Abstract
Background
We evaluated liver transplantation waitlist and posttransplantation outcomes in 18–24 year olds compared to both younger (0–17 year old) and older (25–34 year old) registrants and recipients.
Methods
Utilizing national data from the United Network for Organ Sharing, competing risk, Cox regression and Kaplan-Meier analyses were performed on first-time liver transplant registrants (n=13,979) and recipients (n=8,718) ages 0–34 years old between 2002 to 2015.
Results
Among nonStatus 1A registrants, both 0–17 and 25–34 year olds were less likely to experience drop-out from the waiting list compared to 18–24 year olds (adjusted hazard ratio [AHR] 0–5 year olds=0.36, 6–11=0.29, 12–17=0.48, 18–24=1.00, 25–34=0.82). Although there was no difference in risk of graft failure across all age groups, both younger and older age groups had significantly lower risk of posttransplant mortality compared to 18–24 year olds (AHR for 0–5 year olds=0.53, 6–11=0.48, 12–17=0.70, 18–24=1.00, 25–34=0.77). This may be related to lower likelihood of re-transplantation after graft failure in 18–24 year olds.
Conclusions
This national registry study demonstrates for the first time poorer waitlist and postliver transplant outcomes in young adults ages 18–24 years at the time of listing and transplantation compared to older and younger age groups. Given the potential survival benefit in transplanting young adults and the shortage of solid organs for transplant, future studies are critical to identify and target modifiable risk factors to improve waitlist and long-term posttransplant outcomes in 18–24 year old registrants and recipients.
INTRODUCTION
Age-related differences in liver transplantation outcomes have previously been described in children. In the pediatric population, recipient age of 12–17 years old is an independent risk factor for worse graft survival following liver transplantation when compared to children less than 12 years old.1,2 Previous studies additionally describe young adult recipients of solid organ transplantation as an “at-risk” group where immunosuppression nonadherence, changes in insurance coverage and transition from parent-driven pediatric care to self-driven adult care may lead to worse posttransplant outcomes.3,4
By contrast, a recent study suggests that elements beyond socio-behavioral risk factors may be at play in young adults. Van Arendonk et al examined current recipient age to investigate the idea of a high-risk age window that all pediatric transplant recipients must eventually traverse on their path to adulthood and found no difference in liver graft failure rates comparing recipients with current age of 17–24 years to older and younger age groups.5 Foster et al reported differing results, describing higher liver graft failure rates in recipients with current age of 21–29 years.6 These studies conflict regarding the potential contribution of age-related socio-behavioral risk factors to negative posttransplantation outcomes in young adults. In our study, we examine age at the time of listing and liver transplantation, rather than current age, at a juncture where potential interventions may significantly impact waitlist outcomes and posttransplant graft and patient survival.
Differences also remain in the allocation of organs and use of exception scores between and within pediatric and adult populations. While pediatric and adult registrants of the same medical urgency receive equal priority for livers from adult donors, priority offers are given first to local and regional pediatric registrants when donors are <17 years old.7 Age-related differences also exist in the utilization of nonstandard exception scores in children, where younger children are more likely to be granted these exception scores compared to older children, whereas in adults the majority of exception scores are standard exceptions for hepatocellular carcinoma.8,9
Based on previous work regarding socio-behavioral risk factors in young adults and in the context of the existing allocation policy for liver transplant and use of exception scores, we hypothesized that 18–24 year olds will have a lower use of exception scores, higher risk of death or drop-out from the waitlist, higher rates of graft attrition and worse posttransplant survival compared to both children (0–17 years) and older adults (25–34 years). We used national waitlist and transplant data from the United Network for Organ Sharing (UNOS) to compare patients ages 18–24 years to both immediately older and younger age groups with respect to: 1) Primary indications for liver transplantation; 2) Waitlist outcomes including death, drop-out and transplantation and 3) Posttransplantation graft and patient survival.
MATERIALS AND METHODS
Data source and study population
We received data from UNOS on all patients listed for liver transplantation (registrants) and all patients who underwent transplantation (recipients) in the United States from February 27, 2002, the date of introduction of the Model for End-Stage Liver Disease (MELD) allocation system, to March 31, 2015. We limited our study to first-time transplant registrants (n=16,058) and transplant recipients (n=8,728) aged 0–34 years. We excluded registrants who were listed for multi-organ transplants (n=2,070) or whose MELD score was missing at the time of listing (n=9), leaving 13,979 transplant registrants in the current analysis. We excluded multi-organ recipients (n=4) and recipients missing MELD score data (n=6) at transplant, leaving 8,718 recipients in the current analysis. As inferior graft and patient survival outcomes have been reported for both small-for-size syndrome and large-for-size transplant, graft size mismatch was calculated based on previously described formulas for both living and deceased donors.10–12 This project was approved by the Veterans Affairs Puget Sound Healthcare System Institutional Review Board.
Age categories
Liver transplant registrants and recipients were divided into 5 age groups based on age at the time of listing or transplantation respectively: 0–5, 6–11, 12–17, 18–24 and 25–34 years old.2,13 The 0–5 year age category was selected based on the Organ Procurement and Transplantation Network (OPTN)/Scientific Registry of Transplant Recipients (SRTR) annual data report.13 Additionally, twelve years of age comprises the inflection point when pediatric patients are assigned a MELD, rather than a Pediatric End-Stage Liver Disease (PELD) score. For the purposes of this study, it was critical to define a young adult population. We therefore divided the 18–34 year old age category used in the OPTN/SRTR annual data report and selected 24 years old as the upper age limit for this group based on young adult renal transplantation studies and population-based studies, which identify 18–24 year olds as a high-risk age group in the general population.5,13–15
Modeling the association between age and waitlist outcomes
We used competing risks analysis to examine the association between age at listing (0–5, 6–11, 12–17, 18–24 [the reference category] and 25–34) and the following 3 waitlist competing outcomes: transplantation, death or drop-out from the waitlist.16 Drop-out was defined as any removal from the waitlist except for registrants removed due to “condition improved,” who were censored. Registrants were also censored if they were still alive and awaiting transplantation by the end of the study period on March 31, 2015.17 The competing risks models included the following registrant characteristics ascertained at the time of listing: gender, race or ethnicity, underlying liver disease, ABO blood group, initial listing year, listing PELD/MELD score, whether an exception score was granted (yes/no variable), serum albumin and insurance payer status. Additionally, UNOS region was included as a covariate because the competing risks analysis software does not handle strata.
When comparing registrants with the same medical acuity, previous studies have noted differences in waitlist mortality when further stratified by age, particularly when comparing children and adults.18 We therefore performed clinically relevant sub-group analyses by subdividing transplant registrants based on Status 1A or nonStatus 1A priority and by MELD score <20 or ≥20. Gender inequities have previously been described in adult liver transplant registrants, with females having higher waitlist mortality compared to males.19 We therefore performed sub-group analyses by gender.
Modeling the association between age and posttransplantation graft and patient survival
Graft failure was defined as failure leading to either re-transplantation or resulting in recipient death. Graft survival time was defined as time from initial transplantation until re-transplantation, death or until March 31, 2015 for registrants who were still alive at the end of the study period. Recipient survival time was measured as the number of days from liver transplantation to the date of death; patients who were re-transplanted or were still alive at the end of the study period were censored at that time.
We used a Cox proportional hazards regression model stratified by UNOS region to study the association between age at transplantation and posttransplantation graft survival or patient survival with and without adjusting for recipient and donor characteristics. Additional sub-analyses examined differences in posttransplantation graft and patient survival by gender and 1A Status. Recipient characteristics were the same as those used in waitlist analyses listed above, except they were ascertained at the time of transplantation. The following donor characteristics were included in our models: age, gender, race or ethnicity, body mass index (BMI), ABO match, donor type (living or deceased), cause of donor death, donation after circulatory death, donor organ type, cold ischemia time and organ location (local, regional, national). Observations were stratified by UNOS region. The assumption of proportional hazards was tested and met using weighted residual methods.
In addition to the proportional hazards analysis, we performed an ordered logistic regression analysis among patients who experienced graft loss to determine whether age was associated with graft failure (0–29 days)20 versus early graft loss (between 30–365 days) versus late graft loss (>365 days).21 Logistic regression analysis was used to determine if there was an association between age and re-transplantation after graft failure.
Analytical software
Stata MP version 14.1 (64-bit) (College Station, TX) was used for Cox PH analyses and R version 3.2.2 (64-bit) for competing risks analyses.16,22 A p-value less than 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of liver transplant registrants and recipients by age group
Baseline characteristics at the time of listing [Table 1] were similar to baseline characteristics at transplantation [Table 2]. Adults 18–24 years old had higher mean calculated MELD scores at listing (23.2±12.2) and transplantation (26.2±11.5) and were less likely to have an exception score granted at any time while on the waitlist (18.1%) compared to children and adolescents (0–17 years old). There were no differences in MELD scores at listing or transplantation or in the utilization of exception scores between 18–24 and 25–34 year olds.
Table 1.
Baseline characteristics at the time of listing for transplantation according to age groups (2002–2015)
| REGISTRANT CHARACTERISTICS | AGE GROUPS (years) | |||||
|---|---|---|---|---|---|---|
| 0–5 N=4909 |
6–11 N=1083 |
12–17 N=1600 |
18–24 N=2168 |
25–34 N=4219 |
p-value | |
| Female gender (%) | 50.8% | 49.7% | 54.6% | 54.4% | 50.4% | < 0.01 |
| Race or ethnicity (%) | < 0.001 | |||||
| Caucasian | 48.8% | 55.1% | 59.0% | 63.2% | 63.5% | |
| African American | 15.3% | 14.5% | 17.2% | 18.1% | 16.5% | |
| Hispanic | 25.6% | 21.8% | 17.8% | 12.9% | 13.3% | |
| Other | 10.3% | 8.6% | 6.1% | 5.8% | 6.7% | |
| Weight (kg), mean±standard deviation (SD) | 9.1±5.4 | 30.3±10.7 | 58.9±17.3 | 70.6±15.2 | 77.7±19.7 | <0.001 |
| Body surface area (BSA)a, mean±SD | 0.4±0.1 | 1.0±0.2 | 1.6±0.3 | 1.8±0.2 | 1.9±0.2 | <0.001 |
| Public insurance (%) | 52.3% | 43.8% | 33.0% | 32.3% | 33.7% | < 0.001 |
| Blood group (%) | < 0.001 | |||||
| A | 32.9% | 32.1% | 34.9% | 35.2% | 37.4% | |
| AB | 3.7% | 3.0% | 4.8% | 3.6% | 3.5% | |
| B | 13.1% | 13.2% | 12.2% | 13.6% | 13.6% | |
| O | 50.3% | 51.6% | 48.1% | 47.6% | 45.5% | |
| Calculated PELD/MELD score at listing (no exceptions)b, mean±SD | 13.0±14.4 | 9.1±15.5 | 17.7±12.5 | 23.2±12.2 | 22.4±11.8 | < 0.001 |
| Calculated PELD/MELD categories at listing (no exceptions) (%) | < 0.001 | |||||
| < 10 | 41.60% | 59.40% | 27.10% | 11.90% | 12.20% | |
| 10–14 | 13.50% | 6.60% | 19.10% | 18.30% | 19.30% | |
| 15–19 | 13.70% | 7.80% | 15.10% | 16.80% | 17.70% | |
| 20–24 | 10.8% | 6.8% | 11.6% | 12.4% | 12.7% | |
| 25–29 | 7.7% | 7.3% | 8.3% | 9.8% | 10.0% | |
| 30–34 | 5.2% | 5.3% | 8.8% | 9.5% | 9.0% | |
| 35+ | 7.5% | 6.8% | 10.0% | 21.3% | 19.0% | |
| % of waitlist registrants ever granted an exception score | 35.1% | 33.7% | 27.8% | 12.2% | 12.2% | < 0.001 |
| PELD/MELD score at listing (exception granted)c, mean±SD | 30.7±7.0 | 29.0±6.4 | 26.5±5.8 | 24.2±4.3 | 22.8±3.8 | < 0.001 |
| Waitlist time from listing to outcome (years) | ||||||
| Drop-out, mean±SD | 0.8±1.7 | 1.1±1.7 | 2.1±2.4 | 1.9±2.2 | 1.5±2.0 | < 0.001 |
| Death, mean±SD | 0.3±0.8 | 0.4±0.7 | 0.6±1.6 | 0.7±1.5 | 0.7±1.6 | < 0.01 |
| Transplantation, mean±SD | 0.3±0.6 | 0.4±0.8 | 0.5±0.9 | 0.5±1.1 | 0.5±1.1 | < 0.001 |
| Serum albumin (gm/deciliter), mean ±SD | 3.2±0.7 | 3.3±0.7 | 3.2±0.8 | 3.1±0.8 | 3.0±0.8 | < 0.001 |
| Underlying liver disease (%) | < 0.001 | |||||
| ALF | 13.2% | 26.7% | 27.3% | 30.2% | 21.9% | |
| PSC | 0.4% | 2.8% | 8.5% | 16.2% | 17.8% | |
| Other | 6.3% | 12.7% | 14.4% | 13.3% | 12.0% | |
| Autoimmune hepatitis | 0.3% | 4.1% | 12.4% | 12.3% | 10.5% | |
| Metabolic: inborn errors of metabolism, Alpha-1 antitrypsin deficiency, Wilson disease (excluding NASH) | 12.2% | 16.4% | 11.8% | 8.3% | 4.3% | |
| Cholestatic: biliary atresia | 46.2% | 15.0% | 7.4% | 4.9% | 1.6% | |
| Unknown | 4.4% | 6.2% | 5.6% | 3.2% | 3.4% | |
| Hepatitis C Virus | 0.0% | 0.5% | 2.1% | 2.9% | 7.7% | |
| Cholestatic: other than biliary atresia (Alagille syndrome, total parenteral nutrition cholestasis) | 7.9% | 7.6% | 4.1% | 2.3% | 0.7% | |
| Malignancy: Hepatocellular Carcinoma (HCC) | 0.3% | 1.5% | 2.1% | 1.8% | 1.9% | |
| Malignancy: other than HCC | 8.8% | 6.5% | 3.4% | 1.5% | 2.6% | |
| Hepatitis B Virus | 0.0% | 0.1% | 0.3% | 1.3% | 2.8% | |
| Alcoholic Liver Disease | 0.0% | 0.1% | 0.0% | 0.9% | 8.9% | |
| Nonalcoholic Steatohepatitis (NASH) | 0.0% | 0.1% | 0.3% | 0.6% | 2.3% | |
| Primary Biliary Cirrhosis | 0.0% | 0.0% | 0.4% | 0.6% | 1.7% | |
| Status 1Ad | 11.3% | 20.2% | 21.1% | 29.2% | 19.7% | < 0.001 |
BSA = weight (kg)ˆ0.425 × height (cm)ˆ0.725 × 0.007184
For registrants that did not receive an exception score, the PELD/MELD score at listing was calculated by laboratory values only. Calculated MELD scores range between 6 through 40 and PELD scores range between −11 through 40+. For purposes of comparison, calculated MELD and PELD scores were categorized as <10, 10–14, 15–19, 20–24, 25–29, 30–34, ≥35.
For waiting list analyses, an exception score was only included in the listing PELD/MELD score if an exception was granted within 30 days of listing (10). If a standard exception or additional exception score was granted at any time while the registrant was on the waitlist, this was categorized as a yes/no variable and reported as the percentage of total registrants within each age subgroup.
In August 2005 the liver allocation system changed so that registrants previously listed as Status 1 were split into Status 1A and Status 1B. Registrants listed as Status 1 prior to August 2005 were classified as Status 1A if their primary indication for listing was ALF or acute decompensated Wilson disease.
Table 2.
Baseline characteristics at the time of transplantation according to age groups (2002–2015)
| RECIPIENT CHARACTERISTICS | AGE GROUPS (years) | |||||
|---|---|---|---|---|---|---|
| 0–5 N=3641 |
6–11 N=815 |
12–17 N=982 |
18–24 N=1087 |
25–34 N=2193 |
p-value | |
| Female gender (%) | 50.8% | 46.3% | 54.0% | 54.5% | 48.2% | < 0.001 |
| Race or ethnicity (%) | < 0.001 | |||||
| Caucasian | 49.6% | 57.2% | 57.6% | 62.3% | 63.1% | |
| African American | 15.7% | 12.6% | 19.5% | 19.1% | 18.7% | |
| Hispanic | 24.6% | 21.6% | 17.2% | 13.2% | 12.4% | |
| Other | 10.1% | 8.6% | 5.7% | 5.4% | 5.8% | |
| Weight (kg), mean±SD | 9.8±4.2 | 29.7±9.9 | 58.2±17.1 | 70.5±15.2 | 77.1±19.1 | <0.001 |
| BSA, mean±SD | 0.4±0.1 | 1.0±0.2 | 1.6±0.3 | 1.8±0.2 | 1.9±0.2 | <0.001 |
| Public insurance (%) | 53.9% | 44.2% | 33.6% | 36.8% | 36.8% | < 0.001 |
| Blood group (%) | < 0.01 | |||||
| A | 33.5% | 33.4% | 36.3% | 34.4% | 38.3% | |
| AB | 4.3% | 3.2% | 4.9% | 4.6% | 4.5% | |
| B | 13.5% | 13.1% | 10.4% | 14.8% | 13.0% | |
| O | 48.6% | 50.3% | 48.5% | 46.2% | 44.3% | |
| Calculated PELD/MELD score at transplantation (no exceptions), mean±SD | 15.2±14.5 | 9.6±15.4 | 19.4±12.3 | 26.2±11.5 | 25.8±11.2 | < 0.001 |
| Calculated PELD/MELD categories at transplant (no exceptions) (%) | < 0.001 | |||||
| < 10 | 35.2% | 58.8% | 22.5% | 8.2% | 8.3% | |
| 10–14 | 10.80% | 8.60% | 15.90% | 9.30% | 8.70% | |
| 15–19 | 13.20% | 6.10% | 18.30% | 14.70% | 15.10% | |
| 20–24 | 14.4% | 7.4% | 12.1% | 14.4% | 16.5% | |
| 25–29 | 10.2% | 7.1% | 10.8% | 13.5% | 14.0% | |
| 30–34 | 7.4% | 4.8% | 8.0% | 14.2% | 13.0% | |
| 35+ | 8.8% | 7.2% | 12.3% | 25.8% | 24.4% | |
| % of transplanted patients granted an exception | 38.3% | 39.8% | 33.9% | 18.1% | 15.3% | < 0.001 |
| PELD/MELD score at transplant (exception granted), mean±SD | 32.1±7.9 | 30.1±8.2 | 29.0±6.7 | 27.4±5.8 | 25.7±4.8 | < 0.001 |
| Serum albumin (gm/deciliter), mean ±SD | 3.2±0.8 | 3.3±0.8 | 3.1±0.8 | 3.0±0.8 | 2.9±0.8 | < 0.001 |
| Underlying liver disease (%) | < 0.001 | |||||
| ALF | 10.6% | 22.8% | 24.0% | 29.0% | 22.2% | |
| PSC | 0.3% | 3.1% | 9.6% | 15.7% | 19.1% | |
| Metabolic | 13.1% | 20.9% | 15.5% | 13.1% | 6.5% | |
| Other | 6.4% | 13.6% | 15.7% | 11.7% | 10.8% | |
| Autoimmune hepatitis | 0.2% | 2.2% | 11.4% | 11.7% | 9.0% | |
| Cholestatic: biliary atresia | 49.6% | 15.7% | 6.7% | 3.6% | 1.9% | |
| Malignancy: HCC | 0.5% | 1.7% | 3.6% | 3.0% | 3.1% | |
| Cholestatic: other than biliary atresia | 7.4% | 8.1% | 4.8% | 2.6% | 1.1% | |
| Malignancy: other than HCC | 9.9% | 7.5% | 4.2% | 2.6% | 3.4% | |
| Hepatitis C Virus | 0.1% | 0.6% | 1.7% | 2.5% | 6.9% | |
| Unknown | 1.6% | 3.6% | 1.9% | 1.2% | 1.3% | |
| Hepatitis B Virus | 0.1% | 0.2% | 0.1% | 1.2% | 3.1% | |
| Alcoholic Liver Disease | 0.0% | 0.0% | 0.0% | 0.8% | 7.6% | |
| Primary Biliary Cirrhosis | 0.2% | 0.0% | 0.5% | 0.8% | 1.8% | |
| NASH | 0.0% | 0.0% | 0.3% | 0.6% | 2.2% | |
| Status 1A | 10.9% | 23.1% | 24.6% | 34.7% | 22.3% | < 0.001 |
| DONOR CHARACTERISTICS | ||||||
| Female gender (%) | 44.7% | 41.6% | 39.8% | 42.4% | 44.0% | 0.05 |
| Age (years), mean±SD | 12.0±13.1 | 16.2±12.9 | 24.1±13.9 | 35.1±15.9 | 38.0±16.2 | < 0.001 |
| Race or ethnicity (%) | < 0.001 | |||||
| Caucasian | 56.2% | 57.5% | 65.2% | 66.4% | 65.8% | |
| African American | 18.2% | 16.0% | 13.8% | 15.7% | 16.6% | |
| Hispanic | 21.1% | 21.8% | 17.1% | 15.6% | 14.2% | |
| Other | 4.6% | 4.7% | 3.9% | 2.2% | 3.3% | |
| Body Mass Index (kg/m2), mean±SD | 19.8±4.8 | 20.6±4.8 | 23.1±4.4 | 25.7±5.0 | 26.5±5.9 | < 0.001 |
| BSA, mean±SD | 1.1±0.6 | 1.4±0.5 | 1.7±0.3 | 1.9±0.2 | 1.9±0.3 | <0.001 |
| ABO match (%) | < 0.001 | |||||
| Identical | 81.7% | 83.1% | 83.3% | 82.6% | 86.6% | |
| Compatible | 14.6% | 14.2% | 14.7% | 15.9% | 12.3% | |
| Incompatible | 3.7% | 2.7% | 2.0% | 1.5% | 1.0% | |
| Cold Ischemia time (h), mean ±SD | 6.8±4.0 | 6.9±3.5 | 6.9±4.0 | 6.7±3.7 | 6.6±3.6 | 0.17 |
| Donor type (%) | < 0.001 | |||||
| Living | 14.7% | 9.0% | 6.2% | 8.4% | 7.8% | |
| Deceased | 85.3% | 91.0% | 93.8% | 91.6% | 92.2% | |
| Graft size mismatch, living donor (%) | ||||||
| Small-for-sizea | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Graft size mismatch, deceased donor (%) | ||||||
| Small-for-sizeb | 5.1% | 15.5% | 8.9% | 4.8% | 7.3% | <0.001 |
| Large-for-sizec | 66.2% | 48.8% | 20.2% | 9.2% | 9.0% | <0.001 |
| Liver type (%) | < 0.001 | |||||
| Whole | 50.10% | 67.90% | 86.70% | 89.90% | 90.70% | |
| Split | 21.0% | 12.1% | 5.9% | 1.7% | 1.4% | |
| Reduced | 28.9% | 20.0% | 7.4% | 8.4% | 7.9% | |
| Donor cause of death (%) | < 0.001 | |||||
| Living | 14.7% | 9.0% | 6.2% | 8.4% | 7.8% | |
| Trauma | 45.7% | 49.4% | 52.7% | 41.3% | 34.9% | |
| Anoxia | 28.60% | 25.90% | 17.20% | 15.80% | 18.50% | |
| Cerebrovascular accident | 6.9% | 12.0% | 20.1% | 32.8% | 36.3% | |
| Other | 4.0% | 3.7% | 3.8% | 1.7% | 2.5% | |
| Donation after cardiac death (DCD) (%) | < 0.001 | |||||
| Living | 14.7% | 9.0% | 6.2% | 8.4% | 7.8% | |
| Not DCD | 84.8% | 90.6% | 92.0% | 87.6% | 89.7% | |
| DCD | 0.6% | 0.5% | 1.7% | 4.0% | 2.5% | |
| Organ location (%) | < 0.001 | |||||
| Local | 42.3% | 41.4% | 59.6% | 64.9% | 66.7% | |
| Regional | 38.2% | 48.6% | 36.5% | 31.8% | 29.9% | |
| National | 19.6% | 10.0% | 3.9% | 3.3% | 3.4% | |
Registrants 18–24 years old were more likely to be listed for ALF compared to all other age groups. Among registrants with Status 1A priority, 18–24 year olds were also more likely to be removed from the waiting list secondary to improved condition [Figure 1a]. Despite this, ALF (29.0%) persisted as the highest indication for transplantation in recipients aged 18–24 years compared to all other age groups, followed by PSC (15.7%) and metabolic liver disease (13.1%) [Figure 2a, Table 2].
Figure 1.
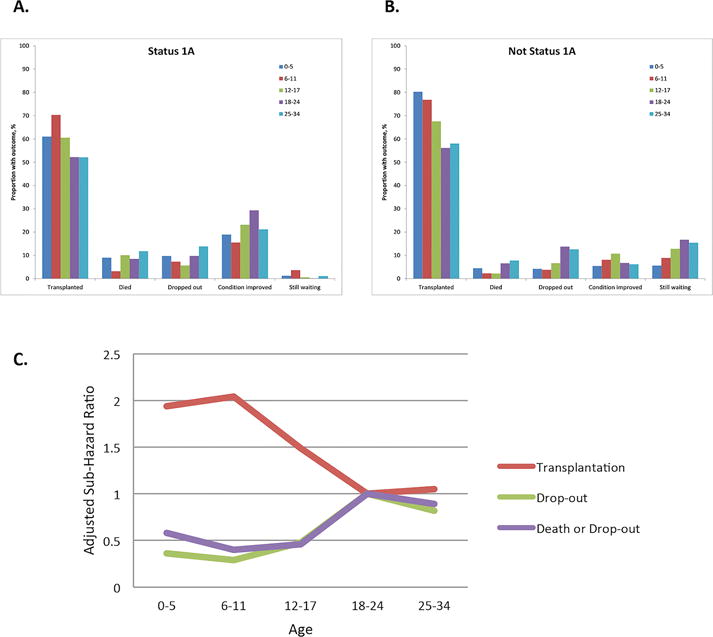
Figure 2.
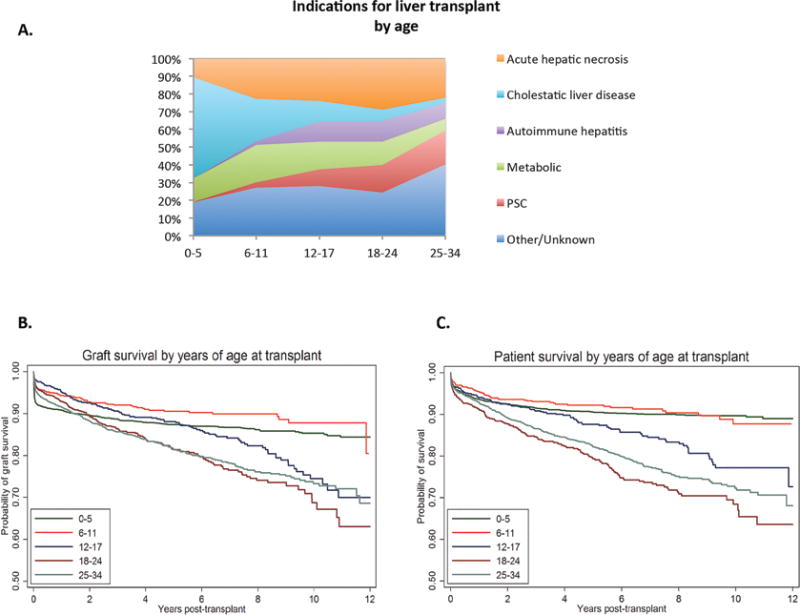
Graft size mismatch was uncommon in 18–24 year olds with 0% having small-for-size grafts in living donor transplantation. In deceased donor transplantation, 18–24 year olds were least likely to receive a small-for-size graft (4.8%) compared to all other age groups and only 9.2% received a large-for-size graft. Very young children ages 0–5 years were much more likely to receive a liver from a live donor (14.7%) or a split liver (21%) compared to all other age groups; by comparison 8.4% of 18–24 year olds received a liver from a live donor and 1.7% received a split liver [Table 2].
Waitlist outcomes by age
Among nonStatus 1A registrants, both younger and older age groups (ages 0–17 and 25–34) were significantly less likely to drop-out from the waiting list compared to 18–24 year olds (adjusted sub-hazard ratio [ASHR] 0.36 for ages 0–5, 0.29 for ages 6–11, 0.48 for ages 12–17, 1.00 for ages 18–24, 0.82 for ages 25–34). NonStatus 1A registrants 0–17 year olds were also significantly less likely to experience the combined outcome of death or drop-out from the waiting list (ASHR 0.58 for ages 0–5, 0.40 for ages 6–11, 0.46 for ages 12–17, 1.00 for ages 18–24, 0.89 for ages 25–34) and were more likely to be transplanted compared to 18–24 year old registrants (ASHR 1.94 for ages 0–5, 2.04 for ages 6–11, 1.49 for ages 12–17, 1.00 for ages 18–24, 1.05 for ages 25–34), while 25–34 year olds had a similar likelihood of the combined outcome of death or drop-out from the waiting list or transplantation [Table 3, Figure 1b and 1c]. In sub-analyses, these associations were strongest in registrants with MELD scores <20 [SDC Tables 1a and 1b]. After adjusting for gender in competing risks analyses examining the association between age at listing and waitlist outcomes in nonStatus 1A registrants, poorer waitlist outcomes for 18–24 year olds persisted [Table 3]. Waitlist time from listing to drop-out, death or transplantation was not significantly different between 18–24 year olds, adolescents (12–17 years old) and older adults (25–34 years old) [Table 1].
Table 3.
Association between age at listing and waitlist outcomes: competing risks analysis for sub-group Not Status 1A
| Patients (N) |
Events (N) |
Sub-Hazard Ratio | Adjusteda Sub-Hazard Ratio | |
|---|---|---|---|---|
| Death | ||||
| Ages 0–5 | 4355 | 196 | 0.68 (0.54–0.87) |
1.05 (0.77–1.42) |
| 6–11 | 864 | 20 | 0.35 (0.21–0.56) |
0.66 (0.4–1.1) |
| 12–17 | 1263 | 28 | 0.33 (0.22–0.51) |
0.41 (0.27–0.63) |
| 18–24 | 1534 | 101 | 1 | 1 |
| 25–34 | 3388 | 263 | 1.18 (0.94–1.48) |
1.02 (0.8–1.31) |
| Transplantation | ||||
| Ages 0–5 | 4355 | 3495 | 2.08 (1.93–2.24) |
1.94 (1.76–2.15) |
| 6–11 | 864 | 664 | 1.82 (1.65–2.01) |
2.04 (1.81–2.29) |
| 12–17 | 1263 | 853 | 1.38 (1.26–1.52) |
1.49 (1.35–1.64) |
| 18–24 | 1534 | 861 | 1 | 1 |
| 25–34 | 3388 | 1966 | 1.06 (0.98–1.15) |
1.05 (0.96–1.14) |
| Drop-out | ||||
| Ages 0–5 | 4355 | 170 | 0.32 (0.26–0.39) |
0.36 (0.27–0.47) |
| 6–11 | 864 | 32 | 0.3 (0.21–0.44) |
0.29 (0.2–0.44) |
| 12–17 | 1263 | 72 | 0.47 (0.36–0.62) |
0.48 (0.37–0.63) |
| 18–24 | 1534 | 185 | 1 | 1 |
| 25–34 | 3388 | 367 | 0.89 (0.75–1.06) |
0.82 (0.69–0.99) |
| Death or Drop-out | ||||
| Ages 0–5 | 4355 | 366 | 0.44 (0.38–0.51) |
0.58 (0.47–0.71) |
| 6–11 | 864 | 52 | 0.31 (0.23–0.42) |
0.4 (0.29–0.55) |
| 12–17 | 1263 | 100 | 0.41 (0.33–0.51) |
0.46 (0.36–0.57) |
| 18–24 | 1534 | 286 | 1 | 1 |
| 25–34 | 3388 | 630 | 1 (0.87–1.15) |
0.89 (0.77–1.03) |
Adjusted for gender, race or ethnicity, underlying liver disease, ABO blood group, initial listing year, listing PELD/MELD score, whether an exception score was granted (yes/no variable), serum albumin level and insurance payor status. UNOS region was included as a covariate.
Among Status 1A registrants, only 6–11 year olds had a lower waitlist mortality and a higher likelihood of transplantation compared to 18–24 year olds [SDC Table 2, Figure 1a].
Association between age and posttransplantation graft survival
After adjusting for recipient and donor characteristics, recipients aged 18–24 years had a similar risk of graft failure compared to all other age groups [Table 4, Figure 2b]. This finding remained unchanged in sub-group analyses of male and female recipients [SDC Tables 3a and 3b] and in recipients listed as Status 1A or Not Status 1A [SDC Table 4a and 4b].
Table 4.
Association between age at transplantation and posttransplant graft survival (Cox Proportional Hazards analysis)
| Age at transplantation | N | Patient-years | Failures | Failure rate (per 100 patient-years) | Unadjusted Hazard Ratio | Adjusteda Hazard Ratio |
|---|---|---|---|---|---|---|
| 0–5 | 3641 | 16677 | 440 | 2.64 | 0.67 (0.56–0.80) |
1.17 (0.88–1.55) |
| 6–11 | 815 | 3757 | 72 | 1.92 | 0.48 (0.37–0.63) |
0.74 (0.53–1.04) |
| 12–17 | 982 | 4361 | 130 | 2.98 | 0.74 (0.59–0.92) |
0.89 (0.69–1.14) |
| 18–24 | 1087 | 4289 | 183 | 4.27 | 1 | 1 |
| 25–34 | 2193 | 8847 | 366 | 4.14 | 0.98 (0.82–1.17) |
0.93 (0.77–1.13) |
Adjusted for all the recipient and donor characteristics at the time of transplantation shown in Table 2:
Recipient characteristics: age, gender, race or ethnicity, insurance payer status, ABO blood group, transplant PELD/MELD score, whether an exception score was granted (yes/no variable), underlying liver disease, Status 1A listing, year of transplantation, serum albumin level
Donor characteristics: age, gender, race or ethnicity, BMI, ABO match, donor type, donation after circulatory death, donor cause of death, cold ischemia time, donor organ type and organ location. Observations were stratified by UNOS region.
However, there were important differences in the timing of graft failure and loss by age group. Among recipients with graft failure in the first 0–29 days after transplant, this event was more likely to occur in children ages 0–11 years old with the highest probability in 0–5 year olds compared to older age groups (proportion 0.60 ages 0–5, 0.43 ages 6–11, 0.18 ages 12–17, 0.21 ages 18–24, 0.28 ages 25–34). Among recipients with late graft loss (>365 days after transplantation), this event was more likely to occur in adolescents (ages 12–17) and adults (ages 18–24 and 25–34) compared to children 0–11 years old (proportion 0.23 ages 0–5, 0.36 ages 6–11, 0.67 ages 12–17, 0.62 ages 18–24, 0.53 ages 25–34). There was no difference in the probability of early graft loss (30–365 days after transplant) across age groups [SDC Table 5].
Association between age and posttransplantation patient survival
Children and adolescents (0–17 years old) and adults 25–34 year olds had significantly lower posttransplant mortality compared to 18–24 year olds (AHR 0.53 for ages 0–5, 0.48 for ages 6–11, 0.70 for ages 12–17, 1.00 for ages 18–24 and 0.77 for ages 25–34) after adjusting for a large number of recipient and donor characteristics. The probability of survival at 1, 2 and 5-years posttransplant was the lowest for recipients ages 18–24 at 0.91, 0.88 and 0.79 respectively compared to all other age groups [Table 5, Figure 2c]. These associations between age-groups and posttransplantation survival were very similar in male and female subgroups [SDC Tables 6a and 6b]. In sub-analyses by medical acuity, these associations were strongest in recipients transplanted as Not Status 1A (AHR 0.55 for ages 0–5, 0.45 for ages 6–11, 0.68 for ages 12–17, 1.00 for ages 18–24 and 0.74 for ages 25–34) [SDC Tables 7a and 7b].
Table 5.
Association between age at transplantation and posttransplant patient survival (Cox Proportional Hazards analysis)
| Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at transplantation | N | Patient-years | Deaths | Mortality (per 100 patient-years) | 1 Year | 2 Years | 5 Years | Unadjusted Hazard Ratio | Adjusteda Hazard Ratio |
| 0–5 | 3641 | 16687 | 307 | 1.84 | 0.94 | 0.92 | 0.9 | 0.41 (0.34–0.48) |
0.53 (0.40–0.72) |
| 6–11 | 815 | 3759 | 62 | 1.65 | 0.95 | 0.94 | 0.92 | 0.36 (0.27–0.48) |
0.48 (0.34–0.67) |
| 12–17 | 982 | 4390 | 123 | 2.8 | 0.94 | 0.92 | 0.88 | 0.60 (0.48–0.75) |
0.70 (0.55–0.91) |
| 18–24 | 1087 | 4301 | 210 | 4.88 | 0.91 | 0.88 | 0.79 | 1 | 1 |
| 25–34 | 2193 | 8890 | 357 | 4.02 | 0.93 | 0.89 | 0.82 | 0.84 (0.71–0.99) |
0.77 (0.64–0.93) |
Adjusted for all the recipient and donor characteristics at the time of transplantation shown in Table 2:
Recipient characteristics: age, gender, race or ethnicity, insurance payer status, ABO blood group, transplant PELD/MELD score, whether an exception score was granted (yes/no variable), underlying liver disease, Status 1A listing, year of transplantation, serum albumin level
Donor characteristics: age, gender, race or ethnicity, BMI, ABO match, donor type, donation after circulatory death, donor cause of death, cold ischemia time, donor organ type and organ location. Observations were stratified by UNOS region.
Re-transplantation after graft failure occurred more commonly in 0–5 year olds (76%, OR 2.53), 6–11 year olds (78%, OR 2.78) and 12–17 year olds (72%, OR 2.00) compared to 18–24 year olds (56%, OR 1.00), while 25–34 year olds had a similar likelihood of re-transplantation after graft failure (63%, OR 1.34) [Table 6]. In sub-analyses by acuity, this association was strongest in recipients transplanted as Not Status 1A [SDC Tables 8a and 8b]. Death after graft failure was also significantly more common in 18–24 year olds (43%) compared to 0–5 (21%), 6–11 (18%), 12–17 (28%) and 25–34 year olds (37%) (χ2 p-value < 0.001). Sub-analyses by gender noted a strikingly higher percentage of females ages 18–24 years olds dying after graft failure (48.9%) than males (37.1%). Females ages 0–17 and 25–34 years were also more likely to be re-transplanted compared to females 18–24 year olds, whereas in males there was no difference in the odds of re-transplantation across all age groups [SDC Table 9a and 9b].
Table 6.
End status of recipients with graft failure by age and odds of re-transplantation after graft failure by age at transplantation (logistic regression)
| Status of recipients with graft failure | |||
|---|---|---|---|
| Age at transplantationa | Alive (%) | Deceased (%) | Retransplanted (%) (ORb, 95% confidence interval) |
|
0–5 N=440 |
11 (2.5%) |
94 (21.4%) |
335 (76.1%) (OR 2.53, 1.76–3.65) |
|
6–11 N=72 |
3 (4.1%) |
13 (18.1%) |
56 (77.8%) (OR 2.78, 1.48–5.21) |
|
12–17 N=130 |
1 (0.8%) |
36 (27.7%) |
93 (71.5%) (OR 2.00, 1.24–3.26) |
|
18–24 N=183 |
2 (1.1%) |
79 (43.2%) |
102 (55.7%) (OR 1.00) |
|
25–34 N=366 |
1 (0.3%) |
135 (36.9%) |
230 (62.8%) (OR 1.34, 0.94–1.93) |
Significant difference found in end status of graft recipients with graft failure by age (χ2 p-value < 0.001)
Odds of re-transplantation after graft failure compared to end status of alive or deceased.
DISCUSSION
This analysis of national registry data demonstrates worse waitlist and posttransplant outcomes in 18–24 year olds compared to both younger and older age groups among liver transplant registrants and recipients in the United States from 2002–2015. Notably, recipients 18–24 years old had the highest mortality following transplant with a 5-year post transplant survival probability of 0.79. Among nonStatus 1A registrants, 18–24 year olds were more likely to experience drop-out from the waitlist. While there was no difference in graft failure across age groups, 18–24 year olds were more likely to have late graft loss, were less likely to be re-transplanted and were more likely to have the outcome of death secondary to graft failure. To our knowledge, this is the first study to examine and demonstrate both poor waitlist and postliver transplant outcomes in the population of young adults aged 18–24 years at the time of listing and transplantation.
ALF was the leading indication for liver transplant in 18–24 year old registrants and recipients. Not surprisingly, given the priority allocated to critically ill Status 1A registrants, there were few differences in waitlist outcomes when comparing both younger and older age groups to 18–24 year old registrants.
Among nonStatus 1A registrants, 0–17 and 25–34 year olds had a significantly lower risk of waitlist drop-out compared to 18–24 year olds and these associations were strongest in those with MELD scores <20. Hsu et al previously demonstrated that younger children are more likely to be granted exception scores compared to older children.8 Additional studies have also found that children and adults who are granted nonstandard exception scores have decreased waitlist mortality and are more likely to be transplanted compared to registrants without nonstandard exception requests.23,24 In support of these trends, we found that adults 18–24 year olds were significantly less likely to be granted an exception score at any time while on the waiting list, had lower MELD exception scores compared to 0–17 year olds and had poorer waitlist outcomes despite having the highest mean calculated MELD score at listing and transplantation. In our study, while the primary indications for liver transplantation were similar between 12–17 year olds and 18–24 year olds, the decline in the use of exception scores at the transition between adolescence and adulthood was dramatic. This likely reflects a culture of advocacy for children by providers most attuned to the utilization of nonstandardized exception scores in the youngest registrants with significant implications on waitlist outcomes.
In the process of transition from late adolescence to adulthood, 18–24 year olds age out of potential allocation benefits allotted to the pediatric population where children with similar medical urgency to adult registrants receive equal priority for adult organs, while also retaining priority offers from pediatric donors.7 We found younger children were more likely to receive split livers or livers from living donors compared to young adults, further expanding the donor pool for children in a time of organ scarcity. The advantage of 1B Status additionally applies only to pediatric registrants <18 years old with chronic liver disease who meet specific criterion, while the adult equivalent of Status 2A was eliminated in 2002.25 Moreover, children with metabolic liver disease causing hyperammonemia may be advanced to 1B status after 30 days; this same advantage is not conferred to 18–24 year olds in whom the 3rd leading indication for liver transplantation is metabolic liver disease.26 As 18–24 year olds age out of the allocation benefits allotted to the pediatric population, we found they are also disadvantaged by a lower utilization of exception scores with higher rates of waitlist drop-out and lower rates of transplantation.
There was no difference in the risk of graft failure for recipients aged 18–24 years at the time of transplant compared to all other age groups. Among those with graft failure, 18–24 year old recipients along with adolescents and older adults were more likely to have late graft loss, compared to children 0–11 years old. Previous studies in children have noted that acute and chronic rejection account for up to 48% of late graft loss, while hepatic artery thrombosis and biliary strictures combined account for an additional 20% of late graft loss in children.20,21 In adults, recurrent disease and chronic rejection primarily account for hepatic causes of late graft loss.27 Immunosuppression nonadherence has been well documented in young adults across all solid organ transplants and may be one potential contributor to late graft loss in this population. Novel solutions continue to target the transition between pediatric and adult care with the aim to decrease chronic rejection leading to re-transplantation or death.2,4,28,29
Most strikingly, we found that adults 18–24 years old had higher posttransplant mortality and a lower probability of survival at 1, 2 and 5 years posttransplant compared to both younger and older age groups. While previous studies have found higher posttransplantation mortality in both adult female recipients19 and adults transplanted for ALF due to suicide, trauma or immunosuppression nonadherence,30 higher posttransplant mortality in 18–24 year olds persisted in our study even after adjusting for gender and 1A Status.
Insights into potential causes of higher posttransplant mortality were obtained from sub-group analyses of recipients with graft failure. Overall, 18–24 year old recipients with death secondary to graft failure comprised a large proportion of total deaths in this age category (38%). Recipients 18–24 year olds with graft failure were less likely to be re-transplanted and had the highest proportion of deaths secondary to graft failure compared to all other age groups, with female recipients accounting primarily for these differences. Gender disparities in posttransplantation outcomes for adult females have previously been described with potential etiologies including poorer graft survival due to lower-quality grafts for women compared to men and a higher risk of posttransplantation renal impairment in females; though these variables were not available for analysis in our study.31
One limitation of our analyses is that specific causes of graft failure, death and the capture of socio-behavioral risk factors can not be further delineated due to the high degree of missing data of these variables in UNOS. This is a known limitation of using a large national database where data cannot be further verified and missing data cannot be retrieved. These limitations, however, are balanced by the strengths of using a large transplant dataset, which enhances study power and the ability to detect meaningful differences while also increasing generalizability through utilization of a national registry.
We describe for the first time worse waitlist and posttransplantation outcomes in 18–24 year olds compared to both older and younger age groups. At a time of organ scarcity and in a population of young adults in whom survival benefit is significant,32 future single or multi-center studies are needed to delineate the potential contribution of socio-behavioral risk factors on posttransplantation outcomes, specific causes of graft failure and barriers to re-transplantation to target interventions and maximize ideal long-term outcomes in young adults 18–24 years old. Finally, the lower utilization of nonstandard exception scores in young adults compared to older adolescents is stark and future collaborative work across the pediatric and adult transplant communities are needed to ensure parity in our allocation system.
Supplementary Material
Acknowledgments
Funding: This research is supported by Research and Development, Veterans Affairs Puget Sound Healthcare System and was based on data derived from the United Network for Organ Sharing (UNOS) in June 2015. This work was supported in part by an NIDDK training grant to the Division of Gastroenterology at the University of Washington (T32 DK007742) and the Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services.
Abbreviations
- ALF
Acute Liver Failure
- AHR
Adjusted Hazard Ratio
- ASHR
Adjusted Sub-Hazard Ratio
- BSA
Body Surface Area
- DCD
Donation after Cardiac Death
- HCC
Hepatocellular Carcinoma
- MELD
Model for End-Stage Liver Disease
- NASH
Nonalcoholic Steatohepatitis
- OPTN
Organ Procurement and Transplantation Network
- PELD
Pediatric End-Stage Liver Disease
- PSC
Primary Sclerosing Cholangitis
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
Footnotes
Authors contribution:
Noelle Ebel: Study conception and design, interpretation of data, drafting the manuscript, approval of the version of the manuscript to be published
Evelyn Hsu: Study conception and design, interpretation of data, critical revision, approval of the version of the manuscript to be published
Kristin Berry: Study conception and design, acquisition of data, analysis of data, interpretation of data, critical revision, approval of the version of the manuscript to be published
Simon Horslen: Study conception and design, interpretation of data, critical revision, approval of the version of the manuscript to be published
George Ioannou: Study conception and design, interpretation of data, critical revision, approval of the version of the manuscript to be published
Disclosures: The authors declare no conflicts of interest.
References
- 1.Bucuvalas JC, Alonso E, Magee JC, Talwalkar J, Hanto D, Doo E. Improving long-term outcomes after liver transplantation in children. Am J Transplant. 2008;8:2506–13. doi: 10.1111/j.1600-6143.2008.02432.x. [DOI] [PubMed] [Google Scholar]
- 2.Dharnidharka VR, Lamb KE, Zheng J, Schechtman KB, Meier-Kriesche HU. Across all solid organs, adolescent age recipients have worse transplant organ survival than younger age children: A US national registry analysis. Pediatr Transplant. 2015;19:471–6. doi: 10.1111/petr.12464. [DOI] [PubMed] [Google Scholar]
- 3.Alonso EM, Ng VL, Anand R, et al. The SPLIT research agenda 2013. Pediatr Transplant. 2013;17:412–22. doi: 10.1111/petr.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annunziato RA, Emre S, Shneider B, Barton C, Dugan CA, Shemesh E. Adherence and medical outcomes in pediatric liver transplant recipients who transition to adult services. Pediatr Transplant. 2007;11:608–14. doi: 10.1111/j.1399-3046.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Arendonk KJ, King EA, Orandi BJ, et al. Loss of pediatric kidney grafts during the “high-risk age window”: insights from pediatric liver and simultaneous liver-kidney recipients. Am J Transplant. 2015;15:445–52. doi: 10.1111/ajt.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster BJ, Dahhou M, Zhang X, Dharnidharka VR, Conway J, Ng VL. High risk of liver allograft failure during late adolescence and young adulthood. Transplantation. 2016;100:577–84. doi: 10.1097/TP.0000000000001009. [DOI] [PubMed] [Google Scholar]
- 7.Questions and answers for transplant candidates about liver allocation policy. United Network for Organ Sharing. www.ohsu.edu/xd/health/services/transplant/liver/pre-transplant/education-and-protocols/upload/liver-allocation.pdf. Accessed August 2016.
- 8.Hsu EK, Shaffer M, Bradford M, Mayer-Hamblett N, Horslen S. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. Am J Transplant. 2015;15:436–44. doi: 10.1111/ajt.13089. [DOI] [PubMed] [Google Scholar]
- 9.Massie AB, Caffo B, Gentry SE, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant. 2011;11:2362–71. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukazawa K, Nishida S. Size mismatch in liver transplantation. J Hepatobiliary Pancreat Sci. 2016;23:457–66. doi: 10.1002/jhbp.371. [DOI] [PubMed] [Google Scholar]
- 11.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–7. doi: 10.1097/00007890-199901270-00024. [DOI] [PubMed] [Google Scholar]
- 12.Fukazawa K, Nishida S, Volsky A, Tzakis AG, Pretto EA., Jr Body surface area index predicts outcome in orthotopic liver transplantation. J Hepatobiliary Pancreat Sci. 2011;18:216–25. doi: 10.1007/s00534-010-0334-9. [DOI] [PubMed] [Google Scholar]
- 13.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2013 annual data report: liver. Am J Transplant. 2015;15(suppl 2):1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 14.White AM, Hingson RW, Pan IJ, Yi HY. Hospitalizations for alcohol and drug overdoses in young adults ages 18–24 in the United States, 1999–2008: results from the Nationwide Inpatient Sample. J Stud Alcohol Drugs. 2011;72:774–86. doi: 10.15288/jsad.2011.72.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neinstein L. The new adolescents: an analysis of health conditions, behaviors, risks, and access to services among emerging young adults. United States: University of Southern California; 2013. http://www.usc.edu/student-affairs/Health_Center/thenewadolescents/doc/TheNewAdolescents_Final_Locked.pdf. Accessed March 2016. [Google Scholar]
- 16.Bob Gray. cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2-7. 2014 http://CRAN.R-project.org/package=cmprsk. Accessed January 2016.
- 17.Schaubel DE, Dykstra DM, Murray S, et al. Analytical approaches for transplant research, 2004. Am J Transplant. 2005;5:950–7. doi: 10.1111/j.1600-6135.2005.00837.x. [DOI] [PubMed] [Google Scholar]
- 18.Freeman RB, Jr, Wiesner RH, Roberts JP, McDiarmid S, Dykstra DM, Merion RM. Improving liver allocation: MELD and PELD. Am J Transplant. 2004;4:114–31. doi: 10.1111/j.1600-6135.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Castro KI, De Martin E, Gambato M, et al. Female gender in the setting of liver transplantation. World J Transplant. 2014;4:229–42. doi: 10.5500/wjt.v4.i4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin SR, Atkison P, Anand R, Lindblad AS, The SPLIT Research Group Studies of Pediatric Liver Transplantation 2002: patient and graft survival and rejection in pediatric recipients of a first liver transplant in the United States and Canada. Pediatr Transplant. 2004;8:273–83. doi: 10.1111/j.1399-3046.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 21.Soltys KA, Mazariegos GV, Squires RH, Sindhi RK, Anand R, The SPLIT Research Group Late graft loss or death in pediatric liver transplantation: an analysis of the SPLIT database. Am J Transplant. 2007;7:2165–71. doi: 10.1111/j.1600-6143.2007.01893.x. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/. Accessed August 2015. [Google Scholar]
- 23.Braun HJ, Perito ER, Dodge JL, Rhee S, Roberts JP. Nonstandard exception requests impact ouccomes for pediatric liver transplant candidates. Am J Transplant. 2016;16:3181–91. doi: 10.1111/ajt.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg DS, Makar G, Bittermann T, French B. Center variation in the use of nonstandardized model for end-stage liver disease exception points. Liver Transpl. 2013;19:1330–42. doi: 10.1002/lt.23732. [DOI] [PubMed] [Google Scholar]
- 25.McDiarmid SV, Goodrich NP, Harper AM, Merion RM. Liver transplantation for Status 1: the consequences of good intentions. Liver Transpl. 2007;13:699–707. doi: 10.1002/lt.21125. [DOI] [PubMed] [Google Scholar]
- 26.McDiarmid S, Gish RG, Horslen S, Mazariegos GV. Model for end-stage liver disease (MELD) exception for unusual metabolic liver diseases. Liver Transpl. 2006;12(12 Suppl 3):S124–7. doi: 10.1002/lt.20973. [DOI] [PubMed] [Google Scholar]
- 27.Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–65. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 28.Shemesh E, Shneider BL, Savitzky JK, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113:825–32. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 29.Fredericks EM, Magee JC, Eder SJ, et al. Quality improvement targeting adherence during the transition from a pediatric to adult liver transplant clinic. J Clin Psychol Med Settings. 2015;22:150–9. doi: 10.1007/s10880-015-9427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014;60:663–70. doi: 10.1016/j.jhep.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar M, Watt KD, Terrault N, Berenguer M. Outcomes in liver transplantation: does sex matter? J Hepatol. 2015;62:946–55. doi: 10.1016/j.jhep.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berry K, Ioannou GN. Comparison of liver transplant-related survival benefit in patients with versus without hepatocellular carcinoma in the United States. Gastroenterology. 2015;149:669–80. doi: 10.1053/j.gastro.2015.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


