Abstract
Acetaminophen (APAP) overdose is a leading cause of acute liver failure worldwide, in which mitochondrial DNA (mtDNA) released by damaged hepatocytes activates neutrophils via the binding of TLR9, further aggravating liver injury. Here, we demonstrated that mtDNA/TLR9 also activates a negative feedback pathway via the induction of microRNA-223 (miR-223) to limit neutrophil over-activation and liver injury. After injection of APAP in mice, levels of miR-223, the most abundant miRNAs in neutrophils, were highly elevated in neutrophils. Disruption of the miR-223 gene exacerbated APAP-induced hepatic neutrophil infiltration, oxidative stress, and injury, and enhanced TLR9 ligand-mediated activation of pro-inflammatory mediators in neutrophils. An additional deletion of the intercellular adhesion molecule 1 (ICAM-1) gene ameliorated APAP-induced neutrophil infiltration and liver injury in miR-223 knockout mice. In vitro experiments revealed that miR-223-deficient neutrophils were more susceptible to TLR9 agonist-mediated induction of pro-inflammatory mediators and NF-κB signaling; whereas overexpression of miR-223 attenuated these effects in neutrophils. Moreover, inhibition of TLR9 signaling via either treatment with a TLR9 inhibitor or via the disruption of TLR9 gene partially but significantly suppressed miR-223 expression in neutrophils post APAP injection. In contrast, activation of TLR9 upregulated miR-223 expression in neutrophils in vivo and in vitro. Mechanistically, activation of TLR9 upregulated miR-223 by enhancing NF-κB binding on miR-223 promoter; while miR-223 attenuated TLR9/NF-κB-mediated inflammation by targeting IKKα expression. Collectively, upregulation of miR-223 plays a key role in terminating the acute neutrophilic response and is a therapeutic target for the treatment of APAP-induced liver failure.
Keywords: liver failure, IKK, NF-κB, hepatocyte, APAP, miR-223
Introduction
Acetaminophen (APAP) is one of the most commonly used antipyretic and analgesic, but its overdose is also a leading cause of acute liver failure.1 APAP is metabolized by hepatic cytochrome P450 2E1 (CYP2E1) into a powerful electrophilic intermediate N-acetyl-p-benzoquinone-imine (NAPQI), which is detoxified by glutathione (GSH). However, excessive NAPQI depletes hepatic GSH and subsequently leads to oxidative stress reactions, mitochondrial DNA (mtDNA) damage, finally resulting in hepatocyte death.1, 2 In addition to APAP-induced direct hepatotoxicity, pro- and anti-inflammatory cascades of the innate immune system are simultaneously activated, the balance of which may further influence the progression and severity of APAP-induced liver injury.3–6 For example, investigators have demonstrated that during APAP-induced liver injury, necrotic hepatocytes release mtDNA, which acts as an agonist for toll-like receptor 9 (TLR9) to induce neutrophil infiltration and liver inflammation that may further exacerbate hepatocellular damage.7–9 In addition, the pathological role of TLR9 activation in inducing neutrophil infiltration and liver injury has also been reported in other liver injury models, including liver ischemia/reperfusion,10–12 chronic-plus-binge ethanol feeding,13 nonalcoholic fatty liver disease,14 and HBsAg transgenic mice.15 However, how the inflammation is terminated in APAP-induced hepatotoxicity remains largely unknown.
MicroRNAs (miRNAs) are endogenous, small, noncoding RNAs of ~22 nucleotides in length that regulate entire intracellular pathways at a posttranscriptional level by specifically binding with the 3′-untranslated region (3′-UTR) of target gene mRNAs.16 Several recent studies reported that a large number of miRNAs were elevated in the serum in mice and in patients after APAP administration,17–21 but the role of miRNAs in APAP-induced liver injury has not been investigated. In the current study, to understand the mechanisms by which neutrophilic inflammation is controlled during APAP-induced liver injury, we were particularly interested in neutrophil-specific miR-223 that was initially reported to regulate the early and late stages of granulopoiesis as well as in the homeostasis of mature neutrophils.22 Later studies suggest that miR-223 acts as an anti-inflammatory mediator, playing an important role in the pathogenesis of various types of diseases.23, 24 In addition, miR-223 has also been found to control liver cell proliferation, survival and cholesterol metabolism in hepatocytes as well as neutrophil functions by targeting a variety of genes.25–28
In the current study, we found that miR-223 expression was highly elevated in neutrophils after APAP injection, and that deletion of miR-223 exacerbated APAP-induced neutrophil infiltration, liver injury, and inflammation. Upregulation of miR-223 was partially dependent on TLR9 activation in APAP-induced liver injury. Mechanistic studies suggest that damaged hepatocytes release hepatic mtDNA, which upregulates miR-223 in neutrophils via the activation of TLR9, forming a negative feedback loop to control APAP-induced neutrophilic inflammation, and subsequently terminating liver inflammation.
Materials and Methods
Mice
MiR-223−/− (miR-223 KO) and ICAM-1−/− mice were purchased from the Jax laboratory (Bar Harbor, ME). Female miR-223+/− and male miR-223−/y mice (the miR-223 locus is on the X chromosome) were bred at the NIAAA FLAC facility to generate male miR-223−/y (male miR-223KO) and wild-type littermate controls. MiR-223−/− mice and ICAM-1−/− mice were bred via several steps to generate miR-223−/−ICAM1−/− double knockout mice. TLR9−/− mice on a C57BL/6 background were kindly provided by Dr. Giorgio Trinchieri (NCI). All mice were maintained in a specific pathogen-free facility and were cared for in accordance with NIH guidelines. The study was approved by the NIAAA Animal Care and Use Committee.
APAP-induced liver injury
Fresh solutions of APAP (Sigma-Aldrich, St. Louis, MO) were prepared immediately before use by dissolving the compound in warmed PBS. All the mice were intraperitoneally injected with 350 mg/kg APAP after overnight-fasting or without fasting. Liver and blood samples were collected at various time points after APAP injection.
TLR9 agonist and antagonist injection
The male mice (body weight 20–22g) were intravenously injected with TLR9 agonist (ODN 1585) or TLR9 agonist control (ODN 1585 control) (Invivogen, CA) at a dose of 50 μg/mouse. Liver and blood samples were collected 6 h after injection, and neutrophils from the liver and blood samples were isolated and subjected to RT-PCR analysis. TLR9 antagonist (ODN2088) (Invivogen, CA) was also used to block TLR9 as described previously.7
Statistical analysis
Data are expressed as the means ± SEM for each group and were analyzed using GraphPad Prism software (v. 5.0a; GraphPad Software, La Jolla, CA). To compare values obtained from two groups, the Student t test was performed. P values of <0.05 were considered significant.
Further details of the materials and methods are provided in Supporting Information.
Results
Upregulation of miR-223 expression in neutrophils in mice after APAP treatment
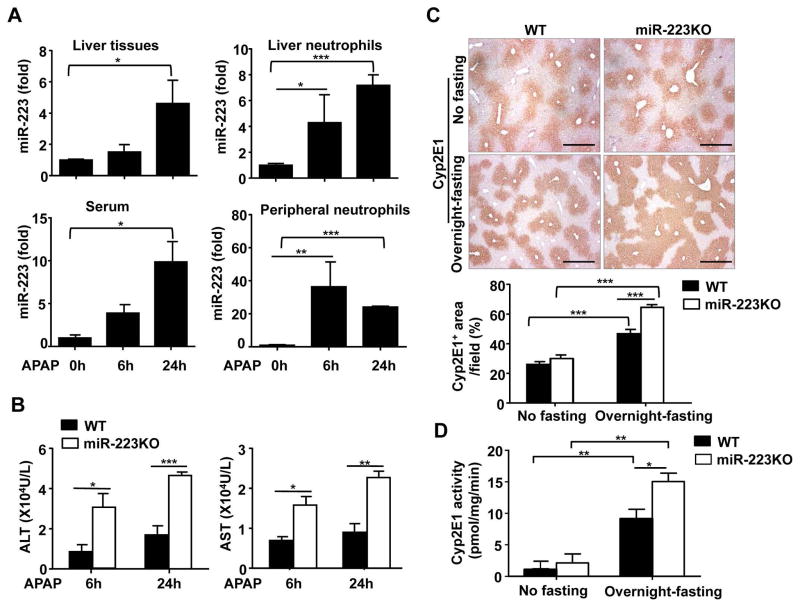
To understand the mechanisms by which hepatic neutrophil infiltration is regulated during APAP-induced liver injury, we examined the serum and hepatic miR-223 expression, a miRNA that was known to regulate neutrophil functions.22 As illustrated in Fig. 1A, hepatic and serum levels of miR-223 were significantly elevated 6h or 24h after APAP treatment. Furthermore, miR-223 expression was markedly upregulated in neutrophils from the liver and blood 6h and 24h after APAP injection (Fig. 1A). Northern blot assay further confirmed the upregulation of miR-223 expression in hepatic neutrophils and circulating neutrophils from APAP-treated mice (Supporting Fig. 1).
Fig. 1. Upregulation of miR-223 and its role in APAP-induced liver injury.
Overnight-fasted WT mice and/or miR-223 KO mice were intraperitoneally injected with 350 mg/kg APAP dissolved in warm PBS. (A) Liver tissues, serum samples, and liver and peripheral neutrophils from WT mice were collected 6h or 24 h after APAP treatment, and subjected to the measurement of miR-223. (B–D) Liver tissues and serum samples were collected 6h or 24h after APAP treatment. Serum ALT and AST levels were measured (panel B). Representative images of liver sections for CYP2E1 protein staining (original magnification X40) are shown in panel C. Hepatic CYP2E1 activity was measured (panel D). Values represent means ± SEM (n=6 in panel A and n=9 in panels B–D). *P< 0.05, **P< 0.01, ***P< 0.001.
Deletion of miR-223 exacerbates APAP-induced liver injury
To better understand the function of miR-223 in APAP-induced liver inflammation and injury, WT and miR-223KO mice were used and treated with APAP. MiR-223 deletion in miR-223KO mice was confirmed by RT-qPCR analyses as shown in supporting Fig 2. Compared with WT mice, overnight-fasted miR-223KO mice showed much higher levels of serum ALT and AST 6h and 24h after APAP injection (Fig. 1B). In addition, H&E staining and immunohistochemistry analyses revealed miR-223KO mice had larger areas of liver necrosis and a greater degree of hepatic neutrophil and macrophage infiltration than WT mice (Supporting Fig. 3A–C).
To test whether the increased susceptibility of miR-223KO mice to APAP-induced liver injury was due to the alteration of APAP metabolism, we examined the expression of CYP2E1, a major metabolizing enzyme in the bioactivation of APAP into NAPQI.29 Interestingly, immunohistochemical staining and real-time qPCR analyses demonstrated that the levels of hepatic CYP2E1 protein, activity, and mRNA were much higher in miR-223KO mice compared with those in WT mice after overnight-fasting (Fig. 1C–D, Supporting Fig. 4A). However, no differences were observed between non-fasted WT and miR-223KO groups (Fig. 1C–D, Supporting Fig. 4A).
The expression and activity of CYP2E1 were reported to be regulated by several transcription factors, such as β-catenin,30 NF- κB,31 and HNF-1α.32 Here, we observed that hepatic β-catenin protein expression was significantly higher in miR-223KO mice compared with WT mice after overnight-fasting. In contrast, no differences in hepatic NF-κB p65 and HNF-1α proteins were observed in WT and miR-223KO mice post overnight fasting (Supporting Fig. 4B). These data indicated that β-catenin may be involved in the upregulation of CYP2E1 in the miR-223KO mice after overnight-fasting, which may cause increased susceptibility of miR-223KO mice to APAP hepatotoxicity.
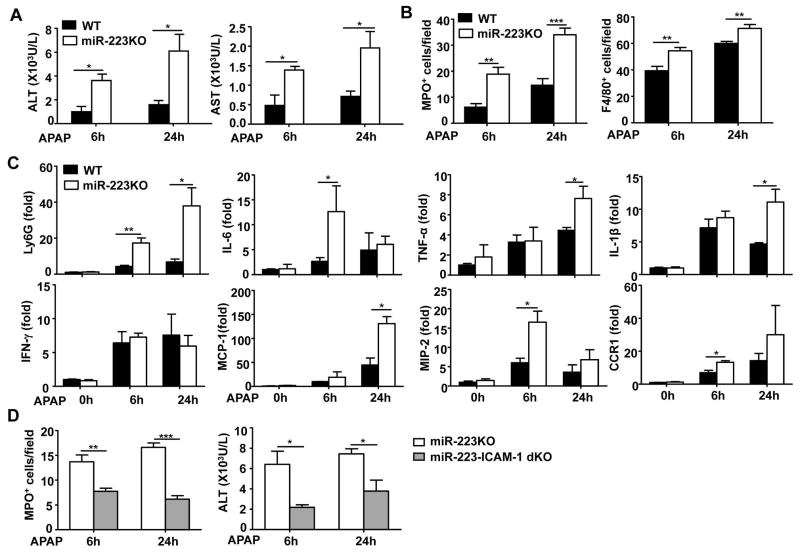
To rule out the involvement of CYP2E1 dysregulation in the increased susceptibility of miR-223KO mice to APAP-induced liver injury, we treated WT and miR-223KO mice with APAP without overnight-fasting, a condition that does not affect hepatic expression of CYP2E1 in miR-223KO and WT mice (as demonstrated in Fig. 1 and supporting Fig. 4A). As expected, injection of APAP caused much lower levels of liver injury in non-fasted WT mice than in fasted WT mice (ALT levels, ~1000 U/L in non-fasted WT mice [Fig. 2A] vs. ~10,000 U/L in fasted WT mice [Fig. 1B]). Interestingly, without fasting, serum ALT and AST levels were also markedly higher in miR-223KO mice than those in WT mice 6h and 24h after APAP challenge (Fig. 2A). H&E staining and immunostaining analyses revealed greater liver necrosis, a much higher number of MPO+ neutrophils and F4/80+ macrophages in miR-223KO mice than in WT mice post APAP injection (Fig. 2B, Supporting Fig. 5 and Supporting Fig. 6). The increased hepatic neutrophil infiltration in APAP-treated miR-223KO mice was also confirmed by real-time PCR analyses (Fig. 2C). In addition, hepatic expression of inflammatory cytokines (IL-6, TNF-α, and IL-1β), chemokines (MCP-1 and MIP-2) in miR-223KO mice was much higher than that in WT mice post APAP injection (Fig. 2C).
Fig. 2. MiR-223KO mice are more susceptible to APAP-induced liver injury and inflammation.
Non-fasted WT and miR-223KO mice were injected with 350 mg/kg APAP dissolved in warm PBS. Liver tissues and serum samples were collected 0, 6 or 24h after APAP treatment. (A) Serum ALT and AST levels were measured. (B) Necrotic area, MPO+ or F4/80+ cells per field were quantified. (C) RT-qPCR analyses of Ly6G and various inflammatory genes. (D) Non-fasted miR-223KO and miR-223/ICAM-1dKO mice were injected with 350 mg/kg APAP dissolved in warm PBS. Liver tissues and serum samples were collected 6h and 24h after APAP treatment. MPO+ neutrophils in the liver were determined, and serum ALT levels were measured. Values represent means ± SEM (n=6 in panels A–C; n=7 in panel D). *P< 0.05, **P< 0.01, ***P< 0.001.
MiR-223 has been implicated in regulating Fas-induced hepatocyte death.25 Thus we wondered whether miR-223 in hepatocytes also affects APAP-induced cell death. To answer this question, the direct role of miR-223 in APAP-induced hepatocyte apoptosis was defined in cultured hepatocytes. As shown in Supporting Figure 7A, 7B, deletion of miR-223 or overexpression of miR-223 in hepatocytes did not significantly affect APAP-induced hepatocyte death.
To further confirm whether neutrophil infiltration contributes to the enhanced liver injury in miR-223KO mice after APAP injection, we performed an additional deletion of the ICAM-1 gene (a key factor for neutrophil infiltration in APAP hepatotoxicity33) in miR-223KO mice to reduce neutrophil infiltration. As illustrated in Fig. 2D, an additional deletion of ICAM-1 markedly reduced hepatic neutrophil number in APAP-treated dKO mice compared with miR-223KO mice. Moreover, dKO mice had much lower serum ALT levels than WT mice post APAP injection. These findings strongly suggest that neutrophils contribute to APAP-induced liver injury in miR-223KO mice.
Next we examined whether miR-223 and ICAM-1 reciprocally regulated each other. As illustrating in supporting Fig. 8A, expression of ICAM-1 mRNAs in the liver and neutrophils was comparable in WT and miR-223KO mice, suggesting that ICAM-1 is not a direct target of miR-223. Interestingly, expression of miR-223 the liver and neutrophils was significantly higher in ICAM-1KO mice than in WT mice (Supporting Fig. 8B), indicating deletion of the ICAM-1 gene affects miR-223 expression. The underlying mechanisms by which ICAM-1 deletion alters miR-223 expression in neutrophils remain unknown and need further studies to be explored.
In summary, miR-223KO mice are more susceptible to APAP-induced liver injury with or without fasting. Because hepatic expression of CYP2E1 protein is comparable in miR-223KO and WT mice without fasting, non-fasted WT and non-fasted miR-223KO mice were used in the rest of paper.
MiR-223KO mice are more susceptible to APAP-induced oxidative stress
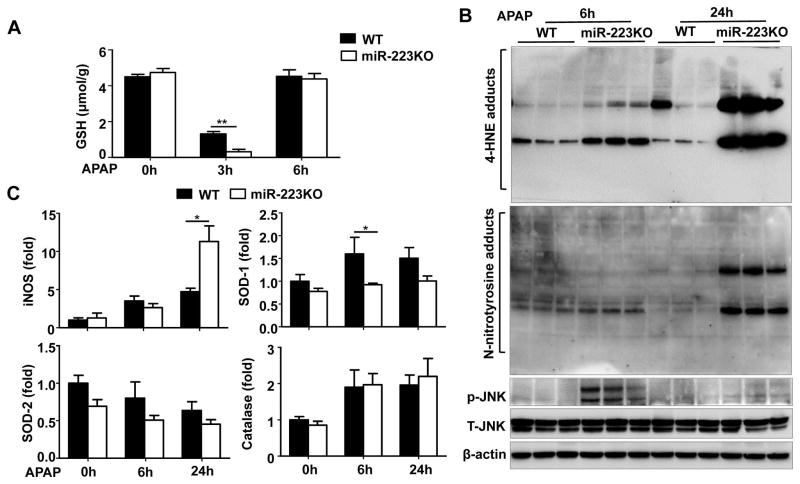
To further examine the mechanisms by which miR-223KO mice are more susceptible to APAP-induced liver injury, hepatic oxidative stress and its related genes were examined. As illustrated in Fig. 3A, prior to the APAP injection, hepatic levels of glutathione (GSH), an important antioxidant, were comparable between WT and miR-223KO mice. Injection of APAP rapidly downregulated GSH levels with a much greater degree in miR-223KO mice than that in WT mice 3h post-APAP challenge. Hepatic levels of 4-HNE (oxidative stress markers) were higher in the APAP-treated miR-223KO mice compared with WT mice (Fig. 3B). Notably, N-nitrotyrosine, which is a reactive nitrogen species formed by the very rapid reaction of superoxide and nitric oxide (NO),34 was also higher in miR-223KO mice compared with WT mice after APAP treatment (Fig. 3B). Additionally, inducible nitric oxide synthase (iNOS), which was reported to promote hepatocytes death by generating the high amount of NO,35, 36 was upregulated in the liver of miR-223KO mice compared to that of WT mice 24h post-APAP injection (Fig. 3C). Finally, hepatic expression of activated p-JNK, which is known to play an important role in APAP-associated oxidative stress,37 was significantly higher in miR-223KO mice 6h after APAP injection compared with WT mice (Fig. 3B).
Fig. 3. MiR-223KO mice are more susceptible to APAP-induced ROS in the liver.
Non-fasted WT and miR-223KO mice were injected with 350 mg/kg APAP. (A) Liver GSH levels were measured. (B) 4-HNE adducts, N-nitrotyrisine adducts, total JNK (T-JNK) and p-JNK expression were detected by western blot. (C) The mRNA expressions of iNOS, SOD-1. SOD-2, and catalase were measured by RT-qPCR. Values represent means ± SEM (n=6). *P< 0.05, **P< 0.01.
In addition, hepatic expression of superoxide dismutase-1 (SOD-1), an ROS detoxification gene, was lower in APAP-treated miR-223KO mice compared with WT mice, whereas hepatic expression of other ROS detoxification genes, such as superoxide dismutase-2 (SOD-2) and catalase were comparable between WT and miR-223KO mice (Fig. 3C).
TLR9 contributes to the increased susceptibility of miR-223KO mice to APAP-induced inflammatory and neutrophilic responses
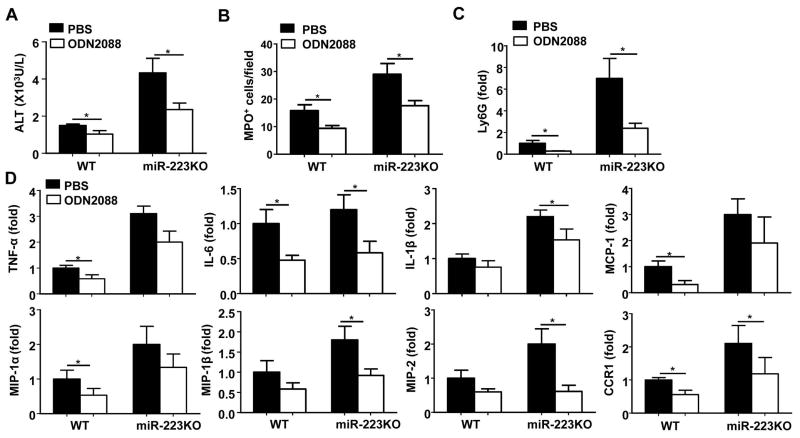
Previous studies have reported that hepatocyte damage induced by APAP can trigger a sterile inflammatory response through release of damage-associated molecular patterns (DAMPs), such as DNAs, which activate neutrophils by targeting TLR9.10, 11 To investigate whether TLR9 is also involved in the increased susceptibility of miR-223KO mice to APAP-induced liver injury and inflammation, miR-223KO mice were treated with the TLR9 antagonist ODN2088 to block TLR9 activation. As illustrated in Fig. 4 and Supporting Fig. 9, treatment with ODN2088 markedly reduced serum ALT levels, hepatic neutrophil infiltration (as demonstrated by RT-PCR analysis of Ly6G mRNA [neutrophil marker] and MPO staining), and hepatic expression of a variety of pro-inflammatory mediators in APAP-treated WT and miR-223KO mice compared to their corresponding controls.
Fig. 4. APAP-induced liver injury and inflammatory responses in WT or miR-223KO mice are dependent on TLR9.
TLR9 antagonist (ODN2088) or PBS were injected into WT or miR-223KO mice immediately after and at 6h after APAP injection, serum and liver samples were collected at 24h after APAP treatment. Serum ALT was measured (panel A). MPO+ cells per field in the liver were determined (panel B). RT-qPCR analysis of Ly6G and various inflammatory genes in the liver (panel C and D). Values represent means ± SEM (n=8). *P< 0.05.
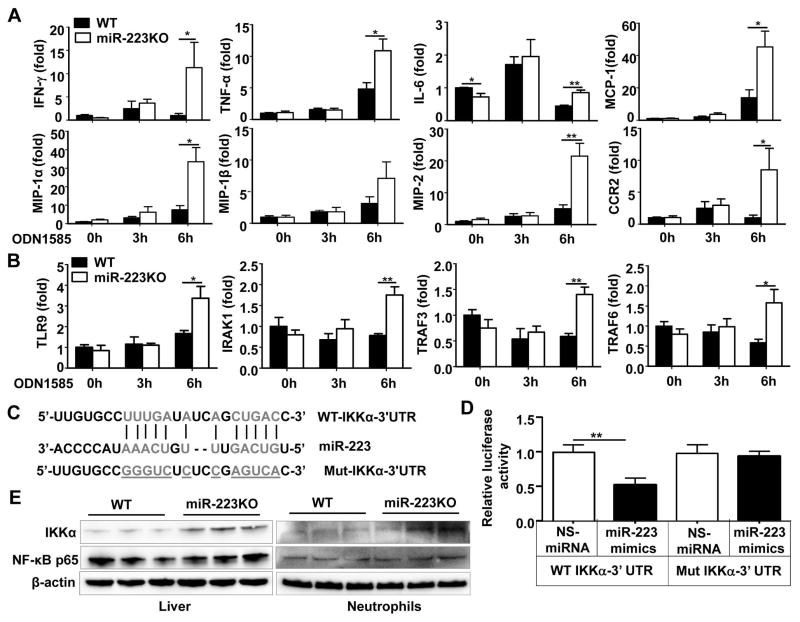
MiR-223-deficient neutrophils are more susceptible to the TLR9 agonist ODN1585-activation of pro-inflammatory mediators and NF-κB signaling
The above data suggest that miR-223KO mice are more susceptible to APAP-induced liver injury and inflammation dependent on TLR9 in vivo. Next we wondered whether miR-223 regulates TLR9-mediated induction of inflammatory mediators in neutrophils. To answer this question, bone marrow neutrophils from WT and miR-223KO mice were isolated and treated with the TLR9 agonist ODN1585 in vitro. As illustrated in Fig. 5A and Supporting Fig. 10, the expression of many inflammatory mediators were significantly higher in the neutrophils from miR-223KO mice after TLR9 agonist stimulation compared to those from WT mice. In addition, expression of TLR9 and its downstream genes (IRAK1, TRAF3 and TRAF6) were also upregulated in bone marrow neutrophils from miR-223KO mice but not in WT neutrophils 6h after TLR9 ligand treatment (Fig. 5B).
Fig. 5. MiR-223 negatively regulates TLR9-mediated NF-κB signaling by targeting IKKα in neutrophils.
(A, B) Bone marrow neutrophils from WT and miR-223KO mice were treated with TLR9 ligand (ODN1585) for 0, 3, and 6h in vitro. RT-qPCR analysis of various inflammatory genes (panel A) and TLR9 and its downstream genes (panel B). (C) The bioinformatics approach analysis of the target prediction of miR-223. (D) Dual luciferase reporter assay was performed to verify binding between miR-223 and IKKα mRNA. The 293T cells were transfected with luciferase vector containing either WT or mutated 3′UTR of IKKα, and also transfected with miR-223 mimics or NS-miRNA. Results of relative luciferase activity are shown. (E) IKKα and NF-κB p65 protein expressions in the liver and bone marrow neutrophils from WT and miR-223KO were detected by western blot. Values represent means ± SEM (n=4 in panels A–B and n=7 in panel D). *P< 0.05, **P< 0.01.
Because NF-κB is a key transcription factor that controls the expression of various inflammatory mediators, and IKKα, which plays a key role in activating NF-κB,38 is a known downstream target of miR-223,39 we hypothesized that miR-223 may inhibit inflammation by regulating IKKα/NF-κB signaling. By using a bioinformatics approach, we confirmed that IKKα contains the potential target sequence of miR-223 (Fig. 5C). Luciferase reporter assay further unveiled that transfection of miR-223 mimics significantly decreased luciferase activity from WT-IKKα-3′UTR construct but not from mutant-IKKα-3′UTR in 293T cells (Fig. 5D) and in dHL-60 cells (Supporting Fig. 11). Western blot analysis revealed that expression of IKKα protein was higher in the liver and bone marrow neutrophils from miR-223KO mice compared to those from WT mice while the expression of p65 protein was comparable between these two groups (Fig. 5E). Interestingly, although IKKα protein levels are higher in the liver and neutrophils from miR-223 KO mice than those from WT neutrophils, expression of IKKα mRNA was comparable in WT and miR-223 mice (Supporting Fig. 12).
To further explore the other downstream targets of miR-223, we used the miRBase database 40 and identified several predicted miR-223 targeted genes, and subsequently performed PCR analyses of these genes in the liver and bone marrow neutrophils. As illustrated in Supporting Fig. 12, expression of many of these miR-223 targeted genes was comparable in WT and miR-223KO mice except the upregulation of STAT3 and the downregulation of NLRP3 (NACHT, LRR and PYD domains-containing protein 3) (Supporting Fig. 12). NALP3 is a component of the inflammasome, thus downregulation of NLRP3 does not explain the enhanced proinflammatory functions in miR-223KO neutrophils, so NLRP3 was not further examined. STAT3 in neutrophils is known to promote inflammation.41 Therefore, we examined STAT3 protein expression. Surprisingly, STAT3 protein expression was not upregulated in the liver or neutrophils from miR-223KO mice compared to those from WT mice, suggesting that STAT3 may not contribute to the anti-inflammatory function of miR-223. Future proteomic analyses are required to identify miR-223 direct or indirect target genes in addition to IKKα that contribute to the anti-inflammatory function of miR-223.
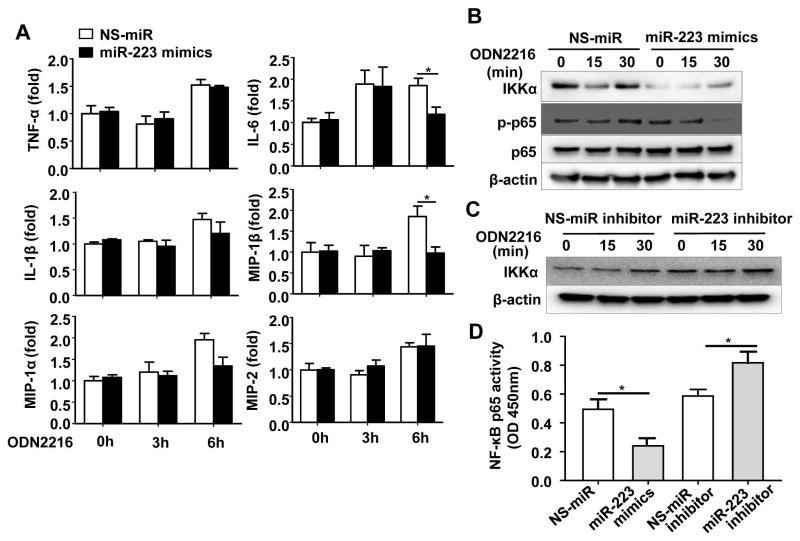
MiR-223 downregulates IKKα protein expression in neutrophils
To further confirm whether miR-223 regulated IKKα expression, dHL-60 cells was transfected with miR-223 mimics or miR-223 inhibitor. MiR-223 expression was significantly increased after transfection with miR-223 mimics, but was markedly downregulated after transfection with miR-223 inhibitor for 24h or 48h in dHL-60 cells (Supporting Fig. 13A), confirming transfection is effective. Fig. 6A showed that overexpression of miR-223 significantly diminished TLR9 agonist-mediated upregulation of IL-6, MIP-1α, and MIP-1β expression in neutrophils. More importantly, overexpression of miR-223 decreased the level of IKKα protein (Fig. 6B). In addition, TLR9 agonist ODN2216 activation of NF-κB (p-p65) at 30 min time point was markedly suppressed in miR-223 mimics-transfected cells compared to that in NS-miRNA-treated cells (Fig. 6B). Interestingly, expression of IKKα mRNA was not altered by miR-223 mimics (Supporting Fig. 13B).
Fig. 6. MiR-223 inhibitsTLR9-mediated NF-κB signaling in human neutrophil-like cells (dHL-60 cells).
dHL-60 cells were transfected with nonspecific miRNA mimics (NS-miR) or miR-223 mimics (panels A, B), nonspecific miR-inhibitor or miR-223 inhibitor (panel C) by Lipofectamine RNAiMAX Reagent according to the manufacturer’s instructions at a final concentration of 20 nM for 48 h. After 48 h, the cells were stimulated with human TLR9 agonist (ODN2216) for the indicated time. (A) RT-qPCR analysis of inflammatory genes. (B, C) Western blot analyses. (D) Transfected dHL-60 cells were stimulated with ODN2216 for 30 min, and NF-κB p65 activity was measured by using an NF-κB p65 transcription factor assay kit. Values represent means ± SEM (n=4–8). *P< 0.05.
Furthermore, we examined the effects of miRNA-223 inhibitor treatment on TLR9 signaling in HL-60 cells. Our data show that inhibition of miR-223 significantly promoted TLR9 agonist-mediated upregulation of IKKα protein expression (Fig. 6C) and mRNA expression of IL-6, MIP-1α, and MIP-1β in dHL-60 cells (Supporting Fig. 13C).
Finally, treatment with miR-223 mimics attenuated TLR9 agonist-mediated NF-κB p65 activation; whereas treatment with miR-223 inhibitor augmented NF-κB activity (Fig. 6D).
Upregulation of miR-223 in neutrophils after APAP-induced liver injury depends on mtDNA/TLR9 and NF-κB activation
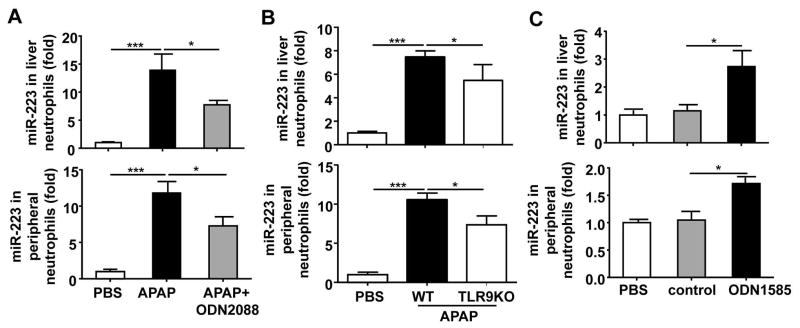
Previous studies have well documented that activation of TLR9 partially contributes to APAP-induced liver injury and inflammation.7, 8 Here we also confirmed that TLR9KO mice have lower levels of serum ALT and neutrophilic NF-κB activity than WT mice after APAP injection (Supporting Fig. 14A, 14B). Interestingly, blockage of TLR9 with ODN2088 also significantly reduced APAP-mediated upregulation of miR-223 expression in neutrophils isolated from the liver and peripheral blood (Fig. 7A). Furthermore, APAP-mediated upregulation of miR-223 in liver neutrophils and peripheral neutrophils was also significantly attenuated in TLR9KO mice (Fig. 7B). Finally, in vivo administration of the TLR9 agonist ODN1585 upregulated the expression of miR-223 in liver neutrophils and peripheral neutrophils (Fig. 7C).
Fig. 7. Upregulation of miR-223 expression in neutrophils during APAP-induced liver injury is mediated via a TLR9-dependent pathway.
(A) TLR9 antagonist (ODN2088) or PBS were injected into WT mice immediately after and at 6h after APAP injection. Liver neutrophils and peripheral neutrophils were isolated 24h after APAP injection and subjected to miR-223 detection. (B) WT and TLR9KO mice were intraperitoneally injected with 350 mg/kg APAP dissolved in warm PBS. Liver neutrophils and peripheral neutrophils were isolated 6h after APAP injection and subjected to miR-223 detection. (C) WT mice were injected with TLR9 agonist (ODN1585) or TLR9 control (ODN 1585 control) for 6h. MiR-223 expression in liver neutrophils and peripheral neutrophils was measured. Values represent means ± SEM (n=8 for panel A and n=7 for panels B–C). *P< 0.05, ***P< 0.001.
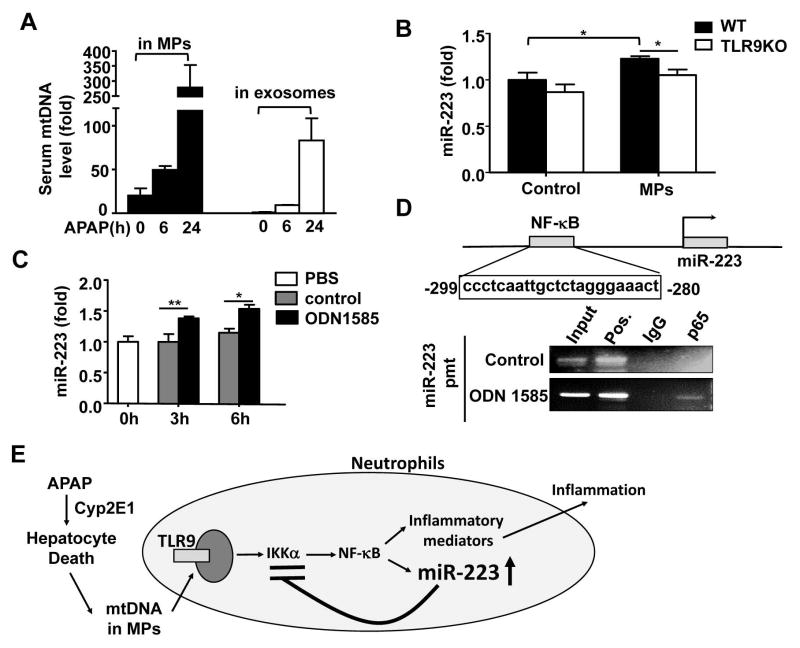
Above data suggest that TLR9 is involved in miR-223 upregulation in neutrophils in vivo post APAP injection. Because mtDNA is a known ligand for TLR9,42 we hypothesized that mtDNA released by damaged hepatocytes upregulates miR-223 expression in neutrophils via activation of TLR9. To test this hypothesis, we first measured serum mtDNA. As illustrated in Fig. 8A, injection of APAP markedly elevated serum levels of mtDNA, and mtDNA were found in microparticles (MPs) and exosomes with much higher levels in MPs. Then we treated neutrophils with MPs from APAP-treated hepatocytes and found MP treatment markedly upregulated expression of miR-223 in WT neutrophils but not in TLR9KO neutrophils (Fig. 8B). Moreover, in vitro treatment with the TLR9 agonist ODN1585 also markedly upregulated miR-223 expression in neutrophils (Fig. 8C).
Fig. 8. Upregulation of miR-223 in neutrophils after APAP-induced liver injury depends on mtDNA/TLR9 and NF-κB activation.
(A) Microparticles and exosomes were isolated from serum after APAP injection, and then mtDNA levels were measured by RT-qPCR. (B) Primary hepatocytes were treated with or without APAP (5 mM) for 24h, and then microparticles were isolated. These microparticles were used to incubate with bone marrow neutrophils for 6h. MiR-223 expression was measured. (C) Bone marrow neutrophils were treated with TLR9 agonist (ODN1585) or control (ODN1585 control) in vitro. MiR-223 expression was measured. (D) The NF-κB binding site is identified in the miR-223 promoter region. Bone marrow neutrophils were treated with a TLR9 agonist (ODN1585) for 1h. A representative ChIP assay blot is shown. Values represent means ± SEM (n=6 in panel A and n=4 in panels B–C). *P< 0.05, **P< 0.01. (E) A model depicting the dichotomy role of TLR9 activation in inducing and terminating inflammation and liver injury after APAP injection. During APAP hepatotoxicity, DNA released from damaged hepatocytes activates NF-κB via a TLR9-dependent pathway and subsequently upregulates expression of many inflammatory genes, thereby inducing liver inflammation and injury. Meanwhile, mtDNA/TLR9/NF-κB signaling also elevates miR-223 expression in neutrophils, which acts a negative feedback loop to control APAP-induced acute liver injury and inflammation by downregulating IKKα expression.
NF-κB is a well-known downstream signal pathway of TLR9 activation.43 Thus we wondered whether TLR9 activation upregulates miR-223 is mediated via the activation of NF-κB. As illustrated in Fig. 8D, DNA sequence analyses identified an NF-κB binding site on the miR-223 promoter, and the ChIP assay demonstrated that NF-κB was recruited to the promoter region of the miR-223 gene upon TLR9 ligand treatment in neutrophils in vitro (Fig. 8D).
The above data clearly indicate that mtDNA-TLR9 contributes to the miR-223 upregulation in neutrophils (Fig. 7, Fig. 8) and liver inflammation (Fig, 4), but the expression of miR-223 in neutrophils and liver inflammation were only partially reduced in mice treated with the TLR9 antagonist ODN2088 or in TLR9KO mice, suggesting other factors also contribute to liver inflammation and miR-223 upregulation in neutrophils. Because both TLR3 and TLR4 activations have been implicated in APAP-induced liver injury,44, 45 we wondered whether activation of these TLRs also upregulated miR-223 in neutrophils. Surprisingly, treatment with the TLR3 ligand Poly I:C and the TLR4 ligand LPS did not alter miR-223 expression in neutrophils (Supporting Fig. 15A), indicating TLR3 and TLR4 activation do not contribute miR-223 upregulation in neutrophils. Moreover, treatment with IL-6 but not IFN-γ or TNF-α significantly upregulated miR-223 expression in neutrophils (Supporting Fig. 15A). Collectively, in addition to TLR9 activation, IL-6 also contributes to upregulated miR-223 expression in neutrophils. Finally, treatment with poly I:C or LPS induced higher levels of IFN-γ, MIP-1β and MIP-2 expression in miR-223 KO neutrophils than in WT neutrophils (Supporting Fig. 15B), suggesting miR-223 affects TLR3 and TLR4 signaling.
Discussion
The novel finding in this paper is that during APAP-induced liver injury, necrotic hepatocytes release mtDNA, which is known to promote neutrophilic inflammation via the activation of TLR9,7, 8 also induces a feedback inhibitory pathway to prevent neutrophil over-activation by upregulating miR-223 expression and subsequently terminate liver inflammation and injury (summarized in Fig. 8E).
Numerous previous studies reported that during APAP-induced liver injury, a large number of miRNAs were elevated in the serum in mice and patients, and most of these miRNAs are derived from hepatocytes.17–21 In the current paper, we demonstrated for the first time that neutrophil-specific miR-223 is highly elevated in serum and in neutrophils in mice post APAP injection. Furthermore, we provided several lines of evidence suggesting that upregulation of miR-223 in neutrophils post APAP injection is partially dependent on TLR9. Firstly, blockage of TLR9 with the TLR9 antagonist treatment or disruption of the TLR9 gene diminished APAP-mediated upregulation of miR-223 in neutrophils. Secondly, activation of TLR9 with the TLR9 agonist upregulated expression of miR-223 in neutrophils in vitro and in vivo. Finally, Chip assays identified an NF-κB binding site on miR-223 promoter in TLR9 agonist-treated neutrophils, providing a mechanistic link between TLR9 activation and miR-223 upregulation in neutrophils. Interestingly, deletion of the TLR9 gene only partially abolished miR-223 upregulation in neutrophils from APAP-treated mice. Further studies indicate that other factors (eg. IL-6) also contribute to miR-223 upregulation in neutrophils.
MiR-223 was first identified as an important regulator for granulopoiesis and homeostasis of mature neutrophils.22 Later, miR-223 was found to be an important anti-inflammatory mediator in suppressing inflammation.23, 24 For example, a recent study suggests that miR-223 attenuates lung neutrophil recruitment in tuberculosis by targeting chemoattractants CXCL2 (MIP-2), CCL3 (MIP-1α), and IL-6.24 In the current study, we also examined the function and significance of miR-223 upregulation during APAP-induced liver injury by using miR-223KO mice. Our findings demonstrated that miR-223KO mice were more susceptible to APAP-induced activation of neutrophils and upregulation of a variety of inflammatory mediators in the liver. In vivo treatment with a TLR9 antagonist reduced expression of many pro-inflammatory mediators in the liver in miR-223KO mice post APAP injection; whereas in vitro treatment with a TLR9 agonist induced higher levels of several pro-inflammatory mediators in neutrophils from miR-223KO mice than those from WT mice. Collectively, upregulation of miR-223 in neutrophils forms a strong inhibitory pathway to prevent neutrophil over-activation during APAP-induced liver damage. Previous studies reported that the anti-inflammatory effect of miR-223 in macrophages is mediated by targeting the NF-κB upstream kinase IKKα and subsequently inhibiting NF-κB.39 In the current paper, our findings suggest that miR-223 also targets IKKα in neutrophils, thereby preventing neutrophil over-activation.
Activation of innate immune system has been suggested to play an important role in APAP-induced liver injury. For example, it was reported that inhibition of neutrophils via the injection of anti-Gr-1 antibody or via the genetic deletion of ICAM-1 protected against APAP hepatotoxicity,9, 33 suggesting neutrophils play an important role in promoting APAP-induced liver injury. However, others believed that neutrophil activation may be a critical event for host defense or injury resolution following APAP overdose, but not a contributing factor to APAP-induced injury.46 In our studies, we found that serum ALT and AST levels showed no difference between WT and miR-223KO mice at the early stages of APAP injection (3h), but miR-223KO mice showed more neutrophil recruitment, systemic inflammation, ROS formation, and higher levels of serum ALT and AST at the later stages of APAP treatment (6h or 24h) than WT mice. Moreover, an additional deletion of the ICAM-1 gene reduced hepatic neutrophil infiltration and APAP-induced liver injury in miR-223KO mice. Taken together, all of these findings strongly suggest that neutrophil infiltration likely contributes to liver injury at the later stages of APAP treatment and miR-223 attenuates liver injury by inhibiting the inflammatory response of neutrophils. In addition, we have previously demonstrated that miR-223 suppresses reactive oxygen species (ROS) production of neutrophils via the inhibition of the IL-6-p47phox pathway during alcoholic liver injury.28 This mechanism is also likely attributed to the increased APAP-induced liver injury in miR-223KO mice.
NETosis is a new form of cell death of activated neutrophils via the release of neutrophil extracellular trap (NET) that are extracelluar scaffolds consisting of nuclear DNA studded with granule proteins, histone, and cytoplasmic antimicrobials, playing a key role in host defense against bacterial infections.47 However, the role of NETosis in tissue injury has not been extensively investigated and remains obscure.48 A recent study showed that development of NET contributes to ischemia/reperfusion-induced liver injury via the TLR4- and TLR9-dependent mechanisms.10 However, whether NETosis and NETs also exacerbate APAP-induced liver injury has not been examined and remains unknown. In the current study, we compared the development of NETs in the livers from APAP-treated WT and miR-223KO mice by measuring citrullination of histone 3 (Cit-H3 staining), which is an important step of NETosis.49 Our data showed that hepatic Cit-H3 staining and protein expression were similar between APAP-treated WT and miR-223 KO mice (Supporting Fig. 16). In addition, in vitro experiments revealed that there were no differences in NET release from PMA-stimulated WT and miR-223KO neutrophils (Supporting Fig. 17). These findings suggest that miR-223 may not affect NET formation and NETosis post APAP injection.
Another interesting finding in the current study was that CYP2E1 upregulation was found in miR-223KO mice compared with WT mice after overnight-fasting. Regulation of CYP2E1 protein expression is quite complex at both transcriptional and post-translational levels and can be influenced by several inflammatory cytokines and transcription factors, including β-catenin,30 NF- κB31 and HNF-1α.32 Among these transcription factors, only β-catenin protein expression was upregulated in miR-223KO mice but not in WT mice after overnight-fasting. These results suggest that deletion of miR-223 increases hepatic expression of β-catenin, followed by the stimulation of CYP2E1 expression after overnight-fasting. However, whether other signaling pathways or factors involved in CYP2E1 regulation in miR-223KO mice after overnight-fasting needs to be further studied. Interestingly, without fasting, hepatic expression of CYP2E1 protein was comparable in WT and miR-223KO mice. The mechanisms by which CYP2E1 protein was upregulated in miR-223KO mice after fasting but not altered in these mice without fasting remain unknown. Interestingly, a recent study showed that hepatic CYP2E1 protein expression is similar in naïve WT and NKT cell-deficient mice, but it is significantly higher in NKT cell-deficient mice than WT mice upon fasting.50 This is probably because compared to WT mice, NKT cell-deficient mice produced much higher levels of ketone bodies that upregulate hepatic expression of CYP2E1 protein.50 Further studies are needed to identify whether ketone body production is also involved in the upregulation of hepatic CYP2E1 protein expression in miR-223 KO mice post starvation.
In summary, on the basis of our findings, we propose a working model in Fig. 8E that neutrophil-specific miR-223 may play a critical role in the control of APAP-induced liver injury by attenuating the inflammatory responses of neutrophils. Thus, miR-223 may be a potential therapeutic target for the treatment of APAP-induced liver damage.
Supplementary Material
Acknowledgments
The authors thank Dr. Young-Eun Cho from the Laboratory of Membrane Biochemistry and Biophysics at the NIAAA, NIH for providing help to measure CYP2E1 activity.
Abbreviations
- ALT
alanine aminotransferase
- APAP
acetaminophen
- AST
aspartate aminotransferase
- CCR
C-C chemokine receptor type
- Con A
Concanavalin A
- CYP2E1
cytochrome P450 2E1
- GSH
glutathione
- H&E
hematoxylin and eosin
- 4-HNE
4-Hydroxynonenal
- ICAM-1
intercellular adhesion molecule 1
- iNOS
inducible nitric oxide synthase
- MCP-1
monocyte chemotactic protein 1
- MIP
macrophage inflammatory protein
- miR-223
microRNA-223
- miR-223KO
miR-223 knockout
- MPs
microparticles
- MPO
myeloperoxidase
- mtDNA
mitochondrial DNA
- NAPQI
N-acetyl-p-benzoquinone-imine
- NETs
neutrophils extracellular traps
- ROS
reactive oxygen species
- TLR9
Toll-like receptor 9
- VCAM-1
vascular cell adhesion molecule 1
- WT
wild-type
Footnotes
Author contributions: YH designed and performed experiments, analyzed data and wrote the paper. DF, ML, YG, TR, HC, SK, YY, YC performed experiments and analyzed data. CJ, HW, JL analyzed data and edited the paper. BG designed experiments, analyzed data and wrote the paper.
Conflict of interest: All authors declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- 1.Jaeschke H. Acetaminophen: Dose-Dependent Drug Hepatotoxicity and Acute Liver Failure in Patients. Dig Dis. 2015;33:464–71. doi: 10.1159/000374090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013;17:587–607. viii. doi: 10.1016/j.cld.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- 4.Mossanen JC, Krenkel O, Ergen C, Govaere O, Liepelt A, Puengel T, et al. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology. 2016;64:1667–1682. doi: 10.1002/hep.28682. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundback P, Lea JD, Sowinska A, Ottosson L, Furst CM, Steen J, et al. A novel high mobility group box 1 neutralizing chimeric antibody attenuates drug-induced liver injury and postinjury inflammation in mice. Hepatology. 2016;64:1699–1710. doi: 10.1002/hep.28736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–14. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques PE, Oliveira AG, Pereira RV, David BA, Gomides LF, Saraiva AM, et al. Hepatic DNA deposition drives drug-induced liver injury and inflammation in mice. Hepatology. 2015;61:348–60. doi: 10.1002/hep.27216. [DOI] [PubMed] [Google Scholar]
- 9.Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012;56:1971–82. doi: 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600–14. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaker ME, Trawick BN, Mehal WZ. The novel TLR9 antagonist COV08-0064 protects from ischemia/reperfusion injury in non-steatotic and steatotic mice livers. Biochem Pharmacol. 2016 doi: 10.1016/j.bcp.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–32. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roh YS, Zhang B, Loomba R, Seki E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am J Physiol Gastrointest Liver Physiol. 2015;309:G30–41. doi: 10.1152/ajpgi.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859–64. doi: 10.1172/JCI83885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou X, Hao X, Zheng M, Xu C, Wang J, Zhou R, et al. CD205-TLR9-IL-12 axis contributes to CpG-induced oversensitive liver injury in HBsAg transgenic mice by promoting the interaction of NKT cells with Kupffer cells. Cell Mol Immunol. 2016 doi: 10.1038/cmi.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DG, Antoine DJ, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54:1767–76. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 18.Ward J, Kanchagar C, Veksler-Lublinsky I, Lee RC, McGill MR, Jaeschke H, et al. Circulating microRNA profiles in human patients with acetaminophen hepatotoxicity or ischemic hepatitis. Proc Natl Acad Sci U S A. 2014;111:12169–74. doi: 10.1073/pnas.1412608111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antoine DJ, Dear JW, Lewis PS, Platt V, Coyle J, Masson M, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–87. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–57. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106:4402–7. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125–9. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 23.Aziz F. The emerging role of miR-223 as novel potential diagnostic and therapeutic target for inflammatory disorders. Cell Immunol. 2016;303:1–6. doi: 10.1016/j.cellimm.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Dorhoi A, Iannaccone M, Farinacci M, Fae KC, Schreiber J, Moura-Alves P, et al. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest. 2013;123:4836–48. doi: 10.1172/JCI67604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qadir XV, Chen W, Han C, Song K, Zhang J, Wu T. miR-223 Deficiency Protects against Fas-Induced Hepatocyte Apoptosis and Liver Injury through Targeting Insulin-Like Growth Factor 1 Receptor. Am J Pathol. 2015;185:3141–51. doi: 10.1016/j.ajpath.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong QW, Lung RW, Law PT, Lai PB, Chan KY, To KF, et al. MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology. 2008;135:257–69. doi: 10.1053/j.gastro.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Vickers KC, Landstreet SR, Levin MG, Shoucri BM, Toth CL, Taylor RC, et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc Natl Acad Sci U S A. 2014;111:14518–23. doi: 10.1073/pnas.1215767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47phox-oxidative stress pathway in neutrophils. Gut. 2016 doi: 10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–7. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 30.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–25. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 31.Zordoky BN, El-Kadi AO. Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr Drug Metab. 2009;10:164–78. doi: 10.2174/138920009787522151. [DOI] [PubMed] [Google Scholar]
- 32.Liu SY, Gonzalez FJ. Role of the liver-enriched transcription factor HNF-1 alpha in expression of the CYP2E1 gene. DNA Cell Biol. 1995;14:285–93. doi: 10.1089/dna.1995.14.285. [DOI] [PubMed] [Google Scholar]
- 33.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–30. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 34.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–76. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 35.Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–95. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- 36.Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, et al. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35:289–98. doi: 10.1053/jhep.2002.30956. [DOI] [PubMed] [Google Scholar]
- 37.Win S, Than TA, Min RW, Aghajan M, Kaplowitz N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology. 2016;63:1987–2003. doi: 10.1002/hep.28486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Israel A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol. 2010;2:a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T, Morgan MJ, Choksi S, Zhang Y, Kim YS, Liu ZG. MicroRNAs modulate the noncanonical transcription factor NF-kappaB pathway by regulating expression of the kinase IKKalpha during macrophage differentiation. Nat Immunol. 2010;11:799–805. doi: 10.1038/ni.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–4. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen-Jackson HT, Li HS, Zhang H, Ohashi E, Watowich SS. G-CSF-activated STAT3 enhances production of the chemokine MIP-2 in bone marrow neutrophils. J Leukoc Biol. 2012;92:1215–25. doi: 10.1189/jlb.0312126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di JM, Pang J, Sun QP, Zhang Y, Fang YQ, Liu XP, et al. Toll-like receptor 9 agonists up-regulates the expression of cyclooxygenase-2 via activation of NF-kappaB in prostate cancer cells. Mol Biol Rep. 2010;37:1849–55. doi: 10.1007/s11033-009-9620-5. [DOI] [PubMed] [Google Scholar]
- 44.Cavassani KA, Moreira AP, Habiel D, Ito T, Coelho AL, Allen RM, et al. Toll like receptor 3 plays a critical role in the progression and severity of acetaminophen-induced hepatotoxicity. PLoS One. 2013;8:e65899. doi: 10.1371/journal.pone.0065899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yohe HC, O’Hara KA, Hunt JA, Kitzmiller TJ, Wood SG, Bement JL, et al. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1269–79. doi: 10.1152/ajpgi.00239.2005. [DOI] [PubMed] [Google Scholar]
- 46.Williams CD, Bajt ML, Sharpe MR, McGill MR, Farhood A, Jaeschke H. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol. 2014;275:122–33. doi: 10.1016/j.taap.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Kockritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med (Berl) 2009;87:775–83. doi: 10.1007/s00109-009-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakazawa D, Kumar S, Desai J, Anders HJ. Neutrophil extracellular traps in tissue pathology. Histol Histopathol. 2017;32:203–213. doi: 10.14670/HH-11-816. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–13. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin-Murphy BV, Kominsky DJ, Orlicky DJ, Donohue TM, Jr, Ju C. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology. 2013;57:1575–84. doi: 10.1002/hep.26134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.