Abstract
Hepatocarcinoma is one of the malignant cancers with significant morbidity and mortality. Immunotherapy has emerged in clinical treatment, owing to the limitation and severe side effects of chemotherapy. In the immune system, natural killer (NK) cells are important effectors required to eliminate malignant tumor cells without the limitation of major histocompatibility complex (MHC) molecule issues. Hence, treatment which could stimulate NK cells is of great interest. Here, we investigated the efficacy of the combined therapy of TT-1 (a mutant of melittin) and interferon-α (IFN-α) on NK cells and human liver cancer HepG-2/Huh7 cells in vitro and in vivo, as well as the mechanism involved. The combination therapy significantly inhibited the growth of HepG-2/Huh7 cells in vivo, but this effect was impaired after depleting NK cells. TT-1 not only up-regulated MHC class I-related chain molecules A (MICA) expression, but also prevented the secretion of soluble MICA (sMICA). Both the mRNA and protein of a disintegrin and metallopeptidase 10 (ADAM 10) in HepG-2/Huh7 cells were decreased after TT-1 treatment. The combined therapy of TT-1 and IFN-α could suppress the growth of HepG-2/Huh7 xenografted tumor effectively via promoting the interaction of NK group 2, member D (NKG2D) and MICA, indicating that TT-1+IFN-α would be a potential approach in treating liver cancer.
Keywords: TT-1, Interferon-α (IFN-α), Natural killer (NK) cells, Hepatocarcinoma, Immunotherapy
1. Introduction
Hepatocellular carcinoma (HCC), one of the fatal malignant tumors, is a leading cause of death among cirrhotic patients (Taïeb et al., 2003). Surgical resection, liver transplantation, ablation, and chemotherapy are common therapeutic methods for HCC (Liu et al., 2016). In most cases, the disease is found in the intermediate or advanced stage rather than at a more treatable stage. Unfortunately, fewer than 20% of patients with late-stage HCC can be treated by surgical resection (Hung, 2005; Yang et al., 2015). We found that the objective response rate (ORR) to a single cytotoxic regimen was merely 0%–10%, with no survival benefit after evaluating different cytotoxic agents for HCC (Guan et al., 2003; Boige et al., 2006; Hebbar et al., 2006; Liu et al., 2015). Immunotherapy, which is a novel therapeutic regimen for malignancy, aims at overcoming the limitations of conventional treatments.
The natural killer (NK) cell, one of the essential effector cells in the immune response, has been adopted in cellular immunotherapy for different cancers (Morisaki et al., 2011). NK cells express a variety of activating receptors, among which NK group 2, member D (NKG2D) has been shown to play a key role in tumor cell rejection and tumor immunosurveillance through binding to ligands such as major histocompatibility complex (MHC) class I-related chain molecules A (MICA) (Cerwenka et al., 2001; Xie et al., 2016). However, the shedding of MICA by tumor cells increases the serum level of soluble MICA (sMICA) and hinders the recognition of HCC by immune cells, resulting in tumor immune escape (Groh et al., 2002; Wang et al., 2016). In vitro studies have shown that sMICA could clearly reduce the expression of NKG2D on NK cells (Wu et al., 2004; 2009). Thus, sMICA is believed to cause the functional impairment of NK cells in MICA+ patients.
Cytokine therapy has been established as one of the main pillars of human cancer immunotherapy (Floros and Tarhini, 2015). Interferon-α (IFN-α), one of the cytokines, mediates immune responses towards Th1 cell, and enhances cytotoxicity and survival of NK cells, resulting in remarkable immunomodulatory effects. IFN-α is approved as a first line treatment for metastatic renal cell carcinoma (RCC), hairy cell leukemia, follicular lymphoma (in combination with Avastin), and as adjuvant treatment for high-risk melanoma (Thompson and Allison, 1997; Parlato et al., 2001). Nevertheless, there are some problems with IFN-α therapy, such as dose-limiting adverse effects and poor tolerability (Ueda et al., 2016). Thus, combination drug therapy of IFN-α and other antitumor drugs has emerged to foil resistance development.
Melittin is a 26-amino acid residue antimicrobial peptide with known antitumor activity. TT-1 (amino acid sequence: KIKAVLKVLTT), a mutant of melittin, was generated by a reduction of the peptide chain length and replacing glycines with lysines (Son et al., 2007; Oršolić, 2012; Sommer et al., 2012). The TT-1 retains the amino-terminal active site region of melittin, and has an increased hydrophobicity but a decreased net charge, which indicates a higher stability and lower toxicity than melittin. In our previous study, TT-1 exhibited a significant inhibitory effect on thyroid cancer cells in vitro, and showed significant anti-tumor activity on thyroid cancer cells in vivo, which suggested a potential inhibitory activity towards HCC cells. Here, we investigated the inhibiting effect of TT-1 on HepG-2/Huh7 cells and the regulatory effects to the secretion of sMICA and the expression of MICA on HepG-2/Huh7 cells. We also evaluated the anticancer efficacy of TT-1+IFN-α in a HepG-2/Huh7 xenografted tumor model. Further, the synergistic immunostimulatory activity of TT-1+IFN-α was verified through cytotoxicity assay.
2. Materials and methods
2.1. Cell culture and reagents
HepG-2/Huh7 and NK cells were obtained from American Type Culture Collection (ATCC, Manassas, USA). HepG-2/Huh7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco, USA). The NK cell line NK92 was cultured in supplemented alpha minimum essential medium (α-MEM), 12.5% (v/v) FBS (Hyclone, Mordlalloc, Australia), 12.5% (v/v) horse serum (Hyclone, Logan, USA), 0.2 mmol/L myo-inositol, 0.02 mmol/L folic acid, 0.1 mmol/L 2-mercaptoethanol (Sigma-Aldrich, St. Louis/MO, USA), and 200 U/ml hIL-2 (Millipore, Temecula, CA, USA). Nude mice (Academy of Military Medical Science, Beijing, China) were housed in a rodent facility at 22 °C and provided with continuous rodent food and water. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma-Aldrich. Monoclonal antibodies against a disintegrin and metallopeptidase 10 (ADAM 10) and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). The anti-MICA-PE antibody was obtained from Miltenyi Biotec (Teterow, Germany) and the anti-asialo GM-1 was purchased from Abcam (Cambridge, UK). The MICA enzyme-linked immunosorbent assay (ELISA) kit was purchased from KeyGen (Nanjing, China).
2.2. Cell proliferation assay
The effects of TT-1 on HepG-2/Huh7 cells were detected by MTT assay. A total of 5×103 HepG-2/Huh7 cells were inoculated to 96-well plates per well, and treated with various concentrations (ranging from 0 to 32 μg/ml) of TT-1 for 48 h. Subsequently, cells were incubated with the MTT solution (5 μg/ml) for 4 h at 37 °C followed by the addition of 150 μl dimethyl sulfoxide (DMSO) per well, and then shaken for 5 min before the absorbance was measured at 490 nm.
2.3. HepG-2/Huh7 xenograft mouse model and administration
HepG-2/Huh7 cells (1×107) were collected and then inoculated intradermally. Twelve days later nude mice were randomly (n=10) divided into four groups, which were the control group (normal saline), the TT-1 (1 mg/kg) group, the IFN-α (1 mg/kg) group, and the TT-1 (1 mg/kg)+IFN-α (1 mg/kg) group. Intra-tumor injection three times a week was adopted. At the indicated time points (the end of the fourth week after the treatment), the mice were sacrificed 24 h after the final administration. Tumor volume (V) was measured every 3 d during the treatment and calculated using the formula: V=(length×width2)/2. Intravenous injection of anti-asialo GM-1 has been shown to eliminate NK cell activity (Habu et al., 1981). To demonstrate that the antitumor efficacy of TT-1+IFN-α was mediated by NK cells via MICA-NKG2D pathway specifically, depletion of NK cells together with TT-1+IFN-α treatment was executed.
2.4. Flow cytometry
The expression of MICA on HepG-2/Huh7 cells after the incubation of TT-1 was detected by flow cytometry. Basically, HepG-2/Huh7 cells were incubated with various concentrations of TT-1 (0.5, 1, and 2 μg/ml), followed by species-specific (1:100) anti-MICA-PE antibody. Finally, the cells were detected by FACSCalibur.
2.5. ELISA
The secretion of MICA from HepG-2/Huh7 cells after the incubation of TT-1 was detected using the commercial ELISA kit. Briefly, HepG-2/Huh7 cells in 96-well plates were treated with various concentrations of TT-1 (from 0 to 16 μg/ml) for 48 h, then the cell culture supernatant was collected and the sMICA level was detected by the ELISA kit.
2.6. RT-PCR
The shedding of MICA by tumor cells was inhibited by silencing ADAM 10 protease (Waldhauer et al., 2008). The effect of TT-1 on the ADAM 10 RNA expression was examined by real-time polymerase chain reaction (RT-PCR). HepG-2/Huh7 cells were treated with TT-1 (ranging from 0 to 2 μg/ml) for 48 h, then the total RNA was extracted using TRIzol reagent and purified with the RNeasy Mini Kit (QIAGEN, CA, USA). Subsequently, RT-PCR was conducted with an ABI PRISM 7300 sequence detection system. The comparative C T (2−ΔΔ C T) method was used to calculate the relative mRNA expression.
2.7. Immunoblotting assay
The effect of TT-1 on ADAM 10 expression was detected by Western blot. HepG-2/Huh7 cells were treated with TT-1 (ranging from 0 to 2 μg/ml) for 48 h, and then homogenized in a lysis buffer (0.5% (v/v) Trition X-100, 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 50 mmol/L Hepes, pH 7.5). Proteins were resolved by electrophoresis then transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were incubated with the anti-ADAM 10 antibody after being blocked, then the membranes were incubated by the secondary antibody. Finally, the membranes were reacted with the electrochemiluminescence (ECL) reagent and exposed to the Bio-Rad detection system. The β-actin was detected to ensure that the proteins were equally loaded.
2.8. Lactate dehydrogenase release cytotoxicity assay
The lactate dehydrogenase (LDH) released from the HepG-2/Huh7 cells was detected by the CytoTox 96 nonradioactive cytotoxicity assay (Promega, Madison, USA). A total of 5×103 HepG-2/Huh7 cells (target cells) were co-cultured with various amounts of NK92 cells (effector cells) in the presence or absence of TT-1 (2 μg/ml) and IFN-α (2 μg/ml) for 4 h at 37 °C. Controls for spontaneous LDH release in effector and target cells, as well as target maximum release, were prepared. The calculation of cytotoxicity percentage was as follows: cytotoxicity (%)=(experimental release−effector spontaneous−target spontaneous)/(target maximum−target spontaneous)×100%.
2.9. Immunohistochemical analysis
We cut the paraffin sections into 5-μm sections and fixed them with 4% paraformaldehyde. For immunohistochemical (IHC) staining, the sections were incubated with MICA/NKG2D antibody (Abcam, Cambridge, UK), followed by the horseradish peroxidase-labeled secondary antibody, and then analyzed with the Vectastain ABC kit (Dako, Copenhagen, Denmark).
2.10. Statistical analysis
All experiments were performed in triplicate. The data were presented as the mean±standard deviation (SD). Statistical analysis was executed by one-way analysis of variance (ANOVA), Dunnett’s multiple comparison, or Student’s t-test, where P<0.05 was considered as a statistically significant result.
3. Results
3.1. Inhibitory effect of TT-1 on proliferation of HepG-2/Huh7 cells
As shown in Fig. 1, the inhibitory effect of TT-1 on HepG-2/Huh7 cells was in a dose-dependent manner with the 50% inhibitory concentrations (IC50) of (3.762±0.285) μg/ml/(5.823±0.138) μg/ml at 72 h. Especially, at 32 μg/ml TT-1, the cell viability of HepG-2/Huh7 cells decreased to 14.8%/26.9%, which showed that TT-1 exhibited potent cytotoxicity to HepG-2/Huh7 cells.
Fig. 1.
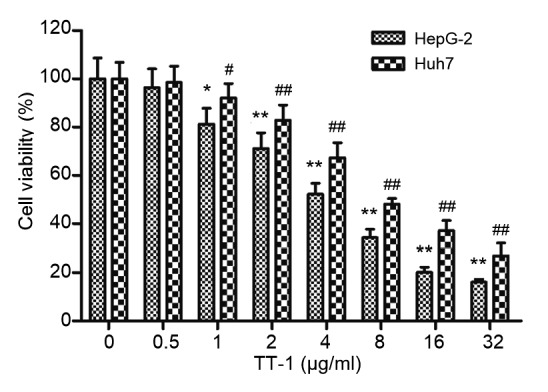
Inhibitory effect of TT-1 on proliferation of HepG-2/Huh7 cells
HepG-2/Huh7 cells were treated with a series of concentrations of TT-1 for 48 h. Cell viability was measured by MTT assay. Values are presented as mean±SD for three independent experiments. * P<0.05, ** P<0.01, # P<0.05, ## P<0.01, versus TT-1-untreated group
3.2. Inhibitory effect of TT-1+IFN-α on tumor growth in HepG-2/Huh7-bearing mice
The HepG-2/Huh7 xenograft model was established successfully. After the treatment, the tumor volumes and weights were measured. As shown in Fig. 2, TT-1 could inhibit the growth of the tumor effectively. IFN-α could suppress the tumor growth slightly, but the tumor volumes and weights were not significantly different from those of other groups. It is noteworthy that the combination of TT-1 and IFN-α could enhance the antitumor activity significantly compared to TT-1. Moreover, the data of tumor volumes and weights indicated that the tumor growth inhibitory activity of TT-1+IFN-α was suppressed remarkably after depleting NK cells using anti-asialo GM-1 antibody (Fig. 3). The control group, in which anti-asialo GM-1 was replaced by an isotype antibody, exhibited a similar tumor inhibitory rate to the TT-1+IFN-α group, which suggested that NK cells played a key role in the immune response. The results above demonstrated that the antitumor efficacy of TT-1+IFN-α was mediated by NK cells specifically via MICA-NKG2D pathway.
Fig. 2.
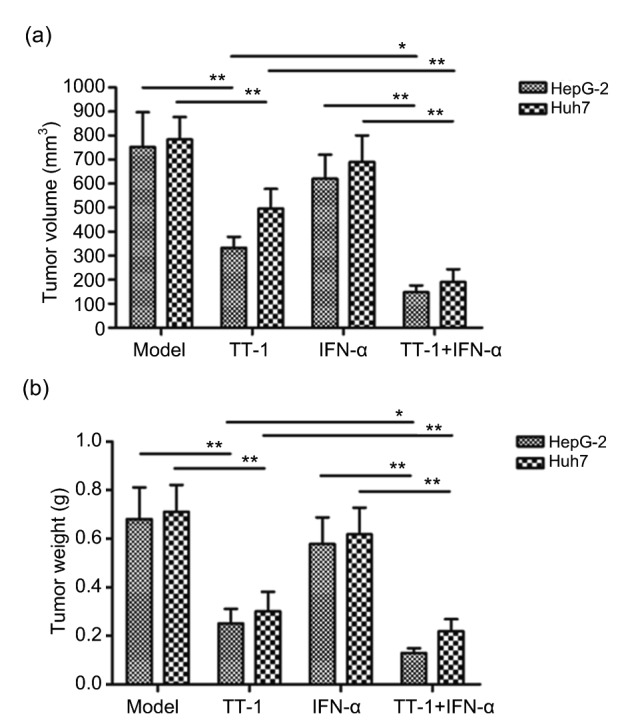
In vivo TT-1+IFN-α efficacy against a HepG-2/Huh7 xenograft
(a) Tumor volumes in HepG-2/Huh7-bearing nude mice. (b) Tumor weight of each group. Each BALB/c nude mouse was subcutaneously injected 1×107 HepG-2/Huh7 cells for different treatments. Treatment began following tumor development, when the measurement of tumor volume also started. Data are presented as mean±SD (n=10). * P<0.05, ** P<0.01
Fig. 3.
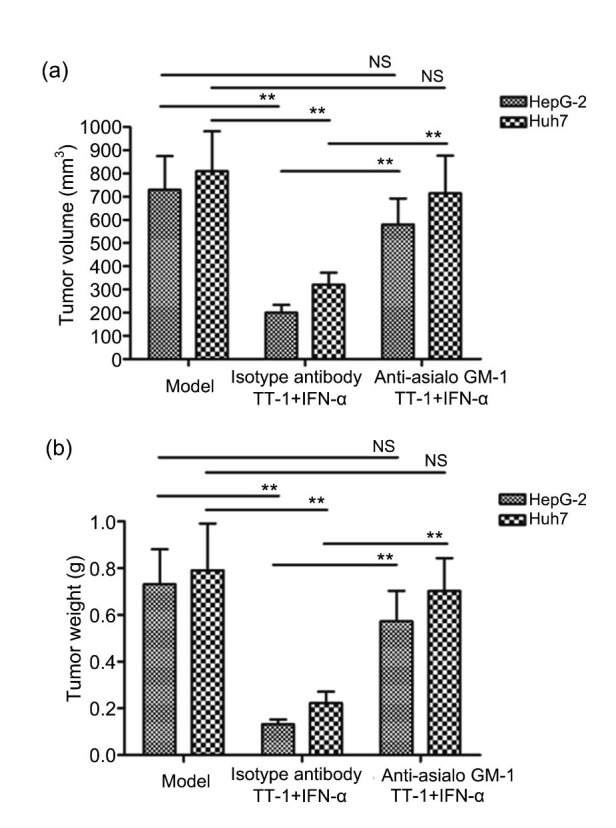
Antitumor activity of TT-1+IFN-α impaired significantly by anti-asialo GM-1
Intravenous injection of anti-asialo GM-1 was carried out to eliminate NK cell activity before the treatment with TT-1+IFN-α, and an isotype antibody was used as control. (a) Tumor volume of each group. (b) Tumor weight of each group. Data are presented as mean±SD (n=10). ** P<0.01. N.S.: no significance
3.3. Effect of TT-1 on the expression of MICA on HepG-2/Huh7 cells
In vivo activity study showed that TT-1 exhibited remarkable antitumor activity when combined with IFN-α which could stimulate the NK cells, implying that TT-1 was likely to up-regulate the MICA expression on HepG-2/Huh7 cells to assist the function of IFN-α. Hence, the flow cytometry was conducted to evaluate the MICA expression. As shown in Fig. 4, the MICA positive rate of HepG-2/Huh7 cells increased with increasing TT-1 concentration. The mean fluorescence intensity (MFI) could reach 40% approximately when the TT-1 concentration was 2 μg/ml. In conclusion, TT-1 treatment could increase the expression of MICA on HepG-2/Huh7 cells as anticipated.
Fig. 4.
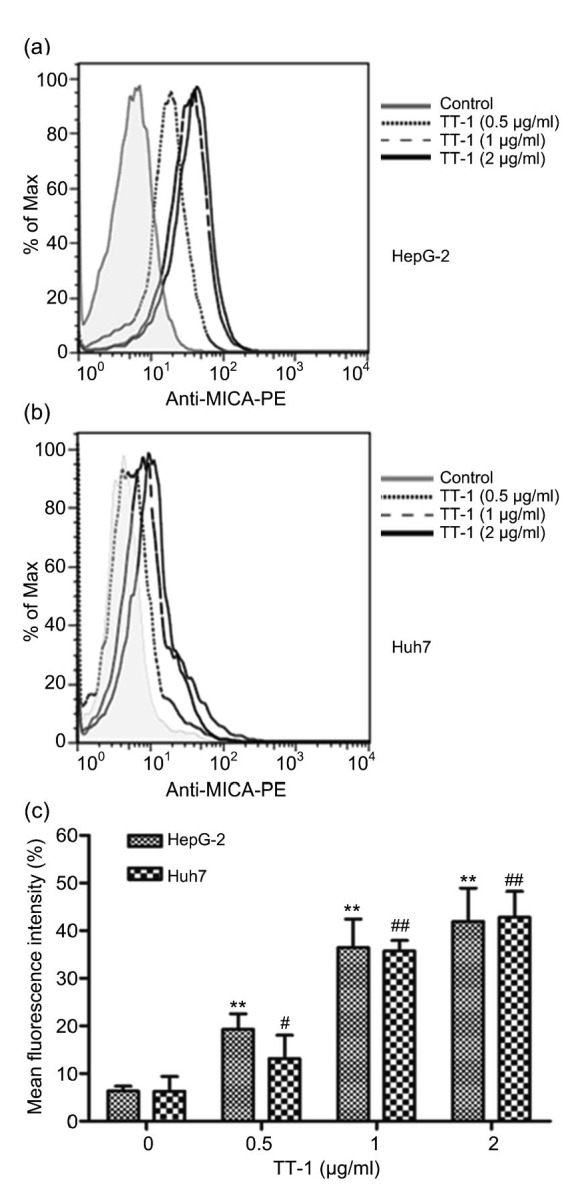
Expression of MICA on HepG-2/Huh7 cells
HepG-2/Huh7 cells were treated with TT-1 (ranging from 0 to 2 μg/ml) for 48 h, and MICA expression was quantified by flow cytometry. (a) Representative histogram of HepG-2 cells. (b) Representative histogram of Huh7 cells. (c) Representative normalized mean fluorescence (mean fluorescence intensity (MFI) of TT-1-treated cells/MFI of control cells). Values are presented as mean±SD for three independent experiments. ** P<0.01, # P<0.05, ## P<0.01, versus TT-1-untreated group
3.4. Effect of TT-1 on the secretion of MICA by HepG-2/Huh7 cells
ELISA assay was executed to demonstrate whether TT-1 could decrease the expression of sMICA at the same time as increasing the expression of MICA on HepG-2/Huh7 cells. The results of ELISA (Fig. 5a) showed that TT-1 inhibited the expression of sMICA in a dose-dependent manner. In order to further confirm this effect of TT-1 on HepG-2/Huh7 cells, the expression of the related ADAM 10 protease was detected on the levels of RNA and protein after TT-1 treatment. As shown in Fig. 5b, the mRNA level of ADAM 10 in HepG-2/Huh7 cells was dose-dependently down-regulated by TT-1. Analysis by t-test showed that a significant difference of ADAM 10 mRNA appeared when the concentration of TT-1 reached 1 μg/ml. Similarly, the Western blot results showed that TT-1 decreased the expression of ADAM 10. Specifically, at 2 μg/ml TT-1, the ratio (ADAM 10:β-actin) of HepG-2/Huh7 was 0.35/0.46 in the group without TT-1. The data above demonstrated that TT-1 could reduce the secretion of MICA by HepG-2/Huh7 cells.
Fig. 5.

Secretion of MICA by HepG-2/Huh7 cells
(a) ELISA detected the sMICA level in the culture supernatant. HepG-2/Huh7 cells were treated with various concentrations of TT-1 (from 0 to 16 μg/ml) for 48 h, then the sMICA level of the culture supernatant was detected using the ELISA kit. (b) Quantitative PCR analysis of ADAM 10 genes in HepG-2/Huh7 cells. HepG-2/Huh7 cells were treated with various concentrations of TT-1 (from 0 to 2 μg/ml) for 48 h, then total RNA was extracted from HepG-2/Huh7 cells, and real-time PCR was conducted. Data are expressed as the fold increase of mRNA expression in TT-1 treated cells relative to untreated cells. (c) Western blot analysis confirmed the down-regulation of ADAM 10 at the protein level. (d) Gray scanning and data statistics of (c). Values are presented as mean±SD for three independent experiments. * P<0.05, ** P<0.01, # P<0.05, ## P<0.01, versus TT-1-untreated group
3.5. Effect of TT-1+IFN-α on the cytotoxicity of NK92 cells
The immunological enhancement mediated by the MICA-NKG2D pathway was evaluated through cytotoxicity assay in vitro. As shown in Fig. 6, the NK cytotoxicity to HepG-2/Huh7 cells increased with increasing effector-target (E:T) ratios. The addition of IFN-α (2 μg/ml) could strengthen the cytotoxicity of NK cells, but there were no obvious changes of the cytotoxicity found when treated with TT-1 (2 μg/ml). It is noteworthy that the cytotoxicity of NK cells increased significantly when treated with the combination of TT-1 (2 μg/ml) and IFN-α (2 μg/ml). Fig. 6a showed that the NK cytotoxicity on HepG-2 cells approached 45% at the 10:1 E:T ratio and 35% at the 5:1 E:T ratio, which were levels considerably higher than those of the control group (where there was neither TT-1 nor IFN-α); Fig. 6b exhibited a similar result in Huh7 cells. The results of cytotoxicity assay were consistent with those of the tumor inhibition experiments in vivo.
Fig. 6.
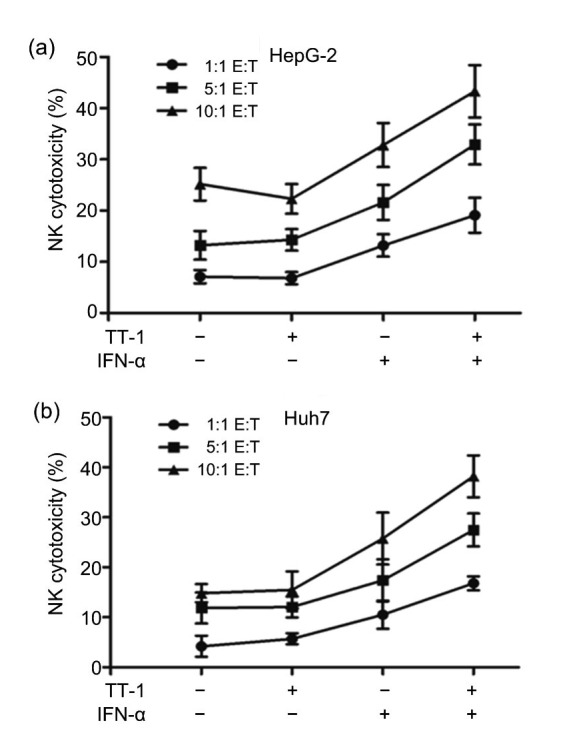
Enhancement of TT-1+IFN-α on the cytotoxicity of NK92 cells HepG-2
(a)/Huh7 (b) cells were co-cultured with various amounts of NK92 cells in the presence or absence TT-1/IFN-α, and cytotoxicity was determined by measuring the released LDH. The 1:1, 5:1, or 10:1 E:T ratio was set respectively. Values are presented as mean±SD for three independent experiments
3.6. Effect of TT-1+IFN-α on the expression of MICA and NKG2D in tumor tissues
IHC analysis was undertaken to examine additional evidence for the conclusions that the antitumor efficacy of TT-1+IFN-α was mediated by NK cells via MICA-NKG2D pathway and that TT-1 could up-regulate the MICA expression on tumor cells to assist the function of IFN-α. Fig. 7a demonstrates that there was a significant augmentation in the numbers and intensity of MICA expression in TT-1+IFN-α-treated tumors compared to untreated groups. IHC analysis revealed that the expression level of NKG2D on infiltrating NK cells was considerably higher in tumor tissues of mice treated with TT-1+IFN-α than in tumors from the untreated mice (Fig. 7b).
Fig. 7.
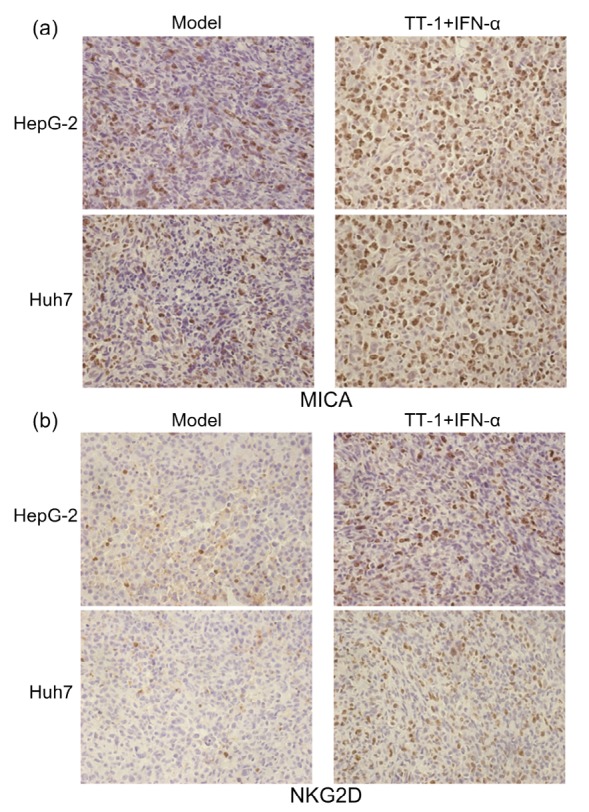
IHC analysis of the expression of MICA and NKG2D in tumor tissues
(a) IHC staining of MICA on paraffin sections of a xenografted tumor. MICA+ cells were identified with an anti-MICA antibody (brown staining). (b) Infiltrated NKG2D+ cells were detected by IHC staining (brown staining) on serial sections, demonstrating more expression of NKG2D with TT-1+IFN-α treatment (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
4. Discussion
The ORR of HCC to chemotherapy has been very frustrating, putting the oncologists in a predicament in the long-term (Dusheiko et al., 1992; Lee et al., 2003). Moreover, conventional radiotherapy and chemotherapy often have severe side effects (Leung et al., 2007). Thus, it is essential to find new therapeutic strategies with low cytotoxicity, but with specificity and availability for chronic treatment.
Antimicrobial peptides with low intrinsic cytotoxicity which could reduce drug resistance have recently attracted significant attention as new antitumor agents. It has been demonstrated previously that melittin (a cationic, hemolytic peptide isolated from honeybee venom) has anti-arthritic, antibacterial, and anti-inflammatory activity (Brown and Kirkwood, 2003). In addition, it has been confirmed that melittin can selectively destroy several tumor cells, such as breast, renal, and lung cells in vitro. To make it more effective in treating cancer cells, we designed a novel peptide, TT-1, based on the amphipathic structure of melittin. In this study, the cytotoxic effect of TT-1 in vitro revealed that TT-1 could dose-dependently suppress the proliferation of HepG-2/Huh7 cells. The antitumor study in vivo also implied the potential inhibition activity of TT-1 on a HepG-2/Huh7 xenografted tumor.
Further, we found that MICA expression on the surface of HepG2/Huh7 cells was increased after exposure of HepG2/Huh7 cells to TT-1. Also TT-1 could reduce the shedding of MICA from HepG2/Huh7 cells. Previous study had demonstrated that the release of sMICA from tumor cells is impaired by the metalloproteinase inhibitors, such as members of matrix metalloproteinase (MMP) families and ADAM proteins (Salih et al., 2002; Waldhauer and Steinle, 2006). Whereas MMPs are mainly involved in the destruction of the extracellular matrix, many ADAMs are membrane-tethered proteases and one of their major functions is the proteolytic release of the extracellular domain of transmembranous proteins from the cell surface (Reiss et al., 2006). Thus, the reduction of ADAM 10 protein expression and ADAM 10 mRNA after TT-1 treatment confirmed the effect of TT-1 on sMICA. The immunomodulatory effects of TT-1 on cancer cells were found for the first time, suggesting that immune cell-activated cytokines could therefore be used combined with TT-1. The cytokine IFN-α with significant immunomodulatory effects selected in this study, combined with TT-1, could really enhance the cytotoxicity and survival of NK cells, and this was confirmed by the NK cytotoxicity assay in vitro, the tumor inhibition experiments in vivo, and the IHC analysis.
Treatment with TT-1 enhanced the susceptibility of HepG-2/Huh7 cells to NK cells treated by IFN-α, which indicated that the interaction between NKG2D and MICA played a key role in NK-mediated lysis of HepG-2/Huh7 cells, and the susceptibility of tumor cells (treated by TT-1) to NK cells might be up-regulated by the increase of MICA. Given the extensive distribution of NKG2D on immune cells and the anticancer significance of interactions between NKG2D and MICA, it is reasonable to speculate that IFN-α may thus enhance NK cell immunotherapies (Zwirner et al., 2007). It is noteworthy that IFN-α is a potent inhibitor of myeloid-derived suppressor cells (MDSCs) which could interfere with the host immune response to tumors. Hence, whether the potent anti-tumor activity of TT-1+IFN-α partially benefited from the host immune response enhanced by the interaction of IFN-α and MDSCs needs further research (Mundy-Bosse et al., 2011).
In conclusion, we have shown that TT-1 could inhibit the proliferation of HepG-2/Huh7 cells and induce MICA expression in HepG-2/Huh7 cells, resulting in the enhancement of the cytotoxic effects of NK cells and a synergistic antitumor effect when combined with IFN-α. Therefore, the combination of TT-1 with IFN-α immunotherapy may have clinical significance for the therapy of HCC.
Footnotes
Project supported by the Outstanding Young Talent Foundation Project of Science and Technology Department in Jilin Province (No. 20170520018JH), China
Compliance with ethics guidelines: Lan-lan WAN, Da-qi ZHANG, Jin-nan ZHANG, and Li-qun REN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Boige V, Taïeb J, Hebbar M, et al. Irinotecan as first-line chemotherapy in patients with advanced hepatocellular carcinoma: a multicenter phase II study with dose adjustment according to baseline serum bilirubin level. Eur J Cancer. 2006;42(4):456–459. doi: 10.1016/j.ejca.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Brown CK, Kirkwood JM. Medical management of melanoma. Surg Clin North Am. 2003;83(2):283–322. doi: 10.1016/S0039-6109(02)00187-1. [DOI] [PubMed] [Google Scholar]
- 3.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo . Proc Natl Acad Sci USA. 2001;98(20):11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dusheiko GM, Hobbs KE, Dick R, et al. Treatment of small hepatocellular carcinomas. Lancet. 1992;340(8814):285–288. doi: 10.1016/0140-6736(92)92367-O. [DOI] [PubMed] [Google Scholar]
- 5.Floros T, Tarhini AA. Anticancer cytokines: biology and clinical effects of IFN-α2, IL-2, IL-15, IL-21, and IL-12. Semin Oncol. 2015;42(4):539–548. doi: 10.1053/j.seminoncol.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groh V, Wu J, Yee C, et al. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 7.Guan Z, Wang Y, Maoleekoonpairoj S, et al. Prospective randomized phase II study of gemcitabine at standard or fixed dose rate schedule in unresectable hepatocellular carcinoma. Br J Cancer. 2003;89(10):1865–1869. doi: 10.1038/sj.bjc.6601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habu S, Fukui H, Shimamura K, et al. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981;127(1):34–38. [PubMed] [Google Scholar]
- 9.Hebbar M, Ernst O, Cattan S, et al. Phase II trial of docetaxel therapy in patients with advanced hepatocellular carcinoma. Oncology. 2006;70(2):154–158. doi: 10.1159/000093007. [DOI] [PubMed] [Google Scholar]
- 10.Hung H. Treatment modalities for hepatocellular carcinoma. Curr Cancer Drug Targets. 2005;5(2):131–138. doi: 10.2174/1568009053202063. [DOI] [PubMed] [Google Scholar]
- 11.Lee WP, Tai DI, Tsai SL, et al. Adenovirus type 5 E1A sensitizes hepatocellular carcinoma cells to gemcitabine. Cancer Res. 2003;63(19):6229–6236. [PubMed] [Google Scholar]
- 12.Leung HW, Yang WH, Lai MY, et al. Inhibition of 12-lipoxygenase during baicalein-induced human lung non-small carcinoma H460 cell apoptosis. Food Chem Toxicol. 2007;45(3):403–411. doi: 10.1016/j.fct.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Yue H, Xu S, et al. First-line gemcitabine and oxaliplatin (GEMOX) plus sorafenib, followed by sorafenib as maintenance therapy, for patients with advanced hepatocellular carcinoma. Int J Clin Oncol. 2015;20(5):952–959. doi: 10.1007/s10147-015-0796-5. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Li Y, Wang R, et al. MiR-130a-3p regulates cell migration and invasion via inhibition of Smad4 in gemcitabine resistant hepatoma cells. J Exp Clin Cancer Res. 2016;35:19. doi: 10.1186/s13046-016-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morisaki T, Onishi H, Koya N, et al. Combinatorial cytotoxicity of gemcitabine and cytokine-activated killer cells in hepatocellular carcinoma via the NKG2D-MICA/B system. Anticancer Res. 2011;31(7):2505–2510. [PubMed] [Google Scholar]
- 16.Mundy-Bosse BL, Lesinski GB, Jaime-Ramirez AC, et al. Myeloid-derived suppressor cell inhibition of the IFN response in tumor-bearing mice. Cancer Res. 2011;71(15):5101–5110. doi: 10.1158/0008-5472.CAN-10-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oršolić N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31(1-2):173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- 18.Parlato S, Santini SM, Lapenta C, et al. Expression of CCR-7, MIP-3β, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98(10):3022–3029. doi: 10.1182/blood.V98.10.3022. [DOI] [PubMed] [Google Scholar]
- 19.Reiss K, Ludwig A, Saftig P. Breaking up the tie: disintegrin-like metalloproteinases as regulators of cell migration in inflammation and invasion. Pharmacol Ther. 2006;111(3):985–1006. doi: 10.1016/j.pharmthera.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169(8):4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 21.Sommer A, Fries A, Cornelsen I, et al. Melittin modulates keratinocyte function through P2 receptor-dependent ADAM activation. J Biol Chem. 2012;287(28):23678–23689. doi: 10.1074/jbc.M112.362756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son DJ, Lee JW, Lee YH, et al. Therapeutic application of anti-arthritis, pain-releasing, and anticancer effects of bee venom and its constituent compounds. Pharmacol Ther. 2007;115(2):246–270. doi: 10.1016/j.pharmthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Taïeb J, Bonyhay L, Golli L, et al. Gemcitabine plus oxaliplatin for patients with advanced hepatocellular carcinoma using two different schedules. Cancer. 2003;98(12):2664–2670. doi: 10.1002/cncr.11869. [DOI] [PubMed] [Google Scholar]
- 24.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–450. doi: 10.1016/S1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 25.Ueda K, Akiba J, Ogasawara S, et al. Growth inhibitory effect of an injectable hyaluronic acid-tyramine hydrogels incorporating human natural interferon-α and sorafenib on renal cell carcinoma cells. Acta Biomater. 2016;29:103–111. doi: 10.1016/j.actbio.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Waldhauer I, Steinle A. Proteolytic release of soluble UL16-binding protein 2 from tumor cells. Cancer Res. 2006;66(5):2520–2526. doi: 10.1158/0008-5472.CAN-05-2520. [DOI] [PubMed] [Google Scholar]
- 27.Waldhauer I, Goehlsdorf D, Gieseke F, et al. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68(15):6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Sun F, Xie W, et al. A bispecific protein rG7S-MICA recruits natural killer cells and enhances NKG2D-mediated immunosurveillance against hepatocellular carcinoma. Cancer Lett. 2016;372(2):166–178. doi: 10.1016/j.canlet.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Wu JD, Higgins LM, Steinle A, et al. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest. 2004;114(4):560–568. doi: 10.1172/JCI200422206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu JD, Atteridge CL, Wang X, et al. Obstructing shedding of the immunostimulatory MHC class I chain-related gene B prevents tumor formation. Clin Cancer Res. 2009;15(2):632–640. doi: 10.1158/1078-0432.CCR-08-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie W, Liu F, Wang Y, et al. VEGFR2 targeted antibody fused with MICA stimulates NKG2D mediated immunosurveillance and exhibits potent anti-tumor activity against breast cancer. Oncotarget. 2016;7(13):16445–16461. doi: 10.18632/oncotarget.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang JT, Tang LH, Liu YQ, et al. Cisplatin combined with hyperthermia kills HepG2 cells in intraoperative blood salvage but preserves the function of erythrocytes. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(5):395–403. doi: 10.1631/jzus.B1400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwirner NW, Fuertes MB, Girart MV, et al. Cytokine-driven regulation of NK cell functions in tumor immunity: role of the MICA-NKG2D system. Cytokine Growth Factor Rev. 2007;18(1-2):159–170. doi: 10.1016/j.cytogfr.2007.01.013. [DOI] [PubMed] [Google Scholar]


