Abstract
We examined children's sleep at age 9 as a predictor of developmental trajectories of cognitive performance from ages 9 to 11 years. The effects of sleep on cognition are not uniform and thus we tested race/ethnicity, socioeconomic status, and sex as moderators of these associations. At the first assessment, 282 children aged 9.44 years (52% boys, 65% European American, 35% African American) participated. Two more waves of data collection spaced one year apart followed. The majority of children (63%) were living at or below the poverty line. Children's sleep was measured objectively with actigraphy and two well-established sleep parameters were derived: duration, indexed by sleep minutes between sleep onset and wake time, and quality, indexed by efficiency. Multiple cognitive functioning domains were examined with the Woodcock Johnson Tests of Cognitive Abilities. Across the sample, higher sleep efficiency, but not duration, was associated with better cognitive performance. Significant moderation effects emerged. Controlling for socioeconomic status, AA children scored lower on general intellectual ability and working memory at age 11 only if they experienced lower sleep efficiency at age 9. Further, boys scored lower on general abilities and processing speed at age 11 only if their sleep efficiency was lower at age 9. Findings indicate that lower sleep efficiency may contribute to lower cognitive functioning especially for AA children and boys. These vulnerabilities appear to emerge early in development and are maintained over time. Results underscore the importance of individual differences in explicating relations between sleep and children's cognitive performance.
Keywords: sleep, actigraphy, cognition, childhood, ethnicity
Sleep problems (short duration, poor quality) along the normative continuum in community samples are linked with lower cognitive performance (Astill, Van der Heijden, Van Ijzendoorn, & Van Someren, 2012). Children from ethnic minority and low income families are at risk for poor cognitive outcomes, and poor sleep may exacerbate that risk (Buckhalt, El-Sheikh, & Keller, 2007). Sex-related effects have less commonly been examined in this literature, but there is some evidence that both girls (Bub, Buckhalt, & El-Sheikh, 2011) and boys (Dewald, Meijer, Oort, Kerkhof, & Bögels, 2010) experience vulnerability for lower cognitive performance as a result of poor sleep. Although cognitive functioning rapidly improves across childhood, few studies have examined how sleep predicts trajectories of change over time. We examined the association between children's sleep and trajectories of cognitive functioning across middle to late childhood, as well as the role of race/ethnicity (referred to as ethnicity henceforth), socioeconomic status (SES), and sex as moderators of these associations.
Sleep is a multi-faceted construct and examination of multiple sleep parameters is warranted (Sadeh, 2015). Towards a rigorous assessment of sleep, we examined two important objective actigraphy-derived variables: sleep duration (actual number of minutes spent asleep between sleep onset and waking up) and quality (efficiency or percentage of the night spent asleep). The terms “sleep problems” and “poor sleep” are used interchangeably throughout the paper and refer to fewer sleep minutes and less efficient sleep examined on a continuum.
A recent meta-analysis of 86 predominantly cross-sectional studies examining children's sleep and cognitive functioning found longer sleep duration to be associated with better executive functioning (Astill et al., 2012). Experimental sleep restriction has been associated with concurrent lower scores on cognitive tasks assessing sustained attention, cognitive processing speed (Louca & Short, 2014) and working memory (Sadeh, Gruber, & Raviv, 2003) among children. Other studies have also found better sleep quality to be linked with better child performance on neurobehavioral tasks (Sadeh, Gruber, & Raviv, 2002), particularly those testing working memory (Steenari et al., 2003), as well as measures of crystallized intelligence including verbal comprehension (Bub et al., 2011).
A handful of studies, most involving two points of assessment, have examined longitudinal relations between sleep and cognitive functioning. Maternal report of persistently short sleep across 5 ages in early childhood has been shown to predict poorer cognitive performance at school-age (Touchette et al., 2007). Another study found increases in self-reported sleepiness (a construct associated with sleep) from ages 8-10 years to predict less growth in verbal comprehension as measured across three waves (Bub et al., 2011). In a study examining cognitive performance at two time points, Buckhalt et al. (2009) found greater self-reported sleepiness, subjective sleep problems, and shorter actigraph-assessed sleep at age 8 to predict lower intellectual ability two years later. We sought to extend prior work by using objective measurement of sleep duration and quality to predict cognitive performance across three time points spanning ages 9 to 11 years. Strengthening the assessment of cognitive performance, we examined general intellectual ability, processing speed, and working memory, which assess crystallized intelligence, decision and perceptual processing speed, and short-term memory retention, respectively.
Although the literature supports relations between sleep and cognitive performance, this association is not uniform and individual differences are likely operative. Studies assessing moderation of the influence of sleep on cognitive outcomes by ethnicity or SES, particularly in longitudinal samples, are scarce. However, examination of such effects is warranted to explicate for whom sleep problems may be especially consequential. African American (AA) and low SES children are at higher risk for poor cognitive functioning than their European American (EA) and higher SES counterparts (Buckhalt et al., 2007; Larson, Russ, Nelson, Olson, & Halfon, 2015). AA children also show less improvement in verbal comprehension and decision speed than EA children across ages 8-10 years when they report increases in sleepiness across this time period (Bub et al., 2011). Cross-sectional work indicates that AA and low SES children score very similarly to EA and higher SES children on measures of processing speed and general intellectual ability when they slept longer, but were significantly negatively affected by shorter sleep durations (Buckhalt et al., 2007). The authors argued that these effects are consistent with a health disparities perspective (Carter-Pokras & Baquet, 2002), whereby minority and low SES children are disproportionately affected by poor sleep as a result of a lifelong accumulation of stressful experiences (Evans & English, 2002).
It is also possible that child sex moderates the relation between sleep and cognitive outcomes, although the research to-date is inconclusive. A meta-analysis indicated that in comparison to studies with older children and those with a high representation of females, investigations with younger and more male participants found stronger associations between poor sleep and lower school performance, suggesting that prepubertal boys may be particularly affected by short and poor quality sleep (Dewald et al., 2010). On the other hand, Bub et al. (2011) found self-reported sleepiness to attenuate improvements in cognitive skills from ages 9 to 11 years old for girls, but not for boys. To explicate effects, further investigations are warranted especially those that employ longitudinal designs and multiple domains of both objective sleep problems and cognitive functioning.
The Present Study
Utilizing multilevel growth modeling, we examined sleep minutes and efficiency at age 9 as predictors of cognitive functioning (general intellectual ability, processing speed, working memory) at age 11 (intercept effects). We also assessed sleep at age 9 as a predictor of developmental trajectories of cognitive performance from ages 9 to 11 years (slope effects). We expected children with longer and more efficient sleep at age 9 to show higher cognitive functioning at age 11 and steeper improvements in cognitive functioning over time. Child ethnicity, SES, and sex were examined as moderators of these associations. Consistent with health disparities perspectives (Buckhalt, 2011), AA and lower SES children were expected to be especially vulnerable to lower cognitive functioning at age 11 and to less improvement in cognitive functioning across time if they experienced poor sleep. Race and SES were entered in the same models to examine the unique effects of each on cognitive performance. Due to mixed findings in the literature, we did not have an a priori hypothesis regarding moderation by sex.
Methods
Participants
Participants were part of a longitudinal study of children's sleep, health, and adjustment across middle and late childhood at a university in the southeastern United States. Data collection took place across three years (2009-2012), with one-year intervals between each time point. At the first study wave, 282 children and families were recruited to participate in the study from local elementary schools. Participants did not have a diagnosis of a learning disability or clinical sleep disorder (e.g., apnea) based on mothers' reports. The sample was 52% boys, 65% European American (EA), and 35% African American (AA). The ethnic composition of the sample is congruent with their representation in the communities from which they were drawn, and Alabama in general. At the second wave, 80% of the original sample remained in the study. Of the children who participated in the study at the second wave, 98% participated at the third. There were no differences between those who remained versus dropped from study at either wave with regard to family demographics or study variables.
Children's mean age at the three time points was 9.44 (SD = .71), 10.37 (SD = .68), and 11.33 years (SD = .69). The sample was 52% boys, 65% European American (EA), and 35% African American (AA). As indicated by family income-to-needs ratio (annual family income divided by the poverty threshold with respect to family size; U.S. Department of Commerce, 2013), the majority of participants (63%) were living at or below the poverty line (ratio ≤ 2), 28% were lower middle class (ratio between 2 and 3), and 9% were middle class (ratio ≥ 3).
Procedure
The study was approved by the institution's review board. At age 9, information regarding children's sleep was collected via an actigraph worn on their non-dominant wrist for seven consecutive nights during the school year. Following sleep data collection, families participated in a laboratory visit during which children completed cognitive assessments and parents filled out questionnaires regarding demographic information. On average, families came to the laboratory 3.43 days (SD = 8.74) following the last night of actigraphy. The same cognitive assessments were repeated at ages 10 and 11.
Measures
Sleep
Nighttime sleep was measured via actigraphy (Motionlogger Octagonal Basic actigraphs, Ambulatory Monitoring Inc., Ardsley, NY), and analyzed via a computer software package (ActionW User's Guide version 2.4, 2002) that uses the well-established Sadeh algorithm (Sadeh, Sharkey, & Carskadon, 1994) . Sleep minutes (number of minutes spent asleep between sleep onset and wake time) and efficiency (percent of minutes spent asleep between sleep onset and wake time) were derived. To validate the actigraphy data, a research assistant called parents nightly during the week of assessment to collect the child's bed- and wake-times and medication use. Nights during which children were taking medication for acute illnesses were not considered valid and were excluded from analyses. Following established guidelines (Acebo et al., 1999; Meltzer, Montgomery-Downs, Insana, & Walsh, 2012), data for children with fewer than 5 nights of valid data (11% of the sample) were also excluded from the analyses.1 There were no significant differences on main study variables or covariates between those children whose data were excluded and those whose data were included. Sleep minutes and efficiency were highly stable across the week, α's =.85 and .90.
Cognitive assessment
Children's cognitive functioning was assessed using the well-validated and individually administered Woodcock Johnson Tests of Cognitive Abilities (WJ III), (McGrew & Woodcock, 2001; Woodcock, McGrew, & Mather, 2001). Three primary domains of cognitive functioning and frequently utilized corresponding scales were examined: Brief Intellectual Ability (BIA), Processing Speed (PS), and Working Memory (WM) (Bub et al., 2011; Buckhalt et al., 2007). BIA is a composite score comprising performance on three tests: Verbal Comprehension (analogies, synonyms & antonyms, vocabulary), Concept Formation (fluid and categorical reasoning), and Visual Matching (perceptual processing). These three tests are indicators of crystallized intelligence, fluid and inductive reasoning, and speeded visual perception and matching speed, respectively. PS is a composite score comprising performance on Decision Speed, which measures object recognition and speeded symbolic/semantic comparisons, and Visual Matching. The WM test measures auditory and working memory by requiring children to repeat a series of numbers and words read to them by an examiner. As BIA taps into crystallized intelligence, scores on this measure are thought to be more stable over time than scores on the PS and WM, which may be more influenced by variation in tiredness (Bub et al., 2011). Because we analyzed cognitive performance longitudinally, we used vertically equated item response theory (IRT)-scaled scores (i.e., W scores) in analyses. Such scores are calibrated on a Rasch model, which indicates a person's deviation from a criterion score and are thus equatable over time (Rasch, 1960). Due to this interval scaling, any changes in cognitive performance scores from age 9 to age 10 can be interpreted in the same way as changes in scores from age 10 to age 11. The W scores are frequently used in the literature to examine performance on the WJIII longitudinally (Bub et al., 2011; McArdle, Ferrer-Caja, Hamagami, & Woodcock, 2002; Tucker-Drob, 2009).
Demographics
During recruitment, mothers reported on child race/ethnicity and sex as well as family income for calculating income-to-needs ratio via a series of questions asked over the phone, including, “What is your child's sex? What is your child's race? What is your family's annual income?” How many persons live within your home?” Henceforth, we refer to income-to-needs ratio as SES for brevity.
Analytic Approach
Consistent with best practices, we used multilevel models to account for the dependent, repeated measures nature of the longitudinal data (Heck & Thomas, 2015 ) using Mplus (Muthen & Muthen, 2012). First, an unconditional growth model was fit for each cognitive outcome, which estimated an intercept (age 11 scores) and slope (change over time from ages 9 to 11) that were allowed to vary between individuals. We set the intercept at age 11 because the study question pertains to children's cognitive outcomes at this age (during the last study wave) as predicted by sleep earlier in development. The unconditional models were used to derive unbiased estimates of basic model parameters and to partition the total variance in repeated measures of cognitive scores into within (level 1) and between (level 2) individual components. In each model we evaluated whether there was statistically significant variability at the between-individual level in intercepts and slopes that would serve as outcomes in conditional models (i.e., models with predictors to account for between individual variability).
Next, we estimated conditional growth models in which time was the level 1 predictor and the sleep variables and individual characteristics (ethnicity, SES, sex) were level 2 predictors of both the intercept (age 11 scores) and the slope (the relationship between time and scores) (Heck & Thomas, 2015). All individual characteristics (ethnicity, SES, sex) were entered in the models simultaneously to examine their unique effects. Separate models were run for sleep minutes and efficiency. When conditional models revealed a significant interaction of interest between the level 2 predictors on the intercept or slope, we plotted the simple slopes at conditional values of the variables of interest (Curran, Bauer, & Willoughby, 2004). In the post-hoc simple slopes analyses continuous predictors were evaluated at ± 1 SD. Missing data in the analytic models was handled with full information maximum likelihood estimation (FIML). The amount of missing data across study variables ranged from 6-13%. This amount is well within the acceptable range for FIML, which has been shown to be the best statistical method for handling missing data because it produces the least biased estimates and lowest Type I error rates (Enders & Bandalos, 2001).
Results
Variable means and SDs as well as correlations among variables are presented in Table 1. AA ethnicity and lower family SES were generally associated with lower BIA and WM scores. Cognitive functioning was highly stable over time and scores within the three domains were moderately correlated with one another. AA ethnicity was moderately associated with lower SES. On average, children slept approximately 458 minutes (7.64 hours) per night, which is comparable to estimates of sleep duration obtained from similar-aged samples using actigraphy (Spruyt, Gozal, Dayyat, Roman, & Molfese, 2011; Steenari et al., 2003; Tremaine, Dorrian, & Blunden, 2010). Table 2 includes descriptive sleep information by sex and race. There was a trend for EA children to sleep longer than AA children.
Table 1. Bivariate correlations, means, and standard deviations for main study variables.
| 1. Ethnicity | 2. SES | 3. Sex | 4. Sleep minutes Age 9 | 5. Sleep efficiency Age 9 | 6. BIA Age 9 | 7. BIA Age 10 | 8. BIA Age 11 | 9. PS Age 9 | 10. PS Age 10 | 11. PS Age 11 | 12. WM Age 9 | 13. WM Age 10 | 14. WM Age 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | ||||||||||||||
| 2. | -.40** | |||||||||||||
| 3. | -.02 | .06 | ||||||||||||
| 4. | -.12 t | .14* | -.11 | |||||||||||
| 5. | -.01 | .13* | -.07 | .81** | ||||||||||
| 6. | -.25** | .25** | -.01 | .01 | .11 t | |||||||||
| 7. | -.28** | .27** | .01 | -.09 | .02 | .84** | ||||||||
| 8. | -.27** | .29** | .01 | .00 | .08 | .82** | .89** | |||||||
| 9. | -.02 | .04 | -.17** | -.02 | .06 | .63** | .48** | .50** | ||||||
| 10. | -.01 | .08 | -.20** | -.08 | .02 | .59** | .63** | .60** | .72** | |||||
| 11. | .03 | .07 | -.10 | .03 | .14 t | .56** | .50** | .59** | .71** | .79** | ||||
| 12. | -.25** | .13* | .01 | .06 | .12 t | .57** | .58** | .58** | .27** | .33** | .33** | |||
| 13. | -.25** | .19** | -.00 | -.02 | .02 | .57** | .62** | .61** | .30** | .37** | .32** | .75** | ||
| 14. | -.31** | .18* | .05 | .09 | .13 t | .52** | .57** | .61** | .31** | .34** | .33** | .72** | .75** | |
|
| ||||||||||||||
| M | .35 | 1.71 | .52 | 458.31 | 88.24 | 494.57 | 502.37 | 507.27 | 497.27 | 503.29 | 508.94 | 499.48 | 506.77 | 509.28 |
| SD | .48 | 1.04 | .50 | 56.17 | 8.05 | 10.34 | 10.27 | 11.20 | 8.78 | 8.01 | 8.71 | 14.81 | 14.48 | 16.02 |
Note. Race/ethnicity is coded as African American = 1, European American = 0. Sex is coded as male = 1, female = 0. BIA = Brief Intellectual Ability, PS = Processing Speed, WM = Working Memory
p < .10
p <.05
p < .01
Table 2. Descriptive sleep information by sex and race.
| Boys | Girls | t-test | European American | African American | t-test | |
|---|---|---|---|---|---|---|
|
| ||||||
| M (SD) | M (SD) | M (SD) | M (SD) | |||
| Sleep minutes | 452.57 (55.09) | 464.33 (56.88) | 1.65 | 462.95 (58.90) | 449.21(49.45) | 1.82 t |
| Sleep efficiency | 87.71 (7.69) | 88.82 (8.41) | 1.12 | 88.31 (8.61) | 88.12 (6.90) | .18 |
Note.
p <.10
p < .05,
p < .01
Brief Intellectual Ability
The unconditional MLM showed that the intercept and slope were both significant, indicating that BIA scores at age 11 were significantly different from zero and that scores increased significantly over time (Table 3). There was significant within individual variability in repeated measures of BIA scores. Additionally, there was significant between individual variability in intercepts and slopes. The intraclass correlation coefficient (ICC) was .66, indicating that 66% of the total variance in BIA scores was at level 2, between individuals, and 34% was at level 1, within individuals. These findings indicated that it was feasible to use predictors (sleep variables, ethnicity, SES, and sex) to attempt to account for the significant between individual variances in intercepts and slopes.
Table 3. Estimates for unconditional growth model for cognitive performance variables with no predictors entered.
| Brief Intellectual | Processing Speed Intercept | Working Memory | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | ||
| Estimate | 507.37**(.67) | 6.33**(.24) | 508.72**(.55) | 5.59**(.24) | 509.40**(.99) | 4.67**(.42) | |
| Between level variance | 96.02**(9.51) | 3.52**(1.34) | 57.57**(7.99) | 3.69*(1.59) | 180.14**(22.02) | 7.53(5.11) | |
| Within level residual variance | 15.50**(1.56) | 15.66**(2.10) | 55.52**(6.91) | ||||
Note. Estimates for intercepts and slopes are unstandardized betas. Race/ethnicity is coded as African American = 1, European American = 0. Sex is coded as male = 1, female = 0.
p < .05,
p < .01
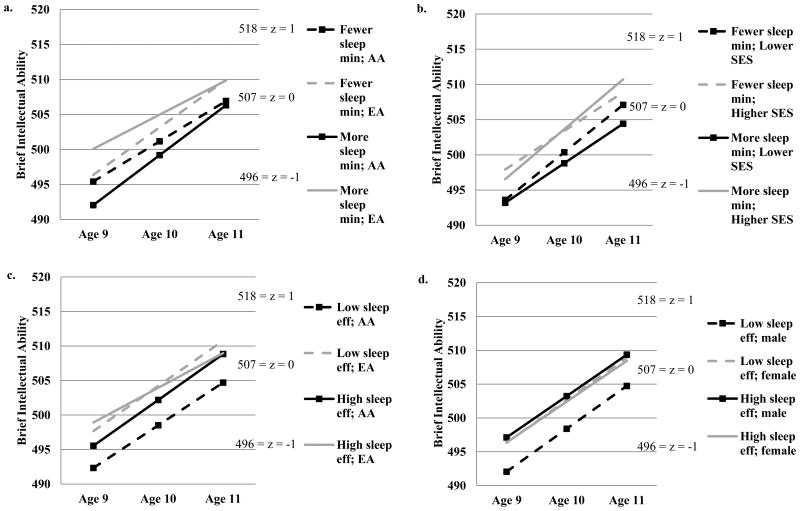
There were several significant effects in the model examining sleep minutes as a predictor of BIA. The unstandardized betas for the estimates of intercept and slope effects are presented in Table 4. Generally, AA children had lower age 11 BIA scores than did EA children, and SES was positively associated with age 11 BIA scores. Two significant interactions with sleep minutes on change in BIA scores over time (slope effects) were also evident. EA children who slept longer at age 9 had consistently high BIA scores (Figure 1a). Nevertheless, almost identical predicted means were observed for EA children at age 11 regardless of sleep at age 9. For AA children, those who slept longer at age 9 showed the greatest improvement in BIA scores; they also had the lowest scores at age 9. However, predicted means for BIA scores for AA children were similar at age 11 regardless of sleep duration at age 9. Additionally, higher SES children tended to have increases in BIA scores regardless of sleep (Figure 1b). However, lower SES children who slept less at age 9 had relatively higher scores at age 11 than their counterparts with longer sleep. These slope effects must be considered in light of the intercept effects indicating that AA and lower SES children tended to show lower cognitive performances at age 11 regardless of sleep minutes. There were no significant direct or interaction effects for sleep minutes predicting BIA scores at age 11 (intercept effects).2
Table 4. Estimates for growth model with sleep minutes predicting the intercept and slope for cognitive performance variables.
| Brief Intellectual Ability | ||
|---|---|---|
|
| ||
| Intercept (SE) | Slope (SE) | |
| Estimate | 507.85** (.72) | 6.24** (.26) |
| Ethnicity | -3.15 t (1.65) | .63 (.61) |
| SES | 1.89** (.74) | .04 (.27) |
| Sex | -1.82 (1.37) | .10 (.51) |
| Sleep minutes | -.00 (.01) | .00 (.00) |
| Sleep mins × ethnicity | -.003 (.04) | .03** (.01) |
| Sleep mins × SES | .02 (.02) | .01* (.00) |
| Sleep mins × sex | .02 (.02) | -.007 (.01) |
|
| ||
| Between level residual variance | 79.61**(8.94) | 3.28*(1.49) |
|
| ||
| Within level residual variance | 14.81**(1.73) | |
Note. Estimates for intercepts and slopes are unstandardized betas. For simplicity, results for Processing Speed and Working Memory are not included because there were no significant main effects or interactions with sleep minutes. Race/ethnicity is coded as African American = 1, European American = 0. Sex is coded as male = 1, female = 0.
p < .10
p < .05,
p < .01
Figure 1.
Change in Brief Intellectual Ability (BIA) scores over time. a.) Moderated by sleep minutes and ethnicity. b.) Moderated by sleep minutes and SES. c.) Moderated by sleep efficiency and ethnicity. d.) Moderated by sleep efficiency and sex.
Note. Eff = efficiency; Min = minutes; AA = African American; EA = European American, SES = socioeconomic status
The model examining sleep efficiency as a predictor of BIA also showed several significant effects (Table 5). Higher sleep efficiency was marginally associated with higher age 11 BIA scores. There were two significant interactions between sleep efficiency and ethnicity, and sleep efficiency and sex, predicting the intercept. AA children with lower sleep efficiency exhibited low levels of performance that were present from age 9 and remained through age 11 (Figure 1c). AA children with higher sleep efficiency had higher predicted means for cognitive performance from ages 9 through 11 than their counterparts with lower sleep efficiency. This indicates that better sleep may function as a protective factor against poor cognitive performance for AA children. However, EA children tended to have better cognitive performance regardless of sleep efficiency. Similarly, boys with lower sleep efficiency had low levels of performance that were evident from ages 9 to 11 (Figure 1d). Higher sleep efficiency seemed to enhance cognitive performance for boys in the sample whereas girls tended to have higher BIA scores independent of their sleep. There were no significant direct or interaction effects for sleep efficiency predicting change over time in BIA scores (slope effects).
Table 5. Estimates for growth model with sleep efficiency predicting the intercept and slope for cognitive performance variables.
| Brief Intellectual Ability | Processing Speed | Working Memory | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | Intercept (SE) | Slope (SE) | |
| Estimate | 507.85** (.67) | 6.19** (.25) | 509.22**(.57) | 5.53** (.22) | 509.47** (1.05) | 4.53** (.45) |
| Ethnicity | -3.11* (1.57) | 63 (.59) | 1.33 (1.35) | .87 (.57) | -6.99** (1.98) | - |
| SES | 1.84** (.70) | .17 (.26) | .77 (.58) | .20 (.26) | .75 (.98) | - |
| Sex | -1.63 (1.32) | .08 (.50) | -3.94** (1.12) | .01 (.44) | -1.03 (1.67) | - |
| Sleep efficiency | 13t (.07) | -.02 (.03) | .11t (.06) | .02 (.02) | .25* (.12) | - |
| Sleep eff × ethnicity | .37* (.18) | .12 (.07) | .02 (.14) | .01 (.05) | .60* (.26) | - |
| Sleep eff × SES | .12 (.09) | .04 (.03) | -.07 (.08) | -.02 (.03) | .16 (.15) | - |
| Sleep eff × sex | .32* (.13) | .01 (.05) | .37** (.12) | .02 (.04) | .30 (.22) | - |
| Between level residual variance | 75.86**(8.51) | 3.22*(1.48) | 52.64**(8.27) | 1.13(1.34) | 153.36**(21.92) | 6.24(5.70) |
| Within level residual variance | 14.87**(1.66) | 14.75**(2.21) | 57.91**(7.79) | |||
Note. Estimates for intercepts and slopes are unstandardized betas. Race/ethnicity is coded as African American = 1, European American = 0. Sex is coded as male = 1, female = 0.
p < .10
p < .05,
p < .01
Processing Speed
Processing Speed scores at age 11 were significantly different from zero, and PS increased significantly over time (Table 3). There was significant within individual variability in PS scores as well as between individual variability at age 11 and change over time. Fifty-six percent of the variance in PS scores was between individuals and 44% was within individuals. These results indicated that variability in age 11 PS scores (intercept) and change over time in PS scores (slope) could be accounted for by level 2 between individual predictors.
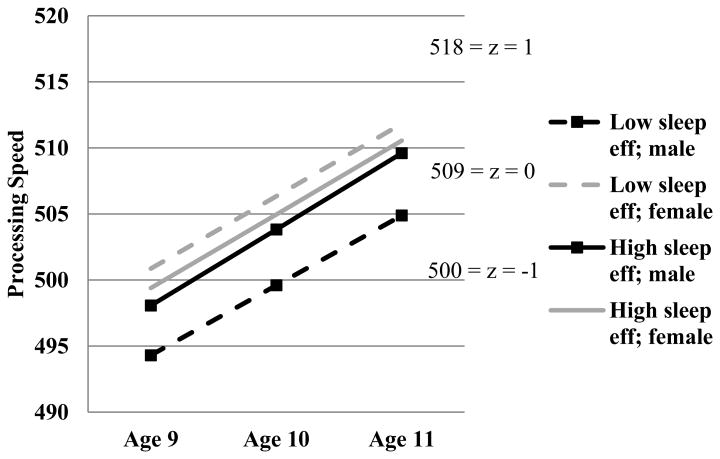
As shown in Table 5, child sex and the interaction between sleep efficiency and sex were significant predictors of age 11 PS, and the effect of sleep efficiency approached statistical significance. Similar to findings for BIA scores, boys with lower sleep efficiency exhibited lower PS scores at age 9 that remained at age 11 (Figure 2). Conversely, boys with higher efficiency, and girls regardless of their sleep, performed better throughout. This suggests that higher sleep efficiency functions as a protective factor for boys. No other effects emerged for sleep minutes or efficiency.
Figure 2.
Sleep efficiency and sex as moderators of change in Processing Speed over time.
Note. Eff = efficiency
Working Memory
The intercept and slope of Working Memory scores were both significantly different from zero and showed that scores increased over time (Table 3). There was significant within individual variability in scores and between individual variability in the intercept. However, there was not significant between individual variability in the slope, which suggested that change over time in WM scores did not vary significantly across children. The ICC indicated that 67% of the variance in WM scores was between individuals while 33% was within individuals. While the ICC clearly showed that the repeated measures were dependent within individuals, the more specific variance estimate for the level 2 slope (change over time) of WM was not significant. Therefore, consistent with best practices we fixed the slope to be the same for all children (Singer & Willett, 2003), which is reflected by the empty slope cells in Table 3.
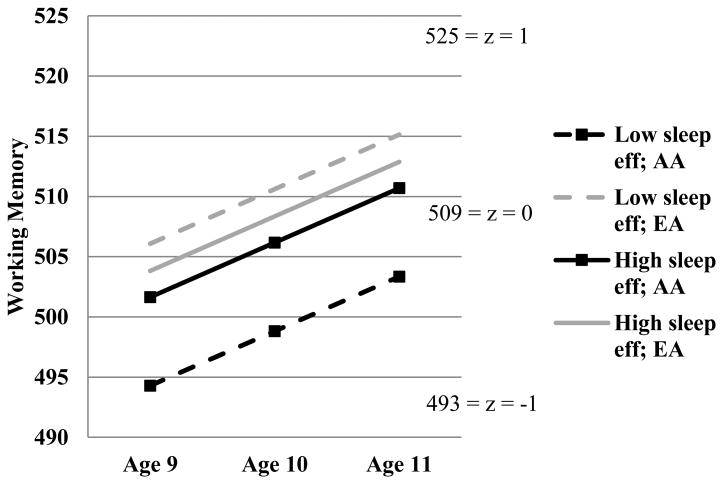
In the conditional model only between individual variability in the intercept (age 11 scores) was predicted (Table 5). As with the BIA and PS models, sleep efficiency was associated with age 11 WM scores. AA children had lower age 11 WM scores, but this effect was superseded by a significant interaction between ethnicity and sleep efficiency. Figure 3 shows that AA children with higher sleep efficiency had higher predicted means for WM than their counterparts with lower sleep efficiency at age 9 that were maintained throughout development. Thus, sleep efficiency may constitute a protective factor against lower cognitive performance for AA children. There were no other effects for sleep minutes or efficiency.
Figure 3.
Sleep efficiency and sex as moderators of change in Working Memory over time.
Note. Eff = efficiency; AA = African American; EA = European American
Supplemental Analyses
All the aforementioned growth models were run with SES and ethnicity entered simultaneously to examine their unique effects. To examine whether findings were the same when each variable was examined independently, we ran additional analyses examining SES and ethnicity in separate models. As SES and ethnicity were moderately correlated, this would allow for an understanding of the extent to which the effects of each were accounted for by the other. In Supplementary Tables 1-2, we have provided the results of the models examining the sleep variables as predictors of the cognitive performance variables, with the effects for SES held to 0 to demonstrate the effects of ethnicity alone. Similarly, in Supplementary Tables 3-4, we show the results with ethnicity instead of SES held to 0. The results were nearly identical to those of the original analyses, though the main effects for ethnicity and SES were stronger without the other included in the model.
Furthermore, to examine whether the significant results for sleep duration held while controlling for sleep efficiency, and vice versa, we re-ran each set of growth modeling analyses with the other sleep variable entered as a covariate. All statistically significant interactions remained, with the exception of one interaction (between sleep efficiency and ethnicity predicting BIA scores) that changed from significant at p < .05 to marginally significant at p = .08.
Discussion
In a racially diverse sample with a high representation of children exposed to socioeconomic adversity, we examined sleep duration and efficiency at age 9 as predictors of cognitive functioning at age 11. We also assessed the sleep variables at age 9 as predictors of developmental trajectories of cognitive performance from ages 9 to 11 years. Ethnicity, SES, and sex were examined as moderators of these relations. Whereas main effects between sleep efficiency and cognitive performance were mainly observed at the trend level, more robust moderation effects emerged. Controlling for SES, AA children and boys were at risk for lower performance when they experienced less efficient sleep. This vulnerability was apparent at age 9 and remained across development. Effects for sleep duration were less consistent, and overall did not indicate a strong influence of sleep minutes at age 9 on later cognitive performance.
Findings contribute to a small literature and demonstrate the importance of efficient sleep for cognitive performance and growth in such performance over time especially for some children. Better quality sleep facilitates neurodevelopmental changes related to brain maturation and learning, such as memory consolidation and increases in synaptic efficiency (Astill et al., 2012), in addition to enabling an alert daytime state that allows for active engagement with the environment (Sadeh, 2007). Prior longitudinal work has shown that mother-reported longer sleep in early childhood (Touchette et al., 2007) and self-report of fewer sleep problems in middle childhood (Bub et al., 2011) predict better cognitive performance. One longitudinal study using objective measures of sleep across two time points showed shorter sleep to predict lower intellectual ability two years later (Buckhalt et al., 2009). Results build on the previous literature in important ways by utilizing three waves of data and showing that objective measurement of sleep efficiency at age 9 is predictive of age 11 cognitive functioning, particularly for AA children and boys. We did not find direct effects of either sleep minutes or efficiency on change in cognitive functioning over time, although others have (Bub et al., 2011; Buckhalt et al., 2009). While speculative, this could be due in part to the greater SES disadvantage in the present sample.
The results included several interactions predicting age 11 BIA, PS, and WM as well as change over time in BIA. SES and ethnicity were moderately correlated. Thus, towards disentangling effects, SES was controlled when examining ethnicity and vice versa. That AA children were at substantially higher risk for lower cognitive performance (general intellectual ability, working memory) when they had less in comparison to more efficient sleep is consistent with a health disparities perspective (Buckhalt, 2011; Carter-Pokras & Baquet, 2002). Minority children are more likely to experience daily stressors related to ethnicity such as discrimination (Evans & English, 2002) that when coupled with additional risk factors like poor sleep may tax children's ability to focus and learn. Although tentative, this explanation is plausible in the historical and cultural context of race relations in the southern United States where the sample was recruited. Importantly, however, more efficient sleep functioned as a protective factor that reduced risk for lower cognitive functioning among AAs. There was also some indication that the AA children who slept longer showed the steepest improvement in cognitive performance, although this effect may be due at least in part to the lower scores for cognitive functioning at age 9. In a similar vein, prior work has shown that objectively assessed longer sleep is protective against externalizing problems (El-Sheikh, Tu, Saini, Fuller-Rowell, & Buckhalt, 2016), and self-reported better quality sleep is protective against internalizing symptoms (Yip, 2015), in the context of experiences of discrimination in adolescence.
The results for SES were less pronounced than those for child ethnicity or sex. Generally, children from higher SES homes showed increases in BIA that were evident regardless of sleep minutes. Surprisingly, low SES children showed greater improvement in cognitive scores when they slept less rather than more at age 9. This effect is difficult to interpret and should be considered tentatively pending further replications. However, the intercept effect of SES on age 11 cognitive performance indicated that in general higher SES children had higher scores than lower SES children regardless of sleep duration or quality. Boys' vulnerability to poorer cognitive outcomes in the context of less efficient sleep is consistent with a meta-analysis indicating that school performance of prepubertal boys was particularly sensitive to fragmented sleep (Dewald et al., 2010), though few studies have examined sex as a moderator of the link between sleep and cognitive outcomes. Prior work has suggested that lower academic performance in school-aged boys compared to girls may be attributable at least in part to higher levels of behavioral problems among boys (DiPrete & Jennings, 2012) resulting from more difficulty with self-regulation (Matthews, Marulis, Williford, 2014; Montroy, Bowles, Skibbe, McClelland, & Morrison, 2016). When coupled with poorer sleep quality, these regulatory difficulties may increase boys' vulnerability to lower cognitive performance. In light of some evidence indicating a gender gap favoring females with respect to measures of academic grades and GPA (Duckworth & Seligman, 2006), the results may suggest that better quality sleep is a potential avenue for reducing this gender disparity.
The results demonstrated that measures of both crystallized intelligence (BIA) and those thought to be more affected by day-to-day variation in fatigue (PS, WM) are negatively influenced by lower sleep efficiency among AA children and boys in particular. A meta-analysis found that sleep deprivation has stronger effects on measures of WM than PS among adults (Lim & Dinges, 2010), prompting the authors to suggest that working memory is more closely tied to real-world cognitive and academic performance. Therefore, our pattern of interaction effects whereby WM performance was more influenced by less efficient sleep in AAs and PS was more affected in boys appears to be consistent with greater documentation of disparities in cognitive-academic functioning between EAs and AAs than between females and males.
The findings for sleep efficiency did not provide evidence of moderation on change in cognitive functioning, suggesting that sleep efficiency earlier in development may have a lasting influence on future cognitive performance. In contrast, interaction effects with sleep minutes only emerged when predicting change in cognitive performance over time. However, the intercept effects for the model examining sleep minutes indicated that AAs and lower SES children tended to have lower cognitive functioning at age 11 regardless of sleep minutes. That more effects emerged for sleep efficiency, even when controlling for sleep duration, may suggest that sleep fragmentation interrupts neurodevelopmental changes during sleep, decreases the amount of time spent in the deeper, more restorative sleep stages (Philip, Stoohs, & Guilleminault, 1994), and/or contributes to mental fatigue, to a greater degree than shortened sleep alone. Effects may be particularly pronounced in children, who spend more time in deep sleep stages than adults (Ohayon, Carskadon, Giuilleminault, & Vitiello, 2004). This contention is consistent with a growing literature that has found more effects for measures of sleep quality than duration on child regulation, adjustment, neurobehavioral functioning, and academic performance (e.g., Bagley & El-Sheikh, 2014; Gruber et al., 2014; Sadeh et al., 2002). A recent experimental study also indicated that repeated nighttime awakenings across 8 hours in bed had the same effects on sustained attention in young adults as restricting sleep to four hours, suggesting a powerful negative influence of sleep fragmentation on cognitive performance (Kahn, Fridenson, Lerer, Bar-Haim, & Sadeh, 2014) .
Furthermore, lower SES and minority children are at greater risk for poor sleep quality, perhaps due to both physical environments that are less conducive to sleep because of crowding, noise, or light (Bagley, Kelly, Buckhalt, & El-Sheikh, 2015), as well as greater stress resulting from experiences related to family adversity or discrimination that make it more difficult to fall and stay asleep (Dahl, 1996; M. El-Sheikh, Buckhalt, Cummings, & Keller, 2007). A recent review implicates poor sleep quality as an important pathway linking race-based stress to poorer performance on cognitive tasks involving attention, working memory, and executive functioning (Levy, Heissel, Richeson, & Adam, 2016). The authors further suggest that subtle experiences of discrimination and racism may accumulate over time to predict chronic sleep problems that exacerbate decrements in cognitive outcomes. High levels of support coping, however, in which the child reports seeking help with a problem from another person, has been found to ameliorate risk for poor sleep efficiency among AA children (El-Sheikh, Kelly, Sadeh, & Buckhalt, 2014), and therefore may represent a point of intervention.
Altogether, the findings underscore the need to establish healthy sleep patterns early in life, and indicate that sleep during childhood may especially benefit cognitive development among AA children and boys. Prior work has shown that interventions targeting young children's sleep, such as those involving adjustments to bedtime routines as well as preventive parent education programs, result in clinically significant improvement in sleep duration and quality (Johnson & Mindell, 2011; Mindell et al., 2006). Thus, sleep may represent an important point of intervention that is particularly responsive to behavioral changes. Furthermore, the results add to an emerging body of literature highlighting the importance of longer and better quality sleep for reducing racial disparities in cognitive performance. Thus, findings suggest that promoting better sleep (e.g., via parent education about sleep hygiene) could potentially be a future direction of public policy and programs aimed at reducing the gap in scholastic achievement between EA and minority children.
Strengths of this study include objective measurement of multiple sleep parameters (minutes, efficiency), a relatively heterogeneous sample with regard to ethnicity and SES, longitudinal assessment of several domains of cognitive functioning via a well-established test battery, and utilization of a longitudinal design with three waves of assessments. However, without information about children's sleep prior to age 9, it is difficult to pinpoint at what age ethnicity- or sex-related differences in performance first emerge. Future work examining these associations in younger samples may be well-suited to identifying a sensitive period for sleep with regard to trajectories of cognitive performance. Extending this developmental window further would also be valuable for understanding whether these trajectories are maintained across adolescence. Additionally, although actigraphy has many advantages (Sadeh, 2011), it also has limitations For example, in comparison to polysomnography, it cannot provide information about sleep stages (Tryon, 2004) . While our results provide meaningful information regarding associations between sleep and cognitive functioning in a diverse community sample, the findings also may not be generalizable to children from other locales or those with clinically significant sleep problems.
Novel findings from the current study show that sleep efficiency predicts cognitive performance two years later. Controlling for SES, higher sleep efficiency was a protective factor associated with better cognitive performance on tests of BIA and WM for AA children, and with better performance of tests of BIA and PS for boys. Vulnerability to poorer performance related to lower sleep efficiency was apparent at the first time point and remained at each age. Sleep duration, however, less clearly impacted cognitive functioning. Together, the results suggest that early intervention emphasizing sleep quality in particular may have lasting effects on cognitive performance.
Supplementary Material
Acknowledgments
This study was supported by Grant R01HL093246 from the National Heart, Lung, and Blood Institute awarded to Mona El-Sheikh. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. We wish to thank our research laboratory staff, particularly Bridget Wingo, for data collection and preparation, as well as the children and parents who participated.
Footnotes
We repeated the analyses using all children's actigraphy information, regardless of the number of nights of available data. All statistically significant results remained.
In follow-up analyses, we ran an additional multilevel growth model testing whether there were intercept effects at age 9 that could explain the slope effects for sleep minutes (i.e., whether there were significant initial differences in age 9 cognitive performance related to sleep minutes that might explain why some groups showed more change over time than others). The results were almost identical to the results predicting the age 11 intercept. As seen at age 11, there was no main effect for sleep minutes and lower SES children had significantly lower scores for cognitive performance at age 9 compared to higher SES children. AA children had significantly lower scores for cognitive performance at age 9 compared to EA children (p < .05), whereas at age 11 the difference between EA and AA children was marginally significant (p < .10). The interactions between sleep minutes and ethnicity and between sleep minutes and SES predicting the age 9 intercept did not reach statistical significance, suggesting that there were not meaningful differences in age 9 cognitive performance scores related to sleep minutes.
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson A, Hafer A, Carskadon MA. Estimating sleep patterns with actigraphy monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep. 1999;22(1):95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- Ardsley NY. ActionW User's Guide version 2.4. Ambulatory Monitoring, Inc; 2002. [Google Scholar]
- Astill RG, Van der Heijden KB, Van Ijzendoorn MH, Van Someren EJ. Sleep, cognition, and behavioral problems in school-age children: A century of research meta-analyzed. Psychological Bulletin. 2012;138(6):1109–1138. doi: 10.1037/a0028204. [DOI] [PubMed] [Google Scholar]
- Bagley EJ, El-Sheikh M. Relations between daytime pre-ejection period reactivity and sleep in late childhood. Journal of Sleep Research. 2014;23(3):337–340. doi: 10.1111/jsr.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EJ, Kelly RJ, Buckhalt JA, El-Sheikh M. What keeps low SES children from sleeping well: The role of pre-sleep worries and sleep environment. Sleep Medicine. 2015;16(4):496–502. doi: 10.1016/j.sleep.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bub KL, Buckhalt JA, El-Sheikh M. Children's sleep and cognitive performance: A cross-domain analysis of change over time. Developmental Psychology. 2011;47(6):1504–1514. doi: 10.1037/a0025535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhalt JA. Insufficient sleep and the socioeconomic status achievement gap. Child Development Perspectives. 2011;5(1):59–65. doi: 10.1111/j.1750-8606.2010.00151.x. [DOI] [Google Scholar]
- Buckhalt JA, El-Sheikh M, Keller PS. Children's sleep and cognitive functioning: Race and socioeconomic status as moderators of effects. Child Development. 2007;78(1):213–231. doi: 10.1111/j.1467-8624.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- Buckhalt JA, El-Sheikh M, Keller PS, Kelly RJ. Concurrent and longitudinal relations between children's sleep and cognitive functioning: The moderating role of parent education. Child Development. 2009;80(3):875–892. doi: 10.1111/j.1467-8624.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- Carter-Pokras O, Baquet C. What is a “health disparity”? Public Health Reports. 2002;117(5):426–434. doi: 10.1093/phr/117.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ, Willoughby MT. Testing main effects and interactions in latent curve analysis. Psychological Methods. 2004;9(2):220–237. doi: 10.1037/1082-989X.9.2.220. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: Development and psychopathology. Development and Psychopathology. 1996;8(1):3–27. doi: 10.1017/S0954579400006945. [DOI] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bögels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews. 2010;14(3):179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- DiPrete TA, Jennings JL. Social and behavioral skills and the gender gap in early educational achievement. Social Science Research. 2012;41(1):1–15. doi: 10.1016/j.ssresearch.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Doane LD, Gress-smith JL, Breitenstein RS. Multi-method assessments of sleep over the transition to college and the associations with depression and anxiety symptoms. Journal of Youth and Adolescence. 2015;44(2):389–404. doi: 10.1007/s10964-014-0150-7. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Seligman ME. Self-discipline gives girls the edge: Gender in self-discipline, grades, and achievement test scores. Journal of Educational Psychology. 2006;98(1):198. doi: 10.1037/0022-0663.98.1.198. [DOI] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Cummings EM, Keller P. Sleep disruptions and emotional insecurity are pathways of risk for children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(1):88–96. doi: 10.1111/j.1469-7610.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Tu KM, Saini EK, Fuller-Rowell TE, Buckhalt JA. Perceived discrimination and youths' adjustment: Sleep as a moderator. Journal of Sleep Research. 2016;25(1):70–77. doi: 10.1111/jsr.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full Information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8(3):430–457. doi: 10.1207/s15328007sem0803_5. [DOI] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Gruber R, Somerville G, Enros P, Paquin S, Kestler M, Gillies-Poitras E. Sleep efficiency (but not sleep duration) of healthy school-age children is associated with grades in math and languages. Sleep Medicine. 2014;15(12):1517–1525. doi: 10.1016/j.sleep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Heck RH, Thomas SL. An introduction to multilevel modeling techniques: MLM and SEM approaches using Mplus. Routledge; 2015. [Google Scholar]
- Johnson C, Mindell JA. Family-based interventions for sleep problems of infants and children. In: El-Sheikh M, editor. Sleep and development: Familial and socio-cultural considerations. New York, NY: Oxford University Press; 2011. pp. 375–402. [Google Scholar]
- Kahn M, Fridenson S, Lerer R, Bar-Haim Y, Sadeh A. Effects of one night of induced night-wakings versus sleep restriction on sustained attention and mood: A pilot study. Sleep Medicine. 2014;15(7):825–832. doi: 10.1016/j.sleep.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Larson K, Russ SA, Nelson BB, Olson LM, Halfon N. Cognitive ability at kindergarten entry and socioeconomic status. Pediatrics. 2015;135(2):e440–e448. doi: 10.1542/peds.2014-0434. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Heissel JA, Richeson JA, Adam EK. Psychological and biological responses to race-based social stress as pathways to disparities in educational outcomes. American Psychologist. 2016;71(6):455. doi: 10.1037/a0040322. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychological Bulletin. 2010;136(3):375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca M, Short MA. The effect of one night's sleep deprivation on adolescent neurobehavioral performance. Sleep. 2014;37(11):1799. doi: 10.5665/sleep.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JS, Marulis LM, Williford AP. Gender processes in school functioning and the mediating role of cognitive self-regulation. Journal of Applied Developmental Psychology. 2014;35(3):128–137. doi:http://dx.doi.org/10.1016/j.appdev.2014.02.003. [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology. 2002;38(1):115. doi: 10.1037/0012-1649.38.1.115. [DOI] [PubMed] [Google Scholar]
- McGrew KS, Woodcock RW. Technical manual Woodcock Johnson III. Itasca, IL: Riverside Publishing Company; 2001. [Google Scholar]
- Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep edicine Reviews. 2012;16(5):463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA, Kuhn B, Lewin DS, Meltzer LJ, Sadeh A, American Academy of Sleep, M Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep. 2006;29(10):1263–1276. [PubMed] [Google Scholar]
- Montroy JJ, Bowles RP, Skibbe LE, McClelland MM, Morrison FJ. The development of self-regulation across early childhood. Developmental Psychology. 2016;52(11):1744–1762. doi: 10.1037/dev0000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén BO, Muthén LK. Mplus (Version 3.01) Los Angeles, CA: Muthén & Muthén; 2004. [Google Scholar]
- Philip P, Stoohs R, Guilleminault C. Short note sleep fragmentation in normals: A model for sleepiness associated with upper airway resistance syndrome. Sleep. 1994;17(3):242–247. [PubMed] [Google Scholar]
- Rasch G. Studies in mathematical psychology: I Probabilistic models for some intelligence and attainment tests. Oxford, England; Nielsen & Lydiche: 1960. [Google Scholar]
- Sadeh A. Consequences of sleep loss or sleep disruption in children. Sleep Medicine Clinics. 2007;2(3):513–520. doi: 10.1016/j.jsmc.2007.05.012. [DOI] [Google Scholar]
- Sadeh A. Sleep assessment methods. In: El-Sheikh M, editor. Sleep and development: Familial and socio-cultural considerations. New York, NY: Oxford University Press; 2011. pp. 355–371. [Google Scholar]
- Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Development. 2002;73(2):405–417. doi: 10.2307/3696365. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development. 2003;74(2):444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Spruyt K, Gozal D, Dayyat E, Roman A, Molfese DL. Sleep assessments in healthy school‐aged children using actigraphy: concordance with polysomnography. Journal of Sleep Research. 2011;20(1 Pt 2):223–232. doi: 10.1111/j.1365-2869.2010.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenari M, Vuontela V, Paavonen EJ, Carlson S, Fjallberg M, Aronen ET. Working memory and sleep in 6- to 13-year-old school children. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(1):85–92. doi: 10.1097/00004583-200301000-00014. doi:0.1097/00004583-200301000-00014. [DOI] [PubMed] [Google Scholar]
- Touchette E, Petit D, Seguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30(9):1213–1219. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaine RB, Dorrian J, Blunden S. Subjective and objective sleep in children and adolescents: Measurement, age, and gender differences. Sleep and Biological Rhythms. 2010;8(4):229–238. doi: 10.1111/j.1479-8425.2010.00452.x. [DOI] [Google Scholar]
- Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27(1):158–165. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM. Differentiation of cognitive abilities across the life span. Developmental Psychology. 2009;45(4):1097. doi: 10.1037/a0015864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Commerce. How the Census Bureau measures poverty. 2013 http://www.census.gov/hhes/www/poverty/about/overview/measure.html.
- Woodcock RW, McGrew KS, Mather M. Woodcock-Johnson III Tests of Cognitive Abilities. New York, NY: Riverside; 2001. [Google Scholar]
- Yip T. The effects of ethnic/racial discrimination and sleep quality on depressive symptoms and self-esteem trajectories among diverse adolescents. Journal of Youth and Adolescence. 2015;44(2):419–430. doi: 10.1007/s10964-014-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.