Abstract
Background
We evaluated the American College of Surgeon’s National Cancer Data Base (NCDB) to describe current hospital-based epidemiologic frequency, survival, and patterns of care of pediatric medulloblastoma.
Methods
We analyzed NCDB 1998–2011 data on medulloblastoma for children ages 0–19 years using logistic and poisson regression, Kaplan-Meier survival estimates, and Cox proportional hazards models.
Results
3,647 cases of medulloblastoma in those aged 0–19 years were identified. Chemotherapy was received by 79% and 74% received radiation, with 65% receiving both therapies. Those who received radiation were more likely to be older than four, while those who received chemotherapy were more likely to be age four and younger. Variables associated with receipt of neither radiation nor chemotherapy included age at diagnosis of <1 year, female gender, being of race other than black or white, having no insurance, and living in a residential area with a low level of high school graduates. Better overall survival was observed as age at diagnosis increased, in females, and having received radiation. Compared to medulloblastoma, NOS, better survival was observed for those with demoplastic medulloblastoma, with worse survival in those with large cell medulloblastoma.
Conclusion
Majority received multi- disciplinary therapy and radiation had the greatest effect on survival. Ages four and under were most likely to receive chemotherapy and least likely to receive radiation. Suboptimal treatment included 17.8% that did not receive chemotherapy, of which 11.8% received neither chemotherapy nor radiation. Disparities associated with medical access were characteristics for not receiving standard treatment, which resulted in poor outcome.
Keywords: Pediatric medulloblastoma, NCDB, radiation therapy, chemotherapy, disparity, surgery
Introduction
Medulloblastoma is the most common brain neoplasm in the pediatric population [1,2]. A disease of the young, 70% of cases affect children 16 years or younger, with the peak incidence occurring at age seven [1–3]. A malignant tumor, classified as World Health Organization (WHO) grade IV, medulloblastoma has a 70% survival at five years from diagnosis compared to 30% survival in the 1960s [4]. The improved survival has been attributed to inclusion of radiation therapy, advances in imaging, and improvements in supportive care [1,5,6]. More recent advances in survival, however, have lagged compared to other common pediatric cancers [7].
Medulloblastoma is an embryonal tumor with neuronal differentiation arising from the cerebellum with an inherent tendency to spread along the neuro-axis [8,9]. This embryonal group includes primitive neuroectodermal tumors, atypical teratoid/rhabdoid tumors, and embryonal tumor with multilayered rosettes [10]. WHO classifies medulloblastoma into four subtypes: desmoplastic/nodular, anaplastic, large cell, and medulloblastoma with extensive nodularity [9]. Recent genomic characterization has identified four intrinsic subtypes, Wnt, Shh, group 3, and group 4 [11–13]. Treament is based on risk stratification including age, extent of surgical resection, presence of metastatic disease, and large cell histology.
Treatment for medulloblastoma is based on multi-disciplinary therapy with maximal safe resection surgery, radiation when tolerable, and multi-agent systemic chemotherapy. Current concerns include the use of radiation therapy in very young children (less than three years) and methods to limit the exposure of radiation to all children, such as proton beam, focal radiation, or lower dose of radiation [5,4,14]. Investigations on quality of life following treatment have also been of special interest and include neurocognitive decline, impairment of growth and endocrine function, hearing loss, and secondary neoplasms [15,16]. There is also a role in refractory cases for high dose chemotherapy with autologous stem cell transplantation [17,18]. Recent inclusion of targeted agents is being considered for specific molecular subtypes [19,20].
As a disease affecting children and young adults, inadequate treatment can result in death from a potentially curative disease [21]. Our analysis of the American College of Surgeons National Cancer Data Base (NCDB) describes current hospital-based epidemiologic frequency, patterns of care, and survival for pediatric medulloblastoma.
Methods
The NCDB, a joint program of the American College of Surgeons and the American Cancer Society, contains cancer patient demographics, tumor identification characteristics, treatment, and outcomes data. NCDB captures nearly 75% of all newly diagnosed cancer cases in the United States from over 1,450 Commission on Cancer accredited hospitals in 49 states. Data reported to the NCDB are retrospective in nature and are in compliance with the privacy requirements of the Health Insurance Portability and Accountability Act of 1996 as reported in the Standards for Privacy of Individually Identifiable Health Information; Final Rule (45 CFR Parts 160 and 164).
A total of 3,647 patients ages 0–19 years diagnosed with medulloblastoma, defined as International Classification of Diseases for Oncology (ICD-O-3) histology/behavior codes 9470/3, 9471/3 and 9474/3 and limited to primary tumor sites C71.0–C71.9, were identified from the NCDB hospital-based cancer registry for years 1998–2011. Fourteen cases included in the analyses were coded as a second or unknown primary. Data were abstracted according to the Facility Oncology Registry Data Standards (FORDS) manual. Follow-up of cases is pursued every 5 years following the initial year of diagnosis (i.e. for a case diagnosed in 1990, follow-up would be done in 1995, 2000, etc. until death or lost to follow-up).
Descriptive statistics were reported overall, and stratified by age group (<5 years and 5–19 years). Primary site was stratified into the following groups: brain stem (C71.7), cerebellum (C71.6), ventricle (C71.5), and others (C71.0–71.4 and 71.8–71.9). If the patient received any therapy (radiation, chemotherapy, and/or surgery) during their first course of treatment, they were classified as “yes” for that particular treatment. The insurance variable identified the patient’s primary insurance carrier at the time of diagnosis and/or treatment. Income and education were defined as the median household income and the number of adults who did not graduate from high school, respectively, in the patient’s residential zip code at the time of diagnosis as derived from the 2000 US Census data.[22] Region was defined by comparing the patient’s residential state and county Federal Information Processing Standard (FIPS) code at diagnosis to the 2003 Rural-Urban Continuum Codes as developed by the US Department of Agriculture Economic Research Service.[22] For our purposes, metro was defined as counties with a population of 250,000 or more; urban had a population of 2,500 to less than 250,000; rural had a population of less than 2,500.
Descriptive demographics were presented using frequency for categorical variables, with differences between groups assessed using Chi-Square tests. Multivariable logistic regression was performed to analyze the factors that may influence receipt of a specific treatment (e.g. radiation treatment, chemotherapy, and neither radiation or chemotherapy). Variables which were statistically significant in any of the full models listed above were included in all of the final models, and model selection was performed to identify critical variables with lower values of Akaike Information Criteria (AIC) suggesting better model fit. Because of the large number of patients with unknown tumor size, this variable was not included in any of the logistic regression models. Due to missing covariates, 484 cases were excluded from the radiation model, 562 cases were excluded from the chemotherapy model, and 647 cases were excluded from the neither treatment model. Poisson regression was employed to assess univariate changes in counts over time, by histology, and by treatment received.
Survival was defined as time from diagnosis until death due to all causes. Data for survival analysis in this paper were only available from 1998 to 2006 due to the 5-year NCDB lag in collecting and reporting survival/follow-up data (n=2,358). Kaplan-Meier estimates of overall survival were calculated. Cox proportional hazards models were employed to assess the risk of mortality according to receiving both radiation and chemotherapy treatments while adjusting for other potential risk factors. These hazard ratios should be interpreted as the average risk of death over the entire time period presented. Due to missing covariate data, 199 cases were excluded from the estimation of the hazard ratios. The level of statistical significance was set at 0.05 for all tests conducted, and all analyses were performed with SAS software version 9.4 for Microsoft Windows on ×64 (SAS Statistical Institute, Cary, NC).
Results
A total of 3,647 children aged 0–19 years with medulloblastoma were included in NCDB between 1998 and 2011 (Table 1). Almost one-third of these were in children less than 5 years of age. The frequency in males (63%) was higher than in females, while whites accounted for 81% of cases. Medulloblastoma, NOS (87%) was the most frequently diagnosed tumor histology, followed by desmoplastic medulloblastoma (9%) and large cell medulloblastoma (4%). Interestingly, over time, the frequency of a large cell or demoplastic medulloblastoma diagnosis significantly increased at a rate of 10% (p=0.001) and 3% (p=0.03) per year, respectively, while the less specific diagnosis of medulloblastoma, NOS significantly decreased by a rate of 2% per year (p=0.0003). Only 4% of patients with medulloblastoma reported no insurance, and only 2% of patients lived in a rural area at the time of diagnosis. At diagnosis, a greater frequency of patients were living in zip codes with the highest categorical median income of $46,000 + (37%) and with the lowest percentage of adults who did not finish high school (32%).
Table 1.
Descriptive characteristics of children aged 0–19 years with medulloblastoma, NCDB 1998–2011 with corresponding Chi-square p-values for differences between age groups.
| Characteristic | Age Group (years) | ||||||
|---|---|---|---|---|---|---|---|
| Total | 0–4 | 5–19 | p-value | ||||
| N | % | N | % | N | % | ||
| Gender | 0.1362 | ||||||
| Male | 2293 | 62.9 | 686 | 61.1 | 1607 | 63.7 | |
| Female | 1354 | 37.1 | 437 | 38.9 | 917 | 36.3 | |
| Race | 0.0225 | ||||||
| White | 2966 | 81.3 | 899 | 80.1 | 2067 | 81.9 | |
| Black | 391 | 10.7 | 117 | 10.4 | 274 | 10.9 | |
| Others | 219 | 6.0 | 74 | 6.6 | 145 | 5.7 | |
| Unknown | 71 | 2.0 | 33 | 2.9 | 38 | 1.5 | |
| Spanish Origin | 0.0073 | ||||||
| Yes | 565 | 15.5 | 201 | 17.9 | 364 | 14.4 | |
| No | 2904 | 79.6 | 859 | 76.5 | 2045 | 81.0 | |
| Unknown | 178 | 4.9 | 63 | 5.6 | 115 | 4.6 | |
| Insurance | <0.0001 | ||||||
| Not Insured | 136 | 3.7 | 34 | 3.0 | 102 | 4.0 | |
| Private Insurance/Managed Care | 2268 | 62.2 | 640 | 57.0 | 1628 | 64.5 | |
| Medicaid | 957 | 26.2 | 361 | 32.1 | 596 | 23.6 | |
| Medicare/Other Government | 119 | 3.3 | 39 | 3.5 | 80 | 3.2 | |
| Unknown | 167 | 4.6 | 49 | 4.4 | 118 | 4.7 | |
| Income | 0.6559 | ||||||
| < $30,000 | 530 | 14.5 | 166 | 14.8 | 364 | 14.4 | |
| $30,000 – $34,999 | 574 | 15.7 | 186 | 16.5 | 388 | 15.4 | |
| $35,000 – $45,999 | 969 | 26.6 | 303 | 27.0 | 666 | 26.4 | |
| $46,000 + | 1333 | 36.6 | 402 | 35.8 | 931 | 36.9 | |
| Unknown | 241 | 6.6 | 66 | 5.9 | 175 | 6.9 | |
| Education | 0.4322 | ||||||
| ≥ 29% (Lowest level of HS Grads) | 696 | 19.1 | 221 | 19.7 | 475 | 18.8 | |
| 20% – 28.9% | 805 | 22.1 | 264 | 23.5 | 541 | 21.4 | |
| 14% – 19.9% | 739 | 20.3 | 218 | 19.4 | 521 | 20.7 | |
| < 14% (Highest level of HS Grads) | 1166 | 32.0 | 354 | 31.5 | 812 | 32.2 | |
| Unknown | 241 | 6.6 | 66 | 5.9 | 175 | 6.9 | |
| Region | 0.0227 | ||||||
| Metro | 2805 | 76.9 | 858 | 76.4 | 1947 | 77.1 | |
| Urban | 535 | 14.7 | 188 | 16.8 | 347 | 13.8 | |
| Rural | 67 | 1.8 | 16 | 1.4 | 51 | 2.0 | |
| Unknown | 240 | 6.6 | 61 | 5.4 | 179 | 7.1 | |
| Primary Site | 0.2820 | ||||||
| Brain stem | 429 | 11.8 | 128 | 11.4 | 301 | 11.9 | |
| Cerebellum | 2448 | 67.1 | 758 | 67.5 | 1690 | 67.0 | |
| Ventricle | 141 | 3.9 | 34 | 3.0 | 107 | 4.2 | |
| Others | 629 | 17.3 | 203 | 18.1 | 426 | 16.9 | |
| Histology | <0.0001 | ||||||
| Medulloblastoma, NOS | 3165 | 86.8 | 900 | 80.1 | 2265 | 89.7 | |
| Desmoplastic Medulloblastoma | 345 | 9.5 | 167 | 14.9 | 178 | 7.1 | |
| Large Cell Medulloblastoma | 137 | 3.8 | 56 | 5.0 | 81 | 3.2 | |
| Chemotherapy | 0.0039 | ||||||
| Yes | 2875 | 78.8 | 922 | 82.1 | 1953 | 77.4 | |
| No | 649 | 17.8 | 165 | 14.7 | 484 | 19.2 | |
| Unknown | 123 | 3.4 | 36 | 3.2 | 87 | 3.4 | |
| Radiation Therapy | <0.0001 | ||||||
| Yes | 2689 | 73.7 | 558 | 49.7 | 2131 | 84.4 | |
| No | 927 | 25.4 | 554 | 49.3 | 373 | 14.8 | |
| Unknown | 31 | 0.9 | 11 | 1.0 | 20 | 0.8 | |
| Combination Therapy | <0.0001 | ||||||
| Both Chemotherapy and Radiation | 2359 | 64.7 | 506 | 45.1 | 1853 | 73.4 | |
| Chemotherapy Only | 516 | 14.2 | 416 | 37.0 | 100 | 4.0 | |
| Radiation Only | 330 | 9.1 | 52 | 4.6 | 278 | 11.0 | |
| Neither Chemotherapy or Radiation | 431 | 11.8 | 146 | 13.0 | 285 | 11.3 | |
| Unknown | 11 | 0.3 | 3 | 0.3 | 8 | 0.3 | |
| Surgical Procedure of the Primary Site | 0.0014 | ||||||
| Yes | 3505 | 96.1 | 61 | 5.4 | 81 | 3.2 | |
| No | 142 | 3.9 | 1062 | 94.6 | 2443 | 96.8 | |
| Tumor Size | 0.0008 | ||||||
| Less than 3 cm | 266 | 7.3 | 77 | 6.9 | 189 | 7.5 | |
| 3–5 cm | 1477 | 40.5 | 412 | 36.7 | 1065 | 42.2 | |
| 5+ cm | 614 | 16.8 | 225 | 20.0 | 389 | 15.4 | |
| Unknown | 1290 | 35.4 | 409 | 36.4 | 881 | 34.9 | |
The vast majority (over 96%) of patients with medulloblastoma received surgery. The frequency of those who received chemotherapy was 79% and those who received radiation therapy was 74%, with 65% of all patients receiving both therapies (Table 1). Over the 1998–2011 diagnosis period, the frequency of patients receiving radiation only decreased at a statistically significantly rate of 4% per year (p=0.005; Fig. 1). There were no changes over time in the frequencies of patients receiving both chemotherapy and radiation, chemotherapy only, or neither chemotherapy nor radiation (p>0.05 for all). When stratified by age, children aged 0–4 years were more likely than those aged 5–19 years to be Hispanic (p=0.01), of ‘other’ race (p=0.02), and live in an urban area (p=0.03). Children 0–4 years were also more likely to be on Medicaid and less likely to have private insurance (p<0.0001). Medulloblastoma, NOS was more frequently diagnosed in children aged 5–19 years (89.7% vs 80.1%), while children aged 0–4 years had a higher frequency of diagnosis of desmoplastic medulloblastoma (14.9% vs 7.1%) and large cell medulloblastoma (5.0% vs 3.2%) than older children (p<0.0001). Children aged 5–19 years were more likely than those aged 0–4 years to have received radiation therapy (84.4% vs 49.7%, p<0.0001), but were less likely to have received chemotherapy (77.4% vs 82.1%, p=0.004). By treatment combination category, children aged 5–19 years were more likely to have received both radiation and chemotherapy (73.4% vs 45.1%) or radiation only (11.0% vs 4.6%), but were less likely to have received chemotherapy only (4.0% vs 37.0%) or neither therapy (11.3% vs 13.0%) than children aged 0–4 years (p<0.001).
Fig. 1.
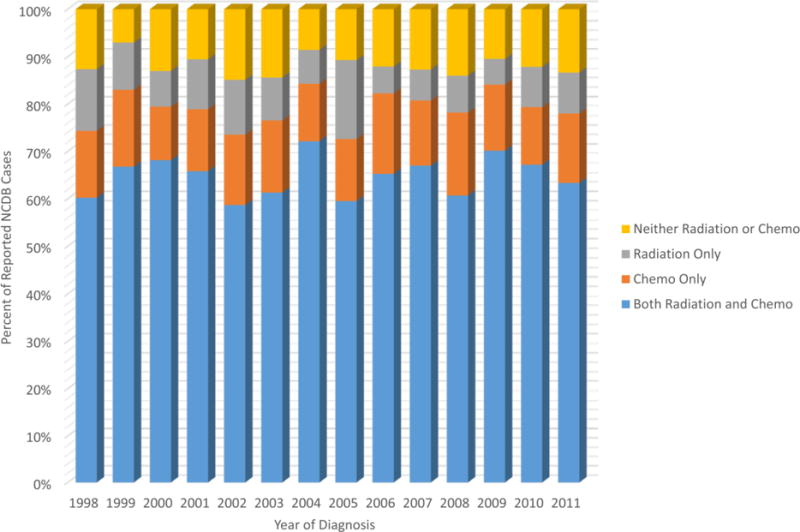
Types of Treatment Received by Year of Diagnosis
To determine the characteristics which led to receiving radiation therapy, chemotherapy, or neither, logistic regression models were created (Table 2). Compared with uninsured patients, private, Medicaid, or Medicare insured patients were 72%, 71%, or more than twice as likely to receive radiation treatment, respectively. Conversely, those under the age of 5, females, and those whose zip code corresponded to the lowest quartile of high school graduates were statistically significantly less likely to receive radiation therapy. Similarly, patients who had any type of insurance (private, Medicaid, or Medicare) were more than twice as likely to receive chemotherapy than those with no insurance. Children in the 1–4 and 5–9 year age groups were also 62% and 28% more likely to receive chemotherapy than those in the 10–19 year age group. While children less than 1 year of age were also 39% more likely to receive chemotherapy than the oldest age group, this difference was not statistically significant, likely due to smaller counts. Non-whites were less likely to receive chemotherapy, as were those whose zip code corresponded to the lowest quartile of high school graduates. Those who received neither radiation therapy nor chemotherapy in the first round of treatment were more likely to be less than 1 year of age (69%), female (35%), of ‘other’ race (74%), and live in a zip code with the lowest percentage of high school graduates (59%). Conversely, those with any type of insurance, whether private, Medicaid, or Medicare, were less likely to have received neither radiation nor chemotherapy.
Table 2.
Characteristics associated with odds of receiving radiation, chemotherapy, or neither in first-line therapy for children aged 0–19 years with medulloblastoma, NCDB 1998–2011.
| Received Radiation* | Received Chemotherapy** | Neither Radiation Nor Chemotherapy*** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | p-value | Odds Ratio | 95% Confidence Interval | p-value | Odds Ratio | 95% Confidence Interval | p-value | |
| Age Group | |||||||||
| <1 year old | 0.03 | 0.01–0.05 | <0.0001 | 1.39 | 0.82–2.35 | 0.22 | 1.69 | 1.01–2.83 | 0.05 |
| 1–4 years old | 0.21 | 0.17–0.27 | <0.0001 | 1.62 | 1.27–2.08 | 0.0001 | 1.03 | 0.78–1.37 | 0.82 |
| 5–9 years old | 1.04 | 0.82–1.31 | 0.77 | 1.28 | 1.03–1.59 | 0.03 | 0.90 | 0.69–1.17 | 0.42 |
| 10–19 years old | 1.00 | 1.00 | 1.00 | ||||||
| Gender | |||||||||
| Male | 1.00 | 1.00 | 1.00 | ||||||
| Female | 0.74 | 0.62–0.88 | 0.001 | 0.88 | 0.73–1.07 | 0.19 | 1.35 | 1.08–1.67 | 0.01 |
| Race | |||||||||
| White | 1.00 | 1.00 | 1.00 | ||||||
| Black | 0.93 | 0.70–1.23 | 0.61 | 0.77 | 0.58–1.03 | 0.08 | 1.19 | 0.85–1.67 | 0.31 |
| Others | 0.78 | 0.54–1.11 | 0.17 | 0.62 | 0.43–0.88 | 0.01 | 1.74 | 1.18–2.58 | 0.01 |
| Insurance | |||||||||
| Not Insured | 1.00 | 1.00 | 1.00 | ||||||
| Private Insurance/Managed Care | 1.72 | 1.11–2.66 | 0.02 | 2.62 | 1.75–3.92 | <0.0001 | 0.39 | 0.25–0.60 | <0.0001 |
| Medicaid | 1.71 | 1.09–2.70 | 0.02 | 2.95 | 1.92–4.52 | <0.0001 | 0.33 | 0.21–0.53 | <0.0001 |
| Medicare/Other Government | 2.43 | 1.25–4.73 | 0.01 | 4.17 | 2.10–8.28 | <0.0001 | 0.21 | 0.09–0.48 | 0.0002 |
| Education | |||||||||
| >= 29% (Lowest level of HS Grads) | 0.75 | 0.58–0.97 | 0.03 | 0.65 | 0.50–0.85 | 0.001 | 1.59 | 1.17–2.16 | 0.003 |
| 20%–28.9% | 0.84 | 0.66–1.07 | 0.16 | 0.85 | 0.65–1.10 | 0.20 | 1.23 | 0.91–1.67 | 0.17 |
| 14%–19.9%% | 0.89 | 0.70–1.14 | 0.36 | 0.86 | 0.66–1.11 | 0.24 | 1.10 | 0.81–1.50 | 0.55 |
| < 14% (Highest level of HS Grads) | 1.00 | 1.00 | 1.00 | ||||||
Due to missing data in the race, education, income, or treatment variables:
484 cases were excluded;
562 cases were excluded;
647 cases were excluded. Models were Due to missing data in the selected using backward selection criteria and AIC values. All ORs are adjusted for the other variables listed above.
Overall five-year survival was 72.6% [95% Confidence Interval (CI): 70.6–74.5] in children aged 0–19 years who were diagnosed during the years 1998 through 2006 (n=2,358). Cox proportional hazard models, including variables which showed significance after model selection, identified several factors that influenced survival rates (Table 3). Compared to those aged 10–19 years (Fig. 2a and Table 3), survival was statistically significantly lower in all younger age groups, with survival being lowest in the youngest age group (aged <1 year) at diagnosis. Five-year survival rates were: 47.4% (<1 year), 64.5% (ages 1–4), 74.2% (ages 5–9), 81.5% (ages 10–19). Females had statistically significantly better survival than males, and blacks had statistically significantly better survival than whites. Those who lived in a zip code with median household incomes less than $30,000 and $35,000–$45,999 both had survival rates that were worse than those with median household incomes in the highest quartile. Children diagnosed with desmoplastic medulloblastoma had one-third better survival, while those with large cell medulloblastoma had 2 times worse survival, than those diagnosed with medulloblastoma, NOS.
Table 3.
Effects of selected characteristics on survival for children aged 0–19 years diagnosed with medulloblastoma, NCDB 1998–2006 (n=2,358).
| Variable | Hazard Ratio* | 95% Confidence Interval | p-value |
|---|---|---|---|
| Age Group | |||
| <1 year old | 3.06 | 2.07–4.53 | <0.0001 |
| 1–4 years old | 1.81 | 1.44–2.27 | <0.0001 |
| 5–9 years old | 1.34 | 1.08–1.66 | 0.01 |
| 10–19 years old | 1.00 | ||
| Gender | |||
| Male | 1.00 | ||
| Female | 0.79 | 0.67–0.94 | 0.01 |
| Race | |||
| White | 1.00 | ||
| Black | 0.71 | 0.53–0.94 | 0.02 |
| Others | 0.72 | 0.48–1.09 | 0.12 |
| Income | |||
| < $30,000 | 1.39 | 1.10–1.75 | 0.01 |
| $30,000 – $34,999 | 1.00 | 0.78–1.27 | 0.98 |
| $35,000 – $45,999 | 1.28 | 1.05–1.55 | 0.01 |
| $46,000 + | 1.00 | ||
| Histology | |||
| Medulloblastoma, NOS | 1.00 | ||
| Desmoplastic Medulloblastoma | 0.69 | 0.51–0.94 | 0.02 |
| Large Cell Medulloblastoma | 2.61 | 1.76–3.89 | <0.0001 |
| Radiation & Chemotherapy | |||
| Both | 1.00 | ||
| Radiation Only | 1.08 | 0.80–1.46 | 0.62 |
| Chemo Only | 1.64 | 1.30–2.06 | <0.0001 |
| Neither | 1.89 | 1.50–2.38 | <0.0001 |
199 subjects were excluded from the analyses due to missing data in covariates. Hazard Ratio adjusted for all variables listed above. Model was selected using Cox proportional hazards with backward selection and AIC values.
Fig. 2.
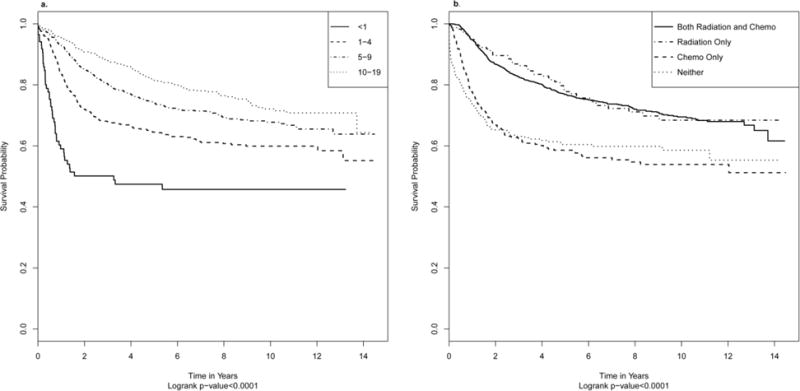
a Overall Survival for Diagnosed Cases 1998–2006 by Age
b Overall Survival for Diagnosed Cases 1998–2006 by Treatment
With regard to treatment, patients who received radiation alone in the first course of treatment had comparable survival rates to those who received both radiation and chemotherapy (Table 3). Alternatively, those who received chemotherapy alone or neither radiation nor chemotherapy had statistically significantly worse survival than those who received both therapies. In post-hoc analyses comparing the survival curves for the four different treatment combinations (both radiation and chemotherapy, radiation alone, chemotherapy alone, and neither; Fig. 2b) survival for those receiving both radiation and chemotherapy and those who received only radiation in the first round of treatment were not significantly different from each other (Hazard Ratio:0.93, 95% CI:0.69–1.25). Similarly, survival for those who received only chemotherapy was not significantly different from those who received neither radiation nor chemotherapy (0.86, 0.65–1.15). Those who received both therapies or radiation only had significantly better survival than those who received chemotherapy alone or neither therapies in the first round of treatment (both vs chemotherapy only: 0.61, 0.49–0.77; both vs neither: 0.53, 0.42–0.67; chemotherapy only vs radiation only: 1.52, 1.07–2.16; neither vs radiation only: 1.76, 1.24–2.49).
Discussion
Medulloblastoma is a common and curable pediatric cancer. Optimal treatment requires advanced care in specialized centers. NCDB is the largest clinical cancer registry and analysis is important to assess what types of therapeutic options have been used and their expected outcomes. The most prominent finding in our investigation is the frequency that patients do not receive multi- disciplinary therapy, specifically radiation and/or chemotherapy. Patients who do not receive radiation have significantly worse outcomes. The patients most likely to not receive radiation are younger, female, or have suspected socioeconomic impediments that limit access to care.
In general we reaffirm the survival benefit of radiation, either as monotherapy or combined with chemotherapy [5,4]. Our results also demonstrate that chemotherapy alone is inferior to radiation, which is consistent with the findings of most prospective treatment trials for medulloblastomas [23,5,4,14,24–27]. The combined use of radiation and chemotherapy, which is the established care for children greater than three years old, however, did not provide statistical benefit compared to radiation alone, although there was a trend towards benefit. The reason for this lack of combined benefit could be that chemotherapy overlaps with the clinical benefit from radiation or the sequence of therapy has yet to be optimized or that the benefits and/or harm are restricted to specific genetic subtypes and become diluted in our analysis.
Radiation has demonstrated a dose dependent effect on outcomes in clinical trials [5,4,24,25]. Acknowledging that radiation is usually avoided in children younger than age three or four due to concerns of treatment related long-term cognitive effects, we sought to identify socio-demographic factors that may prevent patients from receiving radiation treatments. We found as expected that the under five age group was significantly less likely to receive radiation. We also found female gender, lack of insurance, and living in areas with lower education were associated with not receiving radiation. No statistically significant differences were noted by race, although we observe a trend toward increased radiation treatments among blacks or being of race other than black or white. We also found, as demonstrated in clinical trials, the superior benefit of radiation for initial treatment following surgery versus initial use of chemotherapy [23,27].
Chemotherapy without radiation is usually preferred for infants and toddlers under three or four years old [26]. This age group is known to present more frequently with disseminated disease and have inferior outcomes compared to older children [5,4]. Our analysis demonstrates that this early age group is most likely to receive chemotherapy and least likely to receive radiation, in keeping with the literature. We also found that overall 17.8% of children and adolescents did not receive chemotherapy during first-line treatment. This was unexpected as chemotherapy is incorporated as standard of care for all ages in medulloblastoma. In our analysis we further identifed an unfortunate group that received neither radiation nor chemotherapy. This group was associated with infants less than one year old, female gender, lack of insurance, and living in areas with lower education. It is not clear from our data why a significant proportion of children are not receiving optimal multimodal treatment and is a topic for further investigation. This is an opportunity to increase treatment and significantly improve survival.
Our results also confirmed previous clinical studies that report subtype dependent outcomes, specifically poorer outcomes are seen with large cell [5] and more favorable outcomes with desmoplastic [28,26]. These two subtypes, their relation to the recent molecular classification, and incorporation for prognosis and treatment are still evolving. Our analysis also demonstrates that the incidence of these subtypes, large cell and desmoplastic, are increasing, while the less specific medulloblastoma, NOS is decreasing. This is likely due to wider acceptance of subtype classification based on WHO criteria.
Although NCDB is considered nationally applicable, there are limitations in this data. Most pediatric patients will be referred to regional specialty centers, but true access to these centers is not known and this may affect the ability of patients to receive optimal care. Limits of current registry data include lack of information on the dose, volume, or treatment planning for radiation and chemotherapies. There are also concerns on the quality of registry data collected over long periods including changes in histologic classification, although in the time-period of our analysis classification of medulloblastomas has largely remained unchanged [29].
In summary we report patterns of care and outcomes for initial care of medulloblastoma using the largest hospital-based cancer database. Our evaluation reinforces the established benefits of treatment, specifically radiation. We found patterns of treatment based on age in keeping with the literature. Large cell and desmoplastic subtypes of medulloblastoma had outcome differences, which can be used prognostically in future treatment investigations. Finally female gender and disparities associated with access to care emerged as important characteristics for treatment and survival. For a curable cancer in children, it is critical to investigate this non-treatment group in detail.
Acknowledgments
We are grateful to Bridget J. McCarthy, Ph.D. for being a resource for ideas and inspiration as well as providing extremely helpful comments on the manuscript. We would also like to thank Cynthia Phelan, M.D. for critically reviewing the manuscript.
Funding: EVD, TAD, and JLV were supported by the National Cancer Institute (R03CA156561) and EVD is a member of the Biostatistics and Bioinformatics Shared Resource Facility of the University of Kentucky Markey Cancer Center (P30CA177558).
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, Stearns DS, Wolff JE, Wolinsky Y, Letterio JJ, Barnholtz-Sloan JS. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-oncology. 2015;16(Suppl 10):x1–x36. doi: 10.1093/neuonc/nou327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-oncology. 2014;16(Suppl 4):iv1–63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman S, Schellinger KA, Propp JM, McCarthy BJ, Campbell RT, Davis FG. Seasonal variation in incidence of pediatric medulloblastoma in the United States, 1995–2001. Neuroepidemiology. 2007;29(1–2):89–95. doi: 10.1159/000109502. [DOI] [PubMed] [Google Scholar]
- 4.Hughes EN, Shillito J, Sallan SE, Loeffler JS, Cassady JR, Tarbell NJ. Medulloblastoma at the joint center for radiation therapy between 1968 and 1984. The influence of radiation dose on the patterns of failure and survival. Cancer. 1988;61(10):1992–1998. doi: 10.1002/1097-0142(19880515)61:10<1992::aid-cncr2820611011>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. The Lancet Oncology. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 6.Gajjar AJ, Robinson GW. Medulloblastoma-translating discoveries from the bench to the bedside. Nature reviews Clinical oncology. 2014;11(12):714–722. doi: 10.1038/nrclinonc.2014.181. [DOI] [PubMed] [Google Scholar]
- 7.Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer. 2014;120(16):2497–2506. doi: 10.1002/cncr.28748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annual review of pathology. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 9.Louis DN, International Agency for Research on Cancer, World Health Organization . World Health Organization Classification of Tumours. 4. International Agency for Research on Cancer; Lyon: 2007. WHO classification of tumours of the central nervous system. [Google Scholar]
- 10.Paulus W, Kleihues P. Genetic profiling of CNS tumors extends histological classification. Acta neuropathologica. 2010;120(2):269–270. doi: 10.1007/s00401-010-0710-1. [DOI] [PubMed] [Google Scholar]
- 11.Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P, Taylor MD, Pfister SM. Medulloblastomics: the end of the beginning. Nature reviews Cancer. 2012;12(12):818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones DT, Jager N, Kool M, Zichner T, Hutter B, Sultan M, Cho YJ, Pugh TJ, Hovestadt V, Stutz AM, Rausch T, Warnatz HJ, Ryzhova M, Bender S, Sturm D, Pleier S, Cin H, Pfaff E, Sieber L, Wittmann A, Remke M, Witt H, Hutter S, Tzaridis T, Weischenfeldt J, Raeder B, Avci M, Amstislavskiy V, Zapatka M, Weber UD, Wang Q, Lasitschka B, Bartholomae CC, Schmidt M, von Kalle C, Ast V, Lawerenz C, Eils J, Kabbe R, Benes V, van Sluis P, Koster J, Volckmann R, Shih D, Betts MJ, Russell RB, Coco S, Tonini GP, Schuller U, Hans V, Graf N, Kim YJ, Monoranu C, Roggendorf W, Unterberg A, Herold-Mende C, Milde T, Kulozik AE, von Deimling A, Witt O, Maass E, Rossler J, Ebinger M, Schuhmann MU, Fruhwald MC, Hasselblatt M, Jabado N, Rutkowski S, von Bueren AO, Williamson D, Clifford SC, McCabe MG, Collins VP, Wolf S, Wiemann S, Lehrach H, Brors B, Scheurlen W, Felsberg J, Reifenberger G, Northcott PA, Taylor MD, Meyerson M, Pomeroy SL, Yaspo ML, Korbel JO, Korshunov A, Eils R, Pfister SM, Lichter P. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–105. doi: 10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Backlund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta neuropathologica. 2012;123(4):473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(25):4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 15.Gerber NU, Mynarek M, von Hoff K, Friedrich C, Resch A, Rutkowski S. Recent developments and current concepts in medulloblastoma. Cancer treatment reviews. 2014;40(3):356–365. doi: 10.1016/j.ctrv.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Gudrunardottir T, Lannering B, Remke M, Taylor MD, Wells EM, Keating RF, Packer RJ. Treatment developments and the unfolding of the quality of life discussion in childhood medulloblastoma: a review. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2014;30(6):979–990. doi: 10.1007/s00381-014-2388-5. [DOI] [PubMed] [Google Scholar]
- 17.Cohen BH, Geyer JR, Miller DC, Curran JG, Zhou T, Holmes E, Ingles SA, Dunkel IJ, Hilden J, Packer RJ, Pollack IF, Gajjar A, Finlay JL, Children’s Oncology G Pilot Study of Intensive Chemotherapy With Peripheral Hematopoietic Cell Support for Children Less Than 3 Years of Age With Malignant Brain Tumors, the CCG-99703 Phase I/II Study. A Report From the Children’s Oncology Group. Pediatric neurology. 2015;53(1):31–46. doi: 10.1016/j.pediatrneurol.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunkel IJ, Boyett JM, Yates A, Rosenblum M, Garvin JH, Jr, Bostrom BC, Goldman S, Sender LS, Gardner SL, Li H, Allen JC, Finlay JL. High-dose carboplatin, thiotepa, and etoposide with autologous stem-cell rescue for patients with recurrent medulloblastoma. Children’s Cancer Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16(1):222–228. doi: 10.1200/JCO.1998.16.1.222. [DOI] [PubMed] [Google Scholar]
- 19.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. The New England journal of medicine. 2009;361(12):1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieran MW. Targeted treatment for sonic hedgehog-dependent medulloblastoma. Neuro-oncology. 2014;16(8):1037–1047. doi: 10.1093/neuonc/nou109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottardo NG, Hansford JR, McGlade JP, Alvaro F, Ashley DM, Bailey S, Baker DL, Bourdeaut F, Cho YJ, Clay M, Clifford SC, Cohn RJ, Cole CH, Dallas PB, Downie P, Doz F, Ellison DW, Endersby R, Fisher PG, Hassall T, Heath JA, Hii HL, Jones DT, Junckerstorff R, Kellie S, Kool M, Kotecha RS, Lichter P, Laughton SJ, Lee S, McCowage G, Northcott PA, Olson JM, Packer RJ, Pfister SM, Pietsch T, Pizer B, Pomeroy SL, Remke M, Robinson GW, Rutkowski S, Schoep T, Shelat AA, Stewart CF, Sullivan M, Taylor MD, Wainwright B, Walwyn T, Weiss WA, Williamson D, Gajjar A. Medulloblastoma Down Under 2013: a report from the third annual meeting of the International Medulloblastoma Working Group. Acta neuropathologica. 2014;127(2):189–201. doi: 10.1007/s00401-013-1213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Data Base On-Line Data Dictionary. American College of Surgeons, Commission on Cancer; 2014. [Google Scholar]
- 23.Bailey CC, Gnekow A, Wellek S, Jones M, Round C, Brown J, Phillips A, Neidhardt MK. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOP II. Medical and pediatric oncology. 1995;25(3):166–178. doi: 10.1002/mpo.2950250303. [DOI] [PubMed] [Google Scholar]
- 24.Packer RJ, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, Muraszko K, Rorke LB, Wara WM, Cohen BH, Boyett JM. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1999;17(7):2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 25.Packer RJ, Sutton LN, Elterman R, Lange B, Goldwein J, Nicholson HS, Mulne L, Boyett J, D’Angio G, Wechsler-Jentzsch K, et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. Journal of neurosurgery. 1994;81(5):690–698. doi: 10.3171/jns.1994.81.5.0690. [DOI] [PubMed] [Google Scholar]
- 26.Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff JE, Kortmann RD, Kuehl J. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. The New England journal of medicine. 2005;352(10):978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 27.Thomas PR, Deutsch M, Kepner JL, Boyett JM, Krischer J, Aronin P, Albright L, Allen JC, Packer RJ, Linggood R, Mulhern R, Stehbens JA, Langston J, Stanley P, Duffner P, Rorke L, Cherlow J, Friedman HS, Finlay JL, Vietti TJ, Kun LE. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18(16):3004–3011. doi: 10.1200/JCO.2000.18.16.3004. [DOI] [PubMed] [Google Scholar]
- 28.Eberhart CG, Kepner JL, Goldthwaite PT, Kun LE, Duffner PK, Friedman HS, Strother DR, Burger PC. Histopathologic grading of medulloblastomas: a Pediatric Oncology Group study. Cancer. 2002;94(2):552–560. doi: 10.1002/cncr.10189. [DOI] [PubMed] [Google Scholar]
- 29.Burger PC, Scheithauer BW, Armed Forces Institute of Pathology (U.S.), Universities Associated for Research and Education in Pathology . Tumors of the central nervous system Atlas of tumor pathology. Armed Forces Institute of Pathology ;under the auspices of Universities Associated for Research and Education in Pathology Inc.; Washington, D.C.: 1994. 3rd ser., fasc 10. [Google Scholar]


