Abstract
Objective
We hypothesized that the Oncotype Dx® 21-gene Recurrence Score (RS) could guide neoadjuvant systemic therapy (NST) to facilitate breast conserving surgery (BCS) for hormone receptor positive (HR+) breast cancers.
Methods
This study enrolled patients with HR+, HER2-negative, invasive breast cancers not suitable for BCS (size ≥ 2 cm). Core needle biopsy blocks were tested. For tumors with RS < 11, patients received hormonal therapy (NHT); patients with RS >25 tumors received chemotherapy (NCT); patients with RS 11–25 were randomized to NHT or NCT. Primary endpoint was whether 1/3 or more of randomized patients refused assigned treatment.
Results
Sixty-four patients were enrolled. Of 33 patients with RS 11–25, 5 (15%) refused assignment to NCT. This was significantly lower than the 33% target (binomial test, p=0.0292). Results for clinical outcomes (according to treatment received for 55 subjects) included successful BCS for 75% of tumors with RS < 11 receiving NHT, 72% for RS 11 – 25 receiving NHT, 64% for RS 11 – 25 receiving NCT, and 57% for RS > 25 receiving NCT.
Conclusions
Using the RS to guide NST is feasible. These results suggest that for patients with low or intermediate RS, NHT is a potentially effective strategy.
Keywords: Breast cancer, neoadjuvant, chemotherapy, hormonal therapy, gene expression
INTRODUCTION
Systemic therapy for early-stage breast cancer has contributed significantly to improving outcomes over the past 3 decades. More recently, it has been made clear that primary systemic therapy (neoadjuvant therapy) is equal to postoperative or adjuvant therapy in terms of disease-free and overall survival [1,2,3]. Although it was originally introduced as way to make locally advanced inoperable breast cancer operable, it has become an important tool for increasing the feasibility of breast conservation surgery (BCS) in women with operable, but large, breast cancers (relative to overall breast size). Neoadjuvant systemic therapy (NST) has been shown to facilitate BCS for large cancers [1,3,4,5]. While hormone receptor positive (HR+) cancers do respond to neoadjuvant chemotherapy (NCT), pathologic complete responses (pCR) are unlikely, reaching only 10–15% in most trials [4,6,7]. Neoadjuvant hormonal therapy (NHT), on other hand, may make BCS possible with less toxicity than NCT, and it has been shown to induce objective responses in more than two-thirds of selected patients with HR+ cancers [8,9,10]. A recent meta-analysis of studies including NHT concluded that NHT was equally effective for selected HR+ breast cancers as NCT [9]. However, particularly in the United States, NCT is the standard approach for appropriate patients and NHT is seldom utilized [11]. A review of data from the National Cancer Data Base found that from 2004 to 2012, less than 3% of patients 50 years of age or older who had T2–T4 HR+ breast cancers received NHT [12]. Even after the publication of the Z1031 trial data [8], the use of NHT has not changed significantly.
In addition to HR status and histopathologic tumor type, multiple studies have recently shown that gene expression profiles, either microarray-based or RT-PCR based, can predict prognosis as well as the likelihood of responsiveness to chemotherapy [13,14]. These assays were first developed and validated in analyses from legacy adjuvant trials or convenience sample cohorts of patients with long term follow-up and archived tissue. The 21-gene Oncotype DX® Breast Recurrence Score (RS) assay was developed by Genomic Health, Inc. for patients with HR-positive early-stage breast cancer [15,16]. This test provides an individualized RS result from 0 to 100, which correlates with the 10-year risk of distant recurrence as well as a risk category of low, intermediate, or high based on pre-specified cut points: low RS < 18; intermediate RS = 18–30; high RS ≥ 31. Furthermore, the RS result has been shown to predict the likelihood of benefitting from the addition of chemotherapy to hormonal therapy for HR+ tumors in the adjuvant setting [15,16]. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-20 trial (N=2299) showed a small overall benefit for the addition of chemotherapy to hormonal therapy in women with node-negative, HR+ breast cancer. In a subset of 651 patients, which accounted for about one fourth of the larger cohort, only patients with a high RS result showed a statistically significant reduction in the risk of distant recurrence, with an absolute reduction of 28% in the 10-year risk of distant recurrence with the addition of adjuvant chemotherapy. Prior to 2006 (when the Oncotype DX assay was formally incorporated), clinical guidelines such as the National Comprehensive Cancer Network (NCCN) and St. Gallen [17,18,19,20,21] recommended adjuvant chemotherapy be given to patients with HR+, node-negative, early-stage breast cancer, which may account for nearly half of all breast cancer patients in the United States [22,23]. However, based on the RS result, less than 10–15% of such patients would likely benefit from chemotherapy [24]. Indeed, after more than a decade, the 21-gene RS assay has become widely incorporated into clinical diagnostic work-ups by oncologists and their patients to inform decisions about adjuvant chemotherapy [16,25,26,27,28,29,30,31]. Additional data from the SWOG 8814 trial suggest that the RS may also predict the likelihood of benefit from chemotherapy, even for patients with nodal metastases [32].
Recently, there have been multiple small studies incorporating the RS in the neoadjuvant setting. These studies have shown that pCR or clinical complete response (cCR) to chemotherapy almost never occurs in patients with a low RS [13,14,14,33,34]. Furthermore, others have shown that clinical responses with NHT are more likely if the patients have a low RS [35,36]. This fits well with the finding from the NSABP B-14 study showing that a low RS predicted the greatest benefit from adjuvant treatment with tamoxifen as well as the results of the B-20 study showing the absence of benefit from chemotherapy in patients with a low RS [27,16]. While similar observations have also been seen with other tests, including the 70-gene profile and the PAM-50 assays, based on intrinsic subtypes, neither of these 2 assays has formally demonstrated that the assay result predicts the likelihood of chemotherapy benefit in any setting (adjuvant or neoadjuvant) [37,38,39]. One early report using the PAM-50 assay to define intrinsic subtype showed that there was some indication that luminal A patients had little chance of a pCR with chemotherapy, but still had a good prognosis; luminal B tumors, on the other hand, were also unlikely to respond well to chemotherapy, but had a worse prognosis [40,38].
Based on the clinical validation of the 21-gene RS assay to predict the likelihood of chemotherapy benefit in patients with ER+ early-stage breast cancer, we hypothesized that the 21-gene RS assay could be used to guide the decision to treat with NHT versus NCT to facilitate BCS, and we designed this pilot trial to evaluate the feasibility of using this approach in a larger multicenter trial.
METHODS
Patient eligibility
This prospective multi-center study enrolled female patients with HR+ (defined as > 10% tumor staining by immunohistochemistry [IHC]), HER2-negative (according to ASCO/CAP guidelines [41,42], invasive breast cancers (size ≥ 2 cm) which, in the opinion of the surgeon, were not suitable for BCS unless reduced in size by neoadjuvant therapy. Diagnosis had to be made by core needle biopsy. Other key eligibility requirements included: signed and dated IRB-approved consent form that conforms to federal and institutional guidelines; at least 18 years of age; an ECOG performance status of 0 or 1; and ipsilateral axillary lymph nodes evaluated by imaging (MRI or ultrasound) within 6 weeks prior to registration. If indicated for abnormal lymph nodes, fine needle aspirate (FNA) or core needle biopsy was performed. Subjects were evaluated by a treating physician, reviewed and discussed by the multi-disciplinary breast team, and considered to be a candidate for chemotherapy.
Patients were deemed ineligible for the following reasons: FNA alone to diagnose the primary tumor; excisional biopsy or lumpectomy performed prior to registration; surgical axillary staging procedure or sentinel node biopsy performed prior to registration; tumors clinically staged as inflammatory breast cancer; ipsilateral cN2b or cN3 disease (patients with cN1 or cN2a disease were eligible); definitive clinical or radiologic evidence of metastatic disease; synchronous or metachronous contralateral invasive breast cancer (patients with synchronous and/or metachronous contralateral ipsilateral ductal or lobular carcinoma in situ [DCIS or LCIS] were eligible); HER-2 test result of IHC 3+, regardless of FISH results, if performed; any history of ipsilateral invasive breast cancer or DCIS, if treated with radiation therapy; history of non-breast malignancies, except for in situ cancers treated only by local excision and basal cell and squamous cell carcinomas of the skin, within 5 years prior to registration; treatment including radiation therapy, chemotherapy, and/or targeted therapy for the currently diagnosed breast cancer prior to registration; cardiac disease (history of and/or active disease) that would preclude the use of chemotherapy; pregnancy or lactation at the time of registration; other non-malignant systemic disease that would preclude the patient from receiving study treatment or would prevent required follow-up; psychiatric or addictive disorders or other conditions that, in the opinion of the investigator, would preclude the patient from meeting the study requirements; or use of any investigational product within 30 days prior to registration.
Study design
The protocol was approved by each of the 7 local human investigations committees or IRBs, with assurances filed with and approved by the U.S. Department of Health and Human Services. Written informed consent was obtained from all individual participants included in the study.
The study schema is shown in Figure 1. Tissue blocks from the biopsies were sent to the Genomic Health laboratory (Clinical Laboratory Improvement Amendments certified) for RS testing according to standard procedures [16]. Treatment was assigned based on the RS result: patients with RS < 11 were to receive NHT; patients with RS ≥26 were to receive NCT; patients with midrange RS = 11–25 were randomized to NHT or NCT.
Fig. 1.
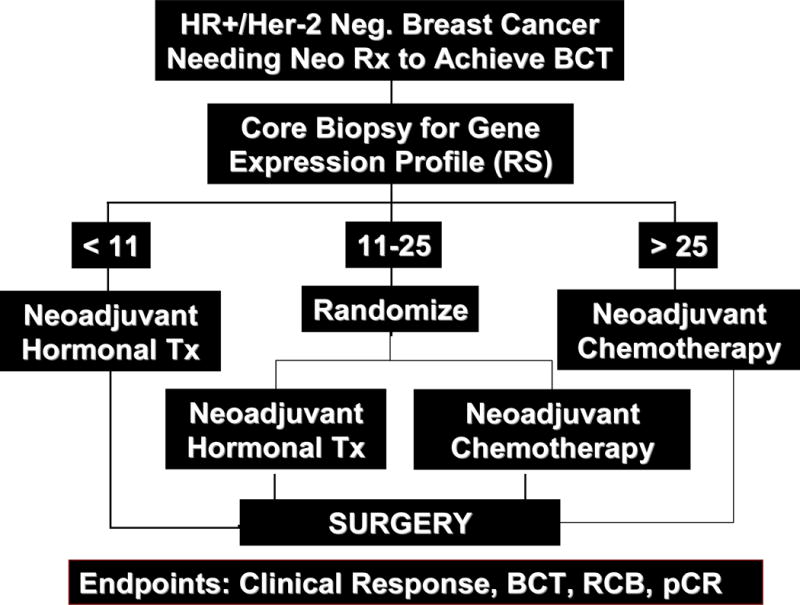
Protocol schema
The primary objective was determining the feasibility of randomizing patients with midrange RS values (11–25) between NHT and NCT. The primary endpoint was to determine whether one-third or more of randomized patients would refuse assigned treatment. Secondary endpoints included: clinical partial and complete response (cPR, cCR) rates using RECIST criteria by either ultrasound or physical exam (using the same method as was used at baseline), overall clinical response rates, pCR in the breast (defined as no histologic evidence of invasive tumor cells in the surgical breast specimen), pCR in the breast and nodes, and successful BCS (partial mastectomy with negative margins followed by radiation therapy).
Patients who were considered potential candidates for BCS were to have the primary tumor site marked in some way prior to initiating chemotherapy or, at least, prior to disappearance of the tumor clinically. This could be achieved with insertion of a radiopaque marker or clip, tattoos of the tumor boundary on the skin (especially for smaller breasts), or by making a transparent template with the tumor site marked on it. Other techniques were acceptable, as long as they provided assurance that the primary tumor site could be located and excised. If a clip was used, a specimen radiograph was to be performed to confirm its removal. For patients assigned to NHT, tamoxifen was used for pre-menopausal women and an aromatase inhibitor (choice of which one up to the investigator) for post-menopausal women. Treatment was to continue for 4 to 6 months, with at least monthly assessments of response, followed by surgery. If tumor progression was noted at any time, the patient could be taken to surgery immediately or switched to cytotoxic chemotherapy at the investigator’s discretion. For patients assigned to NCT, the regimen was chosen at the treating oncologist’s discretion, but in general an anthracycline/taxane based regimen, with 6 to 8 courses of therapy lasting 4 to 6 months was recommended. Response to therapy was to be assessed prior to each course of therapy. If tumor progression was noted at any time during the first therapeutic regimen, then the patient could either be switched to the second planned regimen or could be taken to surgery immediately. If progression occurred during the second regimen, the patient was generally taken to surgery.
Postoperative adjuvant therapy for all subjects was at the discretion of the investigator/treating physician. Adjuvant chemotherapy could be given, using any regimen considered appropriate, generally before irradiation or starting hormonal adjuvant therapy. For all patients, regardless of neoadjuvant therapy, hormonal therapy was to be administered after surgery, chemotherapy (if given), and radiation (if given) for a total of at least 5 years.
Statistical methods
To avoid possible confounding effects and ensure adequate balance between the 2 treatment groups (hormonal vs cytotoxic therapy), randomization was stratified by site. To avoid possible risk for selection bias, a blocked randomization scheme with randomly permuted block sizes (unknown to the investigators) was conducted. The randomization list was generated by the study biostatistician (WW). A random number generator was used to generate a random sequence of the 2 assignments. The treatment assignments were sealed in envelopes, ordered according to the sequence generated and placed securely in the research nurse’s office. A 1-sample binomial test was used to compare the observed refusal rate with one-third, along with its 95% CI. Fisher’s exact test, logistic regression (for a binary endpoint), and/or ordinal regression (for an ordinal endpoint) were used to compare the 4 treatment groups for the secondary endpoints [43]. To compare the 4 groups in terms of each of the clinical characteristics, the Fisher’s exact test (for race, menopausal status, T-stage, N-stage, ER, PR, and tumor grade) and the Kruskal-Wallis test (for age and baseline tumor size) were used. All statistical analyses were performed in SAS 9.4, and all statistical testing was 2-sided and considered significant if a P value was smaller than the type I error of 5%.
RESULTS
Seven U.S. and Canadian centers enrolled 64 patients: 5 were excluded (1 delay in RS result, 1 tissue block lost at participating center, 1 HR testing discrepancy, 2 deemed not eligible [1 for no pregnancy test and 1 for inadequate tissue for RS assay]). The CONSORT diagram is shown in Figure 2. Groups were defined as: A: RS < 11, treated with NHT; B: RS = 11–25, treated with NHT; C: RS = 11–25, treated with NCT; D: RS ≥ 26, treated with NCT. The distributions of RS values, using either the “standard” cutoffs (< 18, 18–30, and ≥ 31) or the study-specific cutoffs (<11, 11–25, ≥26), are shown in Figure 3. Patient and tumor characteristics are shown in Table 1, according to treatment actually received. Not surprisingly, patients in Group A had the highest percentage of low grade tumors (41.7%) and patients in Group D had the highest percentage of high grade tumors (14.3%). Groups C and D had similar percentages of low grade tumors (26.1 % and 23.1%, respectively) and high grade tumors (10.5% and 7.7%, respectively).
Fig. 2.
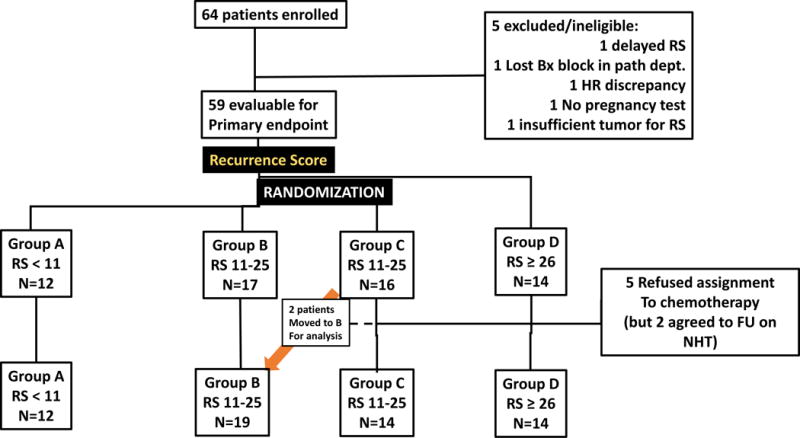
CONSORT diagram of subjects enrolled
Fig. 3.
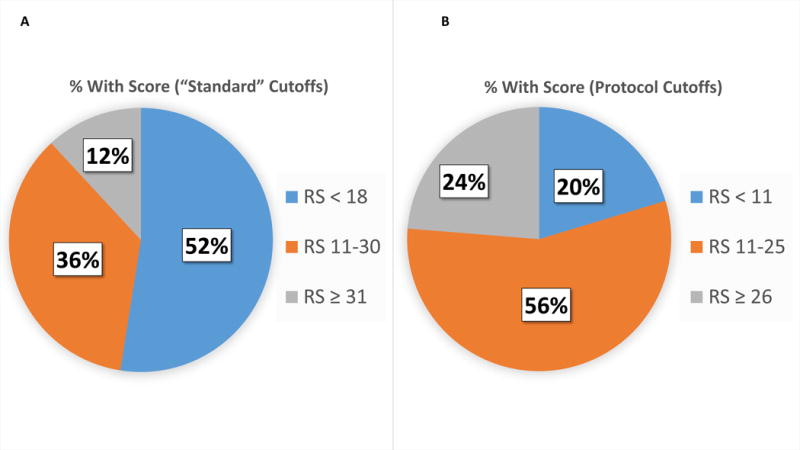
Distribution of patients according to their RS values: based on “standard” cutoffs (A) and cutoffs used to allocate patients in this study (B)
Table 1.
Demographic and clinical characteristics by treatment group
| Group | A N=12 |
B N=19 |
C N=14 |
D N=14 |
P value |
|---|---|---|---|---|---|
| Age | |||||
|
| |||||
| Median | 65 | 64 | 56.5 | 60 | 0.0370 |
| Range | (57, 76) | (41, 80) | (30, 75) | (48, 72) | |
|
| |||||
| Race | |||||
|
| |||||
| Caucasian | 58.3% | 94.7% | 78.6% | 85.7% | 0.0873 |
| African America | 41.7% | 5.3% | 21.4% | 14.3% | |
|
| |||||
| Menopausal status | |||||
|
| |||||
| Pre- | 0% | 15.8% | 38.5% | 7.1% | 0.0492 |
| Post- | 100% | 84.2% | 61.5% | 92.9% | |
| 1 missing | |||||
|
| |||||
| Tumor size (cm) | |||||
|
| |||||
| Median | 3.50 | 3.50 | 4.00 | 3.63 | 0.4529 |
| Mean (standard deviation) | 4.14 (1.53) | 3.59 (1.28) | 4.52 (1.93) | 4.03 (1.49) | |
| 1 missing | |||||
|
| |||||
| T stage | |||||
|
| |||||
| T1 | 0% | 0% | 7.7% | 0% | 0.8301 |
| T2 | 75.0% | 79.0% | 61.5% | 71.4% | |
| T3 | 25.0% | 15.8% | 30.8% | 38.6% | |
| T4 | 0% | 5.3% | 0% | 0% | |
| 1 missing | |||||
|
| |||||
| N stage | |||||
|
| |||||
| N0 | 83.3% | 73.7% | 69.2% | 78.6% | 0.9678 |
| N1 | 16.7% | 30.8% | 30.8% | 21.4% | |
| NX | 0% | 5.3% | 0% | 0% | |
| 1 missing | |||||
|
| |||||
| Hormone receptor status | |||||
|
| |||||
| estrogen receptor positive | 100% | 100% | 100% | 100% | 1.00 |
| progesterone receptor positive | 92.0% | 89.0% | 92.0% | 79.0% | 0.7395 |
| 1 missing | |||||
|
| |||||
| Grades | |||||
|
| |||||
| Low | 41.7% | 27.8% | 23.1% | 0% | 0.1510 |
| Intermediate | 58.3% | 61.1% | 69.2% | 85.7% | |
| High | 0% | 11.1% | 7.7% | 14.3% | |
| 1 missing | 1 missing | ||||
The Kruskal-Wallis test was used for age and tumor size and the Fisher’s exact test was used for the other outcomes.
Of the 33 patients with an RS of 11–25, only 5 (15%; 95% CI =2.9% – 27.4%) refused assignment to NCT (2 chose NHT and finished the study; they are included in the response outcomes). This was significantly lower than the 33% target (binomial test, p=0.0292) stipulated in the primary endpoint.
The results for response and other outcome endpoints are shown in Table 2 (according to treatment received). The total number of patients included in the secondary analyses was 55. One patient had missing data for clinical response. Overall objective clinical response rates were quite high for Groups A, C and D, ranging from 72.7 to 92.9%, with Group D being the highest. Group B patients, with an RS of 11–25 and receiving NHT, had the lowest clinical response rate at 50% (Fisher’s exact test: overall P value = 0.0490). After adjusting for effects from confounders, including age, race, menopausal status, and site, Group B patients had a significantly lower clinical response rate, compared with Group C (Ordinal regression: P = 0.0369). There were also somewhat lower clinical response rates in black versus white women, especially when treated with NCT (Ordinal regression: P=0.0288 for race and P=0.0707 for interaction between race and treatment). Complete responses were more likely in the 2 groups receiving NCT (36.4% in Group C and 28.6% in Group D versus 8.3% for A and 22.2% for B (Fisher’s exact test: P = 0.0422). Only one patient in each of Groups A and B had a breast pCR, and there were none in Group C. In Group D patients, with an RS of 26 or greater and receiving NCT, the breast pCR rate was 21.4% and breast plus nodes pCR rate was 14.3%. None of the other groups had a pCR for the breast plus nodes. Importantly, greater than 70% of the patients in the groups A and B who were treated with NHT achieved successful BCS. Fewer, but still a majority of patients in groups C (63.6%) and D (57.1%) who were treated with NCT achieved successful BCS successfully.
Table 2.
Response results, according to treatment received.
| Treatment Group | Group A RS < 11 NHT N = 12 |
Group B RS = 11–25 NHT N = 18 |
Group C RS = 11–25 NCT N = 11 |
Group D RS ≥ 26 NCT N = 14 |
Overall P value |
|---|---|---|---|---|---|
| cCR | 8.3% | 22.2% | 36.4% | 28.6% | 0.0422 |
| cPR | 75.0% | 27.8% | 36.4% | 64.3% | |
| cCR + cPR | 83.3% | 50.0% | 72.7% | 92.9% | 0.0490 |
| pCR Breast | 8.3% | 6.0% | 0% | 21.4% | NS |
| pCR Breast + Nodes | 0% | 0% | 0% | 14.3% | NS |
| Successful BCS | 75.0% | 72.2% | 63.6% | 57.1% | NS |
RS recurrence score, NHT neoadjuvant hormonal therapy, NCT neoadjuvant chemotherapy, cCR clinical complete response, cPR clinical partial response, pCR pathologic complete response, BCS breast conserving surgery, NS not significant
DISCUSSION
The molecular profiling of breast cancers has now become common practice as a tool for making decisions on whether to use adjuvant chemotherapy after surgery, especially for node-negative and HR+ cancers. A number of different molecular profiles are now commercially available. As of December 2016, the Oncotype Dx assay had been performed 600,000 times, and approximately 20% of the specimens have been core needle biopsies (personal communication from AS at GHI). When the RS assay is run on core biopsy samples, there is a greater than 90% success rate for generating a RS. Presumably, the purpose of most of these specimen submissions was to make a pre-operative decision about whether to choose NCT versus NHT versus primary surgery. Despite these numbers, as noted above, very few patients who would be eligible for NHT actually receive this treatment in the United States.
This pilot trial, along with other reports, demonstrates the feasibility of using molecular profiling of a core biopsy specimen to help with decision making in the neoadjuvant setting, and it provides support for a larger prospective trial, with only 15% of patients with an RS of 11–25 refusing randomized assignment to treatment. The sample sizes in this trial are too small to allow firm conclusions about outcomes, but the clinical response rate and success rate of BCS with NHT for patients with an RS less than 11 were quite reasonable. For patients with an RS of 11–25, the clinical response rate with NCT was higher than for NHT, but the rates of successful BCS were similar. The highest clinical response rates occurred in patients with an RS of 26 or greater receiving NCT, and the only pCRs for breast + nodes observed were in this group. We did not observe any unexpected adverse events that delayed surgery in any of these groups, but it is clear that in terms of making BCS possible, NHT is adequate therapy for patients with an RS of up to 25 and is less toxic than chemotherapy. This agrees with previous studies showing low rates of clinical or pathologic complete responses in patients with a low RS. This study is also in agreement with the prospective study of Yardley et al. [33] showing no pCRs in patients with low RSs. The results reported here are also consistent with data showing that patients with low RSs derive little or no benefit from adding chemotherapy to hormonal therapy; chemotherapy for these patients only adds toxicity. Retrospective studies have demonstrated that patients with low RSs are unlikely to respond well to chemotherapy but are more likely to respond to NHT, while higher RS predicts a greater likelihood of a good response to chemotherapy [36,13,14,34,36,35]. These results also confirm prior studies showing that patients with a low RS have little to no benefit from chemotherapy, similar to the recently published reports of prospective outcomes in patients with RS less than 11 in the adjuvant setting for both node-negative and node-positive patients [44,45,46].
Although we would advocate a larger prospective trial to confirm the outcomes observed here, in the absence of such a trial, these data along with the prior studies cited above do provide support for this strategy for patients who present with tumors that are suboptimal for BCS. Wider use of NHT to make BCS possible in patients with HR-positive tumors and low and intermediate RS should be encouraged, particularly for patients with comorbidities that make NCT less desirable. For patients with low RSs, there seems to be little justification for the added toxicity of chemotherapy, either adjuvant or neoadjuvant. The results reported here for patients with intermediate RSs (showing that NHT is not inferior to NCT in the neoadjuvant setting) may foreshadow the pending results in the adjuvant setting for intermediate RS cancers in the Trial Assigning Individualized Options for Treatment (TAILORx).
CONCLUSIONS
This pilot study demonstrates the feasibility of using the RS to guide NST, with only a 15% refusal rate of assigned treatment in patients with an RS of 11–25. Prospective demonstration that the RS can provide value in neoadjuvant decision making and thus safely and effectively spare patients exposure to the cost and toxicity of chemotherapy is an essential step towards further personalizing care while not sacrificing outcomes. Furthermore, although patients who had an RS of 11–25 and received NHT had a lower rate of objective clinical responses, the rate of successful BCS (> 70%) was similar to the patients with an RS of less than 11 and to patients with an RS of 11–25 treated with chemotherapy. None of the patients with an RS of 11–25 who received NCT had a pCR. These findings should enable physicians to have greater confidence in the value of NHT. These results demonstrate that conducting a similarly designed larger trial is feasible and suggests that for patients with an RS of 25 or less, NHT is a potentially effective strategy. They also support the use of the RS to identify appropriate patients for NCT versus NHT, similar to its previously validated value in the adjuvant setting.
Synopsis.
This multi-center trial explored the possible utility of using a 21-gene recurrence score to determine whether women with T2 or larger breast cancers should be treated with chemotherapy or hormonal therapy. Of 64 patients enrolled 33 had intermediate scores (11–25), and only 5 refused randomized treatment. More than 70% of women with low (< 11) or intermediate scores receiving neoadjuvant hormonal therapy achieved successful breast conservation.
Acknowledgments
This trial was supported by a grant from Genomic Health, Inc. Services in support of the research project were also generated by the VCU Massey Cancer Center, supported in part with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Funding: Supported by a grant from Genomic Health, Inc. Services in support of the research project were also generated by the VCU Massey Cancer Center, supported in part with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
TABLE OF ABBREVIATIONS
- BCS
Breast conservation surgery
- cCR
Clinical complete response
- cPR
Clinical partial response
- DCIS
Ductal carcinoma in situ
- FNA
Fine needle aspirate
- HR+
Hormone receptor positive
- IHC
Immunohistochemistry
- NCCN
National Comprehensive Cancer Network
- NCT
Neoadjuvant chemotherapy
- NHT
Neoadjuvant hormonal therapy
- NSABP
National Surgical Adjuvant Breast and Bowel Project
- NST
Neoadjuvant systemic therapy
- pCR
Pathologic complete response
- RS
Recurrence Score
Footnotes
Preliminary results of this study were presented, in part, at the 39th Annual San Antonio Breast Cancer Symposium, December 8, 2016. The work described in this manuscript is original research.
Trial Registration: ClinicalTrials.gov: NCT01293032; CTRP ID: NCI-2010-02342
Conflict of Interest: HDB has received speaking and advisory board honoraria from Genomic Health, Inc. AS is employed by Genomic Health, Inc. The remaining authors declare that they have no conflict of interest. The trial was funded, in part, by Genomic Health, Inc.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 3.Gianni L, Baselga J, Eiermann W, Porta VG, Semiglazov V, Lluch A, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. J Clin Oncol. 2009;27:2474–2481. doi: 10.1200/JCO.2008.19.2567. [DOI] [PubMed] [Google Scholar]
- 4.Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project (NSABP) Protocol B-27. J Clin Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, Zambetti M. Primary chemotherapy in operable breast cancer: Eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16:93–100. doi: 10.1200/JCO.1998.16.1.93. [DOI] [PubMed] [Google Scholar]
- 6.Bear HD, Tang G, Rastogi P, Geyer CE, Jr, Robidoux A, Atkins JN, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366:310–320. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 8.Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol. 2011;29:2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, et al. Neoadjuvant Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2:1477–1486. doi: 10.1001/jamaoncol.2016.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leal F, Liutti VT, Antunes dos Santos VC, Novis de Figueiredo MA, Macedo LT, Rinck JA, Junior, Sasse AD. Neoadjuvant endocrine therapy for resectable breast cancer: A systematic review and meta-analysis. Breast. 2015;24:406–412. doi: 10.1016/j.breast.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Van Dam PA, van D V, Altintas S, Papadimitriou K, Rolfo C, Trinh XB. Neoadjuvant endocrine treatment in early breast cancer: An overlooked alternative? Eur J Surg Oncol. 2016;42:333–342. doi: 10.1016/j.ejso.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Chiba A, Hoskin TL, Heins CN, Hunt KK, Habermann EB, Boughey JC. Trends in Neoadjuvant Endocrine Therapy Use and Impact on Rates of Breast Conservation in Hormone Receptor-Positive Breast Cancer: A National Cancer Data Base Study. Ann Surg Oncol. 2017;24:418–424. doi: 10.1245/s10434-016-5585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianni L, Baselga J, Eiermann W, Guillem PV, Semiglazov V, Lluch A, et al. Feasibility and tolerability of sequential doxorubicin/paclitaxel followed by cyclophosphamide, methotrexate, and fluorouracil and its effects on tumor response as preoperative therapy. Clin Cancer Res. 2005;11:8715–8721. doi: 10.1158/1078-0432.CCR-05-0539. [DOI] [PubMed] [Google Scholar]
- 14.Chang JC, Makris A, Gutierrez MC, Hilsenbeck SG, Hackett JR, Jeong J, et al. Gene expression patterns in formalin-fixed, paraffin-embedded core biopsies predict docetaxel chemosensitivity in breast cancer patients. Breast Cancer Res Treat. 2008;108:233–240. doi: 10.1007/s10549-007-9590-z. [DOI] [PubMed] [Google Scholar]
- 15.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 16.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. Clinical practice guidelines in oncology - version 2.2006 [Google Scholar]
- 18.Early Breast Cancer Trialists Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–3365. doi: 10.1200/JCO.2003.04.576. [DOI] [PubMed] [Google Scholar]
- 20.Eifel P, Axelson JA, Costa J, Crowley J, Curran WJJ, Deshler A, et al. National Institutes of Health Consensus Development Conference Statement: Adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst. 2001;93:979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 21.Carlson RW, Brown E, Burstein HJ, Gradishar WJ, Hudis CA, Loprinzi C, et al. NCCN Task Force Report: Adjuvant Therapy for Breast Cancer. J Natl Compr Canc Netw. 2006;4(Suppl 1):S1–26. [PubMed] [Google Scholar]
- 22.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, Cronin KA. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106:dju055. doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 24.Swain SM, Nunes R, Yoshizawa C, Rothney M, Sing AP. Quantitative Gene Expression by Recurrence Score in ER-Positive Breast Cancer, by Age. Adv Ther. 2015;32:1222–1236. doi: 10.1007/s12325-015-0268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joh JE, Esposito NN, Kiluk JV, Laronga C, Lee MC, Loftus L, et al. The effect of Oncotype DX recurrence score on treatment recommendations for patients with estrogen receptor-positive early stage breast cancer and correlation with estimation of recurrence risk by breast cancer specialists. Oncologist. 2011;16:1520–1526. doi: 10.1634/theoncologist.2011-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partin JF, Mamounas EP. Impact of the 21-gene recurrence score assay compared with standard clinicopathologic guidelines in adjuvant therapy selection for node-negative, estrogen receptor-positive breast cancer. Ann Surg Oncol. 2011;18:3399–3406. doi: 10.1245/s10434-011-1698-z. [DOI] [PubMed] [Google Scholar]
- 27.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26:721–728. doi: 10.1200/JCO.2007.15.1068. [DOI] [PubMed] [Google Scholar]
- 28.Oratz R, Paul D, Cohn AL, Sedlacek SM. Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. J Oncol Pract. 2007;3:182–186. doi: 10.1200/JOP.0742001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al. Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy. Health Technol Assess. 2006;10:iii–xi. 1–210. doi: 10.3310/hta10340. [DOI] [PubMed] [Google Scholar]
- 30.Issa AM, Chaudhari VS, Marchant GE. The value of multigene predictors of clinical outcome in breast cancer: an analysis of the evidence. Expert Rev Mol Diagn. 2015;15:277–286. doi: 10.1586/14737159.2015.983476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch JA, Berse B, Petkov V, Filipski K, Zhou Y, Khoury MJ, et al. Implementation of the 21-gene recurrence score test in the United States in 2011. Genet Med. 2016;18:982–990. doi: 10.1038/gim.2015.218. [DOI] [PubMed] [Google Scholar]
- 32.Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yardley DA, Peacock NW, Shastry M, Burris HA, III, Bechhold RG, Hendricks CB, et al. A phase II trial of ixabepilone and cyclophosphamide as neoadjuvant therapy for patients with HER2-negative breast cancer: correlation of pathologic complete response with the 21-gene recurrence score. Breast Cancer Res Treat. 2015;154:299–308. doi: 10.1007/s10549-015-3613-y. [DOI] [PubMed] [Google Scholar]
- 34.Soran A, Bhargava R, Johnson R, Ahrendt G, Bonaventura M, Diego E, et al. The impact of Oncotype DX(R) recurrence score of paraffin-embedded core biopsy tissues in predicting response to neoadjuvant chemotherapy in women with breast cancer. Breast Dis. 2016;36:65–71. doi: 10.3233/BD-150199. [DOI] [PubMed] [Google Scholar]
- 35.Akashi-Tanaka S, Shimizu C, Ando M, Shibata T, Katsumata N, Kouno T, et al. 21-Gene expression profile assay on core needle biopsies predicts responses to neoadjuvant endocrine therapy in breast cancer patients. Breast. 2009;18:171–174. doi: 10.1016/j.breast.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Ueno T, Masuda N, Yamanaka T, Saji S, Kuroi K, Sato N, et al. Evaluating the 21-gene assay Recurrence Score(R) as a predictor of clinical response to 24 weeks of neoadjuvant exemestane in estrogen receptor-positive breast cancer. Int J Clin Oncol. 2014;19:607–613. doi: 10.1007/s10147-013-0614-x. [DOI] [PubMed] [Google Scholar]
- 37.Straver ME, Glas AM, Hannemann J, Wesseling J, van d V, Rutgers EJ, Vrancken Peeters MJ, et al. The 70-gene signature as a response predictor for neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2010;119:551–558. doi: 10.1007/s10549-009-0333-1. [DOI] [PubMed] [Google Scholar]
- 38.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwao-Koizumi K, Matoba R, Ueno N, Kim SJ, Ando A, Miyoshi Y, et al. Prediction of docetaxel response in human breast cancer by gene expression profiling. J Clin Oncol. 2005;20:422–431. doi: 10.1200/JCO.2005.09.078. [DOI] [PubMed] [Google Scholar]
- 40.Parker JS, Prat A, Cheang MCU, Lenburg ME, Paik S, Perou CM. Breast cancer molecular subtypes predict response to anthracycline/taxane-based chemotherapy. Cancer Res. 2009;69:598s–598s. [Google Scholar]
- 41.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 42.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 43.Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied Linear Statistical Models. New York: McGraw-Hill; 2005. [Google Scholar]
- 44.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gluz O, Nitz UA, Christgen M, Kates RE, Shak S, Clemens M, et al. West German Study Group Phase III PlanB Trial: First Prospective Outcome Data for the 21-Gene Recurrence Score Assay and Concordance of Prognostic Markers by Central and Local Pathology Assessment. J Clin Oncol. 2016;34:2341–2349. doi: 10.1200/JCO.2015.63.5383. [DOI] [PubMed] [Google Scholar]
- 46.Petkov VI, MIller DP, Howlader N, Gliner N, Howe W, Schussler N, et al. Breast-cancer-specific mortality in patients treated based on the 21-gene assy: a SEER population-based study. npj Breast Cancer. 2016;2:1–9. doi: 10.1038/npjbcancer.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]


