Abstract
The etiology of a number of retinopathies, such as acute zonal occult outer retinopathy (AZOOR), remains undetermined. Candida famata was isolated from conjunctival exudates of a patient diagnosed with AZOOR. This yeast was very abundant, particularly in the more affected eye, while no other pathogens or fungal species were in evidence. Immunological tests revealed the presence of antigen-specific T lymphocytes by using C. famata as a challenge. Moreover, enzyme-linked immunosorbent assay analysis showed the presence of specific antibodies against this yeast in the patient's blood. Delayed hypersensitivity by use of a skin test was also positive. Finally, antifungal treatments led to improvements in several clinical symptoms, including funduscopic analysis. However, despite prolonged treatment with fluconazole and itraconazole, C. famata still appeared in the conjunctival exudates. The new antifungal voriconazole may represent a better choice for treatment.
Yeasts can cause a number of diseases ranging from localized mild infections to deep-seated candidiasis (8, 34). In fact, candidiasis is the most common fungal infection in humans. About 12 to 14 Candida species have been implicated in human infections, with Candida albicans being the most prevalent among the yeast isolates (26, 36). Predisposing factors for candidiasis include prolonged antibiotic treatment, the presence of an indwelling intravenous catheter, intravenous drug use, and severe immunosuppression (2, 7). Different Candida species are part of the natural microbiota and, thus, are commensal organisms in humans (23). This is the case for Candida famata (also known as Debaryomyces hansenii and Torulopsis candida), which is usually found in some foods, including dairy products (3, 11). It was thought that C. famata was nonpathogenic for humans. However, a number of clinical cases in which this yeast was isolated have been reported (25, 46), including ocular endophthalmitis (40) and central nervous system (CNS) infection (39). In addition, different surveys of fungemia in humans have revealed that C. famata is responsible for about 0.2 to 2% of the total cases (1, 24, 35).
Systemic mycoses represent a health threat for at least two main reasons. The first is the relatively limited number of antifungal agents available for systemic use (4). Intravenous amphotericin B does not reach the nervous system well, while some yeast species are resistant to antifungals, such as itraconazole and fluconazole (33, 45, 52). Other oral compounds, such as nystatin and miconazole, are poorly absorbed by the intestinal tract. New antifungals, such as voriconazole and posaconazole, which exhibit greater efficacy against Candida spp., would be more effective than fluconazole or itraconazole against systemic candidiasis (15, 31, 35, 41, 42). The second threat results from the failure to promptly detect a number of fungal species different from the classical fungal species C. albicans, Aspergillus, etc. An example of this is given in the present report, which describes an immunocompetent, otherwise healthy male afflicted by a retinopathy of an as yet unknown etiology. The present findings indicate that this patient was infected with C. famata.
A number of retinopathies present as white dots or whitish material of still undefined composition in the retina (5, 27). The typical retinopathy that belongs to this group is the multiple evanescent white dot syndrome (MEWDS) (22, 30, 48, 50). Closely related to MEWDS is acute zonal occult outer retinopathy (AZOOR) (12, 16, 20, 28, 44). This disease is characterized by a rapid loss of the visual field in the presence of an almost normal funduscopic analysis (14). Both diseases have also been related to other retina dysfunctions, such as multifoccal choroiditis, punctate inner choroidopathy, acute macular retinopathy, and acute idiopathic blind spot enlargement (5, 6, 21, 49, 51). Some of these diseases have been associated with Histoplasma infection (9). The etiologies of all these retinopathies remain undetermined thus far.
CASE REPORT
A Caucasian male (age, 47 years) was diagnosed with AZOOR in 1996. Briefly, the patient started to have vision problems in 1994, characterized by a rapid loss of peripheral vision in the left eye that progressed to both eyes in the next few years. In 1996 funduscopic analysis was almost normal, while the electroretinogram presented clear pathological signs. Extraocular and intraocular motilities and anterior chamber and intraocular pressures were in the normal ranges. As the disease progressed, in 1998 the fluorescein angiogram became more hyperfluoresceinemic, which is also a characteristic of AZOOR in some patients. Besides, the indocyanine angiogram showed a mild captation of the dye around the peripapillary region, particularly in the later stages of this angiography.
Apart from these findings, the patient was healthy and the analytical blood test results, as well as the roentgenograms and nuclear magnetic resonance images, were normal. Although during the early years of the description of AZOOR it was thought to be an immune dysfunction, the different immunological parameters for the patient were within normal limits in 1996. The values obtained for C-reactive protein, rheumatoid factor, antibodies against nuclear antigens or against native DNA, soluble interleukin-2 receptor, antibodies against cardiolipin, the hemolytic activity of complement (C3 and C4), and angiotensin-converting enzyme were normal. The proportions of the different lymphocytic populations in peripheral blood were as follows: CD3, 76%; CD4, 30%; CD8, 44%; and CD9, 9%. Although there was a slight decrease in the CD4/CD8 ratio, the values for total CD4 lymphocytes were normal. Moreover, specific autoantibodies against the retina were absent, as tested by immunofluorescence analyses.
MATERIALS AND METHODS
Yeast growth.
The yeast was grown in YEPD medium (1% yeast extract, 2% peptone, 2% glucose) by incubation at 30°C. The same medium containing agar was used to isolate individual yeast colonies. The identification of the yeast species was carried out by standard biochemical and morphological techniques (54). In addition, yeast identification was performed by PCR, followed by sequencing of the amplified products.
To determine the MICs, the different antifungal compounds tested were initially dissolved in dimethyl sulfoxide and further dilutions were made in RPMI medium (Sigma Chemical Co., St. Louis, Mo.). Different concentrations of each antifungal were added to a 96-well plate in 200 μl of RPMI medium buffered to pH 7.0 with morpholinepropanesulfonic acid (Sigma). The C. famata inoculum was adjusted to a concentration of 0.5 × 103 to 2 × 103 CFU/ml. After incubation at 30°C, the turbidity was estimated by visual reading at 24 and 48 h of incubation. The MIC was defined as the lowest concentration of drug that produced a prominent decrease in turbidity compared with that of the drug-free control.
Immunofluorescence and electron microscopy.
Yeast cells were placed onto glass coverslips. The cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 10 min, and permeabilized with methanol (−20°C) for 2 min. The cells were washed twice in PBS and treated with rabbit anti-C. famata polyclonal antiserum at room temperature for 1 h. Then, goat anti-rabbit immunoglobulin G conjugated to fluorescein antibody was added. The samples were incubated at room temperature for another hour. The cells were then washed, and coverslips were mounted onto the slide with a drop of Mowiol solution. Finally, the cells were observed under a fluorescence microscope. For the ultrastructural analysis of the yeast, we followed the electron microscopy protocol described elsewhere (53), with a few modifications: the cells were fixed for 2 h at room temperature in 2% glutaraldehyde-1% tannic acid in 0.1 M cacodylate buffer (pH 6.8) and were finally embedded in LR white resin (London Resin Co., Ltd.). After postfixation, thin sections were obtained and stained with uranyl acetate and lead citrate. The samples were analyzed with a JEOL 1010 electron microscope.
PCR analysis.
DNA was extracted from the conjunctival exudates by standard procedures (43). The DNA preparations were incubated with oligonucleotides that hybridize to the rRNA genes (29, 32). The oligonucleotides used were 5′-CGTAATGATTAATAG-3′ and 5′-CGTGCGGCCAAGAAC-3′. The amplified product was analyzed by agarose gel electrophoresis.
T-cell proliferation.
Human mononuclear cells were obtained from heparinized venous blood by Ficoll-Hypaque (Pharmacia Fine Chemicals, Uppsala, Sweden) centrifugation. The layer containing mononuclear cells was taken, and the cells were washed thoroughly by centrifugation in Dulbecco's modified Eagle medium (DMEM) and were finally resuspended in DMEM-2% fetal calf serum (FCS). The T cells were further purified by passing the nonadherent population through a nylon-fiber and wool column, as described previously (38). The purity of this population (detected by flow cytometry) was always greater than 95% CD3+ cells. Purified T cells (106/ml in DMEM containing 10% FCS) were seeded in 96-well U-bottom microtiter plates (105/100 μl/well) and stimulated with 1 μg of phytohemagglutinin per ml or with different concentrations of lyophilized C. famata cells. The cultures were incubated for 72 h at 37°C, and cell proliferation was evaluated by measurement of the level of incorporation of [3H]thymidine (Perkin-Elmer Life Science Products, Boston, Mass.) into DNA during the last 16 h of culture.
ELISA analyses.
A C. famata cell suspension was diluted in PBS and seeded in enzyme-linked immunosorbent assay (ELISA) microtiter plates (Maxisorp; Nunc) in a final volume of 100 μl. The plates were incubated overnight at 4°C. Blocking was carried out in PBS containing 3% low-fat dry milk and 0.2% Tween 20 for 1 h at room temperature. Serum from the AZOOR patient and serum from an anonymous donor, used as a T-cell control, were added at different dilutions in PBS-1% low-fat dry milk-0.05% Tween 20 for 10 min and subsequently incubated with goat anti-human immunoglobulin G (heavy and light chains) horseradish peroxidase-conjugated antibodies (Pierce) for 1 h at room temperature and washed five times. Color development was accomplished by incubation with o-phenylenediamine (Sigma) for 30 min and was measured at 450 nm in a microplate reader (EL340; BIO-TEK Instruments).
RESULTS
Isolation and identification of C. famata.
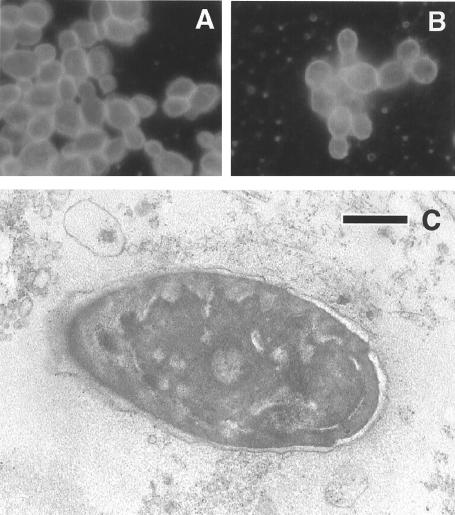
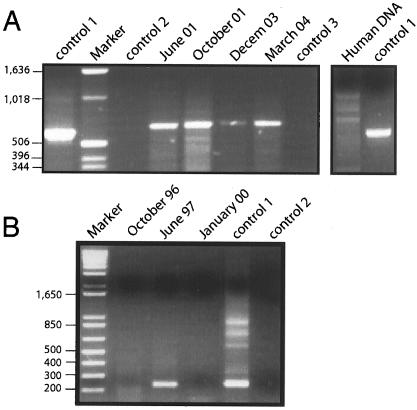
From 1994 to 1999 a number of attempts were made to identify an infectious agent both by blood tests for antibodies and by direct isolation from the conjunctival exudate. The patient's serum was negative for antibodies to the following agents: varicella-zoster virus, Epstein-Barr virus, human herpesvirus 6, parvovirus, coxsackievirus, human immunodeficiency virus, human T-cell leukemia virus types 1 and 2, hepatitis B virus, hepatitis C virus, Mycoplasma pneumoniae, Chlamydia trachomatis, C. psittaci, C. pneumoniae, Rickettsia conorii, Borrelia burgdorferi, and Treponema spp. Some increase in the levels of antibodies against cytomegalovirus was found. No viruses or bacteria apart from the normal flora were detected. Notably, however, in September 1999 C. famata readily grew from the conjunctival exudates when the exudates were placed in yeast growth medium. The results of immunofluorescence analysis for comparison of the isolate with a C. famata isolate obtained from a type culture collection is shown in Fig. 1A and B. Direct examination of the exudates by transmission electron microscopy showed the presence of significant amounts of yeast cells (Fig. 1C). Moreover, PCR analyses of the exudates collected from 1999 revealed the amplification of a distinct fragment that once again gave evidence of the presence of a yeast (Fig. 2A). This finding prompted us to test different serum samples stored at −20°C for the presence of yeast DNA. PCR analysis of these sera obtained at different dates revealed that C. famata DNA was present in the patient's blood in 1997 (Fig. 2B). Amplification followed by sequencing showed no yeast species other than C. famata.
FIG. 1.
Morphological analyses of yeast cells. Comparison of C. famata from the conjunctival exudate (A) with the type culture isolate (B) by immunofluorescence analysis. Immunofluorescence was carried out as described in Materials and Methods. (C) Electron microscopy of yeast cells present in the conjunctival exudates in September 1999. The exudate was placed in PBS and centrifuged. The pellet was processed for electron microscopy. Bar, 200 nm.
FIG. 2.
PCR assays. (A) PCR of different conjunctival exudate samples. DNA extracted from the exudates was analyzed by PCR, as indicated in Materials and Methods. (B) PCR analysis of different blood serum samples. A total of 200 μl of blood serum obtained from the patient on the dates indicated above the lanes was used for the extraction of total DNA. PCR assays were carried out with oligonucleotides that amplify a region of the rRNA internal transcribed spacer genes. Control 1, positive control consisting of DNA extracted from C. famata; control 2, negative PCR control without DNA; control 3, negative control of DNA extraction consisting of phosphate buffer. The numbers to the left of each gel are in base units.
Immunological tests.
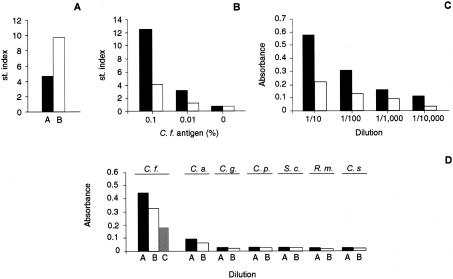
In October 1999, white cells were obtained from fresh blood by use of Ficoll gradients. The level of phytohemagglutinin stimulation of the patient's lymphocytes was lower than that of control lymphocytes from the anonymous donor (Fig. 3A). The stimulation of lymphocytes by sonicated C. famata cells above control values indicated the presence of antigen-specific T lymphocytes (Fig. 3B). The presence of specific antibodies against C. famata was revealed by an ELISA (Fig. 3C). These antibodies did not cross-react with a number of yeast species analyzed (Fig. 3D). Sera from different volunteers did not react with C. famata (data not shown).
FIG. 3.
T-cell proliferation and C. famata antibodies. (A) T cells from the patient described in this report (bar A) and from an anonymous donor (bar B) were obtained in October 1999, as indicated in Materials and Methods. The T cells obtained were incubated with (black bars) or without (empty bars) phytohemagglutinin. (B) T cells from the patient (black bars) or from the donor (empty bars) were incubated with different dilutions of a preparation of C. famata, as indicated. Data are presented as stimulation (st.) indices, defined as follows: counts per minute of [H3]thymidine obtained in the presence of mitogen or antigen divided by the basal counts per minute in their absence. (C) ELISA analysis of the antiserum obtained in October 1999 from the patient (black bars) and an anonymous donor (empty bars). The assay was carried out as described in Materials and Methods. (D) Results of the ELISA of the patient serum used at different dilutions (bars A, 1:100; bars B, 1:200; bars C, 1:400) in the anterior panel for activity against different yeast species. C.f., C. famata; C.a., C. albicans; C.g., Candida glabrata; C.p., Candida parapsilosis; S.c., Saccharomyces cerevisiae; R.m., Rhodotorula mucilaginosa; C.s., Cryptococcus saitoi.
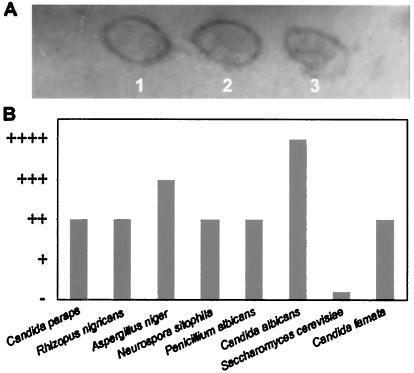
Finally, in October 1999, a delayed hypersensitivity assay was carried out on the patient's skin. After 24 h, a clear positive reaction appeared when the preparation of C. famata was applied (Fig. 4A). Apart from this yeast species, positive reactions to several other fungi were detected, as indicated in Fig. 4B. The patient's skin was the most reactive to C. albicans, while no reaction to Saccharomyces cerevisiae was detected. Together, these data reflect a generalized immune response of the patient to the yeast C. famata.
FIG. 4.
Delayed hypersensitivity test. (A) C. famata was autoclaved and lyophilized before it was mixed with petrolatum under sterile conditions. Petrolatum alone (lane 1) or petrolatum mixed with 0.2 g of C. famata per ml (lane 2) or 0.5 g of C. famata per ml (lane 3) was applied to the patient's skin in October 1999. The photograph was taken 24 h later. (B) Different fungal cells were obtained and mixed with petrolatum at 0.5 g/ml. The different preparations were applied to the patient's skin, and hypersensitivity was observed 24 h after application. Candida paraps, Candida parapsilosis.
Antifungal treatments.
Since C. famata was isolated and the immunological results pointed to infection with this organism, antifungal treatment was started in November 1999. Initially, fluconazole (200 mg daily for 1 month) and then itraconazole (100 mg daily for 3 months) were administered orally. These treatments improved some of the clinical symptoms, including photophobia and the retarded pupillary reflex, and the findings on funduscopic analysis were better. Since the patient's vision itself did not improve after the treatment and C. famata was still abundant in the conjunctival exudates, the two antifungals mentioned above were administered for different periods over the next 2 years. Our analysis showed that the MICs of fluconazole and itraconazole for the initial isolate were 60 and 30 μg/ml, respectively. These values indicate that the sensitivity of the yeast isolate to these two compounds was poor (24, 36, 37). Perhaps this was reflected by the poor response to treatment with these two antifungal compounds, since PCR analyses demonstrated the continuous presence of C. famata in the exudates. However, the infection probably diminished to some extent, considering the improvement in the patient's symptoms. Voriconazole was then approved for clinical use and represented a better choice for the treatment of this infection, as this compound is much more effective against C. famata (MIC, 0.5 μg/ml) (31, 35, 42). Therefore, this treatment was implemented in May 2003. The oral administration of voriconazole (150 mg every 12 h) led to a huge increase in photopsias within 1 h after intake. This suggests that whatever was present in the retina responded to voriconazole within minutes. However, this treatment was discontinued after 3 months due to hepatotoxicity problems. In summary, the antifungal compounds improved the patient's clinical symptoms, although it must be kept in mind that some fungal ocular infections are difficult to treat (18). Clinical trials are urged to establish both the most efficacious compound and the dosage for the treatment of these infections.
DISCUSSION
Despite efforts during the last few years to elucidate the cause of AZOOR, this has not been achieved. The suggestion that AZOOR is an autoimmune disorder has not been substantiated by experimental evidence. In fact, patients have been negative for the presence of autoantibodies, including the patient described in this report, when they have been analyzed for these antibodies. Epstein-Barr virus has been implicated in multifocal choroiditis (47). The possibility that an undefined virus infection provokes AZOOR has also been suggested (13). However, no report of the isolation of a virus related to this disease has ever been published. The infectious character of this retinopathy was suspected soon after its description (14). In this report we describe a single case of AZOOR in a patient infected with C. famata. Additional patients should be investigated to establish a cause-effect relationship between fungal infection and AZOOR. Nevertheless, the present findings open the possibility that a fungal infection may be the cause of this affliction. This idea is consistent with the available evidence that we have about the characteristics of AZOOR. Indeed, some clinical similarities with histoplasmosis syndrome, another fungal infection, were noted in the retina (9, 17). The evolution of AZOOR over the course of years with several recurrences, depending on the patient (14), is consistent with the progress of some fungal infections, since fungi usually grow slowly compared to the rates of growth of some bacteria and viruses, and the infections are recurrent. Chronicity and the recurrence of MEWDS have also been pointed out (48). Some fungal infections are insidious and difficult to diagnose. Ocular infections caused by C. albicans are easily identified by ophthalmologic examination, but this may not be the case with other less inflammatory yeast infections. A number of differences exist, depending on the yeast species. For instance, C. albicans is pathogenic and generates clear inflammatory signs, while C. famata possesses anti-inflammatory properties (10). This may explain, at least in part, the elusive nature of this infection.
Other characteristics of AZOOR also fit well with the idea that it has a fungal etiology. For instance, in addition to the pale, white aspect of the eye fundus, the retention of fluorescein may also reflect the presence of fungal cells or mucous material at the retina and choroidal vessels. Another point of interest is that C. famata may provoke infection of the CNS (39). This may explain why the CNS was also implicated in one case of AZOOR (19).
The isolation of C. famata from the conjunctival exudates and the blood serum of the patient may be directly related to AZOOR or may represent a secondary infection due to an as yet unidentified immunological disorder. Several results are consistent with a direct connection between AZOOR and C. famata infection in the patient described in this report. One of them is that the yeast was more abundant in the exudate from the left eye, which was more affected. Another piece of evidence was the response to antifungal treatments, as discussed below. The recalcitrant nature of fungal infections when they are treated by chemotherapy may explain why the yeast was still present at the conjunctiva even after prolonged treatment.
Little is known about the immune response provoked by non-C. albicans species. The present findings indicate that there was an immune response against C. famata in this AZOOR patient. This was shown both by the increased antibody titer and by the presence of antigen-specific T lymphocytes. Certainly, the immune system of the patient responded to C. famata.
No clear positive effects of corticosteroid treatment were found in some patients (17), particularly those on a long-term treatment regimen. This agrees well with the notion of the infective nature of this disease. The antifungal treatment of the patient described here certainly brought about improvements in several clinical symptoms. Thus far, however, the fungus has not been eradicated from the retina and perhaps other places, including the conjunctival mucosa. We believe that the infection described here has diminished through the implementation of antifungal therapy, but longer and more effective treatments will be necessary. In this sense, contrary to C. albicans, C. famata is less sensitive to fluconazole and itraconazole, thus accounting for their only moderate effectiveness. Voriconazole and, more particularly, posaconazole have greater inhibitory effects on C. famata, at least under laboratory conditions (35). The fact that voriconazole enhanced the photopsias at the retina within minutes after its intake in this patient may reflect the response of the yeast present there. Notably, this enhancement of photopsias was much more pronounced in the left eye, which was more affected than the right eye. In conclusion, the unsuspected possibility that C. famata may be responsible for AZOOR has obvious repercussions in investigations of the etiology of other related retinopathies (21).
Acknowledgments
We express our gratitude to the Organización Nacional de Ciegos Españoles (ONCE) for help and financial support. The institutional grant to Centro de Biología Molecular from Fundación Ramón Areces is acknowledged.
We thank M. Lombardero (Alergia e Inmunología, ALK-Abelló, S.A. Madrid, Spain) for help with the hypersensitivity assays.
REFERENCES
- 1.Al-Hedaithy, S. S. 2003. The yeast species causing fungemia at a univesity hospital in Riyadh, Saudi Arabia, during a 10-year period. Mycoses 46:293-298. [DOI] [PubMed] [Google Scholar]
- 2.Altamura, M., D. Casale, M. Pepe, and A. Tafaro. 2001. Immune responses to fungal infections and therapeutic implications. Curr. Drug Targets Immune Endocr. Metab. Disord. 1:189-197. [DOI] [PubMed] [Google Scholar]
- 3.Andrighetto, C., E. Psomas, N. Tzanetakis, G. Suzzi, and A. Lombardi. 2000. Randomly amplified polymorphic DNA (RAPD) PCR for the identification of yeasts isolated from dairy products. Lett. Appl. Microbiol. 30:5-9. [DOI] [PubMed] [Google Scholar]
- 4.Andriole, V. T. 1998. Current and future therapy of invasive fungal infections. Curr. Clin. Top. Infect. Dis. 18:19-36. [PubMed] [Google Scholar]
- 5.Brown, J., Jr., and J. C. Folk. 1998. Current controversies in the white dot syndromes. Multifocal choroiditis, punctate inner choroidopathy, and the diffuse subretinal fibrosis syndrome. Ocul. Immunol. Inflamm. 6:125-127. [DOI] [PubMed] [Google Scholar]
- 6.Bryan, R. G., K. B. Freund, L. A. Yannuzzi, R. F. Spaide, S. J. Huang, and D. L. Costa. 2002. Multiple evanescent white dot syndrome in patients with multifocal choroiditis. Retina 22:317-322. [DOI] [PubMed] [Google Scholar]
- 7.Clemons, K. V., V. L. Calich, E. Burger, S. G. Filler, M. Grazziutti, J. Murphy, E. Roilides, A. Campa, M. R. Dias, J. E. Edwards, Jr., Y. Fu, G. Fernandes-Bordignon, A. Ibrahim, H. Katsifa, C. G. Lamaignere, L. H. Meloni-Bruneri, J. Rex, C. A. Savary, and C. Xidieh. 2000. Pathogenesis I: interactions of host cells and fungi. Med. Mycol. 38:99-111. [PubMed] [Google Scholar]
- 8.Coleman, D. C., M. G. Rinaldi, K. A. Haynes, J. H. Rex, R. C. Summerbell, E. J. Anaissie, A. Li, and D. J Sullivan. 1998. Importance of Candida species other than Candida albicans as opportunistic pathogens. Med. Mycol. 36:156-165. [PubMed] [Google Scholar]
- 9.Dreyer, R. F., and D. J. Gass. 1984. Multifocal choroiditis and panuveitis. A syndrome that mimics ocular histoplasmosis. Arch. Ophthalmol. 102:1776-1784. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Gonzalez, A., and J. L. Ochoa. 1999. Anti-inflammatory activity of Debaryomyces hansenii Cu,Zn-SOD. Arch. Med. Res. 30:69-73. [DOI] [PubMed] [Google Scholar]
- 11.Gardini, F., G. Suzzi, A. Lombardi, F. Galgano, M. A. Crudele, C. Andrighetto, M. Schirone, and R. Tofalo. 2001. A survey of yeasts in traditional sausages of southern Italy. FEMS Yeast Res. 1:161-167. [DOI] [PubMed] [Google Scholar]
- 12.Gass, J. D. 1993. Acute zonal occult outer retinopathy. Donders Lecture: The Netherlands Ophthalmological Society, Maastricht, Holland, June 19, 1992. J. Clin. Neuroophthalmol. 13:79-97. [PubMed] [Google Scholar]
- 13.Gass, J. D. 2003. Are acute zonal occult outer retinopathy and the white spot syndromes (AZOOR complex) specific autoimmune diseases? Am. J. Ophthalmol. 135:380-381. [DOI] [PubMed] [Google Scholar]
- 14.Gass, J. D., A. Agarwal, and I. U. Scott. 2002. Acute zonal occult outer retinopathy: a long-term follow-up study. Am. J. Ophthalmol. 134:329-339. [DOI] [PubMed] [Google Scholar]
- 15.Hariprasad, S. M., W. F. Mieler, E. R. Holz, H. Gao, J. E. Kim, J. Chi, and R. A. Prince. 2004. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch. Ophthalmol. 122:42-47. [DOI] [PubMed] [Google Scholar]
- 16.Helbig, H., F. Sutter, and A. Tholen. 2001. Acute zonal occult outer retinopathy (AZOOR). Ophthalmologe 98:574-578. [DOI] [PubMed] [Google Scholar]
- 17.Holz, F. G., R. Y. Kim, S. D. Schwartz, C. A. Harper, J. Wroblewski, G. B. Arden, and A. C. Bird. 1994. Acute zonal occult outer retinopathy (AZOOR) associated with multifocal choroidopathy. Eye 8:77-83. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi, Y. 1998. Refractory mycosis in ophthalmology. Nippon Ishinkin Gakkai Zasshi 39:211-212. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson, D. M. 1996. Acute zonal occult outer retinopathy and central nervous system inflammation. J. Neuroophthalmol. 16:172-177. [PubMed] [Google Scholar]
- 20.Jacobson, S. G., D. S. Morales, X. K. Sun, W. J. Feuer, A. V. Cideciyan, J. D. Gass, and A. H. Milam. 1995. Pattern of retinal dysfunction in acute zonal occult outer retinopathy. Ophthalmology 102:1187-1198. [DOI] [PubMed] [Google Scholar]
- 21.Jampol, L. M., and A. Wiredu. 1995. MEWDS, MFC, PIC, AMN, AIBSE, and AZOOR: one disease or many? Retina 15:373-378. [PubMed] [Google Scholar]
- 22.Jampol, L. M., P. A. Sieving, D. Pugh, et al. 1984. Multiple evanescent white dot syndrome. I. Clinical findings. Arch. Ophthalmol. 102:671-674. [DOI] [PubMed] [Google Scholar]
- 23.Kam, A. P., and J. Xu. 2002. Diversity of commensal yeasts within and among healthy hosts. Diagn. Microbiol. Infect. Dis. 43:19-28. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami, S., Y. Ono, Y. Miyazawa, and H. Yamaguchi. 1998. Survey of fungemia cases during the past seventeen years at Teikyo University Hospital. Kansenshogaku Zasshi 72:105-113. [DOI] [PubMed] [Google Scholar]
- 25.Krcméry, V., and A. Kunová. 2000. Candida famata fungemia in a cancer patient: case report. J. Chemother. 12:189-190. [DOI] [PubMed] [Google Scholar]
- 26.Krcmèry, V., and A. J. Barnes. 2002. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J. Hosp. Infect. 50:243-260. [DOI] [PubMed] [Google Scholar]
- 27.Lardenoye, C. W., A. Van der Lelij, W. S. de Loos, W. F. Treffers, and A. Rothova. 1997. Peripheral multifocal chorioretinitis: a distinct clinical entity? Ophthalmology 104:1820-1826. [DOI] [PubMed] [Google Scholar]
- 28.Lee, A. G., and T. C. Prager. 1996. Acute zonal occult outer retinopathy. Acta Ophthalmol. Scand. 74:93-95. [DOI] [PubMed] [Google Scholar]
- 29.Li, Y. L., S. N. Leaw, J. H. Chen, H. C. Chang, and T. C. Chang. 2003. Rapid identification of yeasts commonly found in positive blood cultures by amplification of the internal transcribed spacer regions 1 and 2. Eur. J. Clin. Microbiol. Infect. Dis. 22:693-696. [DOI] [PubMed] [Google Scholar]
- 30.Mamalis, N., and M. J. Daily. 1987. Multiple evanescent white-dot syndrome. A report of eight cases. Ophthalmology 94:1209-1212. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell, M. J., S. A. Messer, R. J. Hollis, L. Boyken, S. Tendolkar, D. J. Diekema, and M. A. Pfaller. 2003. Evaluation of Etest method for determining fluconazole and voriconazole MICs for 279 clinical isolates of Candida species infrequently isolated from blood. J. Clin. Microbiol. 41:1087-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikawa, A., T. Sugita, and T. Shinoda. 1999. Rapid identification of Debaryomyces hansenii/Candida famata by polymerase chain reaction. Med. Mycol. 37:101-104. [PubMed] [Google Scholar]
- 33.Penk, A., and L. Pittrow. 1998. Status of fluconazole in the therapy of endogenous Candida endophthalmitis. Mycoses 41:41-44. [DOI] [PubMed] [Google Scholar]
- 34.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22:S89-S94. [DOI] [PubMed] [Google Scholar]
- 35.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, R. J. Hollis, and R. N. Jones. 2003. In vitro activities of voriconazole, posaconazole, and four licensed systemic antifungal agents against Candida species infrequently isolated from blood. J. Clin. Microbiol. 41:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaller, M. A., R. N. Jones, G. V. Doern, A. C. Fluit, J. Verhoef, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, and R. J. Hollis. 1999. International surveillance of blood stream infections due to Candida species in the European Sentry Program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 35:19-25. [DOI] [PubMed] [Google Scholar]
- 37.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 38.Pimentel-Muiños, F. X., M. A. Muñoz-Fernández, and M. Fresno. 1994. Control of T lymphocyte activation and IL-2 receptor expression by endogenously secreted lymphokines. J. Immunol. 152:5714-5722. [PubMed] [Google Scholar]
- 39.Prinsloo, B., G. F. Weldhagen, and R. W. Blaine. 2003. Candida famata central nervous system infection. S. Afr. Med. J. 93:601-612. [PubMed] [Google Scholar]
- 40.Rao, N. A., A. V. Nerenberg, and D. J. Forster. 1991. Torulopsis candida (Candida famata) endophthalmitis simulating Propionibacterium acnes syndrome. Arch. Ophthalmol. 109:1718-1721. [DOI] [PubMed] [Google Scholar]
- 41.Reis, A., R. Sundmacher, K. Tintelnot, H. Agostini, H. E. Jensen, and C. Althaus. 2000. Successful treatment of ocular invasive mould infection (fusariosis) with the new antifungal agent voriconazole. Br. J. Ophthalmol. 84:932-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubio Calvo, M. C., J. Gil, I. Ramirez de Ocariz, R. Benito, and A. Rezusta. 2003. In vitro activity of fluconazole, voriconazole and posaconazole against Candida spp. Rev. Esp. Quimioter. 16:227-232. [PubMed] [Google Scholar]
- 43.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Smetana, S., S. S. Feman, and C. Kleber. 2003. The retinal features of acute zonal occult outer retinopathy (AZOOR). Arch. Ophthalmol. 121:914-915. [DOI] [PubMed] [Google Scholar]
- 45.Smiddy, W. E. 1998. Treatment outcomes of endogenous fungal endophthalmitis. Curr. Opin. Ophthalmol. 9:66-70. [DOI] [PubMed] [Google Scholar]
- 46.St-Germain, G., and M. Laverdiere. 1986. Torulopsis candida, a new opportunistic pathogen. J. Clin. Microbiol. 24:884-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiedeman, J. S. 1987. Epstein-Barr viral antibodies in multifocal choroiditis and panuveitis. Am. J. Ophthalmol. 103:659-663. [DOI] [PubMed] [Google Scholar]
- 48.Tsai, L., L. M. Jampol, S. Pollock, and J. Olk. 1994. Chronic recurrent multiple evanescent white dot syndrome. Retina 14:160-163. [DOI] [PubMed] [Google Scholar]
- 49.Turbeville, S. D., L. D. Cowan, and J. D. Gass. 2003. Acute macular neuroretinopathy: a review of the literature. Surv. Ophthalmol. 48:1-11. [DOI] [PubMed] [Google Scholar]
- 50.Verougstraete, C. 2001. White spots syndromes. Bull. Soc. Belge Ophthalmol. 279:67-78. [PubMed] [Google Scholar]
- 51.Volpe, N. J., J. R. Rizzo III, and S. Lessell. 2001. Acute idiopathic blind spot enlargement syndrome: a review of 27 new cases. Arch. Ophthalmol. 119:59-63. [PubMed] [Google Scholar]
- 52.White, T. H., K. A. Marr, and R. A. Bowde. 1998. Clinical, cellular and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright, R., M. Basson, L. D'Ari, and J. Rine. 1988. Increased amounts of HMG-CoA reductase induce “karmellae”: a proliferation of stacked membrane pairs surrounding the yeast nucleus. J. Cell Biol. 107:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yarrow, D. 1998. Methods for the isolation, maintenance and identification of yeasts, p. 77-100. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts. A taxonomic study, 4th ed. Elsevier Science, Amsterdam, The Netherlands.