Summary
Members of the actinomycete genus Streptomyces are non-motile, filamentous bacteria that are well known for the production of biomedically relevant secondary metabolites. While considered obligate aerobes, little is known about how these bacteria respond to periods of reduced oxygen availability in their natural habitats, which include soils and ocean sediments. Here we provide evidence that the marine streptomycete strain CNQ-525 can reduce MnO2 via a diffusible mechanism. We investigated the effects of hypoxia on secondary metabolite production and observed a shift away from the antibiotic napyradiomycin towards 8-amino-flaviolin, an intermediate in the napyradiomycin biosynthetic pathway. We purified 8-amino-flaviolin and demonstrated that it is reversibly redox-active (midpoint potential –474.5 mV), indicating that it has the potential to function as an endogenous extracellular electron shuttle. This study provides evidence that environmentally triggered changes in secondary metabolite production may provide clues to the ecological functions of specific compounds, and that Gram-positive bacteria considered to be obligate aerobes may play previously unrecognized roles in biogeochemical cycling through mechanisms that include extracellular electron shuttling.
Introduction
The actinobacterial genus Streptomyces is composed of nearly six hundred species (Labeda, 2011). These non-motile, spore-forming, high G-C Gram-positive bacteria grow as branching filaments that develop into vegetative mycelia. They inhabit heterogeneous environments, such as soils and marine sediments, and are known as plant endophytes, pathogens, and invertebrate mutualists (Seipke et al., 2012). They are considered obligate aerobes and require oxygen as a terminal electron acceptor to support growth (Van Keulen et al., 2007; Sawers et al., 2016). As one of the best-known sources of structurally diverse secondary metabolites, this genus has been heavily exploited by the pharmaceutical industry for the purpose of drug discovery (Watve et al., 2001; Berdy, 2005). Despite being the subject of intensive research, the ecological functions of most streptomycete secondary metabolites remain unknown.
The largely marine-derived streptomycete lineage ‘MAR4’ was originally described as part of a survey of marine actinomycetes (Fenical and Jensen, 2006). Members of this clade are enriched in the production of hybrid isoprenoid (HI) secondary metabolites (Gallagher et al., 2010; Gallagher et al., 2013). HIs are of mixed biosynthetic origin and are defined by the incorporation of at least one isoprene-derived terpenoid moiety into the chemical scaffold. These molecules can include structural features such as quinones and phenazines that make them redox-active and thus have the potential to function as “extracellular electron shuttles” as first reported in Shewanella (Newman and Kolter, 2000). Many HI secondary metabolites also resemble respiratory lipoquinones, which function in electron transport as part of primary metabolism.
Endogenous electron shuttles have been primarily studied in Gram-negative bacteria such as Shewanella and Pseudomonas (Hernandez and Newman, 2001; Rabaey et al., 2007) and are thought to be an adaptation to hypoxic or anoxic conditions. Shuttles allow bacteria to maintain intracellular redox homeostasis in low-oxygen conditions (Price-Whelan et al., 2006) or may connect respiratory quinone pools with extracellular electron acceptors during anaerobic respiration (Brutinel and Gralnick, 2012). In either case, small molecule mediated redox cycling can play a key role in bacterial survival under anoxic conditions and results in the reduction of insoluble extracellular electron acceptors, such as Mn(III,IV) oxides and ferric iron, both of which are prevalent in marine sediments. The ability to respire in the absence of oxygen by reducing a range of alternative electron acceptors such as Fe(III) and Mn(IV) has fundamentally changed the way microbial respiration is viewed (Ishii et al., 2013). This process not only has major implications for biogeochemical cycling (Newman and Kolter, 2000) but also has biotechnological applications for microbial fuel cells and the bioremediation of waters and sediments contaminated with organics, metals, and radionuclides (Von Canstein et al., 2008).
The best studied electron shuttles include flavins and phenazines produced by members of the genera Shewanella (Brutinel and Gralnick, 2012) and Pseudomonas (Wang et al., 2010; Glasser et al., 2014). In the case of P. aeruginosa, the redox-cycling of pigmented phenazine molecules promotes survival, but not growth, in anoxic conditions (Wang et al., 2010; Glasser et al., 2014). Much less is known about the prevalence and mechanisms of metal reduction in Gram-positive bacteria, although contact-dependent electron transfer has been reported (Marshall and May, 2009; Wrighton et al., 2011) and flavins implicated as soluble electron carriers (Dalla Vecchia et al., 2014; Wu et al., 2014) in the few studies targeting these bacteria. To the best of our knowledge, endogenous electron shuttles have not been reported from Gram-positive bacteria that are considered to be obligate aerobes. The redox-active structural features of MAR4 secondary metabolites raise the intriguing possibility that some may play a role in this process.
Streptomycetes in marine sediments and terrestrial soils are likely to encounter periods when oxygen is not available at sufficient levels to maintain a proton gradient. While considered obligate aerobes, some are known to survive periods of anaerobiosis as spores or during vegetative growth (Van Keulen et al., 2007). In S. coelicolor, there is evidence that nitrate respiration maintains spore membrane potential in anoxic conditions (Fischer et al., 2013). It has further been proposed that the induction of Nar3 synthesis in response to oxygen limitation may facilitate the use of nitrate as an alternative electron acceptor in streptomycetes (Falke et al., 2016), however the mechanisms by which vegetative mycelia survive hypoxia remain poorly understood (Van Keulen et al., 2007). Electron shuttling via redox-active secondary metabolites could provide an additional mechanism to facilitate streptomycete survival when oxygen becomes limited.
To identify streptomycete secondary metabolites that may enhance survival in hypoxic conditions, we anticipated their production would increase in response to reduced oxygen availability. To test this, we tracked secondary metabolite production in the MAR4 streptomycete CNQ-525 under oxic and hypoxic conditions. Strain CNQ-525 has previously been shown to produce several distinct classes of HIs, including a suite of prenylated napthoquinones called napyradiomycins, the latter of which display potent antimicrobial and cytotoxic activity (Soria-Mercado et al., 2005; Farnaes et al., 2013). To date, fourteen derivatives of this structural class, including nine new compounds, have been isolated from this strain (Soria-Mercado et al., 2005; Farnaes et al., 2013). The gene cluster responsible for napyradiomycin biosynthesis (the nap pathway) has been described in detail and contains a type III polyketide synthase that is involved in the biosynthesis of the napthoquinone core, a set of genes involved in the biosynthesis and attachment of the terpene component, and several halogenases that attach bromine or chlorine substituents (Winter et al., 2007).
Here we report that Streptomyces strain CNQ-525 is able to reduce MnO2 in the absence of direct cell contact implicating the involvement of a diffusible compound. When grown in low-oxygen conditions, napyradiomycin production decreased while a large increase was observed in another metabolite, which was isolated and identified as 8-amino-flaviolin. This compound is an intermediate in napyradiomycin biosynthesis and was shown to be redox-active making it a candidate electron shuttle. These results demonstrate that the products of a single biosynthetic pathway can shift in response to changing environmental conditions and that the potential functions of some streptomycete secondary metabolites may include enhanced survival when oxygen becomes less readily available.
Results
Extracellular MnO2 reduction
Strain CNQ-525 produces a number of compounds with structural features that suggest they are redox active (Soria-Mercado et al., 2005). It was therefore selected to test the concept that streptomycetes are capable of reducing an extracellular oxidant in the absence of direct cell contact. Strain CNQ-525 was inoculated onto an agar overlay that was physically separated by a 0.2 μm filter from a layer of similar medium containing 50 mM MnO2. Oxidized manganese was selected because Mn(IV) reduction represents an important mechanism for the oxidation of organic compounds in marine environments (Lovley, 1991) and appears as a black solid that becomes colorless and soluble following reduction. Strain CNQ-525 reduced MnO2 as evidenced by the clearing of the black, insoluble material in agar plates (Fig. 1). The fact that the culture was physically separated from the MnO2 indicates that strain CNQ-525 can reduce MnO2 via a diffusible mechanism. These tests were repeated using a medium buffered to pH 7 and similar clearing zones were detected, suggesting they were not simply due to pH effects. To the best of our knowledge, the production of a diffusible compound capable of reducing MnO2 has not previously been reported from any Gram-positive bacteria that are considered to be obligate aerobes.
Fig. 1.
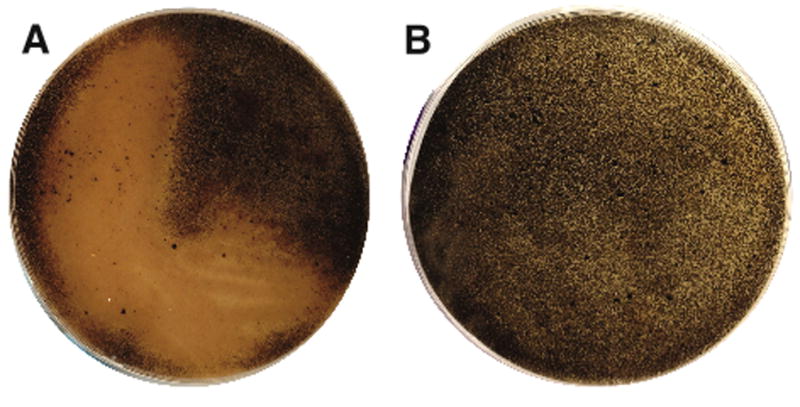
Manganese reduction assay. (A) Strain CNQ-525 reduces MnO2 resulting in a clearing zone around the growing bacteria (underside of plate shown). (B) E. coli DH5α (negative control) shows no evidence of MnO2 reduction despite similar levels of growth (not visible on underside of plate). The cultures are physically separated from the MnO2 by a filter placed below an agar overlay with clearing zones taking a minimum of three weeks to develop.
Secondary metabolite production in oxic and hypoxic conditions
We anticipated that the production of secondary metabolites that enhance survival under hypoxic conditions would increase when oxygen availability was reduced. To test for the effects of oxygen concentration on compound production, Streptomyces sp. CNQ-525 was grown in a bioreactor where dissolved oxygen levels could be carefully controlled. Upon reaching stationary phase following batch growth in 20% of air saturation (approximately 41 μM O2), fresh medium was added to the vessel at a constant dilution rate such that complete volume turnover occurred every 22 hours. Two replicate samples were then taken 24 hours apart to ensure the independence of the measurements. The oxygen concentration was then reduced to 5% of air saturation (approximately 10 μM O2) by increasing the percent nitrogen in the gas mixture and two additional samples taken at least 24 hours apart. The average biomass per unit volume during oxic and hypoxic conditions was not significantly different (p=0.74, paired t-test) (Fig. S1), suggesting that lowering oxygen did not negatively affect CNQ-525 growth. However, there was clear visual evidence for increased production of a purple pigment in the hypoxic conditions (Fig. S2).
Ethyl acetate extracts of strain CNQ-525 grown in steady state oxic conditions yielded numerous compounds including three that were identified as napyradiomycins based on their UV absorption profiles (Fig. 2). A reduction in oxygen concentration in the culture vessel was associated with a large increase in the concentration of one metabolite that strongly absorbed UV light at 540 nm, as would be expected for the purple pigment observed in the bioreactor cultures. In addition, three peaks with UV spectra that matched to napyradiomycins were dramatically reduced in hypoxic conditions. These differences between oxic and hypoxic conditions were consistent in four independent bioreactor runs.
Fig. 2.
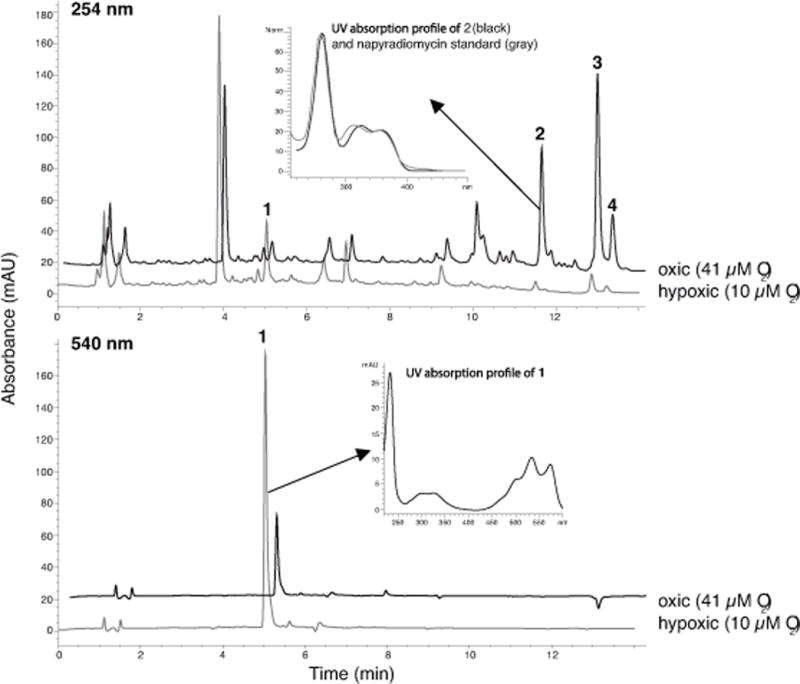
Secondary metabolite production. Representative HPLC traces for crude extracts derived from equal volumes of strain CNQ-525 grown in oxic (black) and hypoxic (gray) conditions measured at 254 and 540 nm. The UV absorption profile for the compound associated with peak 1, which increased in height in hypoxic conditions, is consistent with literature reports for 8-amino-flaviolin (Isogai et al., 2012). The heights of peaks 2–4 consistently decreased in hypoxic conditions and possess UV absorption profiles that closely match previously identified napyradiomycins (Norm = normalized to standard). Changes in the peak heights of 1–4 between hypoxic and oxic conditions were consistent among four individual bioreactor runs. The traces are offset by 1% in × and 10% in y in order to better visualize all peaks.
Isolation, identification, and quantification of 8-amino-flaviolin
The entire volume of the bioreactor vessel and all spent medium were extracted with ethyl acetate and the organic phase separated and solvent removed under vacuum. The compound associated with peak 1 was isolated using reversed-phase preparative HPLC with UV detection at 540 nm. The UV absorption profile of the purified compound (Fig. 2), a purple solid, was consistent with literature reports for 8-amino-2,5,7-trihydroxynapthalene-1,4-dione (8-amino-flaviolin), which was first reported as an intermediate in streptomycete hybrid isoprenoid biosynthesis (Isogai et al., 2012). The observed m/z [M–H]− of 220.0252 (Fig. S3) and associated molecular formula (C10H7NO5) for the purified compound is consistent with that calculated for 8-amino-flaviolin (m/z [M–H]− 220.0251). Furthermore, NMR analysis of the pure compound revealed 10 carbon signals and 2 aromatic proton signals with heteronuclear multiple-bond connectivity (HMBC) correlations that confirmed its structure as 8-amino-flaviolin (Table S1, Figs. S4–S8).
The relative concentrations of 8-amino-flaviolin and napyradiomycins in oxic and hypoxic conditions were determined by calculating the area under the curve for each peak per gram dry weight biomass. The resulting values for the three napyradiomycin peaks were summed to approximate total concentrations for this molecular class. When comparing the oxic and hypoxic conditions, there was a clear shift in production from napyradiomycin to 8-amino-flaviolin when oxygen concentrations were reduced (Fig. 3).
Fig. 3.
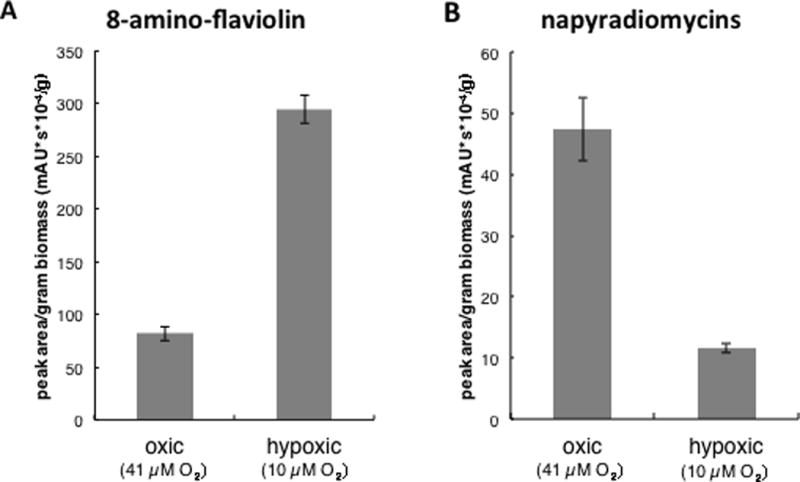
(A) 8-amino-flaviolin and (B) napyradiomycin production by strain CNQ-525 in oxic versus hypoxic conditions. Compound production for each condition is reported as the average of two samples taken 24 hours apart.
Biosynthesis of 8-amino-flaviolin
The structure of 8-amino-flaviolin was originally reported as a precursor in the biosynthesis of furaquinocin (Isogai et al., 2012), a hybrid isoprenoid structurally similar to the napyradiomycins. The three genes required for the biosynthesis of 8-amino-flaviolin were identified as fur1-3, which encode a type III polyketide synthase, a monooxygenase, and an aminotransferase, respectively (Isogai et al., 2012). The napyradiomycin (nap) pathway has been experimentally linked to napyradiomycin production (Winter et al., 2007) and contains the genes napB1-3, which share 72–83% amino acid sequence identity with fur1-3 (Fig. 4). A MultiGeneBlast (Medema et al., 2013) analysis of the CNQ-525 genome using fur1-3 as queries revealed that the only homologs in this strain occur in the nap pathway. These results provide bioinformatic support linking napB1-3 to the biosynthesis of 8-amino-flaviolin in strain CNQ-525 and further evidence that the output of a single biosynthetic pathway shifts in response to oxygen availability.
Fig. 4.
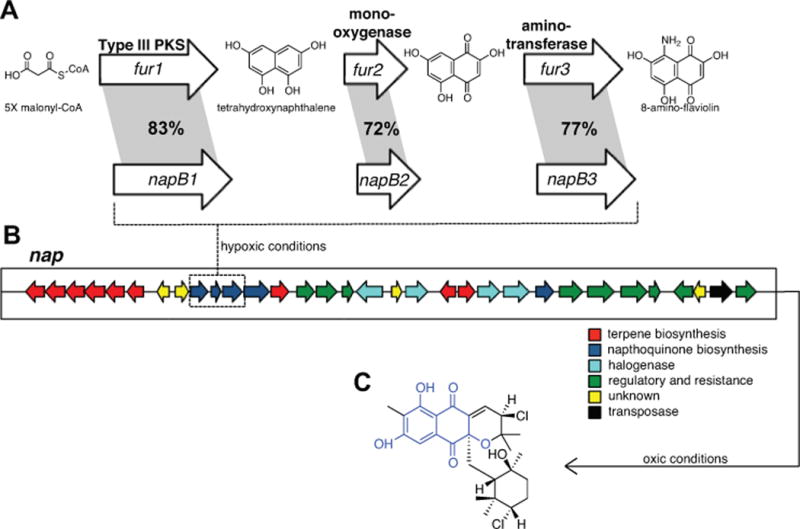
(A) NapB1-3 share a high level of amino acid sequence identity with fur1-3 from the furaquinocin pathway in Streptomyces sp. KO-3988, which is responsible for the biosynthesis of 8-amino-flaviolin. (B) Nap pathway in Streptomyces sp. CNQ-525 (modified from Winter et al., 2007). This pathway favors the biosynthesis of compounds in the napyradiomycin class in oxic conditions. The production of 8-amino-flaviolin is favored in hypoxic conditions in strain CNQ-525. (C) Representative structure of napyradiomycin, with moiety originating from 8-amino-flaviolin highlighted in blue.
Thermodynamic potential of 8-amino-flaviolin
Cyclic voltammetry was used to determine the redox activity of 8-amino-flaviolin. Reduction and re-oxidation peaks were observed in aqueous solution at pH 7 (Fig. 5), confirming that the compound is reversibly redox-active. The re-oxidation peak is slightly smaller in magnitude than the reduction peak, suggesting that the reaction is quasi-reversible. The midpoint between the reduction and re-oxidation peaks (the E1/2 of the molecule), which is approximately equal to the formal reduction potential, was found to be –474.5 mV versus the normal hydrogen electrode. Napyradiomycins were insufficiently water soluble to test for redox activity in aqueous solution.
Fig. 5.
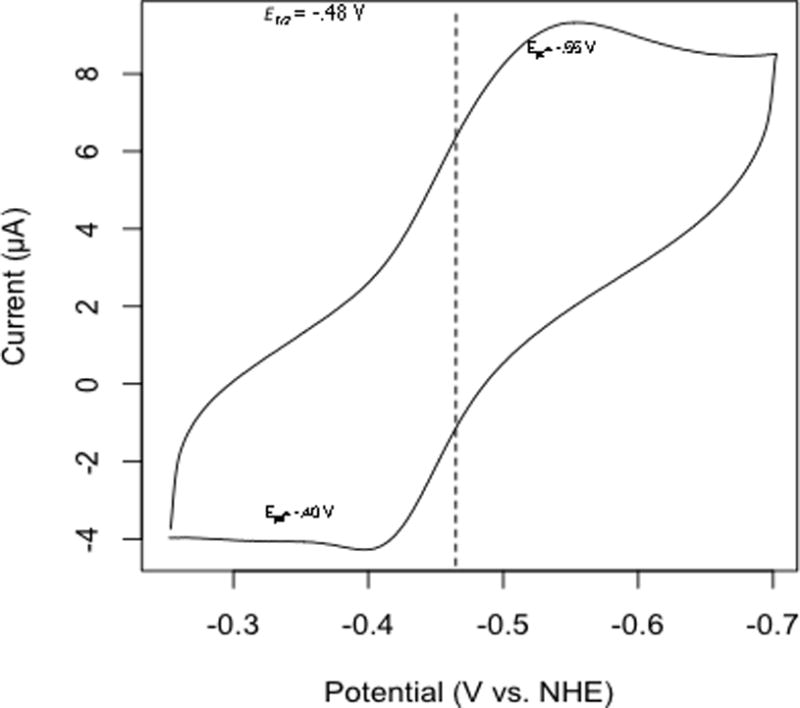
Cyclic voltammogram of 8-amino-flaviolin. The compound was dissolved in an electrolyte solution at pH 7. The E1/2 vs. normal hydrogen electrode (NHE) was calculated using potassium ferricyanide (E°′ = 436 mV vs. NHE at pH 7) as an internal standard (Clark, 1960).
Discussion
Thousands of secondary metabolites have been isolated from Streptomyces spp. (Watve et al., 2001), yet it is rare for these compounds to be connected to their ecological functions. This is largely due to the fact that most interest in these metabolites has been focused on their biomedical or industrial applications. Nonetheless, there is clear evidence that microbial secondary metabolites play important roles in processes such as iron acquisition (Saha et al., 2013), quorum sensing (Waters and Bassler, 2005), and allelopathy (Wietz et al., 2013), and may also play more fundamental roles in bacterial primary metabolism (Price-Whelan et al., 2006). This study took the initial steps towards addressing the hypothesis that environmentally triggered shifts in secondary metabolite production can be used to identify compounds that perform specific ecological functions. If these functions include enhancing the survival of obligate aerobes such as Streptomyces spp. in ocean sediments, it could help establish the mechanism by which these bacteria survive in a vegetative form when oxygen is not readily available (Van Keulen et al., 2007). It also raises the intriguing possibility that some compounds function as extracellular electron shuttles, a process that remains poorly studied among Gram-positive bacteria and, to the best of our knowledge, unknown among obligate aerobes possessing this cell wall type.
As a first step towards addressing the concept that streptomycetes produce compounds that function as extracellular electron shuttles, we demonstrated that the marine streptomycete strain CNQ-525 could reduce an extracellular substrate in the absence of direct cell contact. Although it has long been recognized that bacteria (Trimble and Ehrlich, 1968), including streptomycetes (Gomah et al., 1980; Nogueira et al., 2007), can reduce MnO2 under aerobic conditions, this process had not been linked to a diffusible compound. By physically separating CNQ-525 from the oxidant, we confirmed MnO2 reduction via a diffusible mechanism (Fig. 1). However, links between any specific compounds and this observation have yet to be established.
We next tested the concept that changes in secondary metabolism in response to reduced oxygen availability could help identify candidate electron shuttles. The results from bioreactor cultures of strain CNQ-525 revealed a shift away from the production of potent antibiotics in the napyradiomycin class in favor of 8-amino-flaviolin when oxygen concentrations were reduced. 8-amino-flaviolin is an intermediate in napyradiomycin biosynthesis and is not known to possess antibiotic activity, suggesting it is not being produced for defensive purposes. Hypoxic conditions are well documented along the Eastern Pacific continental margin seafloor (Helly and Levin, 2004) in locations proximate to the site from which strain CNQ-525 was isolated (Soria-Mercado et al., 2005). Thus, it is realistic to expect that bacteria in these habitats will experience periodic hypoxia events. The shift towards the production 8-amino-flaviolin suggests that this compound warrants further study as a candidate electron shuttle.
Extracellular electron shuttles have largely been studied in a limited number of Gram-negative bacteria (Rabaey et al., 2007). In an initial effort to determine if 8-amino-flaviolin has the requisite features of an electron shuttle, we confirmed using cyclic voltammetry that it is redox-active (Fig. 5). The large negative midpoint potential (-474.5 mV vs. NHE) however indicates it is not likely to be reduced by NADH (E°′= −320 mV vs. NHE), which is the proposed reductant of the phenazine shuttle in Pseudomonas aeruginosa (Glasser et al., 2014). If 8-amino-flaviolin were to function as a shuttle, it is possible that one of the hundreds of uncharacterized oxido-reductases observed in streptomycete genomes (Sawers et al., 2016) function as the reductant.
The reduction of oxidized manganese by reduced 8-amino-flaviolin is thermodynamically favorable, supporting the possibility that this compound could be acting as an extracellular oxidant and potentially as a shuttle. However, this strain also produces other compounds, and there was no evidence of a purple pigment in the clearing zone, so further experimental evidence is needed before a link between any specific compound and substrate reduction can be established. Failures to genetically manipulate strain CNQ-525 (pers. comm. B. Moore) and our inability to express the fur1-3 cassette in E. coli (kindly provided by T. Kuzuyama) have hindered these efforts. Napyradiomycins are poor shuttle candidates as their aqueous insolubility, which precluded testing using cyclic voltammetry, suggests they are unlikely to diffuse between cell and extracellular substrate.
The nap pathway yields secondary metabolites with measurably different properties depending upon oxygen availability, which is consistent with the hypothesis they have different ecological functions. While multiple ecological functions have been assigned to individual secondary metabolites (Schmitt et al., 1995; Kubanek et al., 2002), we are not aware of any examples in which a single biosynthetic pathway has yielded different secondary metabolites with biological activities that infer different ecological functions. This observation makes evolutionary sense as shifting the products of a single pathway to meet different environmental challenges conserves genetic resources and provides a rapid method of adaptation. It will be interesting to determine if similar shifts in production occur in other napthoquinone pathways that contain fur1-3 homologs such as the fnq pathway for furanonapthoquinone biosynthesis and the nph pathway for naphtherpin biosynthesis (Isogai et al., 2012), which would suggest a conserved role for 8-amino-flaviolin among napthoquinone producers.
It is well known that culture conditions can dramatically influence secondary metabolite production (Bode et al., 2002), however the ecological significance of these changes are seldom understood. Exceptions include the production of iron-chelating siderophores by streptomycetes, which are under the control of iron-dependent repressors (Flores and Martín, 2004) that ensure production only under iron-limited conditions. Additionally, biosynthetic gene clusters have been identified that produce both intermediates and the end products of the pathway. Examples include the spr pathway, which yields the polyketide intermediates salinipyrone and pacificanone as well as the macrolide rosamycin end product (Agarwal et al., 2015). A similar example is found in the rif pathway, which yields the intermediate saliniketal (Wilson et al., 2010) as well as rifamycin end products (Floss and Yu, 2005). To the best of our knowledge, the results presented here represent the first example in which a specific environmental cue triggers a change in the output of a single biosynthetic pathway.
It remains unclear if a shift in the nap pathway between 8-amino-flaviolin and napyradiomycin biosynthesis is the result of gene regulation or the oxygen requirements of certain enzymes. Based on a literature search, no characterized enzymes downstream of NapB1-B3 have a clear requirement for oxygen, but this possibility cannot be ruled out. Interestingly, the only enzyme in the nap pathway with a demonstrated O2 requirement is NapB2 (Fur2), the monooxygenase linked to 8-amino-flaviolin production (Funa et al., 2005). The increased production of this compound in hypoxic conditions, despite the requirement of molecular oxygen for one of its key biosynthetic enzymes, was unexpected with the most logical explanation being that concentrations remained sufficient to support enzyme function.
Though the functions of the vast majority of bacterial secondary metabolites remain unknown, they are increasingly being shown to play diverse ecological roles (Davies, 2006; Price-Whelan et al., 2006; Romero et al., 2011; Bernier and Surette, 2013). The present study provides a link between an environmental variable and plasticity in the products of a secondary metabolite biosynthetic pathway, and opens the possibility that individual pathways yield compounds with distinct ecological functions tailored to meet the most pressing environmental challenges. If these functions include adaptations to hypoxia, it could provide the mechanism by which streptomycetes survive in the absence of oxygen. The possibility that streptomycetes or other obligate aerobes produce secondary metabolites that function as extracellular electron shuttles suggests that the roles of these bacteria in ocean biogeochemical cycling warrants further study.
Experimental Procedures
Manganese reduction assay
Sterile MnO2 was prepared according to a published protocol (Tebo et al., 2007). Ten mM MnO2 was added to molten A1 agar medium (16 g agar per L) and 10 mL poured into 100 mm × 15 mm plates. An autoclaved 90 mm 0.2 μm filter (Whatman Nuclepore track-etched membrane) was placed on top of the solidified MnO2-containing agar. An additional 15 mL of MnO2-free molten A1 agar was then poured on top of the filter to further separate the bacteria from the MnO2. Strain CNQ-525 and E. coli DH5α (negative control) were streaked onto the surfaces of these plates and incubated at ambient laboratory temperature and oxygen concentrations for a minimum of three weeks before clearing zones became evident. Manganese reduction was observed as zones of clearing in the MnO2 layer. The agar below the filter was checked for sterility by plating following the assays. The assays were repeated using A1 media buffered with 10 mM 4-morpholinepropanesulfonic acid (MOPS). The pH was adjusted to 7 using HCl or NaOH. Following the assay, a pH strip was used to confirm that the pH in the zones of clearing remained neutral.
Chemostat culture
Streptomyces sp. CNQ-525 (16S rRNA accession number EF177816; NCBI Genome Taxonomy ID 418855) was isolated off the coast of La Jolla, California, USA (Soria-Mercado et al., 2005) and stored in 5% glycerol at –80°C. Prior to chemostat inoculation, CNQ-525 was inoculated from a frozen stock into 25 mL of A1 medium (10 g soluble starch, 4 g yeast extract, 2 g peptone, 750 mL seawater, 250 mL deionized water) and cultured at 27°C with shaking at 230 rpm for 7 days.
A 10 mL aliquot of the pre-culture was used to inoculate a BioFlo110 Fermentor (Eppendorf Inc., Enfield, CT, USA) containing 1 L of one-sixth strength A1 medium (1.67 g soluble starch, 0.67 g yeast extract, 0.33 g peptone, 750 mL seawater, 250 mL deionized water). A pH of 7 was maintained throughout the experiment (controlled with 1 M NaOH and 0.2 M H2SO4). The cultures were agitated (400 rpm) at 27°C with a 1.5 Lpm gas flow. dO2 was monitored using a polarographic oxygen sensor (Mettler Toledo, InPro 6800 series) and oxygen levels controlled by adjusting the mix of N2 and air bubbled into the culture. The vessel was inoculated at an O2 concentration of approximately 41 μM (20% of air saturation). Upon reaching stationary phase (approximately 48 h after inoculation), as indicated by a peak in oxygen consumption, fresh medium was added for a dilution rate of 0.045 h−1 (where dilution rate equals medium flow rate divided by culture volume).
While in continuous culture, two 175 mL samples were taken 24 hours apart at an oxygen concentration of approximately 41 μM (20% of air saturation). This time frame was sufficient for at least one complete volume turn over in the vessel and thus the samples can be considered replicates. The oxygen concentration was then lowered to approximately 10 μM (5% of air saturation) and, after equilibrating for at least 48 hours, two replicate 175 mL samples were taken. Dry weights were used to determine culture biomass from triplicate 3 mL sub-samples, which were filtered onto pre-weighed 47 mm glass fiber filters (Pall Life Sciences), dried overnight at 80°C, and weighed. A paired students t-test was implemented using the web tool available at http://www.graphpad.com/quickcalcs/ttest1.cfm. Approx. 10 μL of each sample was plated to ensure the purity of the culture. The remainder of each sample was frozen at –20°C prior to extraction and secondary metabolite analysis.
Secondary metabolite analysis
Each sample was divided into two equal volumes and extracted with 170 mL of ethyl acetate. The organic layer was separated and dried under vacuum. Extracts were analyzed by high performance liquid chromatography HPLC (Hewlett-Packard series 1100) using UV detection, a reversed-phase C18 column (4.6 mm × 100 mm; 5 μm pore size; Phenomenex Luna), and a solvent gradient from 10–100% CH3CN in water with 0.1% trifluoroacetic acid (TFA) with a flow rate of 1 mL/min. UV absorbance was monitored at 254 and 540 nm and the absorbance spectra associated with each peak compared to an in-house spectral library. A peak in the LC trace was assigned to the napyradiomycin class if it matched to a napyradiomycin standard with a match score >950 as calculated using the Agilent Technologies (Santa Clara, CA, USA) ChemStation software.
Compound purification and structure elucidation
Three 1 L bioreactor runs and the associated spent medium reservoirs (~7.5 L) were extracted with an equal volume of ethyl acetate at the end of each run. The combined crude extract was dissolved in methanol and the compound associated with the peak that increased in low-oxygen conditions (major peak in the 540 nm LC trace) was purified by isocratic HPLC (35% acetonitrile in water with 0.1% trifluoroacetic acid, 13 mL/min flow rate) using a Waters 600E multi-solvent delivery system and a reversed-phase C18 column (5 μm; Phenomenex Luna, 250 mm × 10 mm or 250 mm × 21.2 mm columns with 3 mL/min or 13 mL/min flow rates, respectively). The compound was isolated as a purple solid. 1H and 2D NMR spectra (HMBC, HSQC) were recorded at 500 MHz in DMSO-d6 (residual solvent referenced to 2.50 ppm) on a Varian Inova 500 MHz NMR spectrometer. 13C NMR spectra were recorded at 125 MHz in DMSO-d6 (referenced to 39.5 ppm) on a Varian VX 500 NMR spectrometer.
Quantification of relative compound production
Areas under HPLC peaks representing napyradiomycins and 8-amino-flaviolin were calculated based on absorbance measured at 254 and 540 nm, respectively. Peak integration was calculated using the ChemStation software. The values for replicate samples were calculated based on the average of the two subsamples. Compound production per unit biomass was then calculated for the average yields at each fermentation condition.
Electrochemical measurements
Cyclic voltammetry (CV) was performed using an Epsilon e2 potentiostat (BASi) with a scan rate of 100 mV/s. A 3 mm diameter BASi stationary voltammetry glassy carbon electrode was used as the working electrode. The counter electrode was a platinum wire and an Ag/AgCl wire was used as a pseudo-reference electrode. Electrochemical solutions were prepared as a 2 mM solution of 8-amino-flaviolin in a 1 M KNO3 aqueous electrolyte solution buffered with 10 mM 4-morpholinepropanesulfonic acid (MOPS). The pH was adjusted to 7 using HCl or NaOH and the solution sparged with N2 prior to data collection. The starting potential was -0.25 V (vs. NHE) and was scanned towards negative potentials. The redox potential was characterized by the half-wave potential and potassium ferricyanide (E1/2 = 436 mV vs NHE) (Clark, 1960) used as an internal standard.
Supplementary Material
Originality-Significance Statement.
This is the first investigation into the effects of hypoxia on secondary metabolism in bacteria that inhabit marine sediments. It provides evidence that the products of a single biosynthetic pathway shift in response to changes in an environmental variable. The ecological implications of these results are evaluated, laying the groundwork for future studies addressing the hypothesis that compounds produced by obligate aerobes enhance survival when oxygen becomes limiting, possibly by functioning as extracellular electron shuttles. The results provide new insight into the potential ecological functions of secondary metabolites produced by sediment-inhabiting marine bacteria.
Acknowledgments
This work was funded by the NIH under grant RO1GM085770 and the National Science Foundation under grant OCE-1235142 (to P.R.J.). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. K.A.G. was supported by the NIH Training Program in Marine Biotechnology under grant GM067550. Thanks to Dianne Newman, Nicholas Shikuma, and David Lacy for helpful discussions and assistance with the initial electrochemistry experiments. Nastassia Patin is acknowledged for helpful discussion.
References
- Agarwal V, Diethelm S, Ray L, Garg N, Awakawa T, Dorrestein PC, Moore BS. Chemoenzymatic synthesis of acyl coenzyme A substrates enables in situ labeling of small molecules and proteins. Org Lett. 2015;17:4452–4455. doi: 10.1021/acs.orglett.5b02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdy J. Bioactive microbial metabolites. A personal view. J Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- Bernier SP, Surette MG. Concentration-dependent activity of antibiotics in natural environments. Front Microbiol. 2013;4:20. doi: 10.3389/fmicb.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode HB, Bethe B, Höfs R, Zeeck A. Big effects from small changes: possible ways to explore nature’s chemical diversity. ChemBioChem. 2002;3:619–627. doi: 10.1002/1439-7633(20020703)3:7<619::AID-CBIC619>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Brutinel ED, Gralnick JA. Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Appl Microbiol Biotechnol. 2012;93:41–48. doi: 10.1007/s00253-011-3653-0. [DOI] [PubMed] [Google Scholar]
- Clark W. Advertisement: Clark’s oxidation reduction potentials of organic systems. Jf Phys Chem. 1960;64:192–192. [Google Scholar]
- Dalla Vecchia E, Suvorova E, Maillard J, Bernier-Latmani R. Fe (III) reduction during pyruvate fermentation by Desulfotomaculum reducens strain MI‐1. Geobiology. 2014;12:48–61. doi: 10.1111/gbi.12067. [DOI] [PubMed] [Google Scholar]
- Davies J. Are antibiotics naturally antibiotics? J Ind Microbiol Biotechnol. 2006;33:496–499. doi: 10.1007/s10295-006-0112-5. [DOI] [PubMed] [Google Scholar]
- Falke D, Fischer M, Sawers RG. Phosphate and oxygen limitation induce respiratory nitrate reductase 3 synthesis in stationary-phase mycelium of Streptomyces coelicolor A3 (2) Microbiol. 2016;162:1689–1697. doi: 10.1099/mic.0.000349. [DOI] [PubMed] [Google Scholar]
- Farnaes L, Coufal NG, Kauffman CA, Rheingold AL, DiPasquale AG, Jensen PR, Fenical W. Napyradiomycin derivatives, produced by a marine-derived actinomycete, illustrate cytotoxicity by induction of apoptosis. J Nat Prod. 2013;77:15–21. doi: 10.1021/np400466j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenical W, Jensen P. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- Fischer M, Falke D, Sawers RG. A respiratory nitrate reductase active exclusively in resting spores of the obligate aerobe Streptomyces coelicolor A3 (2) Mol Microbiol. 2013;89:1259–1273. doi: 10.1111/mmi.12344. [DOI] [PubMed] [Google Scholar]
- Flores FJ, Martín JF. Iron-regulatory proteins DmdR1 and DmdR2 of Streptomyces coelicolor form two different DNA-protein complexes with iron boxes. Biochem J. 2004;380:497–503. doi: 10.1042/BJ20031945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss H, Yu T. Rifamycin-mode of action, resistance, and biosynthesis. Chem Rev. 2005;105:621–632. doi: 10.1021/cr030112j. [DOI] [PubMed] [Google Scholar]
- Funa N, Funabashi M, Yoshimura E, Horinouchi S. A novel quinone-forming monooxygenase family involved in modification of aromatic polyketides. J Biol Chem. 2005;280:14514–14523. doi: 10.1074/jbc.M500190200. [DOI] [PubMed] [Google Scholar]
- Gallagher KA, Fenical W, Jensen PR. Hybrid isoprenoid secondary metabolite production in terrestrial and marine actinomycetes. Curr Opin Biotechnol. 2010;21:794–800. doi: 10.1016/j.copbio.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Gallagher KA, Rauscher K, Pavan Ioca L, Jensen PR. Phylogenetic and chemical diversity of a hybrid-isoprenoid-producing streptomycete lineage. Appl Environ Microbiol. 2013;79:6894–6902. doi: 10.1128/AEM.01814-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser NR, Kern SE, Newman DK. Phenazine redox cycling enhances anaerobic survival in Pseudomonas aeruginosa by facilitating generation of ATP and a proton‐motive force. Mol Microbiol. 2014;92:399–412. doi: 10.1111/mmi.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomah A, Soliman M, Abdel-Ghaffar A. Manganese mobility in Egyptian soils as affected by inoculation with manganese reducing organisms. Zeitschrift für Pflanzenernährung und Bodenkunde. 1980;143:274–282. [Google Scholar]
- Helly JJ, Levin LA. Global distribution of naturally occurring marine hypoxia on continental margins. Deep Sea Res. 2004;51:1159–1168. [Google Scholar]
- Hernandez M, Newman D. Extracellular electron transfer. Cell Mol Life Sci. 2001;58:1562–1571. doi: 10.1007/PL00000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Suzuki S, Norden-Krichmar T, Tenney A, Chain P, Scholz M, et al. A novel metatranscriptomic approach to identify gene expression dynamics during extracellular electron transfer. Nat Comm. 2013;4:1601. doi: 10.1038/ncomms2615. [DOI] [PubMed] [Google Scholar]
- Isogai S, Nishiyama M, Kuzuyama T. Identification of 8-amino-2, 5, 7-trihydroxynaphthalene-1, 4-dione, a novel intermediate in the biosynthesis of Streptomyces meroterpenoids. Bioorgan Med Chem Lett. 2012;22:5823–5826. doi: 10.1016/j.bmcl.2012.07.084. [DOI] [PubMed] [Google Scholar]
- Kubanek J, Whalen KE, Engel S, Kelly SR, Henkel TP, Fenical W, Pawlik JR. Multiple defensive roles for triterpene glycosides from two Caribbean sponges. Oecologia. 2002;131:125–136. doi: 10.1007/s00442-001-0853-9. [DOI] [PubMed] [Google Scholar]
- Labeda DP. Multilocus sequence analysis of phytopathogenic species of the genus Streptomyces. Inter J Syst Evol Microbiol. 2011;61:2525–2531. doi: 10.1099/ijs.0.028514-0. [DOI] [PubMed] [Google Scholar]
- Lovley DR. Dissimilatory Fe (III) and Mn (IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CW, May HD. Electrochemical evidence of direct electrode reduction by a thermophilic Gram-positive bacterium, Thermincola ferriacetica. Energy Environ Sci. 2009;2:699–705. [Google Scholar]
- Medema MH, Takano E, Breitling R. Detecting sequence homology at the gene cluster level with MultiGeneBlast. Mol Biol Evol. 2013;30:1218–1223. doi: 10.1093/molbev/mst025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DK, Kolter R. A role for excreted quinones in extracellular electron transfer. Nature. 2000;405:94–97. doi: 10.1038/35011098. [DOI] [PubMed] [Google Scholar]
- Nogueira M, Nehls U, Hampp R, Poralla K, Cardoso E. Mycorrhiza and soil bacteria influence extractable iron and manganese in soil and uptake by soybean. Plant and Soil. 2007;298:273–284. [Google Scholar]
- Price-Whelan A, Dietrich LE, Newman D. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol. 2006;2:71–78. doi: 10.1038/nchembio764. [DOI] [PubMed] [Google Scholar]
- Rabaey K, Rodriguez J, Blackall LL, Keller J, Gross P, Batstone D, et al. Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J. 2007;1:9–18. doi: 10.1038/ismej.2007.4. [DOI] [PubMed] [Google Scholar]
- Romero M, Martin-Cuadrado AB, Roca-Rivada A, Cabello AM, Otero A. Quorum quenching in cultivable bacteria from dense marine coastal microbial communities. FEMS Microbiol Ecol. 2011;75:205–217. doi: 10.1111/j.1574-6941.2010.01011.x. [DOI] [PubMed] [Google Scholar]
- Saha R, Saha N, Donofrio RS, Bestervelt LL. Microbial siderophores: a mini review. J Basic Microbiol. 2013;53:303–317. doi: 10.1002/jobm.201100552. [DOI] [PubMed] [Google Scholar]
- Sawers R, Falke D, Fischer M. Oxygen and nitrate respiration in Streptomyces coelicolor A3 (2) Adv Microbial Physiol. 2016;68:1–40. doi: 10.1016/bs.ampbs.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Schmitt TM, Hay ME, Lindquist N. Constraints on chemically mediated coevolution: multiple functions for seaweed secondary metabolites. Ecol. 1995:107–123. [Google Scholar]
- Seipke RF, Kaltenpoth M, Hutchings MI. Streptomyces as symbionts: an emerging and widespread theme? FEMS Microbiol Rev. 2012;36:862–876. doi: 10.1111/j.1574-6976.2011.00313.x. [DOI] [PubMed] [Google Scholar]
- Soria-Mercado I, Prieto-Davo A, Jensen P, Fenical W. Antibiotic terpenoid chloro-dihydroquinones from a new marine actinomycete. J Nat Prod. 2005;68:904–910. doi: 10.1021/np058011z. [DOI] [PubMed] [Google Scholar]
- Tebo B, Clement B, Dick G. Manual of Environmental Microbiology. Washington, DC: ASM Press; 2007. Biotransformations of manganese; pp. 1223–1238. [Google Scholar]
- Trimble R, Ehrlich H. Bacteriology of manganese nodules III. Reduction of MnO2 by two strains of nodule bacteria. Appl Microbiol. 1968;16:695–702. doi: 10.1128/am.16.5.695-702.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keulen G, Alderson J, White J, Sawers RG. The obligate aerobic actinomycete Streptomyces coelicolor A3 (2) survives extended periods of anaerobic stress. Environ Microbiol. 2007;9:3143–3149. doi: 10.1111/j.1462-2920.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- Von Canstein H, Ogawa J, Shimizu S, Lloyd JR. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol. 2008;74:615–623. doi: 10.1128/AEM.01387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kern S, Newman D. Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J Bacteriol. 2010;192:365–369. doi: 10.1128/JB.01188-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Watve MG, Tickoo R, Jog MM, Bhole BD. How many antibiotics are produced by the genus Streptomyces? Arch Microbiol. 2001;176:386–390. doi: 10.1007/s002030100345. [DOI] [PubMed] [Google Scholar]
- Wietz M, Duncan K, Patin N, Jensen P. Antagonistic interactions mediated by marine bacteria: The role of small molecules. J Chem Ecol. 2013;39:879–891. doi: 10.1007/s10886-013-0316-x. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Gulder TAM, Mahmud T, Moore BS. Shared biosynthesis of the saliniketals and rifamycins in Salinispora arenicola is controlled by the sare1259-encoded cytochrome P450. J Am Chem Soc. 2010;132:12757–12765. doi: 10.1021/ja105891a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JM, Moffitt MC, Zazopoulos E, McAlpine JB, Dorrestein PC, Moore BS. Molecular basis for chloronium-mediated meroterpene cyclization: cloning, sequencing, and heterologous expression of the napyradiomycin biosynthetic gene cluster. J Biol Chem. 2007;282:16362–16368. doi: 10.1074/jbc.M611046200. [DOI] [PubMed] [Google Scholar]
- Wrighton K, Thrash J, Melnyk R, Bigi J, Byrne-Bailey K, Remis J, et al. Evidence for direct electron transfer by a Gram-positive bacterium isolated from a microbial fuel cell. Appl Environ Microbiol. 2011;77:7633–7639. doi: 10.1128/AEM.05365-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Xiao Y, Wang L, Zheng Y, Chang K, Zheng Z, et al. Extracellular electron transfer mediated by flavins in gram-positive Bacillus sp. WS-XY1 and yeast Pichia stipitis. Electrochimica Acta. 2014;146:564–567. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


