only for neuronal loss in the hippocampal pyramidal layers [71].
Here we report ~37% loss of CA1 neurons in dtg AβPPswe/PS1ΔE9 mice sacrificed in the age range of 9–13 months. Correlational analysis in female dtg AβPPswe/PS1ΔE9 mice revealed a strong correlation between loss of pyramidal cells in CA1 and increasing amyloid load. In these mice, the prion promoter (PrP) is used to co-express a Swedish mutation of AβPPswe and PS1ΔE9 that leads to the progressive accumulation of mutant Aβ peptides, with a high percentage of the more fibrillar Aβ1–42 peptide beginning at age 4–5 months [62, 63]. By middle age, these dtg AβPPswe/PS1ΔE9 mice manifest a range of AD-like characteristics, including significant loss of catecholaminergic neurons in the LC, dorsal raphe, and ventral tegmental area [43, 44]; stability in the number of TH-positive neurons in substantia nigra [43]; alterations in numbers of synapses in the stratum radiata of the CA1 [44]; reduced branching of cerebral capillaries [64]; and abnormalities in anxiety behavior, spatial and episodic memory [44, 65–67]. These findings have strong relevance to AD because they provide a link between neuron loss in brain regions that project to limbic and associated cortical regions, which are especially vulnerable to amyloid deposition in humans. Thus, the identification of dtg AβPPswe/PS1ΔE9 mice with loss of pyramidal cells in the CA1 region, along with neuronal loss in LC [43, 44] and altered synaptic contacts in CA1 molecular layer [45], highlights a potentially powerful tool to test novel sttategies for the therapeutic management of older men and women with AD.
Based on the finding of significant loss of CA1 neurons in middle-aged dtg AβPPswe/PS1ΔE9 mice, we used these mice to assess the potential of 17αE2 to mitigate this neuronal loss, plaque burden and activation of microglia. These results build on the findings from our earlier studies using 17βE2 in female C57/B16 (B6) mice [21, 54]. In a previous study of female non-tg C57/B16 mice, we reported that age-related loss of circulating estrogen (estrapause) is associated with a significant elevation of microglia in hippocampal molecular layers [54]. To test the hypothesis that this microglia activation may be under the control of circulating 17βE2, we report that 60 days of exogenous 17βE2 treatment via subcutaneous pellets reduces the total number of microglia to a level that is comparable to that of young female mice. Our earlier studies used radioimmunoassay to confirm that the delivery system, i.e., slow-release subcutaneous pellets, does indeed maintain plasma 17βE2 at physiological levels for at least 60 days [21], as claimed by the manufacturer (Innovative Research, Sarasota, FL). A third finding from this earlier study [21] is that subcutaneous delivery of 1.5 mg of 17βE2 over 60 days is associated with minimal morbidity or mortality. In a previous study using this method of delivery in aged female C57BL/6 mice [21], analysis of plasma confirmed that physiological levels of 17βE2 equivalent to that in young female C57BL/6 mice were maintained throughout the treatment period. In the present study, mean uterine weight of 17αE2 treated mice was similar to that of non-OVX (sham-treated) mice, while the uteri weight of OVX mice treated with placebo was siguificantly lower than that for sham mice. This indirect, yet convincing evidence supports the view that slow-release pellets maintained physiological levels of circulating 17αE2 during the treatment period.
The few studies that have investigated the neuro-protective potential of 17βE2 to attenuate AD-type neuropathology in transgenic mouse models have reported conflicting results. Among the likely factors that contribute to these discrepancies is the type of AD-associated mutations expressed, the timing of expression, and the maguitude and type of different Aβ peptides deposited in cortical brain regions. No significant effects of 17βE2 were reported in PDAPP [56], APP670-671/PS1M146L [57], or 3xTg [58] mice, while other studies report significant reductions in amyloid burden in both single and double transgenic mice [36, 59] following 17βE2 treatment. In addition to differences in treatment regimens and choice of transgenic mice, the difficulty in assessing the protective effects of 17βE2 has been hampered by dependence on amyloid load as the primary or sole endpoint [56, 57, 60, 61].
We report that 60 days of high circulating levels of 17αE2, a less feminizing and non-carcinogenic analog of 17βE2, significantly reduced neuronal loss in CA1 in dtg AβPPswe/PS1ΔE9 mice. The present findings support the view that 17αE2 may be at least as neuroprotective as 17βE2, in agreement with the relatively few in vivo studies carried out on 17αE2 [34]. Among those studies is a semi-quantitative study of hippocampal spine densities in rats that indicates 17αE2 stimulates synapse formation on CA1 spines to a greater extent than 17βE2, paralleling previous data on the effects of these two steroids on spatial memory [72]. Importantly, studies that use a single-mutation transgenic mouse model of AD (AβPPswe) demonstrated that 17αE2 is equally or more effective than 17βE2 in altering AβPP processing towards non-amyloidogenic, α-secretase products, leading to a reduction in cerebral Aβ levels as measured by ELISA [36]. In support of these neuroprotective effects in vivo, 17αE2 significantly attenuates the GABAA receptor-induced reduction in hippocampal volume and impaired spatial memory in a neonatal model of early brain injury [24]. As reported by previous studies with 17βE2 [56, 57], we did not find a robust treatment effect on amyloid load. Future studies will be carried out to determine if 17αE2 has a weaker effect on amyloid burden that may have been masked by the limited statistical power or age-range of the mice in this study.
Like 17βE2, 17αE2 appears to protect against a diverse array of lethal and etiologically relevant stressors, including amyloid toxicity, serum withdrawal, oxidative stress, excitotoxicity, and mitochondrial inhibition, among others [73] (for review, see [74]). Unlike 17βE2, however, 17αE2 does not stimulate proliferation of ER-positive tumors.
Hamsters treated with 17βE2 or 17αE2 for seven months showed widespread growth and proliferation of renal tumors after 17βE2 treatment, while hamsters treated with αE2 showed no tumors [75]. Although 17βE2 and 17αE2 share a close structural similarity and some of the same effects on tissue, the fact that these analogs act through different mechanisms and at different cellular locations stresses the need to focus less on the differences between 17βE2 and 17αE2, and rather on the potential of either epimer to provide a safe and effective treatment for middle-aged men and women against the onset and progression of AD.
In addition to protection against neuron loss, 17αE2 treatment appears to have attenuated microglial activation, as evidenced by both quantitative and qualitative endpoints. In female C57BL/6 mice, normal brain aging is associated with activation of neuroglia, possibly due to the loss of estrogen activity during estrapause [25, 51, 54]. Treatment of adult B6 mice with 17βE2 significantly diminishes this age-related activation of neuroglia [21, 51], and reduces neuronal loss through apoptosis [44]. Moreover, the formation of amyloid plaques in AD and dtg AβPPswe/PS1ΔE9 mice are closely associated with activated microglia [3, 76–78]. In response to this deposition of Aβ peptides in brain parenchyma, the activation of microglia leads to the release of pro-inflammatory cytokines [3, 79, 80]. Through a mechanism that is independent of actions on classical ERα and ERβ, 17αE2 inhibits microglia-mediated inflammatory mechanisms in-vivo, which in turn may block the degenerative processes leading to neuronal loss in middle-aged dtg AβPPswe/PS1ΔE9 mice, and perhaps older humans. Understanding these mechanisms and the steps necessary to block them in the brains of middle-aged humans is a critical area for further study. The possibility that 17αE2 may be as neuroprotective as 17βE2, but with over 200-fold less hormonal activity, encourages the development of other E2 analogs with low risk and more potent protection against the onset and progression of AD.
ACKNOWLEDGMENTS
The authors wish to acknowledge support from NIH/NINDS; Grant number: U54 NS42867, R01 AG026478 (RST), the Public Health Service (NIH extramural grants MH076541-02A1, NS039407-06A1), Drs. David R. Borchelt and Michael K. Lee for donation of founders for this study, and the NIA Intramural Program in Baltimore, Maryland. We would also like to acknowledge Dr. John Kwagyan for his assistance with the statistical analysis.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=655).
REFERENCES
- 1.Price JL. Diagnostic criteria for Alzheimer's disease. Neurobiol Aging. 1997;18:S67–S70. doi: 10.1016/s0197-4580(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 2.Berg L, McKeel DW, Jr., Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 3.Meda L, Cassatella MA, Szendrei GI, Otvos L, Jr., Baron P, Villalba M, Ferrari D, Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 4.Xiang Z, Haroutunian V, Ho L, Purohit D, Pasinetti GM. Microglia activation in the brain as inflammatory biomarker of Alzheimer's disease neuropathology and clinical dementia. Dis Markers. 2006;22:95–102. doi: 10.1155/2006/276239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouton PR, Martin U, Calhoun ME, Dal Porno G, Price DL. Cognitive decline strongly correlates with cortical atrophy in Alzheimer's dementia. Neurobiol Aging. 1998;19:371–377. doi: 10.1016/s0197-4580(98)00080-3. [DOI] [PubMed] [Google Scholar]
- 6.McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Jr., Holland D, Koyama A, Brewer JB, Dale AM. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73:457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 8.Giannakopoulrjs P, Kovari E, Gold G, vonGunten A, Hof PR, Bouras C. Pathological substrates of cognitive decline in Alzheimer's disease. Front Neurol Neurosci. 2009;24:20–29. doi: 10.1159/000197881. [DOI] [PubMed] [Google Scholar]
- 9.Manaye KF, McIntire DD, Mann DM, German DC. Locus coeruleus cell loss in the aging human brain: a non-random process. J Comp Neurol. 1995;358:79–87. doi: 10.1002/cne.903580105. [DOI] [PubMed] [Google Scholar]
- 10.Busch C, Bohl J, Ohm TG. Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol Aging. 1997;18:401–406. doi: 10.1016/s0197-4580(97)00035-3. [DOI] [PubMed] [Google Scholar]
- 11.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinal. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 13.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Harm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- 15.Foy MR, Baudry M, Diaz Brinton R, Thompson RF. Estrogen and hippocampal plasticity in rodent models. J Alzheimers Dis. 2008;15:589–603. doi: 10.3233/jad-2008-15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horn Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behl C, Widmann M, Trapp T, Holsboer F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 18.Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- 19.Harms C, Lautenschlager M, Bergk A, Katchanov J, Freyer D, Kapinya K, Herwig U, Megow D, Dirnagl U, Weber JR, Hortnagl H. Differential mechanisms of neuroprotection by 17 beta-estradiol in apoptotic versus necrotic ncurodcgcncration. J Neurosci. 2001;21:2600–2609. doi: 10.1523/JNEUROSCI.21-08-02600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Gastard M, Verina T, Bora S, Mouton PR, Koliatsos VE. Estrogens modulate experimentally induced apoptosis of granule cells in the adult hippocampus. J Comp Neurol. 2001;441:1–8. doi: 10.1002/cne.1393. [DOI] [PubMed] [Google Scholar]
- 21.Lei DL, Long JM, Hengemihle J, O'Neill J, Manaye KF, Ingram DK, Mouton PR. Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice. Neuroscience. 2003;121:659–666. doi: 10.1016/s0306-4522(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 22.Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17alpha-estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–3850. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- 23.Dubai DB, Ran SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubai SB, Merchanthaler L Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- 24.McClean J, Nunez JL. 17alpha-Estradiol is neuroprotective in male and female rats in a model of early brain injury. Exp Neurol. 2008;210:4–1-50. doi: 10.1016/j.expneurol.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- 26.Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res Rev. 2008;57:421–430. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Singh M, Sumien N, Ky ser C, Simpkins JW. Estrogens and progesterone as ncuroprotcctants: what animal models teach us. Front Biosci. 2008;13:1083–1089. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Rrieglstein J. Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab. 1999;19:1263–1269. doi: 10.1097/00004647-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Linford N, Wade C, Dorsa D. The rapid effects of estrogen are implicated in estrogen-mediated neuroprotection. J Neurocytol. 2000;29:367–374. doi: 10.1023/a:1007113323582. [DOI] [PubMed] [Google Scholar]
- 30.Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova AD, Perez EJ, Liu R, Simpkins JW. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc Natl Acad Sci USA. 2003;100:11741–11746. doi: 10.1073/pnas.2032621100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shumaker SA, Legault C, Rapp SR, Thai L, Wallace RB, Ockene JK, Hcndrk SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 32.Anderson GL, Limachcr M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, BrzysM R, Caan B, ChlebowsM R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:17011712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 33.Shumaker SA, Legault C, Kuller L, Rapp SR, Thai L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive Impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 34.Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;1800:1056–1067. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin-Allerhand JA, Lominska CE, Wang J, Smith JD. 17Alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPS WE transgenic mice. J Alzheimers Dig. 2002;4:449–457. doi: 10.3233/jad-2002-4601. [DOI] [PubMed] [Google Scholar]
- 37.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prokai L, Simpkins JW. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther. 2007;114:1–12. doi: 10.1016/j.pharmthera.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh M, Setalo G, Jr., Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neuratrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green PS, Bishop J, Simpkins JW. 17 alpha-estradiol exerts neuroprotective effects on SK-N-SH cells. J Neurosci. 1997;17:511–515. doi: 10.1523/JNEUROSCI.17-02-00511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L, O'Neill K, Brinton RD. Estrogenic agonist activity of ICI 182, 780 (Faslodex) in hippocampal neurons: implications for basic science understanding of estrogen signaling and development of estrogen modulators with a dual therapeutic profile. J Pharmacol Exp Ther. 2006;319:1124–1132. doi: 10.1124/jpet.106.109504. [DOI] [PubMed] [Google Scholar]
- 43.O'Neil JN, Mouton PR, Tizabi Y, Ottinger MA, Lei DL, Ingram DK, Manaye KF. Catecholammergic neuronal loss in locus coeruleus of aged female dtg APP/PS1 mice. J Chem Neuroanat. 2007;34:102–107. doi: 10.1016/j.jchemneu.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Yoo MJ, Savonenko A, Stirling W, Price DL, Borchelt DR, Mamounas L, Lyons WE, Blue ME, Lee MK. Amyloid pathology is associated with progressive monoamin-ergic ncurodegeneration in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2008;28:13805–13814. doi: 10.1523/JNEUROSCI.4218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West MJ, Bach G, Soderman A, Jensen JL. Synaptic contact number and size in stratum radiatum CA1 of APP/PSlDeltaE9 transgenic mice. Neurobiol Aging. 2009;30(11):1756–1776. doi: 10.1016/j.neurobiolaging.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Edossa A, Kalifa S, Polston EK, Mouton PR, Manaye KF. AD-type degeneration of pyramidal cells in hippocampus of dtg APP/PS1 mice. Soc Neurosci Abstr. 2008 Program No. 638. 12. [Google Scholar]
- 47.Manaye KF, Wang PC, O'Neil JN, Huang SY, Xu T, Lei DL, Tizabi Y, Ottinger MA, Ingram DK, Mouton PR. Neu-ropathological quantification of dtg APP/PS1: neuroimaging, stereology, and biochemistry. Age (Dordr) 2007;29:87–96. doi: 10.1007/s11357-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates cortical amyloidosis in a double transgenic mouse model of Alzheimer's disease. Neurosci Lett. 2009;464:184–187. doi: 10.1016/j.neulet.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West MJ, Slomianka L, Gundersen HI. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical frac-tionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 50.Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- 51.Mouton PR. Principles and Practices of Unbiased Stereology: An Introduction For Bioscientists. The Johns Hopkins University Press; Baltimore, Maryland: [Google Scholar]
- 52.Calhoun ME, Wiederhold KH, Abramowaki D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- 53.Phinney AL, Calhoun ME, Wolfer DP, Lipp HP, Zheng H, Jucker M. No hippocampal neuron or synaptic bouton loss in learning-impaired aged beta-amyloid precursor protein-null mice. Neuroscience. 1999;90:1207–1216. doi: 10.1016/s0306-4522(98)00645-9. [DOI] [PubMed] [Google Scholar]
- 54.Mouton PR, Long JM, Lei D-L, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte number in brains of mice. Brain Res. 2002;956:30–35. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- 55.Gundersen HJ, Jensen EB, Kien K, Nielsen J. The efficiency of systematic sampling in stereology – reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 56.Green PS, Bales K, Paul S, Bu G. Estrogen therapy fails to alter amyloid deposition in the PDAFF model of Alzheimer's disease. Endocrinology. 2005;146:2774–2781. doi: 10.1210/en.2004-1433. [DOI] [PubMed] [Google Scholar]
- 57.Heikkinen T, Kalesnykas G, Rissanen A, Tapiola T, Iivonen S, Wang J, Chandhuri J, Tanila H, Miettinen R, Puolivali J. Estrogen treatment improves spatial learning in APP + PS1 mice but does not affect beta amyloid accumulation and plaque formation. Exp Neurol. 2004;187:105–117. doi: 10.1016/j.expneurol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 58.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu H, Wang R, Zhang YW, Zhang X. Estrogen, beta-amyloid metaboUsm/tramcking, and Alzheimer's disease. Ann N YAcad Set. 2006;1089:324–342. doi: 10.1196/annals.1386.036. [DOI] [PubMed] [Google Scholar]
- 60.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Kurt MA, Davies DC, Kidd M, Duff K, Rolph SC, Jennings KH, Howlett DR. Neurodegenerative changes associated with beta-amyloid deposition in the brains of mice carrying mutant amyloid precursor protein and mutant presenilin-1 transgenes. Exp Neurol. 2001;171:59–71. doi: 10.1006/exnr.2001.7717. [DOI] [PubMed] [Google Scholar]
- 62.Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked piesenilin 1 variants elevate Abetal-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 63.Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 64.Lee GD, Aruna JH, Barrett PM, Lei DL, Ingram DK, Mouton PR. Stereological analysis of microvascular parameters in a double transgenic model of Alzheimer's disease. Brain Res Bull. 2005;65:317–322. doi: 10.1016/j.brainresbull.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 65.Lalonde R, Kim HD, Maxwell JA, Fukuchi K. Exploratory activity and spatial learning in 12-month-old APP(695)SWE/co+PS l/Dc1taE9 mice with amyloid plaques. Neurosci Lett. 2005;390:87–92. doi: 10.1016/j.neulet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 66.Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MP, Price DL, Tang F, Markowska AL, Borchelt DR. Episodic-like memory deficits in the APPswe/PSldE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 67.Reiserer RS, Harrison FE, Syverud DC, McDonald MP. Impaired spatial learning in the APPSwe + PSENlDeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 2007;6:54–65. doi: 10.1111/j.1601-183X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 68.Gordon MN, Holcomb LA, Jantzen FT, DiCarlo G, Wilcock D, Boyett KW, Connor K, Mclachrino J, O'Callaghan JP, Morgan D. Time course of the development of Alzheimer-like pathology in the doubly transgenic PS1+APP mouse. Exp Neurol. 2002;173:183–195. doi: 10.1006/exnr.2001.7754. [DOI] [PubMed] [Google Scholar]
- 69.Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Viagtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004;165:1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitz C, Rutten BP, Pielen A, Schafer S, Wirths O, Tremp G, Czech C, Blanchard V, Multhaup G, Rezaie P, Korr H, Steinbusch HW, Pradier L, Bayer TA. Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer's disease. Am J Pathol. 2004;164:1495–1502. doi: 10.1016/S0002-9440(10)63235-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 73.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 74.Dykens JA, Moos WH, Howell N. Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann N Y Acad Sci. 2005;1052:116–135. doi: 10.1196/annals.1347.008. [DOI] [PubMed] [Google Scholar]
- 75.Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem. 2000;75:1447–1454. doi: 10.1046/j.1471-4159.2000.0751447.x. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, Garcia-Segura LM. Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury. Brain Res. 1993;628:271–278. doi: 10.1016/0006-8993(93)90964-o. [DOI] [PubMed] [Google Scholar]
- 77.Fattori E, Lazzaro D, Musiani P, Modcsti A, Alonzi T, Ciliberto G. IL-6 expression in neurons of transgenic mice causes reactive astrocytosis and increase in ramified microglial cells but no neuronal damage. Eur J Neurosci. 1995;7:2441–2449. doi: 10.1111/j.1460-9568.1995.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 78.McGeer EG, McGeer PL. Chronic inflammation in Alzheimer's disease offers therapeutic oppcrtunities. Expert Rev Neurother. 2001;1:53–60. doi: 10.1586/14737175.1.1.53. [DOI] [PubMed] [Google Scholar]
- 79.Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer's disease bela-peplide. J Exp Med. 1998;188:431–438. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frautschy SA, Yang F, Iirizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]