Abstract
Long-term prospective studies of the outcomes associated with HCV infection are rare and critical for assessing the potential impact of HCV treatment. Using liver biopsy as a start point, we looked at development of end stage liver disease (ESLD), hepatocellular carcinoma (HCC) and liver-related death (LRD) according to fibrosis stage, among a cohort of American Indian/Alaska Native persons in Alaska.
Persons were classified as having no/mild (Ishak=0,1), moderate (Ishak=2), or severe (Ishak=3,4) fibrosis or cirrhosis (Ishak=5,6). We examined time until development of ESLD, HCC and LRD and report survival probabilities at 3, 5, 7 and 10-years.
Of 407 persons, 39%(n = 150) had no/mild fibrosis, 32%(n = 131) had moderate fibrosis, 22%(n = 88) had severe fibrosis and 9%(n = 38) had cirrhosis. The average time of follow-up was 7.3 years. Within 5 years of biopsy, 1.7% (95% confidence interval (CI):0.4,6.8) of persons with none/mild fibrosis developed ESLD compared to 7.9% (CI:4.0,15.2), 16.4% (CI:9.6,27.2) and 49.0% (CI:33.0,67.7) with moderate, severe fibrosis, and cirrhosis, respectively (p<0.01). The 5-year outcome of HCC was 1.0% (CI:0.1,7.0), 1.0% (CI 0.1,6.6), 1.1% (CI:0.2,7.7) and 13.4% (CI:4.4,36.7) among persons with none/mild, moderate fibrosis, severe fibrosis and cirrhosis, respectively (p<0.01). Five years following biopsy, 0.0% (CI:0.0,14.8) of persons with none/mild fibrosis had suffered an LRD compared to 1.0% (CI:0.2,7.5) of persons with moderate fibrosis, 4.7% (CI:1.5,14.1) with severe fibrosis and 16.3% (CI:7.0,35.1) with cirrhosis (p<0.01).
Conclusion
For prevention of HCC, LRD and ESLD in the short-term, HCV therapy should target those with more than mild fibrosis.
Keywords: Liver Biopsy, Prospective Cohort, Outcomes, Survival Analysis, HCV Treatment
Introduction
Chronic hepatitis C virus (HCV) infection is a major cause of cirrhosis, hepatocellular carcinoma (HCC) and liver-related death (LRD). An estimated 140 to 170 million persons have been infected with HCV worldwide.(1, 2) In the United States (US), approximately 2 to 3 million persons have chronic HCV infection.(3) Since 1995, The Alaska Native Tribal Health Consortium Liver Disease and Hepatitis Program in collaboration with the Arctic Investigations Program and the Division of Viral Hepatitis of the Centers for Disease Control and Prevention (CDC) have been conducting a population-based prospective cohort study (AK-HepC) of liver-related adverse outcomes of chronic HCV infection in American Indian/Alaska Native (AI/AN) people (4, 5). Rates of HCV-associated HCC among AI/AN persons residing in Alaska have increased significantly since 1980 (6). All AI/AN persons residing in Alaska with positive tests for antibody to HCV plus and HCV RNA are invited to participate. The Alaska Tribal Health System is an integrated system of tribally-owned and operated health care organizations; over 90% of the AI/AN population in Alaska receive care through this system.
The paradigm for the optimal treatment of persons with chronic HCV infection shifted dramatically since the first direct-acting antiviral agents (DAAs) were approved by the FDA in 2011 (7). The standard of care for HCV treatment prior to DAA introduction was Peginterferon and ribavirin. The toxic side effect profile of Peginterferon and ribavirin often resulted in poor drug adherence and discontinuation of therapy (8). In addition, interferon-based therapies had low efficacy within our population and others, particularly in patients infected with genotype 1 (8). Thus, interferon-based therapies were typically reserved for persons with liver fibrosis (9). The high efficacy, tolerability and safety of DAAs has shifted the risk and benefit ratio associated with HCV treatment (7). The American Association for the Study of Liver Diseases (AASLD) practice guidelines now recommend DAA treatment for all HCV-infected persons, except those with a short life expectancy (7). Programs to screen/identify HCV-infected persons and linking those persons to DAA treatment can reduce and could eliminate chronic HCV infection within the developed and developing world.
Although the goal is to treat all HCV-infected persons, the high cost of DAA-therapy combined with the large number of HCV-infected persons might necessitate prioritizing treatment for persons with advanced fibrosis or those at risk for progression to advanced disease. However, it is unknown whether deferring HCV treatment in persons with less severe disease places them at elevated risk for adverse outcomes. Estimates of risk amongst those who have not yet undergone treatment for HCV infection in our cohort can provide guidance regarding timing and severity of liver disease in untreated persons as well as providing information on the number of cases averted as DAA use becomes more widespread. Following percutaneous liver biopsy, we determined the incidence of adverse outcomes including, end stage liver disease (ESLD), or liver failure, HCC and LRD (including liver transplant) based on the stage of liver fibrosis at time of biopsy.
Methods
All of the 130,000 AI/AN persons residing in the state of Alaska are eligible for healthcare with the Alaska Tribal Health System, an integrated system of tribally-owned and operated health care organizations.(4) The Liver Disease and Hepatitis Program provides care for AI/AN persons infected with HCV, by providing daily clinics at the Alaska Native Medical Center in Anchorage and regular field visits to all Tribal Hospitals and regional clinics statewide from Barrow to Ketchikan as well as telemedicine clinics. All AI/AN persons with HCV infection are entered into a registry for clinical management. Patients are recruited from this registry and consented to take part in a formal study of HCV liver disease outcomes. Patients are contacted by mail every 6 months and urged to see their healthcare provider to have blood drawn for a CBC, LFTs, and AFP. All patients identified with cirrhosis or advanced fibrosis are sent an additional letter every 6 months reminding them to obtain a liver ultrasound to screen for HCC. Since the onset of this study all patients have been offered the available FDA approved treatments for HCV at no cost to them. During the period of this analysis, 1994 through 2012, only interferon-based treatment regimens were available. To determine the extent of liver damage and better advise patients regarding the benefits and risks of antiviral therapy, liver biopsy was offered to all persons who were deemed to be good candidates for treatment based on clinical evaluation and presence or absence of other underlying physical conditions that would make interferon based therapy an acceptable risk. We censored participants who received antiviral therapy and had a sustained virological response (SVR) at the date they started therapy. Since 2012, when DAA therapy was licensed, we rarely perform liver biopsy and instead use elastography to determine fibrosis.
Persons with liver biopsy were the primary focus of analysis in this study and in persons with multiple liver biopsies, the most recent biopsy result was used. The Ishak fibrosis score was used to categorize persons into mild (Ishak = 0, 1), moderate (Ishak 2), or advanced fibrosis (Ishak = 3, 4) or cirrhosis (Ishak = 5, 6). Liver biopsies were read and scored for grade of fibrosis by both a staff pathologist and hepatologist. ESLD was defined by prospective clinical determination of one or more of the following: ascites, esophageal varices, hepatic encephalopathy, or coagulopathy (INR ≥1.2 or platelet count <130,000 on at least two occasions more than 1 month apart). The diagnosis of HCC was made by pathological confirmation or on radiographic imaging exam (CT or MRI) consistent with a diagnostically compatible mass lesion. LRD was defined as death with immediate cause from a complication of liver disease or death with liver disease listed as a contributing cause in medical chart review and death certificate and also included persons who underwent liver transplant. Outcomes are not mutually exclusive. Nine persons developed ESLD and one person developed HCC prior to their liver biopsy date and were removed from all analyses. Height and weight were obtained from medical records and obesity was defined as a body mass index ≥ 30 kg/m2. Alcohol use was collected through a standardized questionnaire and heavy alcohol use was defined as > 50 gm/day at the start of follow-up and analyzed in a dichotomous form. HCV infection length was estimated using each person’s history of blood transfusion, intravenous drug use and their anti-HCV results on historical serum; details were previously published (10). Persons co-infected with Hepatitis B Virus and/or HIV were removed from analyses.
Characteristics of persons who were in the liver biopsy cohort compared to all others were tested using the likelihood ratio chi-square test. The date of liver biopsy was the starting point in three separate survival models examining time until development of: 1) ESLD 2) HCC and 3) LRD. The end of follow-up was the date of detection of ESLD, HCC or LRD among those with an outcome. Among those without an outcome, the end of follow-up was the date of their last clinical follow-up or their date of death. For ESLD, we used the date of detection of their first ESLD condition. Kaplan-Meier estimates were used along with their 95% confidence intervals to estimate the probability of the 3 outcomes at 3, 5, 7 and 10 years following liver biopsy. Because of sample size limitations, 10-year estimates were not provided for persons with cirrhosis. P-values are reported using the log-rank statistic. We report univariate and multi-variable hazard ratios (HRs) adjusted for other risk factors, using a Cox Proportional Hazard model. Hazard ratios were adjusted for age, sex and other risk factors found to be significant in the development of these outcomes in a previous study (11). These included HCV genotype, heavy alcohol consumption and obesity. For calculation of hazard ratios, obesity, age, and heavy alcohol consumption at the time of liver biopsy were used. Survival risk estimates stratified by these other risk factors were presented in detail in a previous publication (11) and consequently, we report only the multi-variable hazard ratios from these adjusted models. To account for statistical differences in baseline characteristics between those persons in the biopsy cohort and those persons who were not biopsied but followed in the HepC-AK cohort, we reweighted the observations by the distribution of the significantly different risk factors for the entire cohort (obesity, heavy ETOH consumption, HCV RNA level). All analyses were performed using SAS version 9.3 (Cary, NC). All p-values are 2-sided and a value < 0.05 was considered statistically significant.
Laboratory Testing
Testing for anti-HCV was performed at the ANMC laboratory in Anchorage, AK by EIA2.0 [Abbott Laboratories]), and for HCV RNA by COBAS®Taqman®HCV Test, v2.0 [Roche Molecular Diagnostics]. HCV viral genotype was determined by real time polymerase chain reaction by Quest Diagnostics (Hepatitis C Genotype, LIPA®).
Human Subjects Research Review
The study was approved by the Alaska Area and CDC Institutional Review Boards. All participants provide written informed consent.
Results
Through 2012, 1,132 persons were found to have chronic HCV (HCV RNA positive) and 1080 (95.4%) agreed to participate in the study. The HepC-AK cohort was recruited from 1993 through 2012 with 50% of the cohort being recruited by the year 2000 and 75% by the year 2005. Among the 1080 study participants, 461 liver biopsies were performed for 407 participants. Characteristics of persons in the cohort who received a liver biopsy and those who did not undergo biopsy are shown in Table 1. Those in the liver biopsy cohort were more likely to be obese (p = 0.03), more likely to have an HCV RNA ≥ 5.0 × 105 IU/mL a (p = 0.02) and were less likely to report heavy alcohol use (p < 0.0001) that those who were not biopsied. The median and mean time between entry into the HepC-AK cohort and liver biopsy was 1.5 and 3.1 years, respectively. Among the 407 persons who received a biopsy, 39% (n = 150) had none or mild fibrosis; 32% (n = 131) had moderate fibrosis; 22% (n = 88) had severe fibrosis and 9% (n = 38) had cirrhosis. The average length of follow-up after liver biopsy was 7.3 years/person with a total of 2,973 years of follow-up. Patients were seen an average of 2.5 times per year over the course of their follow-up for this study. The average age at the time of biopsy was 44.2 years and 51% (n = 209) were female. At the time of liver biopsy, 58% (n = 231) had an HCV RNA ≥ 5.0 × 105 IU/mL, the mean length of HCV infection was 18.6 years, 44% (n = 178) were obese and 10% (n = 41) had heavy alcohol consumption There were 36 patients censored at the time they achieved a SVR after HCV treatment and 21 persons that died a non-liver related death.
Table 1.
Characteristics of those not in the liver biopsy cohort and the 407 study participants undergoing liver biopsy in the HepC-AK cohort, 1994–2012.
| Characteristic | Non-Liver Biopsy Cohort Participants (n = 673) | Liver Biopsy Cohort (n = 407) | Level of Liver Disease
|
||||
|---|---|---|---|---|---|---|---|
| Mild Fibrosis (n = 150) | Moderate Fibrosis (n = 131) | Severe Fibrosis (n = 88) | Cirrhosis (n = 38) | ||||
| Gender | % Female | 51% (n = 382) | 51% (n = 209) | 42% (n = 63) | 56% (n = 73) | 42% (n = 37) | 66% (n = 25) |
|
| |||||||
| Age | Mean (min, max) a | 41.2 (14, 82) | 41.2 (11, 70) | 39.5 (18, 70) | 41.0 (14, 65) | 42.9 (11, 64) | 44.0 (28, 62) |
|
| |||||||
| Residence | % in Urban Alaska b | 62% (n = 416) | 56% (n = 227) | 55% (n = 82) | 56% (n = 73) | 52% (n = 46) | 68% (n = 26) |
|
| |||||||
| HCV Genotype | 1 | 64% (n = 430) | 71% (n = 280) | 75% (n = 109) | 68% (n = 87) | 69% (n = 59) | 68% (n = 25) |
| 2 | 20% (n = 136) | 15% (n = 60) | 12% (n = 17) | 20% (n = 25) | 16% (n = 14) | 11% (n = 4) | |
| 3 | 15% (n = 99) | 14% (n = 54) | 12% (n = 17) | 12% (n = 16) | 15% (n = 13) | 22% (n = 8) | |
| 4 | 1% (n = 4) | 0.5% (n = 2) | 1% (n = 2) | 0% (n = 0) | 0% (n = 0) | 0% (n = 0) | |
|
| |||||||
| HCV RNA ≥ 5.0 × 105 IU/mL a, c | 48% (n = 317) d | 55% (n = 218) d | 52% (n = 76) | 43% (n = 55) | 33% (n = 28) | 46% (n = 17) | |
|
| |||||||
| Estimated HCV Infection Length a, e | 13.6 years | 15.7 Years | 13.7 Years | 15.2 Years | 18.2 Years | 19.2 Years | |
|
| |||||||
| Risk Factor | IVDU f | 56% (n = 375) | 59% (n = 239) | 60% (n = 90) | 58% (n = 76) | 55% (n = 48) | 66% (n = 25) |
| BT f | 9% (n = 64) | 14% (n = 57) | 11% (n = 16) | 12% (n = 16) | 23% (n = 20) | 13% (n = 5) | |
| Other | 35% (n = 234) | 27% (n = 111) | 29% (n = 44) | 30% (n = 39) | 23% (n = 20) | 21% (n = 8) | |
|
| |||||||
| Type II Diabetes | 9% (n = 61) | 12% (n = 50) | 5% (n = 7) | 15% (n = 20) | 15% (n = 13) | 26% (n = 10) | |
|
| |||||||
| Obesity (BMI ≥ 30) a | 37% (n = 194) d | 43% (n = 176) d | 39% (n = 59) | 41% (n = 54) | 53% (n = 47) | 42% (n = 16) | |
|
| |||||||
| Heavy Alcohol (> 50 gm/day) a | 19% (n = 125) d | 9% (n = 36) d | 7% (n = 10) | 11% (n = 14) | 9% (n = 8) | 11% (n = 4) | |
|
| |||||||
| Tobacco Smoker | 52% (n = 347) | 49% (n = 198) | 51% (n = 77) | 52% (n = 68) | 43% (n = 38) | 39% (n = 15) | |
|
| |||||||
| Positive for Hepatitis B Core Antibody | 19% (n = 128) | 25% (n = 103) | 23% (n = 35) | 26% (n = 34) | 25% (n = 22) | 32% (n = 12) | |
Characteristics measured at the time of entry into the HepC-AK cohort.
Cities with a population over 10,000.
13 participants were missing and HCV RNA level.
Characteristics with a statistically significant difference between persons in the biopsy cohort and all others.
Estimated length of HCV infection calculated on 90% (n = 961) of cohort.
IVDU is history of intravenous drug use; BT – history of a blood transfusion or blood product exposure before July 1992.
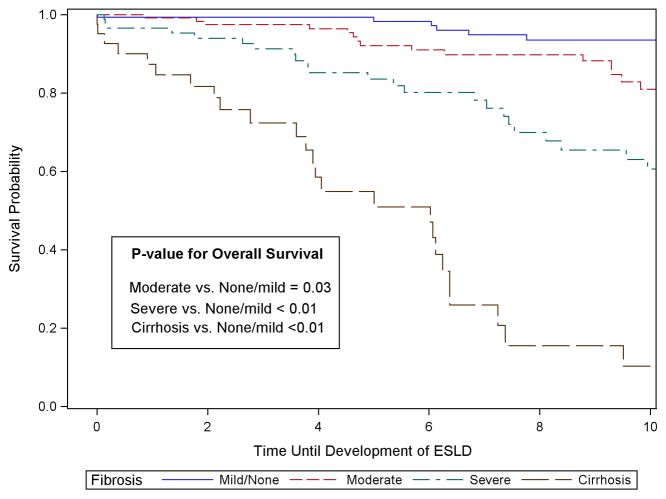
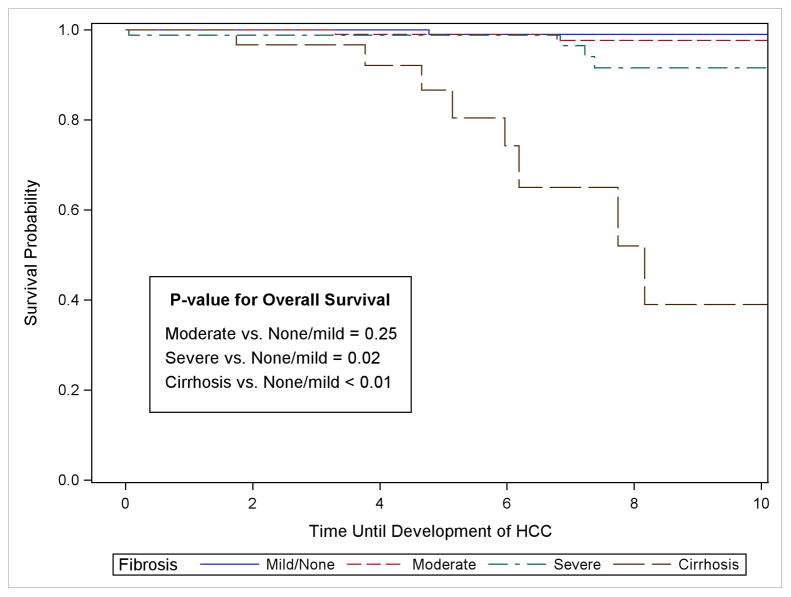
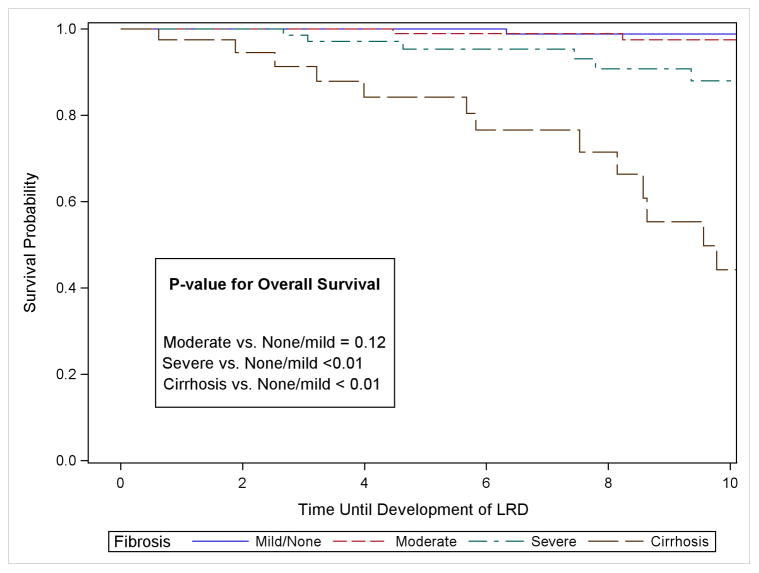
Among the 407 participants, there were 89 cases of ESLD during follow-up, 22 cases of HCC and 37 cases of LRD. For the 89 cases of ESLD, 26 occurred among cirrhotic patients, 31, 20, and 12 occurred among patient with severe, moderate and none-mild fibrosis, respectively. Table 2 displays the proportion of persons who developed ESLD, HCC and LRD by fibrosis stage at 3, 5, 7 and 10 years. The median time to the development of ESLD was 6.1 years following biopsy but ranged from 9.0 years among persons with none-mild fibrosis to a median of 3.9 years among cirrhotic patients. For the 22 HCC cases, 9 were detected among cirrhotic patients, with 7, 4, and 2 among patients with severe, moderate and none-mild fibrosis, respectively. The median time to detection of HCC was 7.0 years ranging from 6.0 among cirrhotic patients to 9.7 among patients with none-mild fibrosis. For LRD, 19 deaths occurred among cirrhotic patients followed by 13, 4 and 1 among patients with severe, moderate and none-mild fibrosis, respectively. The median time to LRD was 8.6 years following liver-biopsy.
Table 2.
Univariate probabilities for occurrence of end stage liver disease (ESLD), hepatocellular carcinoma (HCC), and liver-related death (LRD) for 3, 5, 7 and 10 years following liver biopsy in the HepC-AK cohort according to the biopsy-confirmed fibrosis stage. Ninety-five percent confidence intervals shown in parentheses followed by number at risk.
| Outcome | Time Period | Fibrosis Stage
|
|||
|---|---|---|---|---|---|
| None/Mild (Ishak 0–1) (n = 150) | Moderate (Ishak 2) (n = 131) | Severe (Ishak 3–4) (n = 88) | Cirrhosis (Ishak 5–6) (n = 38) | ||
| ESLD | 3-Year | 0.7% (0.1, 4.6) (n = 121) | 2.5% (0.8, 7.7) (n = 112) | 8.7% (4.2, 17.5) (n = 65) | 27.7% (15.8, 45.6) (n = 21) |
| 5-Year | 1.7% (0.4, 6.8) (n = 96) | 7.9% (4.0, 15.2) (n = 85) | 16.4% (9.6, 27.2) (n = 52) | 49.0% (33.0, 67.7) (n = 15) | |
| 7-Year | 5.1% (2.1, 12.1) (n = 80) | 10.2% (5.6, 18.2) (n = 75) | 23.9% (15.2, 36.4) (n = 40) | 74.1% (56.4, 88.9) (n = 9) | |
| 10-Year | 8.4% (4.0, 17.3) (n = 48) | 19.0% (11.6, 30.3) (n = 42) | 39.3% (27.6, 53.9) (n = 25) | ||
|
| |||||
| # of Cases | 12 | 20 | 31 | 26 | |
|
| |||||
| HCC | 3-Year | 0.0% (0.0, 3.2) (n = 123) | 0.0% (0.0, 3.4) (n = 116) | 1.1% (0.2, 7.7) (n = 68) | 3.3% (0.5, 21.4) (n = 29) |
| 5-Year | 1.0% (0.1, 6.9) (n = 98) | 1.0% (0.1, 6.6) (n = 89) | 1.1% (0.2, 7.7) (n = 56) | 13.4% (4.4, 36.7) (n = 18) | |
| 7-Year | 1.0% (0.1, 6.9) (n = 84) | 2.3% (0.6, 9.1) (n = 72) | 6.0% (1.9, 18.2) (n = 42) | 35.0% (16.5, 64.4) (n = 12) | |
| 10-Year | 1.0% (0.1, 6.9) (n = 52) | 4.6% (1.4, 4.8) (n = 45) | 8.4% (3.1, 21.6) (n = 28) | ||
|
| |||||
| # of Cases | 2 | 4 | 7 | 9 | |
|
| |||||
| LRD | 3-Year | 0.0% (0.0, 3.2) (n = 124) | 0.0% (0.0, 3.4) (n = 118) | 1.4% (0.2, 9.6) (n = 70) | 8.7% (2.9, 24.8) (n = 28) |
| 5-Year | 0.0% (0.0, 3.2) (n = 95) | 1.0% (0.2, 7.5) (n = 90) | 4.7% (1.5, 13.9) (n = 56) | 15.8% (6.8, 34.1) (n = 23) | |
| 7-Year | 1.2% (0.2, 8.1) (n = 84) | 1.0% (0.2, 7.5) (n = 76) | 6.9% (2.6, 17.6) (n = 46) | 23.4% (11.8, 43.4) (n = 20) | |
| 10-Year | 1.2% (0.2, 8.1) (n = 54) | 2.6% (0.6, 10.0) (n = 57) | 12.1% (5.4, 25.6) (n = 31) | ||
|
| |||||
| # of Cases | 1 | 4 | 13 | 19 | |
For ESLD, 3 years following liver biopsy, 27.7% (95% CI: 15.8, 45.6) of patients with cirrhosis had developed the outcome, followed by 8.7% (CI: 4.2, 17.5) of persons with severe fibrosis, 2.5% (CI: 0.8, 7.7) with moderate fibrosis, and 0.7% (CI: 0.1, 4.6) of those with none or mild fibrosis (Table 2). At 5-years post biopsy, the proportion with ESLD increased to 49% (CI: 33.0, 67.7) among those with cirrhosis, 16.4% (CI: 9.6, 27.2) with severe fibrosis and 7.9% (CI: 4.0, 15.2) with moderate fibrosis. After 10 years, the risk of ESLD had increased to 8.4% (CI: 4.0, 17.3) among persons with none-mild fibrosis, 19.0% (CI: 11.6, 30.3) for moderate and 39.3% (CI: 27.6, 53.9) for severe fibrosis. Due to sample size limitations, the 10-year risk was not estimated for cirrhotic patients, but at 7-years, the risk was already 74.1% (CI: 56.4, 88.9). Persons with cirrhosis and severe and moderate fibrosis all had statistically lower survival than those persons with none-mild fibrosis (Figure 1). Examining all the years of follow-up, those with cirrhosis had a hazard ratio (HR) of 19.1 (CI: 9.4, 39.0) compared to those with none-mild fibrosis (Table 3). After adjustment to make all the fibrosis groups similar in terms of age, gender, HCV genotype distribution, obesity and heavy alcohol consumption, the HR remained high at 17.1 (CI: 8.2, 35.5). The unadjusted and adjusted HR for those with severe fibrosis compared to those with none or mild fibrosis was 5.1 (CI: 2.6, 10.2) and 4.1 (CI: 2.0, 8.3), respectively. And for moderate fibrosis the hazard ratio dropped further to 2.0 (CI: 1.0, 4.3) and was no longer statistically significant after multi-variable adjustment, HR = 1.9 (CI: 0.9, 4.0).
Figure 1.
Table 3.
Univariate and multi-variable hazard ratios (HR) for the development of end stage liver disease (ESLD), hepatocellular carcinoma (HCC), and liver-related death (LRD) following liver biopsy in the HepC-AK cohort.
| Ishak Score | Fibrosis Stage | ESLD | HCC | LRD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Univariate HR (95% CI) | Multivariable HR a (95% CI) | Reweighted Multivariable HR b (95% CI) | Univariate HR (95% CI) | Multivariable HR a (95% CI) | Reweighted Multivariable HR b (95% CI) | Univariate HR (95% CI) | Multivariable HR a (95% CI) | Reweighted Multivariable HR b (95% CI) | ||
| 5–6 | Cirrhosis | 19.1 (9.4, 39.0) | 17.1 (8.2, 35.5) | 16.4 (7.9, 34.1) | 44.5 (8.9, 223.1) | 49.7 (9.0, 275.5) | 50.3 (8.5, 299.8) | 116.1 (15.0, 899.2) | 94.0 (11.9, 739.9) | 114.0 (12.0, 1082.3) |
|
| ||||||||||
| 3–4 | Severe | 5.1 (2.6, 10.2) | 4.1 (2.0, 8.3) | 4.1 (2.6, 8.5) | 5.4 (1.0, 26.7) | 4.1 (0.7, 24.1) | 3.7 (0.6, 24.0) | 21.4 (2.7, 167.1) | 13.6 (1.7, 110.0) | 18.7 (2.0, 177.3) |
|
| ||||||||||
| 2 | Moderate | 2.0 (1.0, 4.3) | 1.9 (0.9, 4.0) | 1.8 (0.9, 3.8) | 2.9 (0.5, 16.4) | 3.3 (0.5, 21.0) | 3.2 (0.5, 22.4) | 6.0 (0.7, 55.4) | 5.6 (0.6, 50.9) | 5.9 (0.5, 63.3) |
|
| ||||||||||
| 0–1 | None/Mild | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
Multi-variable HRs were adjusted for HCV genotype, age, sex, obesity, heavy alcohol consumption and HCV RNA ≥ 5.0 × 105 IU.
Reweighted HRs were calculated to account for differences between the persons in the biopsy cohort and all others in the HepC-AK cohort.
For hepatocellular carcinoma, 5 years following liver biopsy, the risk was low for persons with none-mild (1.0%), moderate (1.0%) and severe fibrosis (1.1%) (Table 2). However, in persons with cirrhosis, the 5-year estimates for HCC was 13.4% (CI: 4.4, 36.7). After 10 years, the risk remained low at 1.0% in persons with none-mild fibrosis, but had increased to 4.6% in persons with moderate fibrosis and 8.4% in persons with severe fibrosis. At 7 years, the risk for HCC among cirrhotic patients was 35.0% (CI: 16.5, 64.4). For development of HCC over the entire course of follow-up, persons with cirrhosis and severe fibrosis had significantly lower survival compared to those with none-mild fibrosis (Figure 1), but was not true for those with moderate fibrosis. The increased hazard for cirrhotic patients was 44.5 (CI: 8.9, 223.1) and after adjustment for other risk factors was 49.7 (CI: 9.0, 275.5) (Table 3). For persons with severe fibrosis, the HR was 5.4 (CI: 1.0, 26.7) compared to persons with none-mild fibrosis, but was no longer statistically significant after multivariable adjustment (HR = 4.1, CI: 0.7, 24.1).
Overall survival for the outcome of liver-related death (LRD) was again significantly lower for persons with severe fibrosis and cirrhosis compared to persons with none-mild fibrosis but was not the case for persons with moderate fibrosis (Figure 1). The risk of LRD remained low (<3.0%) for persons with none-mild and moderate fibrosis through 10 years of follow-up (Table 2). In persons with severe fibrosis, the risk of LRD increased to 12.1% (CI: 5.4, 25.6) at 10-years, and for cirrhotic patients, the risk was 23.4% (Ci: 11.8, 43.4) after 7 years of follow-up. The increased risk among cirrhotic patients was 116.1 fold (CI 15.0, 899.2) and 94.0 (CI 11.9, 739.9) before and after adjustment for other risk factors. For persons with severe fibrosis, the univariate and multi-variable HRs were 21.4 (CI 2.7, 167.1) and 13.6 (1.7, 110.0), respectively. Reweighted multi-variable HRs are shown in Table 3. They differed from the unweighted HRs by < 10% with the exception of the cirrhotic and severe fibrosis patients for the outcome of LRD which increased from 94.0 to 114.0 and from 13.6 to 18.7, respectively (Table 3).
Discussion
We examined three adverse outcomes of chronic HCV infection, ESLD (or hepatic decompensation), HCC and LRD separately to determine the incidence of each individually. We found that <2% of persons with no or minimal fibrosis (F 0–1) developed ESLD or HCC and no persons died of a liver-related death during the first 5 years of follow-up after liver biopsy, however by 10 years over 8% had developed ESLD. In contrast, half of persons with compensated cirrhosis developed ESLD, 13.4% HCC and 16% died of a liver-related death during the first 5 years. For persons with advanced fibrosis, Ishak 3–4, 16% developed ESLD, 5% LRD but only 1% with HCC five years out, but by 10-years, 39.3%, 8.4% and 12.1% had developed ESLD, HCC, and LRD, respectively. Persons with moderate fibrosis (Ishak 2, F-2) still had a considerable risk over 5-years, as 8% developed ESLD 1% HCC and 1% LRD. These elevated risks remained high after adjustment for other risk factors and for differences between our biopsied cohort and non-biopsied participants. An important finding of our study is that at 5 years after biopsy, only 1% of persons with either no or mild fibrosis, moderate or advanced fibrosis developed HCC but this rose to 13.4% in those with cirrhosis. This suggests that regular surveillance for HCC is crucial once a person develops cirrhosis but below this fibrosis level, screening may not be effective. Among persons with none-mild fibrosis, after 10 years, the risk of ESLD increased to just over 8% but remained low for the outcomes of HCC and LRD, at approximately 1.0%. This indicates that while treatment may be deferred for up to 5-years in those with no or mild fibrosis on biopsy, by 10-years nearly one in twelve developed liver failure so these persons should be treated in the near future.
During the period analyzed in this study, only interferon (IFN)-based therapy was available and 189 persons were treated.(8) At the time, only approximately 50% of patients eligible agreed to receive IFN-based treatment and most wanted to wait for less toxic direct acting antiviral agents (DAA) to be licensed because of the higher rates of sustained virologic response (SVR) and reduction in severity side effects. Since then, all patients have been offered DAA and over 300 persons have started treatment with an additional 200 evaluated and planned for treatment start in the very near future. While liver biopsy was an integral part of evaluating which persons would best benefit by treatment and which could be deferred during the period of this study, liver biopsy is infrequently used in our program currently. Instead, to satisfy third party payers and assess degree of fibrosis, we now use noninvasive markers of liver fibrosis such as vibration controlled transient elastography, FibroScan, to stage the extent of fibrosis. While the new DAAs have offered high rates of SVR (cure) with minimal side effects, the cost of many of these agents in the US is still high. As a result, insurers, including state run Medicaid programs, have restricted treatment to those with more than mild fibrosis. Indeed, in some US states, only persons with Metavir F4 or F3/F4 will be approved for treatment.(12)
The findings presented here regarding the risk of progression based on the stage of fibrosis is information urgently needed to prioritize those who need treatment immediately versus those who can be deferred with relative safety for a short period of up to 5 years. To our knowledge, only one other large cohort study recently published has investigated progression after liver biopsy.(13) This study by Xu, et al., from four large US integrated health systems, retrospectively examined the estimated risk for hepatic decompensation and HCC 5-years after liver biopsy in 2799 persons with chronic HCV infection. They found that 37% of persons with F4, 19.6% of F3, 4.7% of F2 and 2.3% of F0-F1 developed hepatic decompensation or HCC. While this was an important study showing the risk of ESLD and HCC, combined, after liver biopsy by fibrosis stage, this study was retrospective in nature and not strictly population-based. In addition, in calculating risk ratios, we adjusted for other contributing factors including alcohol consumption, obesity and HCV RNA levels. Our study was prospective in nature, as we carefully followed patients after liver biopsy to determine clinical outcome. Another strength of our study was that the same group of clinicians from our liver disease and hepatitis program followed participants and recorded information in the EHR in a standardized fashion; data in the Xu study were obtained from four large health care organizations consisting of many providers.
Limitations of our study are that the cohort includes only AI/AN persons and our findings may not apply to other racial/ethnic groups. However, we previously found that this cohort closely resembles the US population infected with HCV as found in the NHANES survey in terms of HCV genotype distribution and risk factors for infection (4). Therefore, we believe our rates of adverse events may be applicable to other populations. Our rates of adverse outcomes are higher, although not statistically significantly higher, than those found in the study by Xu et al. and could be related to the closer prospective follow-up after liver biopsy and standardized method of recording events. Furthermore, we have a program for surveillance for HCC with liver ultrasound every 6 months that enhances the detection of HCC early and subclinical cases are recorded in the incidence rates. The liver biopsy cohort only included 38% of the patients that are in the HepC-AK cohort, however when we reweighted the survival estimates to reflect the distribution of risk factors in the entire cohort, the hazard ratios remained relatively similar.
In conclusion, we determined the incidence of adverse events, ESLD, HCC and LRD over time after liver biopsy by stage of liver fibrosis. Our study shows that early treatment is crucial in those with cirrhosis and advanced fibrosis. Furthermore, we think that the risk we found for those with moderate fibrosis also justifies early treatment. In contrast, while in the ideal world, all persons with chronic HCV infection would be treated at time of diagnosis as recommended by current practice guidelines (7), we found that those with no or mild fibrosis could be deferred for up to 5 years, but the risk of ESLD rises substantially after that time and to defer treatment longer could be risky for these persons. Delay in treatment for those with mild fibrosis unfortunately may be necessitated if cost continues to be a major consideration, as is currently the case for many insurers in the US and elsewhere. However, deferment must be accompanied by careful frequent follow-up in order to detect any changes in clinical status to promptly initiate treatment if signs of advancing disease appear. Finally, our study has important findings regarding which patients would benefit most by regular surveillance by liver ultrasound. Our findings demonstrate that surveillance for HCC is crucial for those HCV infected persons with cirrhosis, but based on the low incidence, may not be justified in those with a lesser degree of fibrosis.
Acknowledgments
FUNDING SOURCE: This work was supported by, the Centers for Disease Control and Prevention [U01 PS001097, U01PS004113], and the NIH, National Institute of Allergy and Infectious Disease [AI0066209 and AI48214].
List of Abbreviations
- HCV
hepatitis C virus
- ESLD
end stage liver disease
- HCC
hepatocellular carcinoma
- LRD
liver-related death
- AI/AN
American Indian/Alaska Native
- AK-HepC
Hepatitis C Alaska Cohort Study
- CI
confidence interval
- DAAs
direct-acting antiviral agents
- AASLD
Association for the Study of Liver Diseases
- HRs
hazard ratios
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of the U.S. Centers for Disease Control and Prevention.
CONFLICT OF INTEREST STATEMENT: BS, CH, JP, JG and YB have received research funding from Gilead Sciences for other studies. No commercial funding was involved in this study.
References
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. NEJM. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 3.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The Increasing Burden of Mortality From Viral Hepatitis in the United States Between 1999 and 2007. Ann Intern Med. 2012;156:271–U233. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 4.McMahon BJ, Hennessey TW, Christensen C, Bruden D, Sullivan DG, Homan C, Deubner H, et al. Epidemiology and risk factors for hepatitis C in Alaska natives. Hepatology. 2004;39:325–332. doi: 10.1002/hep.20046. [DOI] [PubMed] [Google Scholar]
- 5.McMahon BJ, Bruden D, Bruce MG, Livingston S, Christensen C, Homan C, Hennessy TW, et al. Adverse Outcomes in Alaska Natives Who Recovered From or Have Chronic Hepatitis C Infection. Gastroenterology. 2010;138:922–931. doi: 10.1053/j.gastro.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 6.Connelly M, Bruce MG, Bulkow L, Snowball M, McMahon BJ. The Changing Epidemiology and Aetiology of Hepatocellular Carcinoma from 1969 through 2013 in Alaska Native People. Liv Intl. 2016:1–7. doi: 10.1111/liv.13173. [DOI] [PubMed] [Google Scholar]
- 7.Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015:932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 8.Livingston S, Townshend-Bulson LJ, Bruden D, Homan C, Gove JE, Plotnik JN, Spradling PR, et al. Results of Interferon-Based Treatments in Alaska Native and American Indian Persons with Chronic Hepatitis C. Int J of Circ Hlth. 2016:75. doi: 10.3402/ijch.v75.30696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, Management, and Treatment of Hepatitis C: An Update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruden D, McMahon B, Thomas T, Christensen C, Homan C, Williams J, Sullivan D, et al. Estimating the Date of Hepatitis C Virus Infection from Patient Interviews and Antibody Tests on Stored Sera. Am J of Gastroenterol. 2004;99(8):2031–8. doi: 10.1111/j.1572-0241.2004.30826.x. [DOI] [PubMed] [Google Scholar]
- 11.Hepatitis C Virus (HCV) Genotype 3 is an Independent Risk Factor for Advanced Fibrosis, End Stage Liver Disease (ESLD), Hepatocellular Carcinoma (HCC), and Liver-related Death (LRD): Alaska Hepatitis C Cohort Study (AK-HepC) McMahon BJ, Bruden D, Townshend-Bulson L, Simons B, et al. Clinical Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.10.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid Reimbursement of Sofosbuvir for the Treatment of Hepatitis C Virus Infection in the United States. Ann Intern Med. 2015;163:215–223. doi: 10.7326/M15-0406. [DOI] [PubMed] [Google Scholar]
- 13.Xu F, Moorman AC, Tong X, Gordon SC, Rupp LB, Lu M, Teshale EH, et al. All-Cause Mortality and Progression Risks to Hepatic Decompensation and Hepatocellular Carcinoma in Patients Infected With Hepatitis C Virus. Clin Infect Dis. 2016;62:289–297. doi: 10.1093/cid/civ860. [DOI] [PubMed] [Google Scholar]