Abstract
Neurons of the cochleovestibular ganglion (CVG) transmit hearing and balance information to the brain. During development, a select population of early otic progenitors express NEUROG1, delaminate from the otocyst, and coalesce to form the neurons that innervate all inner ear sensory regions. At present, the selection process that determines which otic progenitors activate NEUROG1 and adopt a neuroblast fate is incompletely understood. The transcription factor SOX2 has been implicated in otic neurogenesis, but its requirement in the specification of the CVG neurons has not been established. Here we tested SOX2’s requirement during inner ear neuronal specification using a conditional deletion paradigm in the mouse. SOX2 deficiency at otocyst stages caused a near-absence of NEUROG1-expressing neuroblasts, increased cell death in the neurosensory epithelium, and significantly reduced the CVG volume. Interestingly, a milder decrease in neurogenesis was observed in heterozygotes, indicating SOX2 levels are important. Moreover, fate-mapping experiments revealed that the timing of SOX2 expression did not parallel the established vestibular-then-auditory sequence. These results demonstrate that SOX2 is required for the initial events in otic neuronal specification including expression of NEUROG1, although fate-mapping results suggest SOX2 may be required as a competence factor rather than a direct initiator of the neural fate.
Introduction
The vertebrate inner ear is an intricate sensory organ responsible for the perceptions of sound and balance. Critical for transmitting auditory and balance information to higher brain regions is the cochleovestibular ganglion (CVG), consisting of both the spiral (auditory) and vestibular ganglion. In humans, damage or loss of the CVG neurons causes irreversible hearing and balance deficits. Moreover, the success of cochlear implants often depends on the number and relative health of the spiral ganglion neurons1. However, despite their clear physiologic importance, it is not well understood how the inner ear neuronal lineage is specified during otic development. CVG neuroblasts are derived from the otic placode, an embryonic structure that gives rise to most of the derivatives of the inner ear, including the sensory hair cells. Beginning at otic cup stages (E9.5 in the mouse) and continuing to late otocyst stages (~E11.5), neuroblasts delaminate from the anteroventral quadrant of the otic cup/otocyst, proliferate, and differentiate into bipolar neurons that innervate both cochlear and vestibular sensory regions2–5. The initiation of a neuroblast fate is characterized by a cascade of basic helix-loop-helix (bHLH) proneural gene expression, beginning with neurogenin1 (NEUROG1), closely followed by neurogenic differentiation 1 (NEUROD1). NEUROG1, the neural fate-determining gene, is transiently upregulated in otic precursors and rapidly induces the downstream expression of NEUROD1, a molecule required for neuronal maturation, migration, and survival6–8. While it is clear that both these factors are required for neuronal specification and maturation, it is unclear what upstream molecular cues initiate NEUROG1 expression.
The otic neuroblasts derive from the neuro-sensory domain (NSD) in the anteroventral region of the otocyst2, 9–11, a region that expresses the High Mobility Group (HMG) transcription factor SOX212, 13. While SOX2 is best known for its role in maintaining stem and progenitor cell pluripotency14, 15, it also plays a prominent role in neurogenesis16–18. SOX2 is one of the earliest markers of the neural ectoderm and it is highly expressed in neural precursor cells (NPCs), where it supports self-renewal19. The importance of SOX2 for both sensory and neuronal development is highlighted in human patients with SOX2 mutations; these individuals present with a failure of eye formation (anopthalmia), in addition to other neurological symptoms such as hippocampal malformations, severe learning disabilities, epilepsy, and in some cases hearing loss20–22.
Sox2 has been shown to be a critical gene for sensory development in the inner ear23–26, although its requirement in the otic neuronal lineage is less clear. Previous studies have shown that overexpression of SOX2 can induce a neuronal phenotype in otic progenitors25, 27, 28, and in some studies induce NEUROG1 expression13. Interestingly, impairments in the CVG have been observed in some SOX2 loss of function studies26, 28, although given the timing of either the deletion or analysis of the otic ganglion, it was difficult to distinguish primary neuronal defects due to loss of SOX2 vs. secondary CVG defects caused by loss of sensory-derived neurotrophic factors. To establish whether there was a primary defect in neurogenesis due to loss of SOX2, we conditionally deleted Sox2 in the mouse during the period of early inner ear neurogenesis (E8.5-E11.5) and examined the CVG prior to its requirement for sensory derived-neurotrophic factors (E11.75). We found that SOX2 is necessary for the characteristic cascade of proneural gene expression, consisting of NEUROG1 and NEUROD17, 8. Loss of this neuronal gene cascade results in a dramatic reduction of the CVG ganglion by E11.75. Loss of one copy of Sox2 shows a milder reduction in both proneural gene expression and the size of the CVG, indicating that levels of Sox2 are critical for the production of the full complement of otic neurons. These results demonstrate that SOX2 is an essential upstream factor during selection of the CVG neurons, although fate-mapping studies demonstrate that SOX2’s temporal expression does not strictly parallel that of NEUROG1, indicating that SOX2 plays a complex role in neuronal specification in the otocyst.
Results
SOX2 defines a neurosensory competent region of the otocyst
We first examined the extent to which SOX2 expression overlapped with the neurogenic region of the otocyst, defined by NEUROG1 expression29. Timed matings were performed, and embryos were harvested for immunohistochemical analysis at E9.5, E10.5, and E11.5, a time series reflective of the period during which the majority of inner ear neurogenesis has been documented to occur30 (Fig. 1). As expected from previous reports of SOX2 expression13, 23, 31, the SOX2 domain was widespread but generally localized to the more ventral portion of the otocyst at E9.5, where it initially showed a broad anterior-posterior domain. In contrast, NEUROG1 is confined to the anterior half of the otocyst (Fig. 1A,A”) (n = 3). A day later at E10.5, SOX2 was downregulated in the lateral region of the otocyst, but remained strong in the anteromedial region, where NEUROG1 is localized (Fig. 1B,B”) (n = 3). By E11.5, SOX2 expression is focused in the anteroventral regions, and shows strong overlap with NEUROG1 (Fig. 1C,C”) (n = 5). In addition to this region, there is also a SOX2-positive patch in the posterior dorsal region of the otocyst, that does not overlap with NEUROG1 (not shown). This region is likely associated with sensory region generation, which has previously been shown to require SOX223. These results demonstrate that SOX2 and NEUROG1 are overlapping throughout early neurogenesis, although SOX2 initially shows a more broad and diffuse expression pattern. As development proceeds however, SOX2 expression becomes more narrowly focused into two distinct patches: a dorsal posterior region that does not express NEUROG1 (and is therefore likely sensoryrelated) and an anteroventral NSD region that overlaps tightly with NEUROG1.
Figure 1.
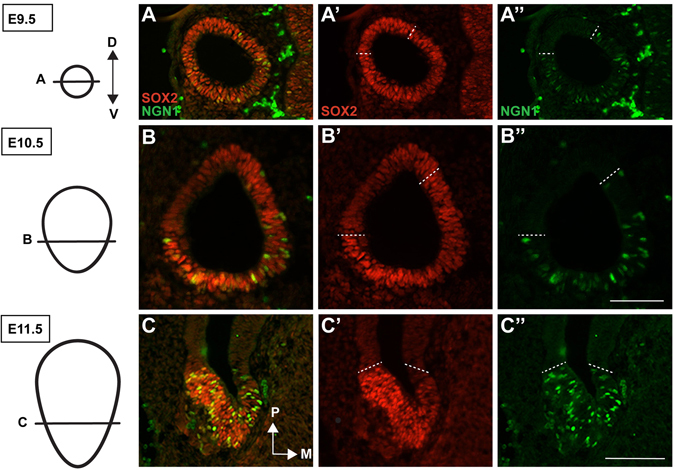
Endogenous SOX2 and NEUROG1 protein show overlapping domains in the mouse otocyst. (A–C”) Sections through the otocyst of wild type mice, over the developmental timeframe E9.5-E11.5, stained with antibodies to SOX2 and NEUROG1. Note the extensive co-localization of both proteins at all time points. The dotted lines demark the dorsal-most region of overlapping protein expression. Scale bars: 100 μm.
SOX2 dose-dependently regulates inner ear neurogenesis
SOX2 coexpression with NEUROG1 within the NSD during early otocyst stages suggests a role for SOX2 in otic neurogenesis, consistent with previous studies13, 26, 28, 32. However, the timeframe in which SOX2 is required for otic neurogenesis has not been established. To examine this more closely, we conditionally deleted SOX2 using a tamoxifen-inducible Sox2-CreERT2 line during the period of otic neuronal specification (E8.5-E11.5)30. In addition to deleting SOX2, we included a tdTomato (tdT) reporter line in the breeding scheme so that we could simultaneously fate-map SOX2-expressing cells. To generate Sox2-deleted animals, we crossed a Sox2-CreERT2 line, in which the SOX2 coding region is replaced by the CreERT2 fusion protein33, to a mouse carrying both the ROSA26-CAGtdT reporter allele34 and the Sox2 flox allele35, thereby generating Sox2 CreERT2/fl /ROSA26 CAGtdT mice (hereafter these mice will only be identified by their Sox2 genotype and not by ROSA26). Previous studies have shown that the Sox2-CreERT2 allele recapitulates endogenous SOX2 expression and is a faithful reporter of SOX2 expression31, 33, 36–38. Using this breeding paradigm, deletion was generally efficient, with very few SOX2-positive cells remaining 48-hours post-injection (Fig. 2) (n = 3). Moreover, the few SOX2-expressing cells that did persist were usually located in the posterior region of the otocyst (Fig. 2B,B’ arrows), a region not associated with neurogenesis.
Figure 2.
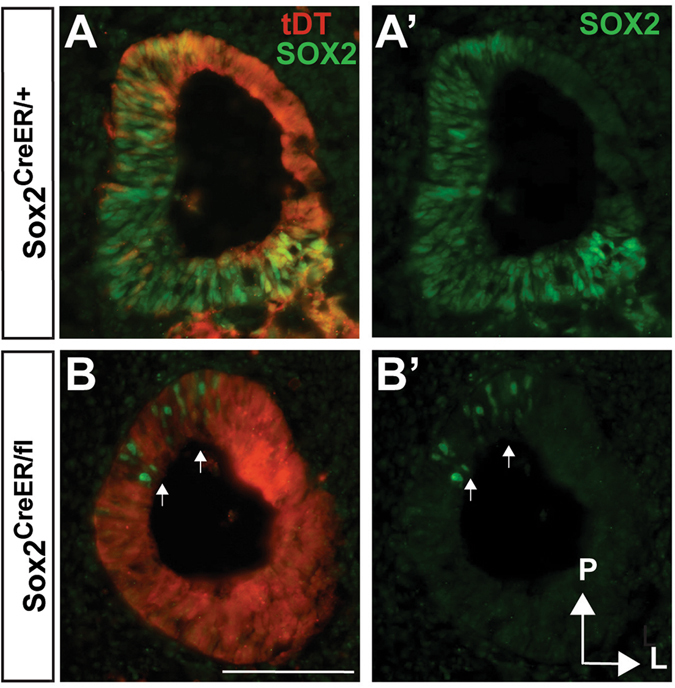
Sox2 is efficiently deleted in the early otocyst using the Sox2-CreER. (A,B) E11.75 otic sections demonstrating both endogenous SOX2 expression and tdT-reported SOX2 expression from E8.5-E11.5 chronic tamoxifen injections. Note that the majority of the otocyst is labeled by tdT because of SOX2’s more widespread expression at earlier developmental times (E8.5-10.5). In comparison to (A’) where widespread SOX2 protein expression is seen in the anterior medial portion of the otocyst (B’) shows effective deletion of Sox2 after chronic tamoxifen administrations in the Sox2 CreER/fl mutant. Arrows point to the few SOX2-expressing cells that persist in the posterior region of the mutant otocyst that is not in the neurogenic region. Scale bar: 100 μm.
To delete Sox2, tamoxifen was injected daily from E8.5 until E11.5, and embryos were harvested at E11.75, and assessed for markers of neurogenesis (Fig. 3A). In controls, numerous NEUROG1-expressing cells were observed in the anteroventral NSD, as expected (Fig. 3B and C). In contrast, SOX2-deficient inner ears were largely devoid of NEUROG1-positive cells as compared to Sox2 +/+ controls (Fig. 3B,C,E and quantified in I; Sox2 +/+ n = 6, Sox2 CreERT2/fl n = 7, p < 0.0001). Interestingly, the number of NEUROG1-expressing cells was also significantly reduced in Sox2 CreERT2/+ heterozygotes compared to controls (Fig. 3B,C,D and I; Sox2 +/+ n = 6, Sox2 CreERT2/+ n = 7, p < 0.0001), indicating Sox2 dosage is important in generating the correct number of otic neurons. These results demonstrate that SOX2 lies upstream of NEUROG1 in otic neurogenesis, and that high levels of SOX2 are required to generate the full complement of CVG neurons.
Figure 3.
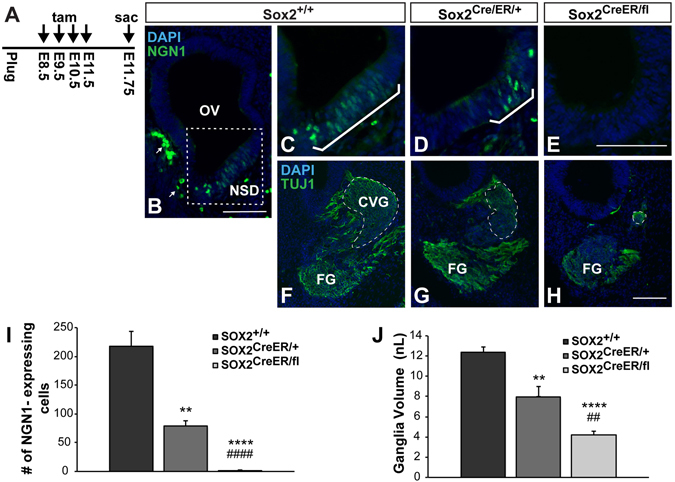
SOX2 dose-dependently regulates inner ear neurogenesis. (A) Chronic tamoxifen injection paradigm used for deleting Sox2 throughout inner ear neurogenesis. (B) Low magnification view of the Sox2 +/+ control otic vesicle, showing the location of the neurosensory domain (dotted box). Arrows point to non-specific staining of blood cells outside the epithelium, which could be easily distinguished from NEUROG1-positive cells based on their cellular shape. (C–E) Representative sections showing that the number of NEUROG1-expressing neuroblasts (brackets) is reduced according to the Sox2 gene dosage. (E) The total number of NEUROG1-positive cells was quantified and found to be significantly decreased in both Sox2 CreER/+ mice (***p = 0.0003) (n = 7) and Sox2 CreER/fl mice (****p < 0.0001) (n = 7) compared to Sox2 +/+ controls (n = 6), and between Sox2 CreER/+ and Sox2 CreER/fl mice (#### p < 0.0001) (one-way ANOVA followed by Student’s t test with a Bonferroni correction). (E-H) Representative sections showing that the CVG (outlined with dashed lines) is similarly reduced according to Sox2 genotype. (I) The total volume of the CVG was quantified from serial sections and found to be significantly decreased in both Sox2 CreER/+ mice (**p = 0.003) and Sox2 CreER/fl mice (****p < 0.0001) compared to Sox2 +/+ controls, and between Sox2 CreER/+ and Sox2 CreER/fl mice ( ## p = 0.004) (one-way ANOVA followed by a Student’s t test with a Bonferroni correction). Error bars represent SEM. OV: Otic vesicle; NSD: Neurosensory domain; CVG: Cochleovestibular ganglion; FG: Facial ganglion. Scale bars: 100 μm.
We next asked whether the reduction in NEUROG1 expression resulted in a smaller or absent CVG in Sox2-deleted inner ears. In order to determine the effects of SOX2 loss or reduction on neuronal formation, we labeled the CVG with a marker for early neurons, neuron-specific class III beta-tubulin (TUJ1), and quantified the total volume in serial sections. Through this analysis, we found a similar trend to that observed in the NEUROG1 cell counts, where the total ganglia volume decreased according to the dosage of Sox2 (Fig. 3F–H and J). We found that the CVG volume was decreased by approximately 70% in Sox2 CreERT2/fl mutants compared to wildtype controls (Fig. 3J; Sox2 +/+ n = 6, Sox2 CreERT2/fl n = 7, p < 0.0001). Moreover, similar to the dose-dependent effects on NEUROG1 expression, we also observed a 30% reduction in CVG volume in the Sox2 CreERT2/fl heterozygotes compared to controls (Fig. 3F,G and J; Sox2 +/+ n = 6, Sox2 CreERT2/+ n = 7, p = 0.003). These results demonstrate that the loss of NEUROG1-expressing cells in the Sox2-deficient otocyst results in a significant reduction of the CVG ganglion, indicating that SOX2 is required for early specification of the otic neural progenitors.
Absence of SOX2 impairs downstream expression of NEUROD1
In cranial sensory neurons, Neurog1 has been placed at the top of a hierarchy of a signaling cascade that initiates a sequence of gene expression required for their formation, such that loss of Neurog1 abolishes expression of all subsequent genes in the pathway6. However the dependence of later proneuronal genes on Neurog1 has not been thoroughly investigated in the ear. To address this, we next asked how loss of Sox2 during early otocyst stages affects downstream proneuronal genes, by analyzing NEUROD1, which acts as a potent neuralizing agent39, in addition to promoting migration40 and maturation6. Using the previously described experimental paradigm, Sox2 +/+ control ears and Sox2 CreERT2/fl Sox2-deleted ears were analyzed for expression of NEUROD1 and SOX2. We examined and quantified NEUROD1 expression at E11.75 and found, similar to NEUROG1, a significant reduction of NEUROD1-expressing cells compared to controls (Fig. 4). In Sox2 +/+ control ears we saw robust expression of NEUROD1 in neuroblasts within the epithelium, as well as NEUROD1-positive migrating neuroblasts detected adjacent to the sensory epithelium, and in the ganglia (Fig. 4A), consistent with NEUROD1’s reported involvement in the multiple stages of neuronal maturation7, 39, 41. NEUROD1 and SOX2 were co-localized in almost 100% of the neuroblasts within the epithelium (Fig. 4A,A’,A” and C), supporting previous findings in the chick13. Importantly, in Sox2-deficient samples, the total number of NEUROD1-positive cells is reduced by approximately 90% (Fig. 4B,B” and C; Sox2 +/+ n = 3, Sox2 CreERT2/fl n = 4, p < 0.0001). To determine whether the loss of Sox2 affected NEUROD1 expression preferentially in the epithelium or during migration, we quantified these areas separately. A severe reduction was observed in both epithelial and migrating neuroblasts in the mutant otocysts (Fig. 4D).
Figure 4.
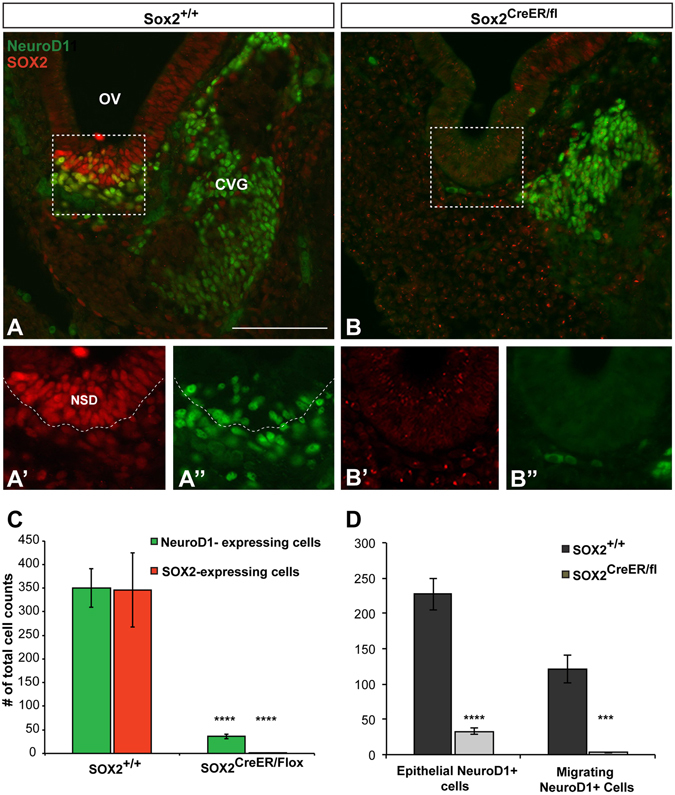
SOX2 is required for the downstream expression of NeuroD1. (A) Sox2 +/+ control section showing the antereoventral portion of the otic vesicle from which neuroblasts delaminate. Boxed region refers to the area in which cell counts were performed. (A’ and A”) show a higher magnification of the neuroblasts contained within the boxed region. Note the extensive colabeling of SOX2 (red) and NEUROD1 (green). Dotted line shows the boundary between the neurosensory epithelium and the mesenchyme. (B) Representative section showing the absence of SOX2 and NEUROD1 protein expression in a Sox2-deficient mutant. (B’ and B”) Show a higher magnification of the boxed region from (B). Note the absence of immunofluorescence for both SOX2 and NEUROD1. To note, the handful of green cells seen in the mesenchyme did not meet inclusion criteria for neuroblasts and were not counted. (C) The total number of NEUROD1 and SOX2-positive neuroblasts was quantified, and both markers were found to be significantly decreased in Sox2 CreER/fl mice (n = 4) compared to Sox2 +/+ controls (n = 3) (****p < 0.0001) (Student’s t test). (D) Total NEUROD1 cell counts were separated based on either being located in the neurosensory epithelium, or migrating into the mesenchyme; in both cases the number of NEUROD1-positive cells were significantly reduced in the Sox2 CreER/fl mice (epithelial NEUROD1: ****p < 0.0001 migrating NEUROD1: ***p = 0.0002). Error bars represent SEM. OV: Otic Vesicle; CVG: Cochleovestibular ganglion; FG: Facial ganglion. Scale bars: 100 μm.
Neurosensory progenitors die in the absence of SOX2
In both the neural retina and hippocampus, SOX2 has been shown to be important for neuronal progenitor survival19, 42, 43. In order to determine whether SOX2 is similarly required for otic progenitor survival, we investigated whether there was increased cell death in the Sox2-deficient otocysts. For these experiments a single injection was delivered at E9.5, and embryos were harvested at E11.5 and stained for activated caspase 3, a marker of apoptotic cell death, as well as SOX2 in order to assess the extent of Sox2-depletion in the mutant (Fig. 5A). Upon analyzing activated caspase 3-stained samples, we found increased labeling in the Sox2 CreERT2/fl samples, particularly in the NSD region (Fig. 5C and C’). To quantify this increase, total caspase 3-positive cells were counted in all samples, and a significant increase was seen in the Sox2-deficient mutant (n = 7) compared to controls (Fig. 5D; Sox2 +/+ n = 6, p = 0.0005, Sox2 CreERT2/+ n = 6, p = 0.0002). To ascertain whether the dying cells were likely to be presumptive neuroblasts, total caspase 3-positive counts were divided into posterior dorsal non-neurosensory domains (non-NSD) versus anteroventral neurosensory domains (NSD). This analysis revealed while cell death was mildly increased in the non-NSD, a more dramatic increase was observed in the NSD (Fig. 5E), indicating that a large percentage of the dying cells were likely to be presumptive neuroblasts.
Figure 5.
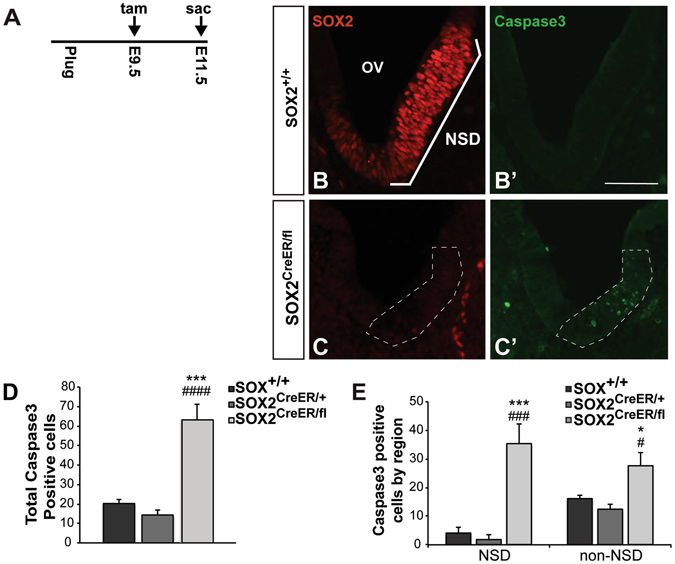
Progenitors die in the absence of SOX2. (A) Tamoxifen injection paradigm used for assessing the effect of SOX2 on cell survival. (B–C’) Representative sections showing SOX2 endogenous protein and activated caspase 3 immunostaining in a Sox2 +/+ control and Sox2 CreER/fl mutant. Note the robust increase of caspase 3 in the Sox2-deleted mutant (outlined with dashed lines). (D) The total number of caspase 3-positive cells was quantified and found to be significantly increased in the Sox2 CreER/fl mice (n = 7) compared to Sox2 +/+ (n = 6) (***p = 0.0005) and Sox2 CreER/+ mice (n = 6) (### p = 0.0002) (one-way ANOVA followed by a Student’s t test with a Bonferroni correction) NSD: Neurosensory domain. Scale bar: 50 μm.
Sox2 does not recapitulate early NEUROG1 fate mapping
It is well established during otic development that the vestibular sensory regions develop first, followed by the organ of Corti in the cochlea5, 44, 45. A previous fate-mapping study using a NEUROG1-Cre has demonstrated that otic neuronal maturation parallels the sensory maturation, with the cells destined for the vestibular ganglia expressing NEUROG1 initially, followed by cells destined for the spiral ganglion46. We wondered whether SOX2 would show a similar temporal pattern of expression in the vestibular ganglia (VG) versus spiral ganglia (SG). By crossing the Sox2-CreERT2 allele with the ROSA26 CAGtdTomato reporter, we fate-mapped SOX2 expression using Cre-mediated recombination by administering tamoxifen at either E8.5 (when vestibular ganglia begin expressing NEUROG1) or E12.5 (when primarily spiral ganglia express NEUROG1). Embryos were collected at E14.5 (Fig. 6A and D) at which point the ganglia are mature enough to be identified (VG vs SG). Unexpectedly, we found that early (E8.5) SOX2 fate-mapping robustly labeled both the spiral ganglia (Fig. 6B,B”) (n = 3) as well as (expectedly) the vestibular ganglia (Fig. 6C,C”) (n = 2). This finding is in contrast from NEUROG1 fate-mapping results46 and suggests that SOX2 is expressed in spiral ganglia precursors well before NEUROG1 expression is initiated.
Figure 6.
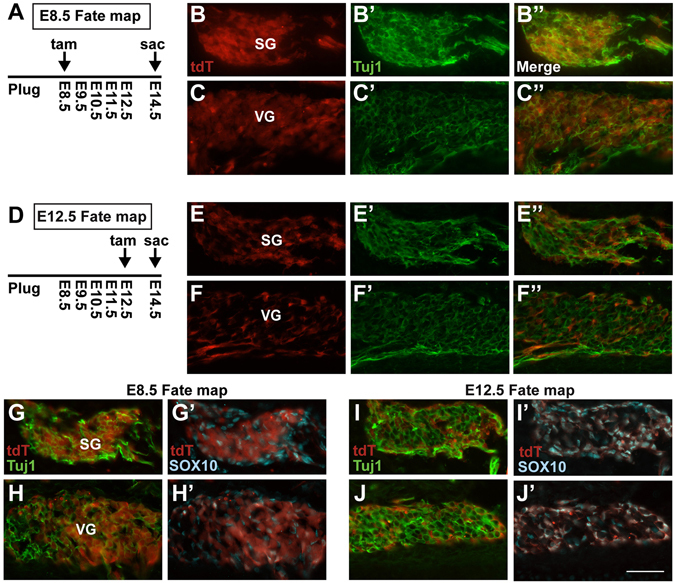
Early (E8.5) SOX2 expression maps to both the cochlea and vestibular ganglia whereas later (E12.5) SOX2 expression maps to the glia. (A) Schematic of the early (E8.5) SOX2 fate-mapping timeline. (B,B”) Representative sections showing E8.5 tdT fate-mapped SOX2 expression in the SG and VG (C,C”) at E14.5. Note that tdT reporting specifically labels the neuronal cell bodies in both ganglia. (D) Schematic of the later (E12.5) SOX2 fate-mapping timeline. (E,E”) Representative sections showing E12.5 fate-mapped SOX2 expression reported with tdT in both the SG and (F,F”) VG at E14.5. Note that tdT reporting does not overlap with the neuronal cell bodies, but instead appears adjacent to the TUJ1 neuronal marker. (G,H’) Representative image showing that E8.5 SOX2-reported progenitors contribute to neuronal cell bodies in both the SG and VG at E14.5, and does not overlap with the glia cell marker, SOX10. In contrast (I,J’) show representative images where E12.5 SOX2 fate-mapped progenitors contribute nearly exclusively to SOX10 labeled glial cells in both the SG and VG. Scale bar: 50 μm.
We also examined SOX2 reporter expression in the vestibular and spiral ganglia at a later time point, by injecting tamoxifen at E12.5 injection and again harvesting at E14.5. Results of these experiments show many labeled cells in the both the vestibular and spiral ganglion (Fig. 6F) (n = 2), although many did not appear to overlap with the neuronal maker TUJ1 (Fig. 6E” and F”). Since previous studies have also reported SOX2 in the neural-crest derived glia in the ganglia47, 48, we co-labeled tdT-expressing cells with a glial marker, SOX1049–51. Results of this experiment showed that while the majority of cells expressing SOX2 at E8.5 contribute to the CVG neurons (Fig. 6G’,H’), the majority of cells expressing SOX2 at E12.5 become glia (Fig. 6I’J’), as also noted by31, 52. Together, these results demonstrate that SOX2 is expressed early throughout both the vestibular and spiral ganglia, in contrast to NEUROG1 fate-mapping results46.
Discussion
SOX2 has been suggested to play a role in promoting inner ear neurogenesis, through both gain and loss-of-function studies, although its exact role in this process has not been clearly elucidated. Our results fill this knowledge gap by demonstrating that SOX2 is necessary during the otic neuroblast specification stage for the formation of the full complement of otic neurons. Specifically, we demonstrate that SOX2 is required for expression of NEUROG1 and NEUROD1, bHLH transcription factors necessary for otic neural development6–8. Not surprisingly, given the near-absence of NEUROG1-postive neuroblasts in SOX2-deficient inner ears, the volume of mutant CVG was significantly reduced. Interestingly, our data indicates that SOX2 levels are important for generating the correct number of inner ear neuroblasts. Furthermore, our results place the requirement for SOX2 early in the otic neurogenic signaling cascade, as it is required for the expression of NEUROG1, the earliest marker of the neuroblast fate. However, our SOX2 fate-mapping results do not demonstrate the same temporal pattern of expression in the vestibular and spiral ganglion exhibited by NEUROG146, suggesting that SOX2 may be more of a competence factor than the sole initiator of NEUROG1 expression.
Our results regarding the role of SOX2 in otic neurogenesis are consistent with findings in the brain and other sensory systems, such as the hippocampus and neural retina, that have established a requirement for SOX2 in promoting neural competence19, 42, 43, 53. It is currently unclear whether SOX2 directly activates the neural program in the otocyst, or is required as a competence factor. In support of the former, overexpression studies in the chick have found that ectopic expression of SOX2 can expand the NEUROG1-positive domain13, and can increase the number of otic neurons32. In the mouse, overexpression of SOX2 at later stages in the organ of Corti leads to cells preferentially differentiating as neurons, although these neurons did not express otic-specific neural markers and failed to express later maturation markers28. Our fate mapping studies by contrast, indicate that SOX2 is expressed well before NEUROG1, at least in spiral ganglion neuroblasts46, suggesting a role as a competence factor. It is possible that the ability of SOX2 to initiate NEUROG1 expression is dependent on protein levels, which fate-mapping does not provide insight into. However, other studies indicate that SOX2 cannot activate the neuronal fate independently. For example, studies by Ahmed et al.27, demonstrated that ectopic neural differentiation was enhanced when SOX2 was included in combination with the transcription factors SIX1 and EYA1, but was ineffective in inducing neurogenesis on its own. Studies in the lens and embryonic stem cells additionally have shown that SOX2 must partner with specific factors to exert its transcriptional effects54. More recent studies in the adult hippocampus have suggested that SOX2 primes the epigenetic landscape by maintaining a bivalent chromatin state and allowing neural differentiation programs to be activated55. This type of role has yet to be shown during development, but would be consistent with our results.
Our study found that mice carrying only one copy of Sox2 produced fewer NEUROG1-positive cells, and impaired neurogenesis in the inner ear. Sox2 haploinsuffiency has been demonstrated to cause the human condition SOX2 Anophthalmia Syndrome, a disorder characterized by anophthalmia, learning disabilities, seizures, and postnatal growth failure22, 56, 57. In some cases hearing loss has also been reported22, 58. Additionally, a number of animal studies have also found that Sox2 haploinsufficiency produces defects in the eye43, the anterior pituitary58, and anterior foregut59. Two distinct mechanisms of transcriptional regulation by SOX factors have been proposed, including protein-protein interactions and modification of chromatin structure17. With regards to SOX2 dependence on protein-protein interactions54, 60, it is possible that levels of SOX2 influence which co-factors it partners with, which could produce differences in target gene expression. Alternatively, differences in protein levels may lead to functional consequences by modifying SOX2’s ability to alter DNA confirmation, leading to reduced accessibility for transcriptional complexes. While the molecular mechanism by which Sox2 dosage regulates cell fate remains to be fully elucidated, our finding of reduced numbers of otic neurons due to Sox2 haploinsufficiency is consistent with findings in other systems, including human patients with SOX2 mutations, and highlights the importance of protein levels for effective SOX2 function.
Previously, studies have shown that SOX2 is required for the generation of the sensory regions of the inner ear23, 24. Here, for the first time, we demonstrate that SOX2 is also required for the specification of the otic neurons. Based on these findings, it is tempting to speculate that SOX2 is required for an early neuro-sensory progenitor, which in its absence, leads to failure of both neural and sensory inner ear components. However, lineage and fate-mapping studies in the ear have not supported the idea of a general neuro-sensory progenitor, although evidence has been shown for a subset of neuro-sensory progenitors that give rise to both neurons and sensory cells in the utricle and saccule30, 61. In the absence of evidence for a general neuro-sensory progenitor, it is likely that SOX2 plays specific and distinct roles in both sensory and neural development; however, we currently do not understand the mechanism that determines whether SOX2 specifies neurons or sensory regions. Given that partners of SOX2 can vastly influence which target genes are activated54, it is likely that neural vs. sensory may well be dictated by SOX2 co-factors. Factors that have been implicated as interacting with SOX2 include EYA1 and SIX127, 62, both of which are associated with neuronal as well as sensory hair cell formation. Ahmed et al.27, suggested that interaction with the SWI/SNF chromatin remodeling factors BRG1 and BAF leads to neuronal-specific induction. Another factor that may play a role in determining whether SOX2 acts to specify neural vs. sensory is the Notch signaling pathway. Lateral inhibition has been shown to select a neuronal fate, in which cells that have low Notch activity and high Dll1 express NEUROG1 and become otic neuroblasts6, 11, 63, 64. This suggests that Notch-negative and SOX2-positive progenitors will become neurons, whereas Notch-positive and SOX2-positive cells will likely adopt a sensory fate. Consistent with this idea, forced activation of Notch during neurogenesis leads to ectopic hair cells in the otic ganglion65, suggesting that Notch activation diverts progenitors from a neuronal fate into a sensory cell fate.
Our results demonstrate that, consistent with other studies, loss of SOX2 leads to cell death26, 55, 66. Potentially, SOX2 could function directly as a prosurvival factor. However, it may be more likely that SOX2 is needed to trigger expression of downstream genes necessary for neuroblast specification, and in the absence of the expression of these genes, progenitors undergo cell death. This idea is consistent with studies that showed massive apoptosis in the otic epithelium caused by deletion of either Neurog1 or NeuroD1 6, 7, a result the authors hypothesized stemmed from the lack of specification. Similarly, in our findings, given the reduction of NEUROG1 expressing cells in Sox2-deleted mutants, it is possible that neural progenitors died from a failure to be specified. Our results are distinct from studies that showed the absence of a ganglia28 or neuronal death26 in the CVG in more mature Sox2-deleted mutants ears. In those studies, Sox2-deficient ears were analyzed at later stages, at which point there is also a sensory defect. Thus, the absence/death of neurons at these stages may be attributed to the absence of hair cell targets in the epithelium that provide neurotrophic support rather than a direct reliance on SOX2 for survival. As our study focuses on the role of SOX2 during early otic stages using early specification markers, our results establish a direct requirement for SOX2 in specification of the otic neuroblasts that give rise to the CVG. Further studies will need to be performed to establish whether there is a direct requirement for SOX2 after delamination, although several studies (including ours) have demonstrated an abrupt downregulation of SOX2 in the neuroblasts after delamination until E18/P013, 52.
Our study demonstrates that while SOX2 is required for specifying the vast majority of NEUROG1-expressing neuroblasts, some CVG neurons remain. What then accounts for the remaining neurons? It is possible that the intervals between tamoxifen injections allow enough SOX2 expression to initiate a neuronal program in some cells. However it is also plausible that some neurons develop independently of SOX2 and other factors contribute to the formation of the CVG. Similar to in the neural tube, there could be redundancy between SOXB1 factors19. Alternatively, other transcription factors, such as the aforementioned EYA1/SIX1 transcriptional complex, could be responsible for the formation of a portion of the neurons. As discussed, EYA1/SIX1 factors can promote neurogenesis independently of SOX227. Thus, it is possible that a small subset of inner ear neurons can be produced in a SOX2-independent manner.
In conclusion, we have demonstrated that SOX2 is required for the formation of the full complement of CVG neurons, and acts in a dose-dependent manner. These results complement evidence from overexpression studies that have implicated SOX2 in otic neurogenesis13, 28, 32. Our fate mapping results show that SOX2 is expressed well in advance of NEUROG1 in the spiral ganglion, raising the possibility that it may be acting as a competence factor rather than a direct regulator. In humans, loss or dysfunction of the spiral ganglion neurons causes permanent hearing impairment, and health of the spiral ganglion is an important feature in cochlear implant success. Thus, deciphering the molecular cascade that leads to inner ear neurogenesis is an important goal for designing future regeneration therapies.
Methods
All experimental procedures were performed in accordance with guidelines and regulations of the University of Rochester Medical Center. All animal experiments were approved by the University of Rochester’s Committee on Animal Resources.
Mice and tamoxifen treatment
The following mouse strains were used: Sox2-Cre ERT2 33, ROSA26-CAGTdtomato 34, Sox2 flox 35, NEUROG1-Cre 67. Genotyping was performed by PCR using the following primers: Sox2-CreER T2 and NEUROG1-Cre were both detected with the same primer pair: Cre1F (TGA TGA GGT TCG CAA GAA CC) and Cre1R (CCA TGA GTG AAC GAA CCT GG) yield a 350 bp band. Rosa26-CAGTdtomato: tdTomatoF: (CTG TTC CTG TAC GGC ATG G) and tdTomatoR (GGC ATT AAA GCA GCG TAT CC) homozygous mice yield a 196 bp band. Sox2 flox: Sox2Fl WT1 (TGG AAT CAG GCT GCC GAG AAT CC), Sox2Fl WT2 (TCG TTC TGG CAA CAA GTG CTA AAG C), and Sox2FlMut (CTG CCA TAG CCA CTC GAG AAG). Heterozygous mice have both a wildtype 427 bp band and a mutant 546 bp band, whereas homozygous mice have only the 546 bp band. Timed matings were determined by checking for vaginal plugs, and the morning of the plug considered E0.5. Pregnant dams were injected with tamoxifen (3 mg/40 g body weight) and progesterone (2 mg/40 g body weight) at various developmental time points.
Immunohistochemistry
Embryos were fixed in 4% PFA in PBS buffer at 4 °C for 2.5–4 hours, cryoprotected, and sectioned at a thickness of 16 μm. The sections were incubated with primary antibodies overnight at 4 °C and then incubated with secondary antibodies for 2 hours at room temperature. The primary antibodies used were goat polyclonal α-NEUROG1 (1:700, Santa Cruz), rat monoclonal α-RFP (1:1000, ChromoTek), rabbit polyclonal α-SOX2 (1:500, abcam), mouse monoclonal α-TUJ1 (1:1000, Covance), goat polyclonal α-NEUROD1 (1:1000, Santa Cruz), rabbit α-activated caspase 3 (1:1000, R&D Systems), goat polyclonal α-SOX2 (1:700, Santa Cruz), rabbit polyclonal α-SOX2 (1:500, Millipore), and goat polyclonal SOX10 (1:100, Santa Cruz). The secondary antibodies used were Alexa Fluor 488 donkey α-goat (1:1000), Alexa Fluor 488 donkey α-rabbit (1:1000), Alexa Fluor 555 donkey α-rabbit (1:1000), Alexa Fluor 555 donkey α-rat (1:1000), Alexa Fluor 647 donkey α-mouse (1:1000), Alexa Fluor 647 donkey α-goat (1:1000).
Quantifications and statistical analysis
NEUROG1 cell counts and ganglia volume measurements. NEUROG1-positive cells were counted using Axiovision software (Carl Zeiss). Criterion was set such that cells were counted as NEUROG1-positive only if they had the characteristic teardrop nuclear shape of NEUROG1 and sufficient brightness. The area of the ganglion was measured by tracing the TUJ1 positive ganglia using the outline spline tool in Axiovision software. The total area for each ganglion was converted into a volume by multiplying by the section thickness, 16 μm. NEUROD1 cell counts. NEUROD1-positive cells were counted using Axiovision software (Carl Zeiss). A bounded box measuring 100 μm2 was placed at the most anterior point of the otocyst and the total number of NEUROD1 + cells in the otic epithelium and surrounding mesenchyme constrained by the bounding box were quantified. NEUROD1 + cells were further categorized by their location (mesenchyme vs epithelium) and co-localization with SOX2.
Caspase 3 cell counts. Activated caspase 3 was counted using Axiovision software (Carl Zeiss). Total caspase 3 counts were segregated based on whether the positively labeled cells were located in the anteroventral neurosensory region of the otocyst, that in the controls expressed SOX2 and in the mutant was tdT-positive (termed neurosensory domain (NSD)), versus caspase3 positive cells located in more posterior dorsal regions of the otocyst (termed non-neurosensory domain (non-NSD)).
ANOVA and Student’s t-tests were used for statistical analysis. Prism Graphpad 6.0 was used for all statistics.
Acknowledgements
The authors would like to thank Dr. Bernd Fritzsch for careful reading of the manuscript and providing thoughtful comments. This work was supported by grants from the National Institutes of Health to AEK (RO1 DC009250) and to ARS (F31 DC015153), and a departmental grant from the foundation Research to Prevent Blindness (RPB).
Author Contributions
A.S. and A.K. designed research; A.S., D.S., and J.G. performed research; A.S., D.S., J.G. and A.K. analyzed data; A.S. and A.K. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geleoc GS, Holt JR. Sound strategies for hearing restoration. Science. 2014;344:1241062. doi: 10.1126/science.1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. The Journal of comparative neurology. 1983;215:359–369. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- 3.Lu CC, Appler JM, Houseman EA, Goodrich LV. Developmental profiling of spiral ganglion neurons reveals insights into auditory circuit assembly. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:10903–10918. doi: 10.1523/JNEUROSCI.2358-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farinas I, et al. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell D, et al. Spatial and temporal segregation of auditory and vestibular neurons in the otic placode. Developmental biology. 2008;322:109–120. doi: 10.1016/j.ydbio.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/S0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 7.Kim WY, et al. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. Journal of the Association for Research in Otolaryngology: JARO. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Current opinion in neurobiology. 2002;12:35–42. doi: 10.1016/S0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- 10.Fritzsch, B. et al. Development and evolution of inner ear sensory epithelia and their innervation. Journal of neurobiology53, 143–156, doi:10.1002/neu.10098 (2002). [DOI] [PMC free article] [PubMed]
- 11.Adam J, et al. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- 12.Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. The Journal of comparative neurology. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- 13.Evsen L, Sugahara S, Uchikawa M, Kondoh H, Wu DK. Progression of neurogenesis in the inner ear requires inhibition of Sox2 transcription by neurogenin1 and neurod1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:3879–3890. doi: 10.1523/JNEUROSCI.4030-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avilion AA, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes & development. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist’s view of neural development. Trends in neurosciences. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. The international journal of biochemistry & cell biology. 2010;42:421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Wegner M. SOX after SOX: SOXession regulates neurogenesis. Genes & development. 2011;25:2423–2428. doi: 10.1101/gad.181487.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/S0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 20.Sisodiya SM, et al. Role of SOX2 mutations in human hippocampal malformations and epilepsy. Epilepsia. 2006;47:534–542. doi: 10.1111/j.1528-1167.2006.00464.x. [DOI] [PubMed] [Google Scholar]
- 21.Fantes J, et al. Mutations in SOX2 cause anophthalmia. Nature genetics. 2003;33:461–463. doi: 10.1038/ng1120. [DOI] [PubMed] [Google Scholar]
- 22.Hagstrom SA, et al. SOX2 mutation causes anophthalmia, hearing loss, and brain anomalies. American journal of medical genetics. Part A. 2005;138A:95–98. doi: 10.1002/ajmg.a.30803. [DOI] [PubMed] [Google Scholar]
- 23.Kiernan AE, et al. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- 24.Kempfle JS, Turban JL, Edge AS. Sox2 in the differentiation of cochlear progenitor cells. Scientific reports. 2016;6:23293. doi: 10.1038/srep23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neves J, Uchikawa M, Bigas A, Giraldez F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PloS one. 2012;7:e30871. doi: 10.1371/journal.pone.0030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dvorakova M, et al. Incomplete and delayed Sox2 deletion defines residual ear neurosensory development and maintenance. Scientific reports. 2016;6:38253. doi: 10.1038/srep38253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed M, Xu J, Xu PX. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development. 2012;139:1965–1977. doi: 10.1242/dev.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 induces neuronal formation in the developing mammalian cochlea. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:714–722. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- 30.Raft S, et al. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- 31.Gu R, et al. Lineage tracing of Sox2-expressing progenitor cells in the mouse inner ear reveals a broad contribution to non-sensory tissues and insights into the origin of the organ of Corti. Developmental biology. 2016 doi: 10.1016/j.ydbio.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neves J, Parada C, Chamizo M, Giraldez F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development. 2011;138:735–744. doi: 10.1242/dev.060657. [DOI] [PubMed] [Google Scholar]
- 33.Arnold K, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell stem cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaham O, et al. Pax6 is essential for lens fiber cell differentiation. Development. 2009;136:2567–2578. doi: 10.1242/dev.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi F, Hu L, Edge AS. Generation of hair cells in neonatal mice by beta-catenin overexpression in Lgr5-positive cochlear progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walters BJ, Yamashita T, Zuo J. Sox2-CreER mice are useful for fate mapping of mature, but not neonatal, cochlear supporting cells in hair cell regeneration studies. Scientific reports. 2015;5:11621. doi: 10.1038/srep11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem cell reports. 2014;2:311–322. doi: 10.1016/j.stemcr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JE, et al. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- 40.Pataskar A, et al. NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program. The EMBO journal. 2016;35:24–45. doi: 10.15252/embj.201591206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng M, Yang H, Xie X, Liang G, Gan L. Comparative expression analysis of POU4F1, POU4F2 and ISL1 in developing mouse cochleovestibular ganglion neurons. Gene expression patterns: GEP. 2014;15:31–37. doi: 10.1016/j.gep.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Favaro R, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nature neuroscience. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- 43.Taranova OV, et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes & development. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu DK, Oh SH. Sensory organ generation in the chick inner ear. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koundakjian EJ, Appler JL, Goodrich LV. Auditory neurons make stereotyped wiring decisions before maturation of their targets. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:14078–14088. doi: 10.1523/JNEUROSCI.3765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang H, et al. Sox2 up-regulation and glial cell proliferation following degeneration of spiral ganglion neurons in the adult mouse inner ear. Journal of the Association for Research in Otolaryngology: JARO. 2011;12:151–171. doi: 10.1007/s10162-010-0244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang H, et al. Neural stem/progenitor cell properties of glial cells in the adult mouse auditory nerve. Scientific reports. 2015;5:13383. doi: 10.1038/srep13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breuskin I, et al. Glial but not neuronal development in the cochleo-vestibular ganglion requires Sox10. Journal of neurochemistry. 2010;114:1827–1839. doi: 10.1111/j.1471-4159.2010.06897.x. [DOI] [PubMed] [Google Scholar]
- 50.Britsch S, et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes & development. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paratore C, Goerich DE, Suter U, Wegner M, Sommer L. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development. 2001;128:3949–3961. doi: 10.1242/dev.128.20.3949. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura K, Noda T, Dabdoub A. Dynamic Expression of Sox2, Gata3, and Prox1 during Primary Auditory Neuron Development in the Mammalian Cochlea. PloS one. 2017;12:e0170568. doi: 10.1371/journal.pone.0170568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavallaro M, et al. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008;135:541–557. doi: 10.1242/dev.010801. [DOI] [PubMed] [Google Scholar]
- 54.Kondoh H, Kamachi Y. SOX-partner code for cell specification: Regulatory target selection and underlying molecular mechanisms. The international journal of biochemistry & cell biology. 2010;42:391–399. doi: 10.1016/j.biocel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Amador-Arjona, A. et al. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America, doi:10.1073/pnas.1421480112 (2015). [DOI] [PMC free article] [PubMed]
- 56.Bakrania P, et al. SOX2 anophthalmia syndrome: 12 new cases demonstrating broader phenotype and high frequency of large gene deletions. The British journal of ophthalmology. 2007;91:1471–1476. doi: 10.1136/bjo.2007.117929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ragge, N. K. et al. SOX2 anophthalmia syndrome. American journal of medical genetics. Part A135, 1–7, discussion 8, doi:10.1002/ajmg.a.30642 (2005). [DOI] [PubMed]
- 58.Kelberman D, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. The Journal of clinical investigation. 2006;116:2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Que J, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends in genetics: TIG. 2000;16:182–187. doi: 10.1016/S0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- 61.Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–1697. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed M, et al. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Developmental cell. 2012;22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison A, Hodgetts C, Gossler A, Hrabe de Angelis M, Lewis J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mechanisms of development. 1999;84:169–172. doi: 10.1016/S0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 64.Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- 65.Pan W, et al. Ectopic expression of activated notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:16146–16157. doi: 10.1523/JNEUROSCI.3150-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cimadamore F, et al. Human ESC-derived neural crest model reveals a key role for SOX2 in sensory neurogenesis. Cell stem cell. 2011;8:538–551. doi: 10.1016/j.stem.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quinones HI, Savage TK, Battiste J, Johnson JE. Neurogenin 1 (Neurog1) expression in the ventral neural tube is mediated by a distinct enhancer and preferentially marks ventral interneuron lineages. Developmental biology. 2010;340:283–292. doi: 10.1016/j.ydbio.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


