Abstract
Essentially, all neonates are exposed to infections, antibiotics, or vaccines early in their lives. This is especially true for those neonates born underweight or premature. In contrast to septic adults and children who are at an increased risk for subsequent infections, exposure to infection during the neonatal period is not associated with an increased risk of subsequent infection and may be paradoxically associated with reductions in late-onset sepsis (LOS) in the most premature infants. Perinatal inflammation is also associated with a decreased incidence of asthma and atopy later in life. Conversely, septic neonates are at increased risk of impaired long-term neurodevelopment. While the positive effects of antibiotics in the setting of infection are irrefutable, prolonged administration of broad-spectrum, empiric antibiotics in neonates without documented infection is associated with increased risk of LOS, necrotizing enterocolitis, or death. Vaccines provide a unique opportunity to prevent infection-associated disease; unfortunately, vaccinations have been largely unsuccessful when administered in the first month of life with the exception of vaccines against hepatitis B and tuberculosis. Future vaccines will require the use of novel adjuvants to overcome this challenge. This review describes the influence of infections, antibiotics, and vaccines during the first days of life, as well as the influence on future health and disease. We will also discuss potential immunomodulating therapies, which may serve to train the preterm immune system and reduce subsequent infectious burden without subjecting neonates to the risks accompanied by virulent pathogens.
Keywords: innate immunity, inflammation, infectious disease, sepsis, vaccination, immune agonists
Introduction
Neonates, especially those born preterm (<37 weeks gestation), are prone to infections and sepsis given their diminished adaptive and innate immunity, decreased pro-inflammatory response, and attenuated antigen presentation and signaling (1). This unique immunological profile is possibly a result of the intrauterine fetal environment in which there is a need for immune tolerance to maternal antigens (2); however, this lack of a substantial immune response places the neonate at significant risk to microbes in the extrauterine environment. In the United States, early-onset sepsis (EOS; defined as sepsis occurring in the first 72 h of life) occurs at approximately 0.76–1 case per 1,000 live births with an increased incidence among very low birth weight (VLWB; <1,500 g) and preterm neonates (3–6). Due to concerns for infectious complications among preterm neonates, empiric antibiotics are almost universally administered shortly after birth (7). Meanwhile, healthy term neonates are administered hepatitis B vaccination in the first month of life, generally during the birth hospitalization or first clinic visit. How the presence of infections as well as the use of antibiotics and vaccines in the early neonatal period influences future health and disease remains an extremely complex and expanding topic. Herein, we review the impact of early life immune system exposures and discuss the use of immunomodulatory therapies to positively augment host protective immunity.
Early-Life Exposures
Infections
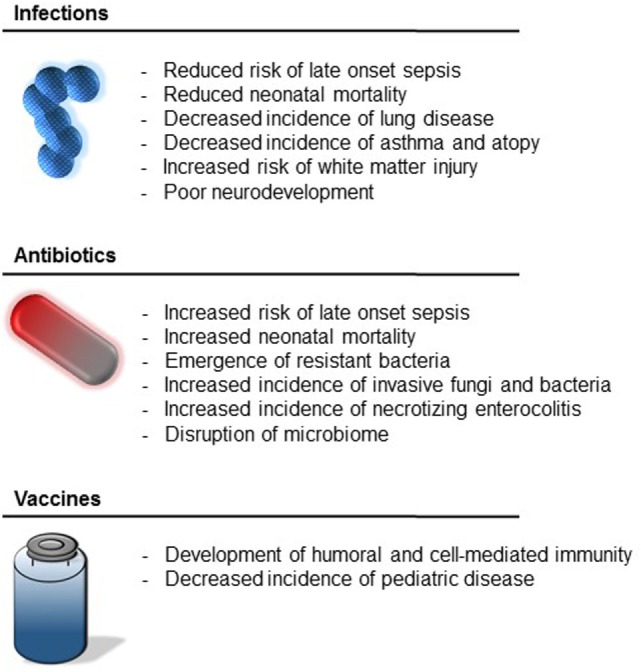
Early-onset sepsis is generally acquired via maternal ascending vaginal infection (8) (Table 1). Untreated genital tract colonization with group B streptococcus (GBS), prolonged rupture of membranes, and chorioamnionitis are known risk factors for the development of EOS (9–12). Importantly, two large, retrospective studies of very low birth weight (VLBW) neonates failed to identify an association between the development of EOS and the risk for subsequent or late-onset sepsis (LOS; defined as sepsis occurring after 72 h of life) (13, 14). Interestingly, neonates who were born at <25 weeks gestation and survived EOS showed a significant reduction in risk of LOS or death by 120 days (14). Extremely preterm neonates that are able to survive EOS may simply have a more robust immune response and thus, have a bias to equally fair well with subsequent infections. An alternative explanation offered by the authors is that the early immune stimulus may transform the preterm neonatal immune system from a relative state of tolerance to a level of competence that is better suited to defend against pathogens in the extrauterine environment (14).
Table 1.
Early-onset sepsis (EOS) and late-onset sepsis (LOS) characteristics.
| EOS | LOS | |
|---|---|---|
| Age | <72 h | >72 h |
| Source | Maternal genital tract | Nosocomial |
| Pathogen | GBS, Escherichia coli | Coagulase-negative staphylococcus |
| Risk factors | Maternal infections, prolonged ROM, chorioamnionitis | Prolonged mechanical ventilation and intravascular access |
| Incidence | 1.7% among VLBW neonates | 21% among VLBW neonates |
Chorioamnionitis likewise has been shown to be associated with reductions in respiratory distress syndrome, chronic lung disease, and mortality in preterm neonates (16–19) (Figure 1). The mechanism by which chorioamnionitis decreases the incidence of pulmonary disease in preterm neonates is likely via increased levels of interleukin (IL)-1 and IL-6, which stimulate pulmonary surfactant production and promote fetal lung maturation (20–23). Meanwhile, the observed difference in mortality among neonates born to mothers with chorioamnionitis may be explained in part by the fact that these neonates develop significantly fewer cases of LOS, which has been associated with prolonged hospital stays and death (15, 24).
Figure 1.
Impact of early-life exposures.
The presence of early exposure to inflammation, bacteria, and infections may have lasting beneficial effects. Sepsis among preterm neonates appears protective to the development of childhood asthma (25). Birth cohort studies investigating the impact of endotoxin [bacterial lipopolysaccharide (LPS)] exposure during infancy on the risk of later wheezing, atopy, and asthma have had largely mixed results (26–32). More recently, Lynch et al. demonstrated that children who had reduced bacterial exposure in the first year of life were more likely to develop atopy at age 3 years (33). In the September 2015 issue of Science, Schuijs et al. published their results utilizing a murine model of asthma to investigate the impact of chronic exposure to low-dose endotoxin and concluded that endotoxin protects mice from asthma development by increasing the synthesis of the enzyme A20 (a nuclear factor-κB attenuator) in airway epithelial cells (34).
Early exposure to inflammation and infection is not without harmful and devastating effects. VLBW neonates with EOS are about threefold more likely to die than those without EOS with an overall mortality of 35–37% by 120 days (3, 35). Moreover, a meta-analysis of 17 studies demonstrated that sepsis was associated with poor long-term neurodevelopment among VLBW neonates including cerebral palsy (36). Likewise, chorioamnionitis appears to be associated with cystic periventricular leukomalacia in preterm neonates, encephalopathy in term neonates, and cerebral palsy in both preterm and term neonates (37–41). Injury to the preterm brain is believed to result from a multi-hit mechanism in which the neonate is first exposed to inflammation and cytokine release in utero, leading to increased susceptibility to subsequent perinatal and postnatal insults (42). This model is supported by the work of Korzeniewski et al. who demonstrated the cumulative contributions of chronic placental inflammation, acute fetal inflammation, and postnatal inflammatory events on neonatal white matter injury (43). Microglial activation has a central role in this process via excitotoxic, inflammatory, and free radical injury to the developing central nervous system (44). The aforementioned hypothesis has been further validated in rat models of the generation of neuroinflammation in which rat pups subjected to both endotoxin and hypoxic ischemia demonstrated white water injury but sham and endotoxin alone groups did not (45). The group that received both endotoxin and hypoxic ischemia notably had an increase in activated microglia and tumor necrosis factor (TNF)-alpha expression compared to the other groups. The investigation of potential therapies to treat theses prenatal insults by targeting activated microglia and astrocytes is ongoing and includes the administration of dendrimer-based N-acetyl-l-cysteine treatment in the postnatal period, which has been shown to suppress neuroinflammation and improve motor function in newborn rabbits with cerebral palsy (46).
Antibiotics
The first exposure to antibiotics often occurs prior to birth in the form of intrapartum antibiotic prophylaxis against GBS, treatment of suspected chorioamnionitis, or antibiotic prophylaxis for women undergoing elective or emergency Cesarean sections. The use of intrapartum antibiotics is steadily increasing; for example, Van Dyke et al. demonstrated that the percentage of pregnant women receiving intrapartum increased from 26.8% in 1998–1999 to 31.7% in 2003–2004 (47). In an era of widespread prophylactic treatment of GBS for colonized pregnant women, the incidence of invasive early-onset GBS disease has decreased by more than 80%; however, the incidence of late-onset invasive GBS disease has remained unchanged (48). The use of maternal antibiotic prophylaxis is not without risks including the emergence of antimicrobial resistance invasive GBS and other neonatal pathogens. Between 1996 and 2003, clindamycin and erythromycin resistance has significantly increased in invasive GBS isolates (49). The incidence of early-onset Escherichia coli sepsis has also significantly increased in VLBW neonates with 64–85% of recent cases having resistance to ampicillin (35, 50). Not unexpectedly, neonates with ampicillin-resistant E. coli infections were more likely to be born from mothers who received intrapartum ampicillin (35, 50–52). Moreover, a multicenter case–control study during 1995–1996 demonstrated that cases of resistance E. coli infection were more often preterm (91 vs 20%, p < 0.001) and had significantly greater morality (40.9 vs 0%, p = 0.017), compared to cases of susceptible E. coli infections (51).
Among underweight and preterm neonates, the use of empiric antibiotics has essentially become standard of practice with antibiotics being the most prescribed medications in the neonatal intensive care unit (53). This phenomenon is largely due to the difficulty of accurately diagnosing neonatal sepsis in symptomatic neonates with developmental immaturity. A retrospective cohort analysis of 5,693 extremely low birth weight (ELBW; <1,000 g) neonates demonstrated that 98% of neonates received antibiotic treatment in the first three postnatal days, while <2% of neonates had positive blood cultures and clinical symptoms of EOS (7). The majority of neonates in the cohort received >5 days of empiric antibiotics despite having negative cultures; each additional day of empiric treatment was associated with a 4% increase in the odds of necrotizing enterocolitis (NEC) and a 16% increase in the odds of death (7). Similarly, Kuppala et al. demonstrated prolonged administration of empirical antibiotics was associated with increased LOS and the composite outcome of LOS, NEC, or death (54). These short-term deleterious outcomes, as well as an increased incidence of invasive candidiasis, may be the result of intestinal microbiome modification, including decreased microbial diversity, which is associated with broad-spectrum antibiotic use in ELBW and preterm neonates (55–57). The disruption of the microbiome may lead to long-term health consequences including decreased absorption of nutrients and vitamin production, as well as increased risk of infections, asthma, diabetes, and obesity. Further discussion on the effect of antibiotics on the microbiome and the role of dysbiosis in pediatric disease is beyond the scope of this mini review, but the interested reader is directed to a number of outstanding recent reviews (58–60).
Vaccines
Vaccines provide a unique opportunity to prevent infection-associated disease. Hepatitis B vaccine is the only vaccine currently recommended in the first month of life by the United States Department of Health and Human Services, Centers for Disease Control and Prevention and is often administered during the birth hospitalization for healthy, term neonates (61, 62). Essentiality, all infants administrated hepatitis B vaccine respond with hepatitis B surface antigen-specific humoral and cell-mediated immunity following completion of the primary vaccine series (63). Although antibody titers decrease over time, immunological memory persists with vaccinated responders mounting a rapid anti-hepatitis B surface antibody response to a vaccine challenge (63). This immunological memory has had a dramatic impact on reducing hepatitis B infection and disease worldwide. After the implementation of universal hepatitis B vaccination program in Taiwan, the seroprevalence rate of hepatitis B surface antigen in children decreased from 10 to 0.7% (64). Likewise, universal vaccination significantly reduced the incidence of pediatric fulminant hepatitis and hepatocellular carcinoma (64). In addition to its clear beneficial effects, early vaccination for hepatitis B remains remarkably safe. Over one billion doses of hepatitis B vaccine have been administered worldwide with few true adverse reactions, and no evidence of an association with sudden infant death syndrome, multiple sclerosis, or chronic fatigue syndrome (65).
Contrasting with the success of the hepatitis B vaccine, the use of other vaccines early in life has been more challenging and frequently less successful. The administration of vaccines against influenza, measles, and mumps during infancy has been unsuccessful given the poor generation of host antibodies (66, 67). Likewise, infants demonstrate decreased cell proliferation and IFN-γ production in response to the polio vaccine, compared to adults (68). This relative resistance to the development of life-long adaptive immunity early in life has impeded the use of many current vaccines in neonates. Generally, this has been attributed to the absence of a strong type 1 T helper cellular response to the antigen. The use of immune adjuvants appears to be one of the best methods to elicit a stronger immune response and overcome this limitation. Currently, aluminum salts, oil-in-water emulsions (MF59, AS03, AF03), virosomes, and AS04 [monophosphoryl lipid A (MPLA) preparation with aluminum salt] are being used as adjuvants in vaccines approved for use in the United States and/or Europe (69).
Implications for Future Therapies
The first step in being able to combat invading pathogens relies on their proper recognition by host cellular populations. This occurs via complement in blood and pattern recognition receptors including toll-like receptors (TLRs), C-type lectin receptors, nucleotide-binding oligomerization domain (NOD)-like receptors, beta integrins, and others on cells responsible for immune surveillance (70). Specifically, TLRs are located on and within numerous cell populations, including immune, epithelial, and endothelial cell populations. TLRs continuously survey the environment to recognize microbial components and intracellular signals of infection and/or cellular damage. Activation leads to downstream signaling, transcriptional changes, and the eventual secretion of inflammatory cytokines, type I IFN, chemokines, and antimicrobial peptides, which together function to target, localize, and kill the invading pathogen (70). In neonatal murine models, CpG oligodeoxynucleotides (TLR 9 agonist) have shown promise in improving survival to Listeria monocytogenes, Cryptosporidium parvum, and neurotropic Tacaribe arenavirus infections (71–73). In addition, LPS (TLR 4 agonist) and resiquimod (TLR 7/8 agonist) were shown to augment innate immunity, reduce bacteremia, and improve survival to polymicrobial sepsis (74); nevertheless, LPS is highly toxic and thus not suitable for clinical use. In ex vivo human newborn cord blood studies, novel agonists VTX-294 (TLR 8 agonist) and Hybrid-2 (TLR 7/8 agonist) demonstrated a greater cytokine-inducing potency compared to resiquimod (75, 76). Moreover, VTX-294 acted in synergy with MPLA (TLR 4 agonist) to induce an even greater production of TNF and IL-1β (75). Finally, Dowling et al. recently demonstrated that the TLR 7/8 agonist 3M-052 synergistically enhances type 1 immunity from newborn leukocytes when combined with pneumococcal conjugate vaccine (PCV13) in vitro and accelerates neonatal serotype-specific antibody response and pneumococcal opsonophagocytic killing (77).
In addition to increasing the immune responsiveness to the targeted pathogen, the use of TLR agonists in vaccines may provide additional non-specific immune benefits. As a particular example, the bacillus Calmette–Guerin (BCG) vaccine against tuberculosis is the most commonly administered vaccine worldwide and possesses inherent TLR 2/4/8 activity (78). In under-resourced areas of the world, BCG vaccinations are frequently given to neonates on the day of birth due to the absence of consistent postnatal care. Neonatal BCG vaccination has been shown to induce an adult-like immune response characterized by a predominant production of IFN-γ by CD4+ T lymphocytes (79). Administration of BCG vaccine at birth in Guinea-Bissau led to a 41% reduction in all-cause mortality at 12 months among VLBW neonates (80). This reduction was attributed not to reduced tuberculosis but to fewer cases of neonatal sepsis and respiratory infections. It is likely that the success of this vaccine in early life is due to the induction of a strong immune response by the engagement of multiple TLRs simultaneously by products of the Bacillus (81). These findings require further investigation and may lead to the development of novel immune agonists that can augment the host immune response early in life with an associated reduction in the infectious burden in neonates. The human adult literature on the use of TLR agonists as modulators of the innate immune response and as therapeutic strategies for the management of sepsis is vast and beyond the scope of this mini review, but there are several recent outstanding reviews (82–84).
The basal expression of TLRs, accessory proteins, and adaptor proteins on neonatal mononuclear cells is similar to adults; nevertheless, the early gene activation secondary to ligation of these receptors appears to be reduced in neonates due to impaired MyD88 and p38 signaling (85, 86). Understanding TLR biology is important for developing new compounds and ligands, which can activate these receptors and their signaling pathways. Alternative approaches using activators of the inflammasome in combination with TLR agonists may be considered. Future TLR ligands must be able to induce a sufficient immune response while remaining safe in newborns. This balance has made the development of innate immune agonists a difficult task. Therapeutic use of immunotherapies with agonists, which result in the development of antimicrobial resistance, holds great promise to be used prophylactically in the most susceptible population (i.e., VLBW and preterm neonates), in combination with live attenuated organisms to foster development of long-lasting antigen-specific immunity. As mentioned previously, engagement of multiple TLRs at the same time brings greater proliferation and higher cytokine production; however, the clinical applications of TLRs agonists have been limited to local delivery to minimize immune response-related toxicity (87). New vaccine strategies taking advantage of the inclusion of TLR and NOD agonists are currently being investigated to activate dendritic cells, enhance antigen presentation, and improve the host protective immune response (88–93). These novel vaccines require further investigations particularly in the neonatal population to prevent and treat infectious diseases among our most vulnerable patients.
Conclusion
The impact of infections, antibiotics, and vaccines during the early neonatal period, and their influence on future health and disease remains an important and evolving area of research. A better understanding of the immediate and long-term effects of these exposures may lead to novel therapeutics with the ability to drastically reduce infectious complications and mortality in the neonatal period as well as promote longstanding health.
Author Contributions
SR, JR, JW, LM, and SL drafted and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by grants awarded by the National Institute of General Medical Sciences (K08 GM106143, R01 GM097531, and P50 GM111152).
References
- 1.Raymond SL, Stortz JA, Mira JC, Larson SD, Wynn JL, Moldawer LL. Immunological defects in neonatal sepsis and potential therapeutic approaches. Front Pediatr (2017) 5:14. 10.3389/fped.2017.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol (2013) 30(2):105–12. 10.1055/s-0032-1333412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J (2005) 24(7):635–9. 10.1097/01.inf.0000168749.82105.64 [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Wolkowiez M, Moran C, Benjamin DK, Cotten CM, Clark RH, Benjamin DK, Jr, et al. Early and late onset sepsis in late preterm infants. Pediatr Infect Dis J (2009) 28(12):1052–6. 10.1097/INF.0b013e3181acf6bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics (2011) 127(5):817–26. 10.1542/peds.2010-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weston EJ, Pondo T, Lewis MM, Martell-Cleary P, Morin C, Jewell B, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatr Infect Dis J (2011) 30(11):937–41. 10.1097/INF.0b013e318223bad2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics (2009) 123(1):58–66. 10.1542/peds.2007-3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am (2013) 60(2):367–89. 10.1016/j.pcl.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korbage de Araujo MC, Schultz R, do Rosario Dias de Oliveira L, Ramos JL, Vaz FA. A risk factor for early-onset infection in premature newborns: invasion of chorioamniotic tissues by leukocytes. Early Hum Dev (1999) 56(1):1–15. 10.1016/S0378-3782(99)00027-4 [DOI] [PubMed] [Google Scholar]
- 10.Koenig JM, Keenan WJ. Group B Streptococcus and early-onset sepsis in the era of maternal prophylaxis. Pediatr Clin North Am (2009) 56(3):689–708. 10.1016/j.pcl.2009.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botet F, Figueras J, Carbonell-Estrany X, Arca G, The Castrillo Study Group . Effect of maternal clinical chorioamnionitis on neonatal morbidity in very-low birthweight infants: a case-control study. J Perinat Med (2010) 38(3):269–73. 10.1515/jpm.2010.029 [DOI] [PubMed] [Google Scholar]
- 12.Polin RA, Committee on Fetus and Newborn . Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics (2012) 129(5):1006–15. 10.1542/peds.2012-0541 [DOI] [PubMed] [Google Scholar]
- 13.Lin CB, Hornik CP, Clark R, Cotten CM, Benjamin DK, Jr, Cohen-Wolkoweiz M, et al. Very low birth weight neonates who survive early-onset sepsis do not have an increased risk of developing late-onset sepsis. Early Hum Dev (2012) 88(11):905–9. 10.1016/j.earlhumdev.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynn JL, Hansen NI, Das A, Cotten CM, Goldberg RN, Sanchez PJ, et al. Early sepsis does not increase the risk of late sepsis in very low birth weight neonates. J Pediatr (2013) 162(5):942–8.e1–3. 10.1016/j.jpeds.2012.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics (2002) 110(2 Pt 1):285–91. 10.1542/peds.110.2.285 [DOI] [PubMed] [Google Scholar]
- 16.Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol (2004) 190(1):147–51. 10.1016/j.ajog.2003.07.012 [DOI] [PubMed] [Google Scholar]
- 17.Dempsey E, Chen MF, Kokottis T, Vallerand D, Usher R. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am J Perinatol (2005) 22(3):155–9. 10.1055/s-2005-865020 [DOI] [PubMed] [Google Scholar]
- 18.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics (2009) 123(5):1314–9. 10.1542/peds.2008-0656 [DOI] [PubMed] [Google Scholar]
- 19.Lahra MM, Beeby PJ, Jeffery HE. Maternal versus fetal inflammation and respiratory distress syndrome: a 10-year hospital cohort study. Arch Dis Child Fetal Neonatal Ed (2009) 94(1):F13–6. 10.1136/adc.2007.135889 [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi T, Matsuzaki N, Kameda T, Shimoya K, Jo T, Saji F, et al. The enhanced production of placental interleukin-1 during labor and intrauterine infection. Am J Obstet Gynecol (1991) 165(1):131–7. 10.1016/0002-9378(91)90241-I [DOI] [PubMed] [Google Scholar]
- 21.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics (1996) 97(2):210–5. [PubMed] [Google Scholar]
- 22.Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest (1997) 99(12):2992–9. 10.1172/jci119494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimoya K, Taniguchi T, Matsuzaki N, Moriyama A, Murata Y, Kitajima H, et al. Chorioamnionitis decreased incidence of respiratory distress syndrome by elevating fetal interleukin-6 serum concentration. Hum Reprod (2000) 15(10):2234–40. 10.1093/humrep/15.10.2234 [DOI] [PubMed] [Google Scholar]
- 24.Strunk T, Doherty D, Jacques A, Simmer K, Richmond P, Kohan R, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics (2012) 129(1):e134–41. 10.1542/peds.2010-3493 [DOI] [PubMed] [Google Scholar]
- 25.Grischkan J, Storfer-Isser A, Rosen CL, Larkin EK, Kirchner HL, South A, et al. Variation in childhood asthma among former preterm infants. J Pediatr (2004) 144(3):321–6. 10.1016/j.jpeds.2003.11.029 [DOI] [PubMed] [Google Scholar]
- 26.Gehring U, Bolte G, Borte M, Bischof W, Fahlbusch B, Wichmann HE, et al. Exposure to endotoxin decreases the risk of atopic eczema in infancy: a cohort study. J Allergy Clin Immunol (2001) 108(5):847–54. 10.1067/mai.2001.119026 [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med (2001) 163(2):322–8. 10.1164/ajrccm.163.2.2002088 [DOI] [PubMed] [Google Scholar]
- 28.Bolte G, Bischof W, Borte M, Lehmann I, Wichmann HE, Heinrich J, et al. Early endotoxin exposure and atopy development in infants: results of a birth cohort study. Clin Exp Allergy (2003) 33(6):770–6. 10.1046/j.1365-2222.2003.01665.x [DOI] [PubMed] [Google Scholar]
- 29.Gillespie J, Wickens K, Siebers R, Howden-Chapman P, Town I, Epton M, et al. Endotoxin exposure, wheezing, and rash in infancy in a New Zealand birth cohort. J Allergy Clin Immunol (2006) 118(6):1265–70. 10.1016/j.jaci.2006.07.051 [DOI] [PubMed] [Google Scholar]
- 30.Celedon JC, Milton DK, Ramsey CD, Litonjua AA, Ryan L, Platts-Mills TA, et al. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol (2007) 120(1):144–9. 10.1016/j.jaci.2007.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karvonen AM, Hyvarinen A, Gehring U, Korppi M, Doekes G, Riedler J, et al. Exposure to microbial agents in house dust and wheezing, atopic dermatitis and atopic sensitization in early childhood: a birth cohort study in rural areas. Clin Exp Allergy (2012) 42(8):1246–56. 10.1111/j.1365-2222.2012.04002.x [DOI] [PubMed] [Google Scholar]
- 32.Illi S, Weber J, Zutavern A, Genuneit J, Schierl R, Strunz-Lehner C, et al. Perinatal influences on the development of asthma and atopy in childhood. Ann Allergy Asthma Immunol (2014) 112(2):132–9.e1. 10.1016/j.anai.2013.11.019 [DOI] [PubMed] [Google Scholar]
- 33.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol (2014) 134(3):593–601.e12. 10.1016/j.jaci.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science (2015) 349(6252):1106–10. 10.1126/science.aac6623 [DOI] [PubMed] [Google Scholar]
- 35.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med (2002) 347(4):240–7. 10.1056/NEJMoa012657 [DOI] [PubMed] [Google Scholar]
- 36.Alshaikh B, Yusuf K, Sauve R. Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: systematic review and meta-analysis. J Perinatol (2013) 33(7):558–64. 10.1038/jp.2012.167 [DOI] [PubMed] [Google Scholar]
- 37.Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev (2002) 8(1):25–9. 10.1002/mrdd.10003 [DOI] [PubMed] [Google Scholar]
- 38.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA (2003) 290(20):2677–84. 10.1001/jama.290.20.2677 [DOI] [PubMed] [Google Scholar]
- 39.Blume HK, Li CI, Loch CM, Koepsell TD. Intrapartum fever and chorioamnionitis as risks for encephalopathy in term newborns: a case-control study. Dev Med Child Neurol (2008) 50(1):19–24. 10.1111/j.1469-8749.2007.02007.x [DOI] [PubMed] [Google Scholar]
- 40.Leviton A, Allred EN, Kuban KC, Hecht JL, Onderdonk AB, O’Shea TM, et al. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. The ELGAN study. Pediatr Res (2010) 67(1):95–101. 10.1203/PDR.0b013e3181bf5fab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol (2010) 116(2 Pt 1):387–92. 10.1097/AOG.0b013e3181e90046 [DOI] [PubMed] [Google Scholar]
- 42.Kaindl AM, Favrais G, Gressens P. Molecular mechanisms involved in injury to the preterm brain. J Child Neurol (2009) 24(9):1112–8. 10.1177/0883073809337920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korzeniewski SJ, Romero R, Cortez J, Pappas A, Schwartz AG, Kim CJ, et al. A “multi-hit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J Perinat Med (2014) 42(6):731–43. 10.1515/jpm-2014-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol (2009) 24(9):1119–26. 10.1177/0883073809338066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang LW, Chang YC, Lin CY, Hong JS, Huang CC. Low-dose lipopolysaccharide selectively sensitizes hypoxic ischemia-induced white matter injury in the immature brain. Pediatr Res (2010) 68(1):41–7. 10.1203/00006450-201011001-00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med (2012) 4(130):130ra146. 10.1126/scitranslmed.3003162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, et al. Evaluation of universal antenatal screening for group B Streptococcus. N Engl J Med (2009) 360(25):2626–36. 10.1056/NEJMoa0806820 [DOI] [PubMed] [Google Scholar]
- 48.Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine (2013) 31(Suppl 4):D20–6. 10.1016/j.vaccine.2012.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castor ML, Whitney CG, Como-Sabetti K, Facklam RR, Ferrieri P, Bartkus JM, et al. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol (2008) 2008:727505. 10.1155/2008/727505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics (2008) 121(4):689–96. 10.1542/peds.2007-2171 [DOI] [PubMed] [Google Scholar]
- 51.Schuchat A, Zywicki SS, Dinsmoor MJ, Mercer B, Romaguera J, O’Sullivan MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics (2000) 105(1 Pt 1):21–6. 10.1542/peds.105.1.21 [DOI] [PubMed] [Google Scholar]
- 52.Bergin SP, Thaden JT, Ericson JE, Cross H, Messina J, Clark RH, et al. Neonatal Escherichia coli bloodstream infections: clinical outcomes and impact of initial antibiotic therapy. Pediatr Infect Dis J (2015) 34(9):933–6. 10.1097/inf.0000000000000769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics (2006) 117(6):1979–87. 10.1542/peds.2005-1707 [DOI] [PubMed] [Google Scholar]
- 54.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr (2011) 159(5):720–5. 10.1016/j.jpeds.2011.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed (1999) 80(3):F167–73. 10.1136/fn.80.3.F167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK, Jr, et al. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics (2006) 118(2):717–22. 10.1542/peds.2005-2677 [DOI] [PubMed] [Google Scholar]
- 57.Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr (2014) 165(1):23–9. 10.1016/j.jpeds.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol (2014) 5:427. 10.3389/fimmu.2014.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol (2015) 6:1543. 10.3389/fmicb.2015.01543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med (2016) 8(1):39. 10.1186/s13073-016-0294-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.CDC. Vaccine Information Statement Hepatitis B Vaccine. U.S. Department of Health and Human Services Centers for Disease Control and Prevention; (2016). Available from: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/hep-b.pdf [Google Scholar]
- 62.CDC. 2017 Recommended Immunization for Children from Birth through 6 Years Old. U.S. Department of Health and Human Services Center for Disease Control and Prevention; (2017). Available from: https://www.cdc.gov/vaccines/parents/downloads/parent-ver-sch-0-6yrs.pdf [Google Scholar]
- 63.Fitzsimons D, Francois G, Hall A, McMahon B, Meheus A, Zanetti A, et al. Long-term efficacy of hepatitis B vaccine, booster policy, and impact of hepatitis B virus mutants. Vaccine (2005) 23(32):4158–66. 10.1016/j.vaccine.2005.03.017 [DOI] [PubMed] [Google Scholar]
- 64.Chang MH. Impact of hepatitis B vaccination on hepatitis B disease and nucleic acid testing in high-prevalence populations. J Clin Virol (2006) 36(Suppl 1):S45–50. 10.1016/S1386-6532(06)80008-9 [DOI] [PubMed] [Google Scholar]
- 65.Zuckerman AJ. Safety of hepatitis B vaccines. Travel Med Infect Dis (2004) 2(2):81–4. 10.1016/j.tmaid.2004.03.009 [DOI] [PubMed] [Google Scholar]
- 66.Gans H, DeHovitz R, Forghani B, Beeler J, Maldonado Y, Arvin AM. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine (2003) 21(24):3398–405. 10.1016/S0264-410X(03)00341-4 [DOI] [PubMed] [Google Scholar]
- 67.Halasa NB, Gerber MA, Chen Q, Wright PF, Edwards KM. Safety and immunogenicity of trivalent inactivated influenza vaccine in infants. J Infect Dis (2008) 197(10):1448–54. 10.1086/587643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vekemans J, Ota MO, Wang EC, Kidd M, Borysiewicz LK, Whittle H, et al. T cell responses to vaccines in infants: defective IFNgamma production after oral polio vaccination. Clin Exp Immunol (2002) 127(3):495–8. 10.1046/j.1365-2249.2002.01788.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med (2013) 19(12):1597–608. 10.1038/nm.3409 [DOI] [PubMed] [Google Scholar]
- 70.Raymond SL, Holden DC, Mira JC, Stortz JA, Loftus TJ, Mohr AM, et al. Microbial recognition and danger signals in sepsis and trauma. Biochim Biophys Acta (2017). 10.1016/j.bbadis.2017.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito S, Ishii KJ, Gursel M, Shirotra H, Ihata A, Klinman DM. CpG oligodeoxynucleotides enhance neonatal resistance to Listeria infection. J Immunol (2005) 174(2):777–82. 10.4049/jimmunol.174.2.777 [DOI] [PubMed] [Google Scholar]
- 72.Barrier M, Lacroix-Lamande S, Mancassola R, Auray G, Bernardet N, Chausse AM, et al. Oral and intraperitoneal administration of phosphorothioate oligodeoxynucleotides leads to control of Cryptosporidium parvum infection in neonatal mice. J Infect Dis (2006) 193(10):1400–7. 10.1086/503748 [DOI] [PubMed] [Google Scholar]
- 73.Pedras-Vasconcelos JA, Goucher D, Puig M, Tonelli LH, Wang V, Ito S, et al. CpG oligodeoxynucleotides protect newborn mice from a lethal challenge with the neurotropic Tacaribe arenavirus. J Immunol (2006) 176(8):4940–9. 10.4049/jimmunol.176.8.4940 [DOI] [PubMed] [Google Scholar]
- 74.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood (2008) 112(5):1750–8. 10.1182/blood-2008-01-130500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dowling DJ, Tan Z, Prokopowicz ZM, Palmer CD, Matthews MA, Dietsch GN, et al. The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes. PLoS One (2013) 8(3):e58164. 10.1371/journal.pone.0058164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganapathi L, Van Haren S, Dowling DJ, Bergelson I, Shukla NM, Malladi SS, et al. The imidazoquinoline toll-like receptor-7/8 agonist hybrid-2 potently induces cytokine production by human newborn and adult leukocytes. PLoS One (2015) 10(8):e0134640. 10.1371/journal.pone.0134640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dowling DJ, van Haren SD, Scheid A, Bergelson I, Kim D, Mancuso CJ, et al. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight (2017) 2(6):e91020. 10.1172/jci.insight.91020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med (2011) 3(90):90s27. 10.1126/scitranslmed.3001880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vekemans J, Amedei A, Ota MO, D’Elios MM, Goetghebuer T, Ismaili J, et al. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol (2001) 31(5):1531–5. [DOI] [PubMed] [Google Scholar]
- 80.Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis (2011) 204(2):245–52. 10.1093/infdis/jir240 [DOI] [PubMed] [Google Scholar]
- 81.Talat Iqbal N, Hussain R. Non-specific immunity of BCG vaccine: a perspective of BCG immunotherapy. Trials Vaccinol (2014) 3:143–9. 10.1016/j.trivac.2014.08.002 [DOI] [Google Scholar]
- 82.Hennessy EJ, Parker AE, O’Neill LA. Targeting toll-like receptors: emerging therapeutics? Nat Rev Drug Discov (2010) 9(4):293–307. 10.1038/nrd3203 [DOI] [PubMed] [Google Scholar]
- 83.Savva A, Roger T. Targeting toll-like receptors: promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front Immunol (2013) 4:387. 10.3389/fimmu.2013.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mifsud EJ, Tan AC, Jackson DC. TLR agonists as modulators of the innate immune response and their potential as agents against infectious disease. Front Immunol (2014) 5:79. 10.3389/fimmu.2014.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun (2004) 72(3):1223–9. 10.1128/IAI.72.3.1223-1229.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Hertani W, Yan SR, Byers DM, Bortolussi R. Human newborn polymorphonuclear neutrophils exhibit decreased levels of MyD88 and attenuated p38 phosphorylation in response to lipopolysaccharide. Clin Invest Med (2007) 30(2):E44–53. 10.25011/cim.v30i2.979 [DOI] [PubMed] [Google Scholar]
- 87.Wu TY. Strategies for designing synthetic immune agonists. Immunology (2016) 148(4):315–25. 10.1111/imm.12622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, et al. Synergistic stimulation of human monocytes and dendritic cells by toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol (2005) 35(8):2459–70. 10.1002/eji.200526286 [DOI] [PubMed] [Google Scholar]
- 89.Khan S, Bijker MS, Weterings JJ, Tanke HJ, Adema GJ, van Hall T, et al. Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J Biol Chem (2007) 282(29):21145–59. 10.1074/jbc.M701705200 [DOI] [PubMed] [Google Scholar]
- 90.Schlosser E, Mueller M, Fischer S, Basta S, Busch DH, Gander B, et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine (2008) 26(13):1626–37. 10.1016/j.vaccine.2008.01.030 [DOI] [PubMed] [Google Scholar]
- 91.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med (2009) 206(7):1589–602. 10.1084/jem.20090247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oh JZ, Kedl RM. The capacity to induce cross-presentation dictates the success of a TLR7 agonist-conjugate vaccine for eliciting cellular immunity. J Immunol (2010) 185(8):4602–8. 10.4049/jimmunol.1001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cardinaud S, Urrutia A, Rouers A, Coulon PG, Kervevan J, Richetta C, et al. Triggering of TLR-3, -4, NOD2, and DC-SIGN reduces viral replication and increases T-cell activation capacity of HIV-infected human dendritic cells. Eur J Immunol (2017) 47(5):818–29. 10.1002/eji.201646603 [DOI] [PubMed] [Google Scholar]


