Abstract
Background
A review of data from large clinical trials reported more than 90% of subjects significantly improved their bone mineral density (BMD) at the lumbar spine (LS) with teriparatide (TPTD) (bone 39:1268–1275, 1). However, our clinical experience suggests that many patients may be non-responders, raising questions as to the true efficacy of TPTD in improving BMD in osteoporotic patients.
Questions/Purposes
The purpose of the study is to determine the rate of improvement in BMD following 18–24 months of teriparatide (TPTD) in patients with osteoporosis within an orthopedic hospital setting.
Methods
This is a retrospective chart review of patients with osteoporosis who completed 18–24 months of TPTD therapy. The primary endpoint was the change in BMD at lumbar spine (LS) and hip-femoral neck (FN) and total hip (TH) following treatment. Secondary endpoints included the effect of prior bisphosphonate therapy, age, body mass index (BMI) and family history of fracture on BMD response, and the changes in bone-specific markers during active treatment.
Results
Seventy-eight women and men with mean T-scores at the LS = −2.63 met the inclusion criteria. The overall group showed a 10.7% increase in LS-BMD after 24 months of TPTD. Eighty-three percent were considered responders defined as ≥3.0% increase in LS-BMD. Non-responders (16.7%) had mean LS-BMD change = −1.41%. No difference in baseline vitamin D, calcium, creatinine, BMI, age, gender, prior fracture history, or bisphosphonate use was observed between responders and non-responders. No consistent pattern of change in measures of bone markers was noted between responders and non-responders.
Conclusion
Eighty-three percent of patients with osteoporosis showed a >3% increase in BMD after TPTD treatment. Baseline parameters, prior bisphosphonate therapy, and the changes in bone markers showed no correlation with final BMD outcome.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-016-9537-1) contains supplementary material, which is available to authorized users.
Keywords: bone, osteoporosis, teriparatide treatment, clinical outcome, bone markers
Introduction
A review of data from several clinical trials reported more than 90% of subjects showed a greater than 3% increase in areal bone density (BMD) at the lumbar spine (LS) with teriparatide (TPTD) treatment [14]. In one large clinical trial, only 8 out of 354 patients (2%) failed to achieve an increase of 3% in the LS BMD after 24 months of daily teriparatide treatment. These patients were identified as non-responders [26]. Given the duration, effort, and expense of TPTD therapy, there is a strong incentive to ascertain response rates in specific patient populations and strive to improve therapeutic outcomes. Early identification of patients likely to show a poor outcome to TPTD therapy alone might benefit by a change in therapy as recent evidence from combined therapeutic interventions have been shown to augment or prolong the anabolic effect of TPTD [2, 3, 10, 18, 25, 32–34]. In principle, the primary goal of osteoporosis therapy is to reduce fracture risk. However, fracture incidence is low and a surrogate measure such as areal BMD has been shown to have sufficient precision to follow overall response to therapy (11–21). The utility of following BMD was especially true with regard to TPTD since BMD changes were shown to account for up to 41% of the vertebral fracture reduction effect (19).
In the current study, the response rates to TPTD therapy in postmenopausal women and men with osteoporosis presenting to an orthopedic hospital were examined. Baseline parameters reported to affect response rate were assessed. Bone-specific markers were monitored during therapy in an effort to correlate osteoblast and osteoclast activity with response rate.
Patients and Methods
The charts of 78 osteoporotic patients treated with TPTD from a single clinician’s practice that met the inclusion criteria of a Hospital for Special Surgery approved IRB retrospective study were reviewed. To be included, patients had to receive TPTD, 20mcg/day by subcutaneous injection for 18 to 24 months and have Dual-energy X-ray absorptiometry (DXA) scans the beginning and end of treatment. Patients with concurrent bisphosphonate, denosumab, or strontium use, underlying metabolic bone disorders, degenerative disc disease, or confounding lumbar scoliosis were excluded from the study. All subjects had bone markers monitored every 4 months. The specific markers included bone specific alkaline phosphatase (BSAP) measured by a two-site immunoradiometric assay (interassay CV, 7.4–7.9%; Hybritech, Beckman Beckman-Coulter, Brea, CA, USA and urinary cross-linked N-telopeptide of type I collagen (NTx) by a competitive-inhibition ELISA (interassay CV, 6.7–14.8%; Ostex, Seattle, WA, USA). Areal BMD measured by DXA-Hologic (Bedford, MA, USA) or GE-Lunar (Madison, WI, USA) equipment. The % change in standardized BMD (sBMD) values [26, 35] before and after 18–24 months of treatment were compared for each patient.
Patient data that were collected and assessed included age, BMI, gender, medical co-morbidities, tobacco history, prior bisphosphonate use, BMD at lumbar spine, femoral neck, total hip (LS, FN, TH), fracture history, serum calcium, creatinine, 25 hydroxy vitamin D (25OHD), and estrogen or selective estrogen receptor modulator (SERM) history. Patients were calcium and vitamin D replete, which was verified at the quarterly encounters to ensure that they maintained a total calcium intake between 1000 and 1500 mg/day from diet and supplements. 25-OH vitamin D levels were checked during TPTD treatment with the goal of maintaining the level above 30 ng/ml.
Statistical Analyses
All patients were included in the outcome analyses since prior studies had demonstrated that response to TPTD was independent of age, history of prior fracture, prior bisphosphonate therapy, or gender [23, 24, 27]. Demographics, baseline, and end of study characteristics were summarized across treatment groups using descriptive statistics. Baseline and end of study characteristics were compared using paired t test or Wilcoxon signed rank test. Percentage of change in sBMD between prior bisphosphate users and non-users was compared using two-sample t test or Wilcoxon rank sum test.
At the inception of the study, patients were identified as “responders” if they showed a 3% or greater increase in areal BMD at the lumbar spine from start to the completion of TPTD treatment. “Non-responders” were patients who showed less than a 3% gain in BMD at the lumbar spine at the end of TPTD treatment based on previously established criteria of least significant change (LSC) [5, 7, 9, 11, 14, 16, 19–21, 23, 24, 26–28, 31]. Baseline and follow-up characteristics were summarized and compared between responders and non-responders. The comparison methods included two-sample t test or Wilcoxon rank sum test for continuous variables and Chi-squared test or Fisher’s exact test for categorical variables.
Linear mixed effect model with random intercept was used to compare the mean NTx over time between TPTD responders and non-responders after controlling for time and other clinical confounders and/or effect modifiers (age, gender, history of prior fracture or family history of fracture) [15, 22, 35]. The same model was also applied to compare the mean BSAP over time between responders and non-responders. Stepwise selection method and Akaike Information Criteria (AIC) were used for the model selection. The model with smallest AIC was chosen as the final model. All the statistical analyses were calculated by SAS 9.2.
Results
The patients in this study were predominately female in their mid sixties with osteoporosis as assessed by DEXA scan. BMD measurements recorded at the initiation of TPTD treatment and at the end (18–24 months) improved for the entire population (Table 1). The changes in bone density at the LS, FN, and TH for the entire patient group were statistically significant. The patients remained calcium and vitamin D replete for the duration of the study.
Table 1.
Baseline and end of study parameters for entire patient population
| Baseline Mean (std.) |
End of Treatment Mean (std.) |
p value* | |
|---|---|---|---|
| Age (years) | 62.62 (8.53) | 64.62 | N/A |
| Male (%) | 10.26% | 10.26% | N/A |
| BMI | 22.42 (4.00) | 22.11 (8.07) | 0.47 |
| sBMD_LS (g/cm2) | 0.83 (0.16) | 0.91 (0.20) | <0.001 |
| T score-LS | −2.63 (1.38) | −1.98 (1.68) | <0.001 |
| sBMD-FN (g/cm2) | 0.67 (0.11) | 0.70 (0.11) | <0.001 |
| sBMD-TH (g/cm2) | 0.73 (0.11) | 0.75 (0.10) | <0.001 |
| 25OHD (ng/Ml) | 38.37 (11.40) | 41.10 (10.23) | 0.29 |
| Serum Ca (mg/dL) | 9.73 (0.47) | 9.61 (0.45) | 0.14 |
| NTx (Nm BCE/Mm Cr) | 37.04 (33.94) | 46.75 (26.69) | 0.048 |
| BSAP (units/L) | 14.95 (8.75) | 20.04 (11.21) | 0.08 |
For patients who had never received bisphosphonate therapy before TPTD (n = 28), a mean increase in bone mineral density of 12.6% at LS, 4.3% at FN, and 3% at TH was seen. Patients who had received bisphosphonate therapy in the past (n = 50), a mean increase in BMD of 9.65% at LS, 3.98% at FN, and 4.34% at TH was seen. The differences in the change in BMD between the two groups were not statistically significant at lumbar spine, femoral neck, and total hip.
Thirteen (16.7%) patients were TPTD non-responders in that they showed less than a 3% change in LS BMD, (mean = −1.4%), (median = −1.29%) with a mean duration of TPTD treatment of 24 months. Sixty-five (83.3%) patients were TPTD responders and gained more than 3% in lumbar spine BMD, (mean = 13.1%) (median = 9.84%) with a mean TPTD treatment duration of 23.29 months. The % change in BMD at the LS between non-responders and responders was highly significant (p < 0.0001). The % change in BMD between non-responders and responders was also significant at the FN (p = 0.04) but was not statistically significant at the TH. The baseline characteristics of the non-responders and responders are summarized in Table 2.
Table 2.
Baseline and follow-up characteristics for non-responders and responders
| Non-responder (N = 13) mean (std.) |
Responder (N = 65) mean (std.) |
p value* | |
|---|---|---|---|
| Age (years) | 63.08 (6.70) | 62.52 (8.89) | 0.91 |
| Male (%) | 0% | 12.31% | 0.34 |
| Bisphosphates user (%) | 76.92% | 61.54% | 0.36 |
| Total months on any bisphosphates before Forteo | 51.10 (48.98) | 71.74 (38.73) | 0.11 |
| Total months of bisphosphates free period before Forteo | 9.50 (14.03) | 16.49 (24.57) | 0.86 |
| Fracture yes (%) | 76.92% | 69.23% | 0.74 |
| Fracture during therapy yes (%) | 16.67% | 4.65% | 0.20 |
| BMI_Bbaseline | 20.84 (2.80) | 22.74 (4.15) | 0.17 |
| sBMD_LS initial (g/cm2) | 0.78 (0.07) | 0.83 (0.18) | 0.53 |
| sBMD_LS final (g/cm2) | 0.77 (0.07) | 0.94 (0.20) | 0.0008 |
| % change in sBMD_LS | −1.41 (3.52) | 13.15 (11.58) | <0.0001 |
| T score initial (g/cm2) | −2.98 (0.60) | −2.56 (1.49) | 0.45 |
| T score final (g/cm2) | −3.28 (0.91) | −1.72 (1.69) | 0.0005 |
| sBMD_FN initial (g/cm2) | 0.69 (0.09) | 0.66 (0.11) | 0.53 |
| sBMD_FN final (g/cm2) | 0.67 (0.08) | 0.70 (0.12) | 0.35 |
| % change in sBMD_FN | −0.11 (4.33) | 4.92 (8.48) | 0.04 |
| sBMD_TH Initial (g/cm2) | 0.72 (0.09) | 0.73 (0.12) | 1.00 |
| sBMD_TH final (g/cm2) | 0.71 (0.07) | 0.75 (0.10) | 0.40 |
| % change in sBMD_TH | 1.25 (6.04) | 4.17 (7.24) | 0.39 |
| 25OHD Initial (ng/Ml) | 42.80 (13.08) | 37.51 (10.98) | 0.29 |
| 25OHD Final (ng/Ml) | 42.10 (7.46) | 40.89 (10.81) | 0.53 |
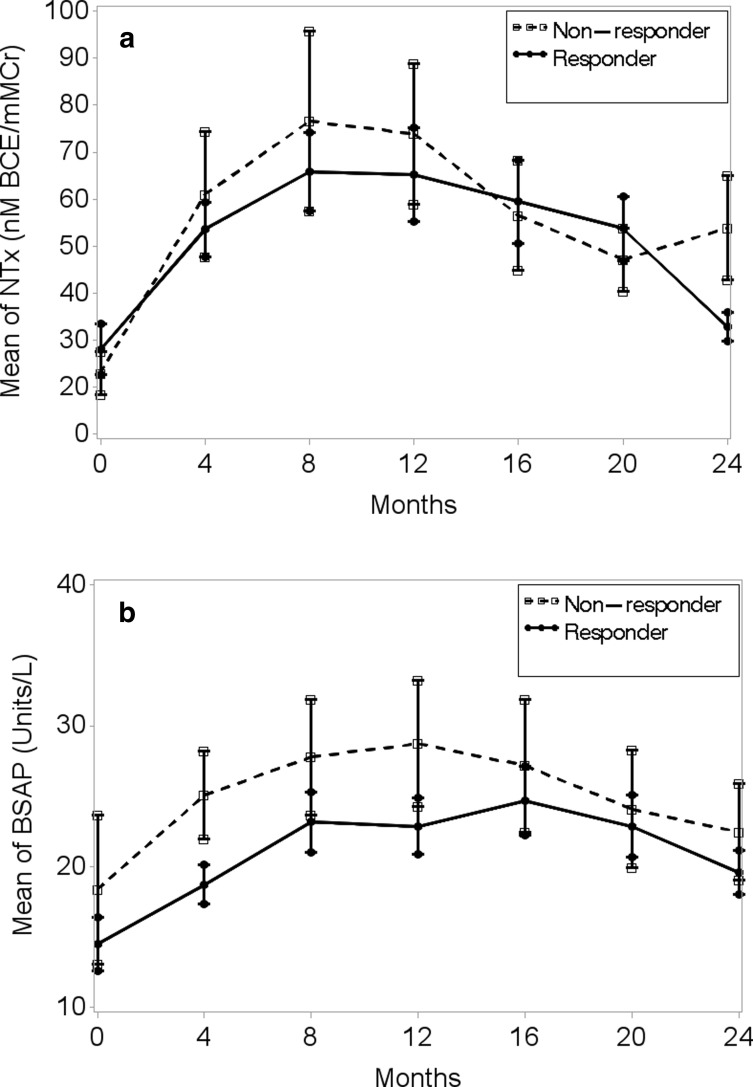
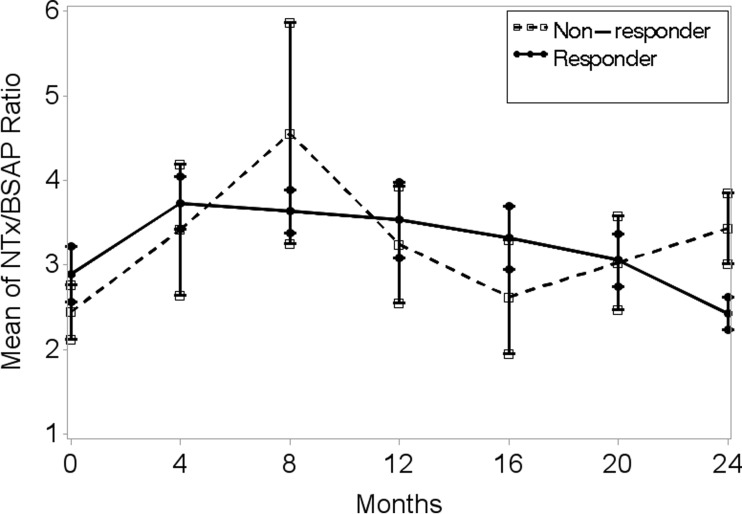
A peak response in bone marker NTx was observed at 4–8 months in both non-responders and responders while the peak response in BSAP was observed at 8–12 months in non-responders and at 4–8 months in responders (Table 3 and Fig. 1a, b). During the 24 months of TPTD, BSAP remained higher in the non-responders. However, the magnitude of BSAP was significantly higher in the non-responders compared to TPTD responders only at 4 months (p = 0.04). The magnitude of NTX was significantly higher in the TPTD non-responders compared to the responders only at the end of TPTD at 24 months. Although the differences were not statistically significant, the NTx/BSAP ratio was higher in the TPTD non-responders at 4–12 months, indicative of greater resorptive activity compared to bone formation (Fig. 2).
Table 3.
NTx and BSAP over time for non-responders and responders
| Non-responder (N = 13) mean (std.) |
Responder (N = 65) mean (std.) |
p value* | |
|---|---|---|---|
| NTx initial (Nm BCE/Mm Cr) | 32.90 (15.00) | 37.98 (37.00) | 0.88 |
| NTx 4 months (Nm BCE/Mm Cr) | 71.00 (40.18) | 63.58 (42.63) | 0.44 |
| NTx 8 months (Nm BCE/Mm Cr) | 86.56 (57.44) | 75.84 (55.32) | 0.52 |
| NTx 12 months (Nm BCE/Mm Cr) | 83.91 (49.86) | 75.16 (72.36) | 0.29 |
| NTx 16 months (Nm BCE/Mm Cr) | 66.50 (36.98) | 69.41 (55.37) | 0.88 |
| NTx 20 months (Nm BCE/Mm Cr) | 57.10 (22.32) | 63.70 (41.64) | 0.81 |
| NTx 24 months (Nm BCE/Mm Cr) | 63.83 (38.40) | 42.73 (21.74) | 0.02 |
| BSAP initial (units/L) | 18.37 (9.17) | 14.47 (8.82) | 0.35 |
| BSAP 4 months (units/L) | 25.06 (8.86) | 18.70 (9.96) | 0.04 |
| BSAP 8 months (units/L) | 27.79 (10.87) | 23.17 (14.20) | 0.17 |
| BSAP 12 months (units/L) | 28.71 (14.18) | 22.84 (13.69) | 0.15 |
| BSAP 16 months (units/L) | 27.16 (13.33) | 24.65 (14.84) | 0.50 |
| BSAP 20 months (units/L) | 24.08 (12.60) | 22.84 (13.74) | 0.67 |
| BSAP 24 months (units/L) | 22.45 (10.88) | 19.57 (11.31) | 0.31 |
Fig. 1.
a A peak response in bone marker NTx was observed at 4–8 months in both non-responders and responders while the peak response in BSAP was observed at 8–12 months in non-responders and at 4–8 months in responders. b The magnitude of BSAP was significantly higher in the non-responders compared to TPTD responders only at 4 months (p = 0.04).
Fig. 2.
Although the differences were not statistically significant, the NTx/BSAP ratio was higher in the TPTD non-responders at 4–12 months, indicative of greater resorptive activity compared to bone formation.
Two separate linear mixed effect models with random intercept were fitted for NTx and BSAP, controlling for time, time2 (time-squared), responders/non-responders, age, gender, history of prior fracture, and family history of fracture [15, 22, 35]. Holding other factors constant, both NTx and BSAP changed over time (p < 0.001 in both models); but no statistically significant differences were found between responders and non-responders over time (time taken as continuous variable) in either NTx (p = 0.79) or BSAP (p = 0.27).
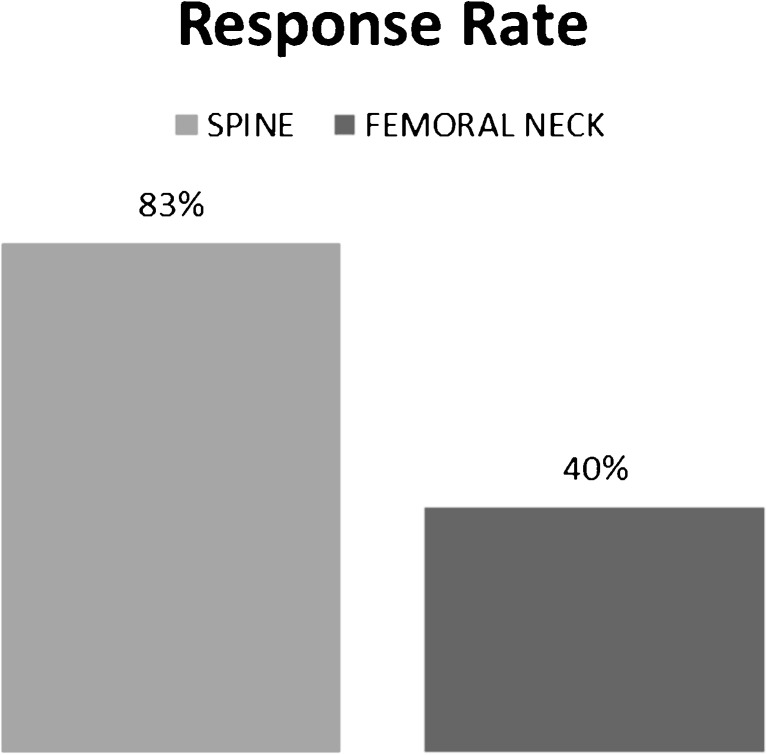
The percentage of patients showing a greater than 3% increase in BMD (responder rates) after 2 years of TPTD treatment in our study was 83% using the spine as outcome, but only 40% at the femoral neck (Fig. 3.)
Fig. 3.
Eighty-three percent of the lumbar spine and 40% of the femoral neck showed significant response to teriparatide therapy with more than 3% increase in BMD.
Discussion
Not every patient receiving 18–24 months of TPTD showed a significant increase in lumbar spine bone density. In the current study, 83% of patients were TPTD responders, demonstrating a greater than 3% increase in BMD, a value pre-selected to exceed the least significant change (LSC) used by prior investigators in distinguishing responders and non-responders based on LS BMD changes with TPTD treatment [9, 26].
In the current study, 16.7% of the patients were identified as non-responders, which is a significantly higher rate of non-responders to TPTD than previously reported [14, 26]. None of the initial characteristics including age, BMI, BMD, vitamin D status, or prior bisphosphonate use predicted response to TPTD. Interestingly, with regard to gender, all 8 men in the study were responders. Since so few men were in the study, this result could not be judged to be statistically significant.
Bone markers showed the typical increases with TPTD treatment that have been described in earlier studies [2–4, 8–10, 12, 17, 18, 23–27, 29, 32–34]. In general, both BSAP and NTx were higher in the TPTD non-responders compared to the responders.
The NTx marker measures a fragment of the cross-linking bridge between the type I collagen chains in bone. High values of the NTx reflect accelerated type I collagen breakdown associated with increased bone resorption [13]. BSAP is primarily derived from osteoblasts. Increased levels of BSAP reflect increased bone synthesis and anabolic activity. A rise in BSAP has been demonstrated during early anabolic response to TPTD treatment, which is subsequently followed by a rise in the resorptive markers [4]. This early osteoblast-mediated anabolic response to TPTD, which precedes the osteoclast-mediated resorption of type I collagen describes the so-called anabolic window [1, 6, 30]. Previous studies of TPTD combination therapy were aimed at improving BMD anabolic response by prolonging the anabolic window during which bone formation exceeds bone resorption [1, 6, 25, 30]. Based on the current study results, there were no significant differences in the BSAP or NTX markers between the TPTD responders and non-responders over time.
Identifying TPTD non-responders early in therapy would have great practical utility especially as there was a significant percentage of patients who failed to respond with a net increase in bone formation, 16.7% in our study. Previous reports suggest sequential addition of an anti-remodeling agent consistently leads to an increase in net bone formation [2, 3, 25] as does concurrent TPTD and zolendronate or denosumab [10, 18, 33, 34]. In the Muschitz et al. study, the accentuated BMD anabolic response with TPTD-alendronate therapy compared to TPTD monotherapy was accompanied by early suppression of bone resorptive marker CTx to pre-TPTD treatment levels along with partial suppression of P1NP [25]. This finding supported the hypothesis of re-opening of the anabolic window of TPTD, which is characterized by greater bone formation than resorption, resulting in net gain of BMD. In the more recent TPTD-denosumab combination trial, Tsai et al. demonstrated augmented BMD anabolic response with concurrent TPTD-denosumab therapy compared to TPTD or denosumab monotherapy, which was accompanied by complete suppression of CTx and only partial suppression of markers of bone formation, osteocalcin, and P1NP [34]. Furthermore, combined denosumab-TPTD therapy produced favorable changes in cortical parameters, such as less porosity, increased cortical thickness, and volume along with apparent filling of resorptive cavities at the endocortical surface [33].
Clinicians often rely on bone turnover markers to confirm that patients are responding to osteoporosis therapy, in this case, TPTD. Although our study results showed significantly higher BSAP levels in the TPTD non-responders at 4 months, there were no significant differences between the TPTD responders and non-responders over time (24 months). This finding suggests that the changes seen in the bone turnover markers during TPTD therapy may not correlate well with clinical outcome, such as improvement in BMD.
There are several limitations to the current study. First, the data are derived from a retrospective, open-label, small patient number cohort. However, the study being open label would not have influenced the changes in BTM and BMD. Those performing the laboratory measurements were blinded with regard to treatment. Furthermore, the primary endpoint of a BMD change greater than 3% for the study was predetermined and no differences in baseline characteristics or laboratory values were demonstrable in the responder and non-responder patient populations.
Second, there was limitation in racial and ethnic diversity in the study population. This was an unavoidable consequence of the patient population coming from a single physician’s practice and reflects the typical regional and referral patient population to this academic practice for treatment of osteoporosis. In fact, it is a strength that the patients/subjects were drawn from a real-life medical practice in which the choice to treat with TPTD was based solely on the clinical presentation.
Third, these changes in bone markers do not provide a true insight into biological mechanism of BMD changes. Finally, too few fractures occurred during the study so no correlation of BTM or BMD changes with fracture incidence can be made. However, it is important to remember that the strongest correlation of BMD change with fracture risk is seen with TPTD treatment more than with any of the other therapeutic interventions for osteoporosis [7].
It would appear that there is a complex balance of osteoclast-mediated resorptive activity and osteoblast-mediated bone formation modulating the anabolic response. The current study suggests that the bone turnover markers BSAP and NTx do not correlate well with clinical response to teriparatide therapy, and puts into question the role of monitoring BTM during therapy. Recently proposed TPTD combination therapy with denosumab or zolendronate may be an effective treatment option for patients who do not respond to TPTD monotherapy. However, additional studies dedicated to identifying TPTD non-responders early in therapy are necessary so that this subset of osteoporotic patients can be treated earlier and more effectively with combination or sequential therapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(DOCX 13 kb)
Acknowledgements
The authors thank Mr. Erik Nielsen for his assistance in collating the early patient data.
Compliance with Ethical Standards
Conflict of Interest
So-Young Kim, MD, Meng Zhang, PhD, and Richard Bockman, MD, PhD, have declared that they have no conflict of interest.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was waived from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Work performed at Hospital for Special Surgery.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-016-9537-1) contains supplementary material, which is available to authorized users.
References
- 1.Bilezikian JP, Rubin MR, Finkelstein JS. Parathyroid hormone as an anabolic therapy for women and men. J Endocrinol Invest. 2005;28:41–49. [PubMed] [Google Scholar]
- 2.Black DM, Bilezikian JP, Ensrud KE, et al. Rosen CJ; PaTH Study Investigators. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med. 2005;353(6):555–65. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 4.Blumsohn A, Marin F, Nickelsen T, et al. González de la Vera J, Boonen S, Liu-Léage S, Barker C, Eastell R; EUROFORS Study Group. Early changes in biochemical markers of bone turnover and their relationship with bone mineral density changes after 24 months of treatment with teriparatide. Osteoporos Int. 2011;22(6):1935–46. doi: 10.1007/s00198-010-1379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnick SL, Johnston CC, Kleerekoper M, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4:105–10. doi: 10.1385/JCD:4:2:105. [DOI] [PubMed] [Google Scholar]
- 6.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Miller PD, Delmas PD, et al. Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21:1785–1790. doi: 10.1359/jbmr.060802. [DOI] [PubMed] [Google Scholar]
- 8.Chen P, Satterwhite JH, Licata AA, et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20:962–70. doi: 10.1359/JBMR.050105. [DOI] [PubMed] [Google Scholar]
- 9.Cohen A, Stein EM, Recker RR, et al. Teriparatide for Idiopathic Osteoporosis in Premenopausal Women: A Pilot Study. J Clin Endocrinol Metab. 2013;98:1971–1981. doi: 10.1210/jc.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:503–511. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281–9. doi: 10.1016/S0002-9343(01)01124-X. [DOI] [PubMed] [Google Scholar]
- 12.Dobnig H, Sipos A, Jiang Y, et al. Early changes in biochemical markers of bone formation correlate with improvements in bone structure during teriparatide therapy. J Clin Endocrinol Metab. 2005;90:3970–3977. doi: 10.1210/jc.2003-1703. [DOI] [PubMed] [Google Scholar]
- 13.Eastell R, Robins SP, Colwell T, et al. Evaluation of bone turnover in type I osteoporosis using biochemical markers specific for both bone formation and bone resorption. Osteoporos Int. 1993;3:255–260. doi: 10.1007/BF01623829. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher JC, Rosen CJ, Chen P, et al. Response rate of bone mineral density to teriparatide in postmenopausal women with osteoporosis. Bone. 2006;39:1268–1275. doi: 10.1016/j.bone.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons RD, Hedeker D, DuToit S. Advances in Analysis of Longitudinal Data. Annu Rev Clin Psychol. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gluer CC. Monitoring skeletal changes by radiological techniques. J Bone Miner Res. 1999;14:1952–62. doi: 10.1359/jbmr.1999.14.11.1952. [DOI] [PubMed] [Google Scholar]
- 17.Kurland ES, Cosman F, McMahon DJ, et al. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000;85:3069–3076. doi: 10.1210/jcem.85.9.6818. [DOI] [PubMed] [Google Scholar]
- 18.Leder BZ, Tsai JN, Neer, RM, Uihlein AV, Wallace PM, Burnett-Bowie SM. Response to Therapy With Teriparatide, Denosumab, or Both in Postmenopausal Women in the DATA (Denosumab and Teriparatide Administration) Study Randomized Controlled Trial. Journal of Clinical Densitometry: Assessment & Management of Musculoskeletal Health 2016; 1–6. [DOI] [PubMed]
- 19.Lenchik L, Kiebzak GM, Blunt BA. What is the role of serial bone mineral density measurements in patient management? J Clin Densitom. 2002;5:S29–38. doi: 10.1385/JCD:5:3S:S29. [DOI] [PubMed] [Google Scholar]
- 20.Lewiecki EM. Non-responders to osteoporosis therapy. J Clin Densitom. 2003;6:307–14. doi: 10.1385/JCD:6:4:307. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Meredith MP, Hoseyni MS. A method to assess the proportion of treatment effect explained by a surrogate endpoint. Stat Med. 2001;20:3175–88. doi: 10.1002/sim.984. [DOI] [PubMed] [Google Scholar]
- 22.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 23.Ma YL, Bryant HU, Zeng Q, et al. New bone formation with teriparatide [human parathyroid hormone-(1–34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology. 2003;144:2008–2015. doi: 10.1210/en.2002-221061. [DOI] [PubMed] [Google Scholar]
- 24.Marcus R, Wang O, Satterwhite J, et al. The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res. 2003;18:18–23. doi: 10.1359/jbmr.2003.18.1.18. [DOI] [PubMed] [Google Scholar]
- 25.Muschitz C, Kocijan R, Fahrleitner-Pammer A, et al. Antiresorptives overlapping ongoing teriparatide treatment result in additional increases in bone mineral density. J Bone Miner Res. 2013;28(1):196–205. doi: 10.1002/jbmr.1716. [DOI] [PubMed] [Google Scholar]
- 26.Niimi R, Kono T, Nishihara A, et al. A retrospective analysis of nonresponse to daily teriparatide treatment. Osteoporos Int. 2016;27(9):2845–53. doi: 10.1007/s00198-016-3581-z. [DOI] [PubMed] [Google Scholar]
- 27.Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003;18:9–17. doi: 10.1359/jbmr.2003.18.1.9. [DOI] [PubMed] [Google Scholar]
- 28.Ravaud P, Reny JL, Giraudeau B, et al. Individual smallest detectable difference in bone mineral density measurements. J Bone Miner Res. 1999;14:1449–56. doi: 10.1359/jbmr.1999.14.8.1449. [DOI] [PubMed] [Google Scholar]
- 29.Recker RR, Marin F, Ish-Shalom S, et al. Comparative effects of teriparatide and strontium ranelate on bone biopsies and biochemical markers of bone turnover in postmenopausal women with osteoporosis. J Bone Miner Res. 2009;24:1358–1368. doi: 10.1359/jbmr.090315. [DOI] [PubMed] [Google Scholar]
- 30.Rubin MR, Bilezikian JP. The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med. 2003;19:415–432. doi: 10.1016/S0749-0690(02)00074-5. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar S, Mitlak BH, Wong M, et al. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res. 2002;17:1–10. doi: 10.1359/jbmr.2002.17.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Schafer AL, Sellmeyer DE, Palermo L, et al. Six months of parathyroid Hormone (1–84) administered concurrently versus sequentially with monthly ibandronate over two years: the PTH and ibandronate combination study (PICS) randomized trial. J Clin Endocrinol Metab. 2012;97(10):3522–9. doi: 10.1210/jc.2012-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai JN, Uihlein AV, Burnett-Bowie SA, et al. Comparative Effects of Teriparatide, Denosumab, and Combination Therapy on Peripheral Compartmental Bone Density, Microarchitecture, and Estimated Strength: the DATA-HRpQCT Study. J Bone Miner Res. 2015;30(1):39–45. doi: 10.1002/jbmr.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomized trial. Lancet. 2013;382:50–6. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. doi: 10.2307/2531248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(DOCX 13 kb)