Abstract
The ability of cancer cells to survive and grow under hypoxic conditions has been known for decades, but the mechanisms remain poorly understood. Under certain conditions, cancer cells undergo changes in their bioenergetic profile to favor mitochondrial respiration by activating the peroxisome proliferator-activated receptor gamma (PGC-1α) and upregulating mitochondrial biogenesis. In this study, we hypothesized that augmented mitochondrial biogenesis plays a critical role for cancer cells to survive hypoxia. Consistent with this hypothesis, both hypoxic human hepatocellular carcinoma (HCC) tumors and HCC cell lines subjected to hypoxia increase mitochondrial biogenesis. Silencing of PGC-1α in hypoxic HCC cell lines halts their proliferation. Mechanistic investigations in vitro indicated that intracellular High Mobility Group Box (HMGB)-1 protein, a nuclear protein overexpressed in HCC, is essential for the process. Silencing of HMGB1 in hypoxic HCC cell lines resulted in a significant decrease in PGC-1α activation and mitochondrial biogenesis. Without HMGB1, hypoxic HCC cells had significantly reduced ATP production and decreased cellular proliferation and increased apoptosis. In a diethynitrosamine (DEN)-induced murine model of HCC, genetic blocking of HMGB1 in the hypoxic tumors resulted in a significant decrease in tumor growth. Tumors lacking HMGB1 had a significant reduction in mitochondrial biogenesis and a significant increase in mitochondrial dysfunction. Further in vitro mechanistic experiments indicated that, during hypoxia, HMGB1 translocates from the nucleus to the cytoplasm and binds to cytoplasmic Toll-like receptor (TLR)-9. This binding leads to the activation of p38 and subsequent phosphorylation of PGC-1α with resultant upregulation of mitochondrial biogenesis.
Conclusion
Taken together, our findings suggest that during hypoxia HMGB1 upregulates mitochondrial biogenesis in HCC cancer cells promoting tumor survival and proliferation.
Keywords: cancer metabolism, oxidative phosphorylation, cancer energy, mitochondrial dysfunction, Liver cancer
INTRODUCTION
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide (1). The incidence in the United States is rising and the overall 5-year survival is only 16%. Reasons for this poor prognosis include severe underlying liver disease, delay in diagnosis and the lack of effective therapy (2). But most importantly, the dismal outcomes are closely related to the aggressive and highly proliferative capacity of HCC cells (3), which has, in turn, been linked to the continuous oxidative stress and chronic inflammation generated in the hepatic tumor microenvironment (4). In HCC, regions of hypoxia are commonly found throughout the rapidly growing tumor with areas of necrosis, irregular blood flow and poor oxygen diffusion (5, 6). The link between hypoxia and tumor progression has long been recognized. Indeed, the extent of tumor hypoxia has been shown to confer worse prognosis in patients with solid tumors (7) but mechanisms by which HCC tumors thrive despite the harsh hypoxic microenvironment remain incompletely understood.
It has long been accepted that dividing cancer cells meet their metabolic demands through the ‘Warburg Effect’, the process of aerobic glycolysis (8, 9). The energy generated through aerobic glycolysis was once thought to be sufficient to supply the anabolic energy demands of a rapidly dividing cancer cell (8, 9). More recently, however, cancer cells have been shown to supplement the energy provided by aerobic glycolysis by upregulating mitochondrial respiration for a major fraction of their ATP (10). Thus cancer cells seem to have the capacity to reprogram their bioenergetic profiles in response to unfavorable microenvironmental conditions (10–13). In fact, cancer cells in the process of metastasis and invasion exhibit downregulation of glycolysis pathways and enhanced oxidative phosphorylation by inducing mitochondrial biogenesis (13). Whether HCC growth under severe hypoxic conditions depends on mitochondrial biogenesis is unknown.
Mitochondrial biogenesis is a potential alternative for cancer cells to employ to reprogram their metabolic pathways under adverse conditions. Mitochondrial biogenesis would allow the cancer cells to increase oxidative phosphorylation in mitochondria by growth and division of existing mitochondria. Even though evidence shows that metabolic reprogramming towards oxidative phosphorylation and mitochondrial biogenesis is important for metastasis (13) and tumor growth (11), the exact mechanisms governing it remain unclear.
High Mobility Group Box 1 (HMGB1) is a highly conserved DNA-binding nuclear protein that acts as a damage associated molecular pattern (DAMP) protein in response to stress (14). HMGB1 plays a major role in conditions of inflammation accompanied by mitochondrial abnormalities, including neurodegeneration, aging and cancer (15). HMGB1 is overexpressed in HCC tumors and serum of patients with HCC (16). The hypoxic environment generated in solid tumors, including HCC, induces the translocation of HMGB1 from the nucleus of tumor cells to the cytoplasm and thence to the extracellular space (6, 16). We have previously shown that cytoplasmic HMGB1 activates Toll-like receptor (TLR)-9 in hypoxic HCC cells and that the interaction is critical for cancer cell proliferation and survival under stressful conditions (6). TLR9 is an endosomal receptor that is widely overexpressed in many tumors including HCC where hypoxia is common and it is also overexpressed in HCC cell lines subjected to hypoxia (6, 17, 18). Of interest, TLR9 has been previously shown to be important in inducing mitochondrial biogenesis in hepatocytes under experimental septic conditions (19). Whether HMGB1/TLR9 interactions in hypoxic cancer cells can also foster mitochondrial biogenesis and possibly enhance tumor growth has not previously been studied.
In this study we demonstrate that HCC cells adapt to hypoxic conditions by upregulating their mitochondrial biogenesis. During hypoxia, HMGB1 quickly translocates to the cytosol and interacts with TLR9. The activation of TLR9 results in triggering the signaling cascade involved in mitochondrial biogenesis, with subsequent increased cancer proliferation under harsh hypoxic conditions both in vitro and in vivo.
METHODS
Patient samples and cell lines
All human materials used were obtained under an approved Institutional Review Board protocol. Written informed consent was received from all participants prior to inclusion. Tumor and adjacent non-tumor tissues were collected from patients undergoing surgery for HCC at the University of Pittsburgh. Hepa-1-6 and Huh7 cell lines were purchased from ATCC (Manassas, VA). Cell lines were amplified in our lab and stored in liquid nitrogen to ensure that cells used for experiments were passaged for fewer than 6 months. Cell lines were tested biannually for identity by appearance and growth curve analysis and validated to be mycoplasma free. Cancer cells were transferred to a hypoxia chamber (1% O2) when needed.
Animals
Animal protocols were approved by the institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guidelines. Male wild-type (HMGB1loxP/loxP) mice and hepatocyte HMGB1 depleted (Alb-HMGB1−/−) mice were bred at our facility. All mice developed were on a C57BL/6 genetic background. The generation of the control mice (HMGB1loxP/loxP) and the Alb-HMGB1−/− mice and the confirmation and characterization of the mice are described in detail in our previous work (20). Briefly, the HMGB1loxP allele was created by inserting loxP sites within intron 1 and 2, flanking exon 2 of HMGB1. Homozygous HMGB1loxP mice were generated using Ozgene (Bently, WA). HMGB1loxP/loxP mice were interbred with stud males (HMGB1loxP/−;Alb-cre) to generate the desired phenotype. Mice homozygous for Cre recombinase linked to the albumin (alb) promoter are commercially available from Jackson.
Diethylnitrosamine (DEN) model of murine HCC
Two week-old male pups, Control (HMGB1loxP/loxP) and Alb-HMGB1−/−, were injected with DEN 25 mg/kg (in 0.9% saline) intraperitoneally. Mice were sacrificed six months later and the livers were harvested for analysis.
Silencing of HMGB1 and PGC-1α in cancer cells
HMGB1 and PGC-1α siRNA (Santa Cruz Biotechnology) were transfected into cancer cells using transfection reagent (Santa Cruz) according to manufacturer’s instruction. Control sequences purchased from Santa Cruz were included as a negative control. After 48h cells were collected and treated accordingly.
Measurement of Oxygen Consumption rate (OCR)
Cancer cells were plated in XF96 cell culture (Seahorse bioscience) microplates at the density of 10K per well, total volume of 200ul per well. Cells were incubated at 37°C overnight and then treated with 24 hours of hypoxia (1% O2). OCR was measured by the XF96e machine. After measurement of basal OCR, cells were treated with 1.0uM oligomycin, 1.0uM FCCP and 10μM rotenone to generate a bioenergetic profile. All rates are shown with rotenone rate subtracted to correct for non-mitochondrial oxygen consumption (21).
Adenosine Triphosphate (ATP) measurement
Quantification of cellular ATP levels was performed using ATP assay kit (Abcam) as per manufacturer’s instructions (20).
Mitochondrial DNA (mtDNA) Quantification
Total DNA was harvested from cancer cells using DNeasy Blood and Tissue Kit. Quantitative PCRs were performed in triplicates using MicroAmp fast 96-well reaction plate (Applied Biosystems). Each reaction contained 50ng of DNA, 10ul of Power SYBER-Green PCR Master Mix (Applied Biosystem), 1ul of each sense and anti-sense primer, total of 20ul per well. The copy number of DNA encoding cytochrome c oxidase I (COX I) was measured and normalized by mouse β-actin using quantitative real-time PCR.
Statistical analysis
Results are expressed as either standard error of the mean (SEM) or mean standard deviation (SD). Group comparisons were performed using One-way ANOVA with post-hoc Tukey honestly significant difference (HSD) analysis and Student’s t-test. p <0.05 was considered to be statistically significant. Experimental data were collected from at least three independent experiments to avoid cell culture variation.
RESULTS
HCC cells exhibit enhanced mitochondrial biogenesis during hypoxia
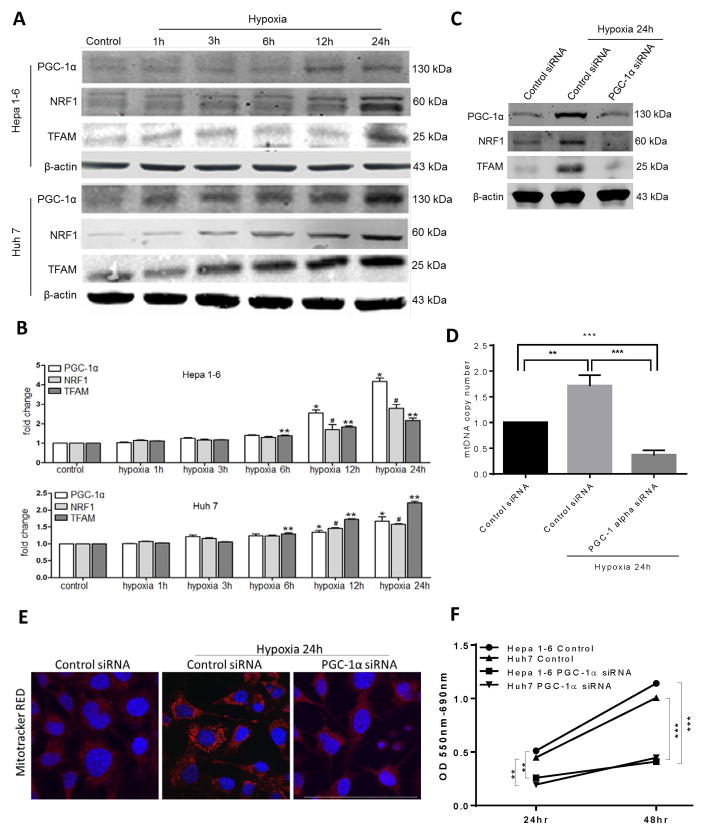
Some cancer cells can undergo metabolic reprogramming with upregulation of mitochondrial biogenesis and more reliance on oxidative phosphorylation under certain conditions (13). Peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α is a key transcription co-activator for mitochondrial biogenesis. After translocation to the nucleus, PGC-1α and nuclear respiratory factor 1 (NRF1) control the expression of transcription factor A (Tfam) (22). Tfam then translocates to the mitochondria to initiate mitochondrial DNA replication and biogenesis. We first looked at whether hypoxic HCC cells utilize this pathway to survive and grow under stress. Hypoxic murine HCC cells, Hepa1-6, and human HCC cell line, Huh7, are able to grow at comparable rates to respective HCC cells at normal conditions despite conditions of hypoxia (Supplementary Fig. 1A). Subjecting HCC cell lines to a time course of hypoxia (1% O2), we observed a time-dependent upregulation in the expression of PGC-1α, NRF1 and Tfam in both HCC cell lines as evidenced by both Western Blots (Fig 1A) and RT PCR analysis (Fig 1B). Of note, there was no significant difference in the expression of PGC-1α when HCC cells were subjected to atmospheric normoxia (21% O2) compared to physioxia (5% O2) (Supplementary Figure 1B). When PGC-1α was silenced using PGC-1α siRNA, there was a significant decrease in the expression of the proteins associated with mitochondrial biogenesis (PGC-1α, NRF1 and Tfam) (Fig 1C) and a significant reduction in mitochondrial biogenesis as assessed by reduced cellular mitochondrial DNA content (Fig 1D). Following 24 hours of hypoxia, there was a significant increase in mitochondrial density in hypoxic HCC cell lines compared to cells under normoxic culture conditions (Fig 1E). When PGC-1α was silenced, there was no increase in mitochondrial density (Fig 1E). Intracellular ROS production, a consequence of mitochondrial dysfunction when biogenesis is impaired, can induce apoptosis in cancer cells (23). Indeed, there was a significant increase in accumulation of reactive oxygen species (ROS) (Supplementary Figure 1C) and a decrease in proliferation (Fig 1F) of PGC-1α-silenced HCC cell lines subjected to hypoxia compared to HCC cell lines treated with control siRNA.
Figure 1. HCC cells exhibit enhanced mitochondrial biogenesis during hypoxia.
Upregulation of mitochondrial biogenesis associated proteins PGC-1α, TFAM and NRF1 in HCC cancer cell lines, Hepa1-6 and Huh7, subjected to a time course of hypoxia as seen by (A) Western Blot and (B) RT-PCR analysis. *, **, #p<0.05, comparisons made to control. (C) There was a significant decrease in the expression of the above proteins when PGC-1α was silenced in the hypoxic Hep1-6 cells compared to a scramble control siRNA. (D) Mitochondrial DNA (mtDNA) content in Hep1-6 cells undergoing 24h of hypoxia treated with either PGC-1α siRNA or control siRNA compared with control cells under normoxic conditions. **P<0.01, ***P<0.001. (E) Mitochondrial density was also measured using confocal microscopy. Mitotracker staining demonstrated increased mitochondrial density in Hepa1-6 cells subjected to 24h of hypoxia (0.32×103 μm2/nucleus in Control normoxia versus 2.32×103 μm2/nucleus in Control hypoxia versus 0.26×103 μm2/nucleus in HMGB1-siRNA hypoxia, p<0.05; Mitotracker (red), Nuclei (blue); Scale Bar: 50μm). Conversely, there was no increase in mitochondrial density when PGC-1α was silenced. (F) As a result of decreased mitochondrial biogenesis, MTT assay shows a significant decrease in proliferation among the Hep1-6 cells treated with PGC-1α siRNA compared to control cells.
HMGB1 facilitates PGC1-α expression and mitochondrial biogenesis in hypoxic cancer cells
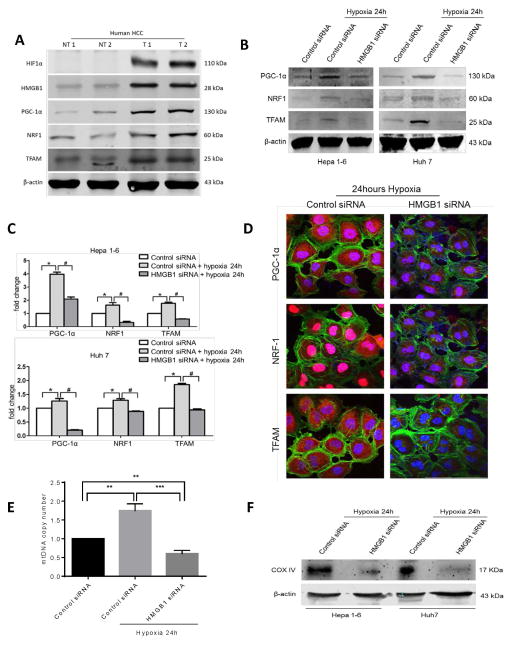
We next sought to explore the mechanism behind hypoxic-driven upregulation of mitochondrial biogenesis. HMGB1 is a nuclear protein that translocates to the cytoplasm in stressed cancer cells and is critical for cancer cell adaptation to hypoxic stress (6). We, therefore, next examined the role of HMGB1 in upregulating mitochondrial biogenesis in cancer cells. We compared the expression of HMGB1, PGC-1α, NRF1, Tfam and HIF-1 α in 15 paired human HCC tissue samples and their corresponding non-tumor liver tissue by Western blot analysis. There was increased expression of HMGB1, PGC-1α, NRF1 and Tfam in the hypoxic tumor tissue (expressing HIF-1α) compared to non-tumor tissue. Two representative pairs of the 15 pairs tested are shown in Fig 2A. In vitro, we silenced HMGB1 expression in both Hepa1-6 and HuH7 HCC cell lines using mouse and human HMGB1 siRNA (Supplementary Fig 2A). Western Blot, quantitative PCR analyses and immunofluorescent staining revealed significant downregulation of PGC-1α, NRF1 and Tfam in hypoxic HCC cells when HMGB1 was silenced (Fig 2B, Fig 2C and Fig 2D). There was no difference in PGC-1α expression between HMGB1 siRNA treated and control cells under normoxic conditions (Supplementary Fig 2B). Measurement of mitochondrial DNA copies revealed decreased mitochondrial density and thus inability of HMGB1 deficient cancer cells to upregulate mitochondrial biogenesis under hypoxic stress (Fig 2E). Similarly, we found decreased mitochondrial mass in HMGB1 silenced HCC cells by measuring levels of complex IV compared to control HCC cells after hypoxia (Fig 2F).
Figure 2. HMGB1 facilitates PGC1-α expression and mitochondrial biogenesis in hypoxic cancer cells.
(A) HIF-1α, HMGB1, PGC-1α, NRF1 and TFAM levels were measured using Western blot analysis in paired human HCC samples and their non-tumor counterparts. In humans there is increased HMGB1 expression and upregulation of mitochondrial biogenesis associated proteins within the hypoxic tumors (NT, nontumor liver; T, Tumor). Selected samples are representative of 15 unique paired samples. (B) There was a significant decrease in the expression of PGC-1α, NRF1 and TFAM when HMGB1 was silenced in the HCC cell lines compared to a scramble control siRNA when cells were subjected to 24h of hypoxia. (C) Real-time PCR analyses of the relative expression of the mitochondrial biogenesis associated genes in hypoxic control HCC cells normalized to normoxic control HCC cells, and hypoxic HMGB1 siRNA HCC cells. *, #p<0.05. (D) Using confocal microscopy, there is a significant decrease in the expression of the mitochondrial biogenesis associated protein in HMGB1 silenced Huh7 cells compared to control siRNA Huh7 cells after 24 hours of hypoxia. Mean PGC1-α area (μm2/nucleus): 16.7 in control versus 4.5 in HMGB1 siRNA; Mean NRF1 area (μm2/nucleus): 1.03×103 in control versus 0.16×103 in HMGB1 siRNA; Mean TFAM area (μm2/nucleus): 66.1 in control versus 12.8 in HMGB1 siRNA; p<0.05. PGC-1α, NRF1, TFAM (red), Nuclei (blue), β-actin (green); Scale Bar: 100μm. (E) Mitochondrial DNA (mtDNA) content in Hep1-6 cells undergoing 24h of hypoxia treated with either HMGB1 siRNA or control siRNA compared with control cells under normoxic conditions (arbitrarily set to 1). **P<0.01, ***P<0.001. (F) Mitochondrial density was also measured by Western blot analysis of mitochondrial protein COX IV in Hep1-6 and Huh7 cells treated with either control or HMGB1 siRNA.
Silencing HMGB1 expression in HCC cells impairs mitochondrial functional integrity and cell survival under hypoxic conditions
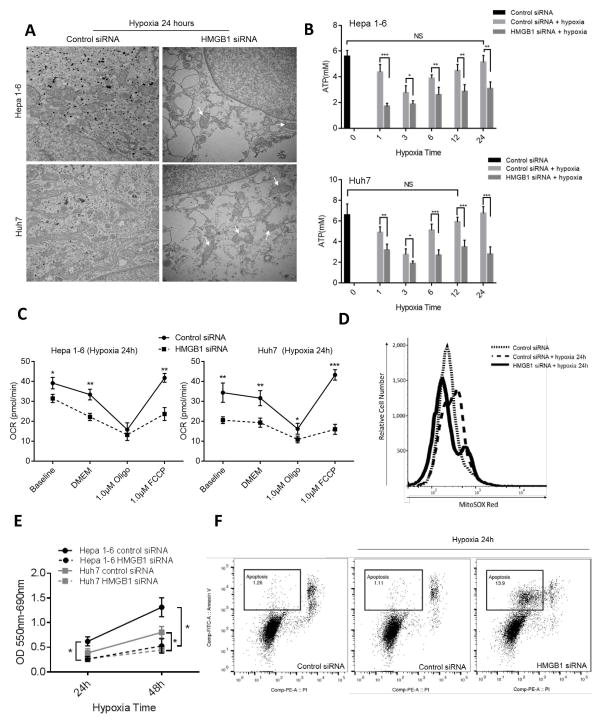
We next sought to study the effect of loss of silencing HMGB1 and its consequent loss of mitochondrial biogenesis has on the adaptation of cancer cells to hypoxia. We therefore studied the effect of HMGB1 siRNA on mitochondrial morphology, ATP production, oxygen consumption rate (OCR), ROS production and degree of proliferation and apoptosis of HCC cell lines exposed to hypoxia. Transmission electron microscopy (TEM) revealed reduced numbers of mitochondria and swollen mitochondria with disorganized cristae, suggesting impaired mitochondrial respiration when HMGB1 deficient cancer cells were subjected to hypoxia (Fig 3A). As shown in Fig 3B, ATP production levels in hypoxic tumor cells fall soon after the onset of hypoxia; then gradually increased to levels comparable to normoxic cancer cells. When HMGB1 is silenced, however, ATP levels do not recover and remain low.
Figure 3. Loss of HMGB1 results in decreased biogenesis and decreased survival of HCC cells under stress.
(A) Transmission electron microscopy of HCC cells treated with control or HMGB1 siRNA showing significantly decreased numbers of mitochondria with disrupted cristae (white arrows) in the hypoxic HMGB1-deficient Hepa1-6 and Huh7 cells. (B) Intracellular ATP levels in control and HMGB1 siRNA HCC cell lines in normoxia and at different time exposure to hypoxia. There is a significant decrease in ATP production between control and HMGB1 siRNA HCC cells at each time point of hypoxia. Control cells regain their baseline normoxic ATP production capacity at 12 and 24 hours in Huh7 and Hep1-6 cells, respectively. *P<0.05, **P<0.01, ***P<0.001, ns: Not Significant (C) Oxygen consumption rate (OCR) measurements in permeabilized control and HMGB1 siRNA treated HCC cells after 24 hours of hypoxia. Mitochondrial respiratory capacity is diminished in HMGB1-deficient cells (D) Cancer cells labeled with MitoSOX subjected to flow cytometric analysis show increased intracellular ROS when HMGB1 is silenced. (E) MTT assay at different time points for hypoxic Hepa1-6 and Huh7 cells after treatment with either control or HMGB1 siRNA. HMGB1 siRNA cells show significantly decreased proliferation at 24 and 48 hours. (F) Flow cytometry analysis after staining for annexin V to detect apoptosis in the different groups shows increased apoptosis after hypoxia in the HMGB1 deficient Hep1-6 cells with decreased mitochondrial biogenesis compared to control cells (13.9% vs. 1.11%).
We verified the impaired mitochondrial respiration by measuring OCR in a series of mitochondrial stress tests performed on HMGB1-depleted and control HCC cells using an extracellular flux analyzer. These experiments indicated that mitochondrial respiratory capacity was diminished when HMGB1 was suppressed (Fig 3C). Basal respiration rate was significantly decreased in hypoxic HMGB1 siRNA cells compared with control cells. In addition, there was a substantial decrease in maximal capacity (FCCP-stimulated OCR) when HMGB1 was silenced. Of note, we again found no significant changes in basal respiration rate between HMGB1 siRNA cells and control cells under normoxic conditions (Supplementary Fig. 2C). As expected in cells with impaired mitochondrial respiration, flow cytometry (Fig 3D) and immunofluorescent staining (Supplementary Fig. 2D) both showed a significant increase in ROS cellular production when HMGB1-silenced cells were stimulated with hypoxia. As a consequence of the metabolic derangements in the absence of HMGB1 and mitochondrial biogenesis, there was a significant decrease in cancer cell proliferation (Fig 3E) and a significant increase in apoptosis as seen of flow cytometry analysis (Fig 3F) and increased levels of cleaved caspase 3 (Supplementary Fig. 2E). Of note, we also found that when there was a decrease in the migratory and invasive ability of the HMGB1 deficient cancer cell lines that are not able to upregulate their mitochondrial biogenesis in response to hypoxia (Supplementary Fig. 2F). Similar to HMGB1-depleted cells, when PGC-1α is silenced in Hepa1-6 cells, there is an increased rate of apoptosis in the hypoxic cells (Supplementary Fig. 3A). The mitochondrial respiratory capacity was diminished when PGC-1α was suppressed. Basal respiration rate was significantly decreased in hypoxic PGC-1α siRNA cells compared with control cells. In addition, there was a substantial decrease in maximal capacity (FCCP-stimulated OCR) when PGC-1α was silenced (Supplementary Fig. 3B). Also, ATP levels do not recover and remain low when PGC-1α siRNA treated cells are subjected to hypoxia compared to control cells (Supplementary Fig. 3C). These experiments indicate that mitochondrial respiratory capacity and mitochondrial biogenesis are diminished when HMGB1 or PGC-1α was suppressed and that this translated into a lesser ability to survive harsh hypoxic stress.
HMGB1 loss in hepatocyte causes decreased tumor growth in mice in response to DEN
Our above findings demonstrate that HMGB1-induced mitochondrial biogenesis is critical for continued cancer cell survival under conditions of hypoxia in vitro. As hypoxia is prominent in HCC tumors, we next evaluated the in vivo behavior of tumors in the absence of HMGB1. We therefore created hepatocyte depleted HMGB1 knock out mice using Cre-LoxP technology (20).
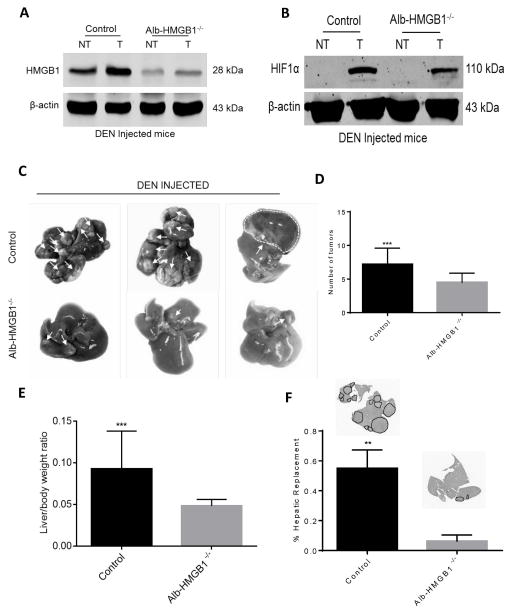
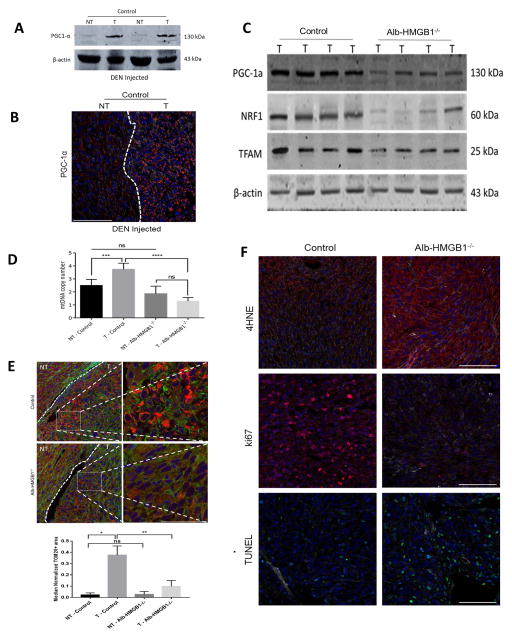
Control (HMGB1loxP/loxP) and Alb-HMGB1−/− mice were then subjected to DEN to promote formation of HCC. Both groups developed HCC. Six months after DEN injection, we confirmed that HMGB1 is depleted in both the tumor and non-tumor liver tissue in Alb-HMGB1−/−mice (Fig 4A). Fig 4A also shows that HMGB1 is overexpressed in tumor compared to non-tumor tissue in HMGB1 control mice. As anticipated, hypoxia was prominent in the tumors of both HMGB1 control and Alb-HMGB1−/−mice (Fig 4B). However, compared to HMGB1 control, Alb-HMGB1−/− mice exhibited smaller and less numerous tumors (Fig 4C and Fig 4D). In addition, Alb-HMGB1−/− mice had significantly decreased tumor load after DEN as evidenced by the liver-to-body weight ratio and the tumor hepatic replacement area (Figure 4E and 4F).
Figure 4. HMGB1 loss in hepatocytes causes decreased tumor growth in mice in response to Diethylnitrosamine (DEN).
(A) HMGB1 levels were significantly increased in tumor tissue compared to liver background in HMGB1 control (HMGB1loxP/loxP) mice injected with DEN 6 months prior. (NT, nontumor liver; T, Tumor). HMGB1 expression is nearly absent in tumor and non-tumor liver tissue in hepatocyte depleted HMGB1 knockout mice (Alb-HMGB1−/−). (B) HIF1α levels are increased in the tumors of both HMGB1 control and Alb-HMGB1−/− mice. (C) Representative images of hepatic nodules (white arrows) after 6 months of DEN treatment in Alb-HMGB1−/− and control mice (D) Alb-HMGB1−/− mice treated with DEN had significantly smaller and less numerous surface nodules compared with HMGB1 control mice (mean 4.5±0.3 nodules in Alb-HMGB1−/− versus 7.2±0.6 nodules in control HMGB1 mice; p<0.001). Alb-HMGB1−/− mice had a significant decrease in tumor burden compared to HMGB1 control mice as seen by (E) liver-to-body ratio (48% decrease in Alb-HMGB1−/− mice, p<0.001) and (F) percentage hepatic replacement by DEN-induced HCC tumors (mean percentage replacement 6.3±1.8% in control vs 55±5.5% in Alb-HMGB1−/−, p<0.01; area occupied by tumors represented as black dashed line). Data represent mean±SEM; n=16 mice/group. The above data are each representative of three experiments with similar results. NS: not significant, **P<0.01, ***P<0.001.
Alb-HMGB1−/− tumors after DEN show decreased adaptation to hypoxia and decreased mitochondrial biogenesis
Fig. 5A and Fig. 5B show that hypoxic DEN-induced HCC tumors in control mice showed significant upregulation of mitochondrial biogenesis as evidenced by increased expression of PGC1-α compared to normal liver background. In contrast, mitochondrial biogenesis pathways were not upregulated in the slow growing smaller tumors of Alb-HMGB1−/− mice (Fig. 5C). Of note, there is no significant difference in PGC1-α expression in non-tumor tissue of control mice compared to Alb-HMGB1−/−mice (Supplementary Fig. 4 A and B). Also, there was no difference in TOM20 staining between non-tumor tissue of control and Alb-HMGB1−/−mice (Supplementary Fig. 4C). There was a decrease in mitochondrial density in the hypoxic Alb-HMGB1−/− tumors with decreased mtDNA copies and decreased staining for mitochondrial marker TOM20 compared to control tumors (Fig. 5D and Fig 5E). In addition, there was a decrease in ND1 and COX3 expression in tumors of Alb-HMGB1−/−mice compared to controls (Supplementary Fig. 5A). Moreover, there was a significant decrease in the expression of PGC-1α, NRF, and TFAM in Alb-HMGB1−/− tumors compared to control (Supplementary Fig. 5B and C).
Figure 5. DEN-induced HCC tumors in Alb-HMGB1−/− mice show decreased mitochondrial biogenesis and decreased adaptation to hypoxia.
(A and B) DEN-induced HCC tumors exhibit increased expression of PGC-1α compared to background liver. (C) PGC-1α, NRF1 and TFAM protein tumor levels measured in HMGB1 control and in Alb-HMGB1−/− mice showing decreased expression in tumors lacking HMGB1. (D) There was a significant decreased in mtDNA copy numbers measured by RT-PCR in tumors lacking HMGB1. (T, Tumor; NT, Non-tumor). ***p<0.01, ****p<0.001, ns: not significant. (E) Confocal microscopy shows decreased mitochondrial density (TOM20 levels) in HMGB1 deficient tumors. The median normalized TOM20+ area in control non-tumors was 0.025 (Range 0.001–0.038) versus 0.384 (Range 0.261–0.474) in control tumors. When looking at Alb-HMGB1−/− tumors, the median normalized TOM20+ area in Alb-HMGB1−/− non-tumors was 0.019 (Range 0.0004–0.056) versus 0.073 (Range 0.0053–0.192) in Alb-HMGB1−/− tumors. Representative magnified (60X–1.75z) images included of both control and Alb-HMGB1−/− tumors (Scale Bars 50μm). (T, Tumor; NT, Non-tumor). *p<0.05, **p<0.01, ns: not significant. Scale Bars 100μm, Nuclei (blue), Actin (green), TOM20(red). The values were based on analysis of tumors from 6 mice for each group. (F) There is marked increase in oxidative stress and formation of 4-HNE in Alb-HMGB1−/− tumors compared to HMGB1 control tumors. Median (Range) 4-HNE area (μm2) normalized by actin area (μm2): 0.046 (0.002–0.067) in control mice versus 0.108 (0.051–0.3550) in Alb-HMGB1−/−, p<0.05. Alb-HMGB1−/− tumors had significantly ameliorated proliferation rates and increased apoptosis as evident by immunofluorescent staining for Ki67 (median 112 (57–143) Ki67+ cells/106 μm2 in control mice versus 28 (4–47) Ki67+ cells/106 μm2 Alb-HMGB1−/− mice; p<0.01) and TUNEL (median 8.7 (2-12.3) TUNEL+ cells/103μm2 in control mice versus 15.4 (8–32.7) TUNEL+ cells/103 μm2 in Alb-HMGB1−/− mice; p<0.05), respectively. Nuclei (blue), Actin (white), PGC-1α/4HNE/Ki67 (red), TUNEL (green), Scale Bars 100μm. n=6 mice/group
To examine whether Alb-HMGB1−/− tumors exhibit more oxidative damage in the setting of hypoxia and diminished mitochondrial biogenesis, we measured 4-HNE Michael adducts, a marker of oxidative stress. We found a marked increase in formation of 4-HNE in Alb-HMGB1−/− tumors compared to control (Fig. 5F). This was associated with decreased expression of the tumor proliferation marker Ki67 and an increase in the fragmented DNA and increased expression of cleaved caspase 3 both of which are characteristics of apoptosis (Fig 5F and Supplementary Fig. 5D). Altogether, the above results suggest that the loss of HMGB1 in HCC cells results in decreased mitochondrial biogenesis in response to hypoxia, with consequent increased oxidative stress, decreased proliferation and increased apoptosis. It also suggests that hypoxia and tumor growth act to provide mutually supportive environments in vivo.
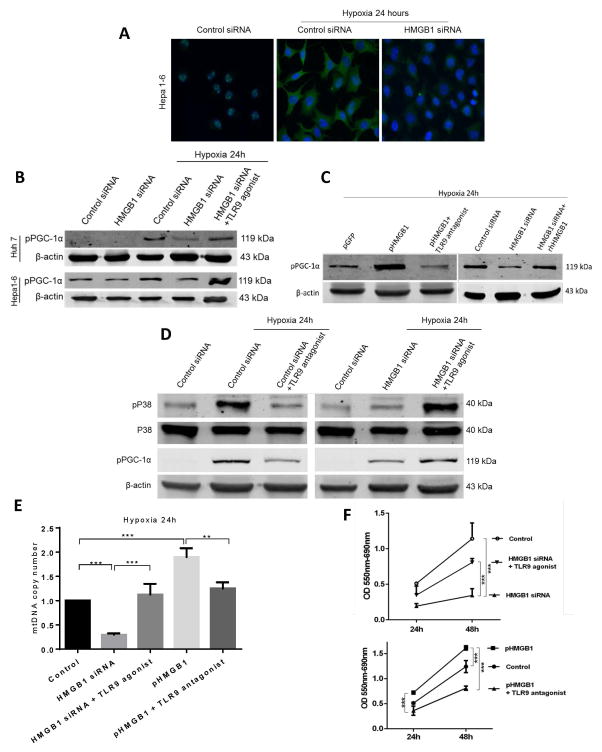
HMGB1 induces mitochondrial biogenesis in hypoxic cancer cells by translocating to the cytoplasm and activating TLR9 dependent pathways
We have shown that both in vitro and in vivo hypoxia leads HCC cells to upregulate PGC-1α expression and mitochondrial biogenesis in an HMGB1-dependent manner. However, the exact intracellular pathways initiated by HMGB1 to induce mitochondrial biogenesis have not been defined. Toll-like receptor (TLR) 9, a known cytoplasmic receptor for HMGB1, is overexpressed in HCC tumors and is known to promote cancer growth in response to hypoxia (6, 24–26). We therefore investigated whether HMGB1 stimulates TLR9 pathways to activate PGC-1α and induce mitochondrial biogenesis. We have previously shown that HMGB1 translocates from the nucleus to the cytoplasm in hypoxic cancer cells but not in normoxic cells (6). To confirm this, we subjected Hep1-6 cells to hypoxia and demonstrated that HMGB1 translocates to the cytoplasm, an effect silenced by HMGB1 siRNA (Fig. 6A). Supplementary Fig. 6A confirms our previous observation that HMGB1 associates with TLR9 in Hep1-6 cell lines subjected to hypoxia (6).
Figure 6. HMGB1 induces mitochondrial biogenesis in hypoxic cancer cells via a TLR9 dependent pathway.
(A) HMGB1 localizes in the nucleus in normoxic Hep1-6 cells. HMGB1 translocates to the cytoplasm of Hep1-6 cells during hypoxia. There is a decrease in cytoplasmic HMGB1 in hypoxic HMGB1 siRNA treated Hep1-6 cells. (B) PGC1-α phosphorylation (pPGC-1 α) was determined in Hepa1-6 and Huh7 cells treated with control or HMGB1 siRNA under normoxic or hypoxic conditions. PGC1-α activation was increased when control HCC cells were subjected to hypoxia but not in HMGB-deficient cells. Adding a TLR9 agonist bypasses the deficiency in HMGB1 and induces PGC-1α expression under the hypoxic conditions. (C) Hypoxic pHMGB1 Hep1-6 cells (stable overexpression of HMGB1) showed an increase in pPGC1-α compared to control pGFP Hep1-6 cells. PGC1-α activation was decreased when pHMGB1 Hep1-6 cells were pretreated with a TLR9 antagonist. The addition of extracellular recombinant HMGB1 increased PGC1-α activation in HMGB1-siRNA treated hypoxic Hepa1-6 cells (D) Levels of phosphorylated p38 and PGC1-α were determined for the different groups. P38 phosphorylation and subsequently pPGC1-α was decreased in HMGB1 deficient or TLR9 antagonist treated cells. Adding a TLR9 agonist rescues the activation of both p38 and PGC1-α in the HMGB1-deficient cells. (E) mtDNA content in the different groups after exposure to 24 hours of hypoxia shows an increase in mitochondrial density in the experimental groups that had an increase in PGC1-α activation when subjected to hypoxia. (F) MTT assays in the different groups shows that cancer cell proliferation is directly correlated with the degree of PGC1-α activation and upregulation of mitochondrial biogenesis.
PGC-1α activity and consequently the upregulation of mitochondrial biogenesis is not only determined by PGC-1α expression levels, but also by a number of post-translational modifications, such as phosphorylation (27–29). Fig. 6B shows that hypoxia is accompanied by increased phosphorylated PGC-1α (pPGC-1α) in Hep1-6 and Huh7 tumor cells, and that this upregulation is silenced by HMGB1 siRNA. However, activation of TLR9 by the TLR9 agonist markedly upregulates pPGC-1α even in the presence of HMGB1 specific siRNA. Of note, there were no differences in the levels of PGC-1α phosphorylation in normoxic HCC cells treated with control or HMGB1 siRNA (Fig 6B and Supplementary Fig. 6B). Fig. 6C demonstrates that stable HMGB1 expressing Hepa1-6 cells displayed a significant increase in activated PGC-1α after hypoxia compared to control cells (pGFP). Yet, the enhanced effect of pHMGB1 on pPGC-1α expression in Hep1-6 can be blocked by adding a TLR9 antagonist (Fig. 6C and Supplementary Fig. 6C). When recombinant HMGB1 (1μg/μl) was added to the HMGB1-siRNA treated cells, PGC-1α phosphorylation increased similar to control cells and thus mitochondrial biogenesis was rescued (Fig. 6C). Of note, the addition of the TLR9 agonist in combination with recombinant HMGB1 to HMGB1-depleted hypoxic cancer cells did not further increase PGC-1α activation as TLR9 receptors may have already been saturated with the recombinant HMGB1 (Data not shown). Furthermore, pretreatment of rhHMGB1 with DNase (Roche) did not alter the results of the above experiments suggesting that it is a direct effect of rhHMGB1 rather than any attached DNA during preparation (data not shown).
HMGB1 activation of TLR9 can initiate mitogen-activated protein kinase (MAPK) signaling (26) and activation of the p38 mitogen-activated protein kinase (MAPK) pathway has been previously shown to promote PGC-1α expression and mitochondrial biogenesis (22, 28, 29). Fig. 6D shows that hypoxic Hep1-6 cells exhibit an increase in the activation of both p38 and PGC-1α when subjected to hypoxia. Either the silencing of HMGB1 expression or the use of a TLR9 antagonist significantly decreases both p38 and PGC-1α activation (Fig. 6D). The ability of the TLR9 agonist to activate both p38 and PGC-1α is however retained. The levels of PGC-1α expression paralleled the levels of PGC-1α phosphorylation in all of the above experimental groups as evidenced by confocal microscopy (Supplementary Figure 7A). Furthermore, similar to HMGB1-depleted cells, there was a significant decrease in PGC-1α activation in hypoxic TLR9 siRNA treated cells compared to cells treated with control siRNA (Supplementary Figure 7B).
Fig. 6E and Fig. 6F indicate that increased mitochondrial biogenesis in these systems lead to increased mtDNA density and increased cell survival and growth. Taken together one can conclude that HMGB1 leaves the nucleus of hypoxic tumor cells and serves as an agonist of cytoplasmic TLR9, which acts with p38 to phosphorylate PGC-1a thereby facilitating mitochondrial biogenesis and cancer cell survival.
DISCUSSION
The death rate in the USA due to HCC is increasing faster than any other type of cancer (30). The inherent ability of HCC cells to thrive and proliferate despite hypoxic conditions is repeatedly cited as a major reason for this dreadful trend (6, 31). The ability of some tumors to shift their bioenergetic profile from glycolysis to oxidative phosphorylation in order to satisfy the energetic needs of a rapidly growing population of cells may offer a partial explanation for tumor growth in apparently adverse conditions. Understanding the mechanism by which this energy generating switch takes place would offer potential therapeutic targets in an area in which medical therapy is now lacking. We have presented here evidence that mitochondrial biogenesis plays a critical role in the ability of HCC cancer cells to survive hypoxia. Both human HCC tumors and HCC cell lines showed evidence of mitochondrial biogenesis by upregulation PGC-1a under hypoxic conditions. Furthermore, silencing of PGC-1α in hypoxic HCC cell lines halts proliferation. Mechanistic investigations in vitro indicated that intracellular HMGB1 was essential for increased mitochondrial biogenesis and tumor growth; silencing of HMGB1 in hypoxic HCC cell lines resulted in a significant decrease in PGC-1α activation, mitochondrial biogenesis, oxidative phosphorylation, ATP production, and mitochondrial integrity. Cellular proliferation decreased and apoptosis increased. In a diethynitrosamine (DEN)-induced murine in vivo model of HCC, genetic blocking of HMGB1 in the hypoxic tumors resulted in a significant decrease in tumor growth. Further in vitro mechanistic experiments indicated that, during hypoxia, HMGB1 translocates from the nucleus to the cytoplasm and binds to cytoplasmic Toll-like receptor (TLR)-9. This binding leads to the activation of p38 and subsequent phosphorylation of PGC-1α with resultant upregulation of mitochondrial biogenesis. Taken together, our findings suggest HMGB1 upregulates mitochondrial biogenesis in HCC cancer cells promoting tumor survival and proliferation through the stimulation of TLR9 signaling pathways (Figure 7). Although more studies are required to better understand the role of mitochondrial biogenesis in tumor progression, these results demonstrate that cancer growth under hypoxic conditions is largely dependent on mitochondrial biogenesis and identify HMGB1, TLR9 and PGC-1α as potential targets for therapeutic intervention.
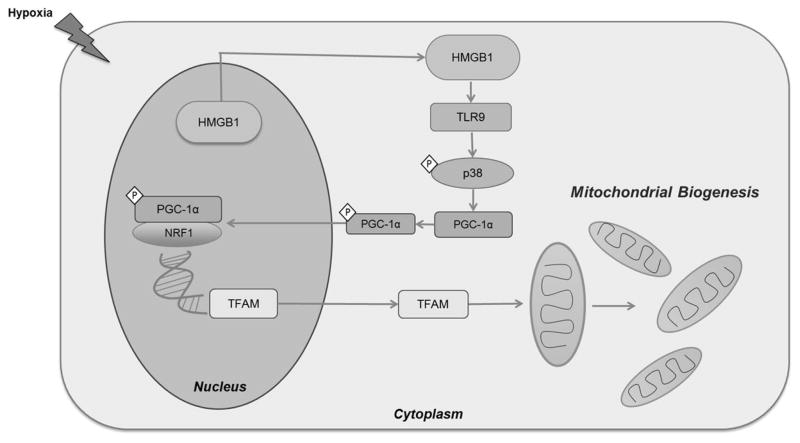
Figure 7. Schematic diagram illustrating the proposed role of HMGB1 and TLR9 in activating mitochondrial biogenesis in hypoxic cancer cells.
During hypoxia, HMGB1 translocated from the nucleus to the cytoplasm of HCC cells. In the cytoplasm, HMGB1 binds and activated TLR9 receptor. This consequently activates TLR9 associated MAP kinases including p38. This leads to the phosphorylation and activation of PGC1-α. Activated PGC1-α then translocates to the nucleus, binds to NRF and both act and transcription co-activators of TFAM. TFAM then translocated to the mitochondria to initiate the process of mitochondrial biogenesis.
The traditional view about cancer metabolism, namely that it depends on aerobic glycolysis to support the energy requirement for continued growth requires revision. In order to provide sufficient ATP some compensatory mechanism is required especially for solid tumors like HCC that prosper under hypoxic conditions. To meet the energy supply, the cells must increase the number of the mitochondria to produce more ATP. Recently, other researchers have highlighted the crucial role of mitochondrial biogenesis in several different cells facing stress. For instance, hepatocyte survival and maintenance of function in conditions of sepsis or in conditions of ischemia is dependent on mitochondrial biogenesis (19, 32). Furthermore, mitochondrial biogenesis is an essential repair mechanism tool utilized by ischemic brain cells (33). Thus, the elevation of PGC-1α expression and mitochondrial number found in other cells and in HCC cells in our study could also be a mechanism by which the cancer cells attempt to increase their energy output.
As solid tumors grow they create internal and peritumoral areas of hypoxia. Our findings suggest that in zones of tumor hypoxia cancer cells can upregulate their mitochondrial biogenesis to overcome the harsh environment and offer several advantages needed to pull through and thrive. For example, hypoxic cancer cells can accumulate ROS, which if not cleared, can lead the cancer cell to undergo apoptosis (34). Our findings suggest that mitochondrial biogenesis is critical to maintain mitochondrial proficiency and the ability to detoxify ROS to increase cancer cell viability during hypoxia. Another advantage is that hypoxic HCC cells can upregulate their mitochondrial biogenesis to transit to other areas with less hypoxia. LeBleu et al. have previously shown that by upregulating mitochondrial biogenesis, mammary epithelial cell lines enhance their migratory and invasive properties (13). Thus, upregulation of mitochondrial biogenesis and respiration may represent a mechanism HCC cells utilize to form intrahepatic and extrahepatic metastases in more favorable and less hypoxic environments. Increased proliferation also increases tumors’ ability to acquire further mutations which in turn leads to tumor heterogeneity and the emergence of neoantigens to which the host has had no immunologic experience (35). In addition, the changes in the energy profile of cancer cells probably impacts the surrounding tumor stroma which in turn may reciprocally impact the cancer cell’s growth. Furthermore, PGC-1α activation and mitochondrial biogenesis can support chemotherapy resistance in colorectal cancer cells (12) and this may also apply to HCC cells. Tumor heterogeneity could also contribute to chemotherapy resistance encountered in hypoxic tumors in general and HCC specifically. Therefore, further investigations to gain a better understanding of the advantages acquired when HCC cells upregulate mitochondrial biogenesis is warranted.
These experiments support the hypothesis that HMGB1 signaling is critical to the induction of biogenesis. This is not surprising, as this DAMP protein is known to play number of pleiotropic roles in inflammation, healing and cellular biology (14). HMGB1 can be mobilized and released from the nucleus of cancer cells during hypoxia and pass through the cytoplasm into the peritumoral tissue (6, 16). In cancer, HMGB1 has been described to play both protumorigenic and anti-tumorigenic roles (36). HMGB1 when present in the nucleus stabilizes the chromosomes and maintains telomeres length and can act as an anti-tumorigenesis protein. On the other hand, when secreted by cancer cells, extracellular HMGB1 had a predominately protumorigenic role of stimulating tumor growth and survival by promoting invasion, metastasis and angiogenesis (36). Indeed, our lab has previously shown that HMGB1 induced caspase-1 activation promoted invasion in hypoxia (16). As mitochondrial biogenesis is also critical for increasing tumor invasiveness (13), HMGB1 enhances the invasive potential of stressed cancer cells by supplying energy through its cytoplasmic role of upregulating mitochondrial biogenesis and by providing the tools by activating caspase 1 and the downstream effectors through its extracellular function (16).
The role of cytoplasmic HMGB1 in cancer cells had not been adequately defined in regards to tumor behavior. The novel finding in our experiments is the dependence of hypoxia-induced biogenesis on cytoplasmic HMGB1 and its receptor TLR9 in cancer cells. In HCC, HMGB1 seems to behave predominately as a protumorigenic protein both in hypoxic HCC cell lines and in vivo. We also provide further evidence for a mechanism by which HMGB1 contributes to HCC growth, i.e. by activation of endosomal TLR9 signaling to upregulate mitochondrial biogenesis. Of note, we have previously shown that during stress, mitochondria release its DNA (mtDNA) and complex with HMGB1 to activate TLR9 pathways to promote tumor proliferation by activating STAT pathways (6). In this paper, we show that activation of the TLR9 pathways can also induce mitochondrial biogenesis. This brings up an interesting future direction of whether mitochondria themselves can release cytoplasmic signals in the form of mitochondrial DNA to activate their own biogenesis.
During hepatic surgery to resect HCC, the liver is routinely subjected to injury due to ischemia resulting from the interruption of the hepatic blood supply that is often necessary to control blood loss (37). The hypoxia resulting may induce mitochondrial biogenesis in micrometastatic intrahepatic metastatic disease and may explain the suboptimal rates of disease free survival after resection (38). Death of tumor cells induced by chemotherapy and chemoembolization most likely leads to necrosis, hypoxia, HMGB1 release, mitochondrial biogenesis and tumor proliferation. Potentially palliative techniques such as ablation by heat, ethanol or cold depends for its effectiveness on tumor hypoxia; but what may result is mitochondrial biogenesis and more vigorous regrowth. Therefore, the combination of surgery and strategies to block mitochondrial biogenesis may prove to be a promising adjunct treatment to improve outcomes after resection of all but the smallest HCC tumors.
In summary, hypoxia-induced mitochondrial biogenesis in cancer cells, induced by the HMGB1-TLR9 pathway, acts to protect against cell damage and provide a survival mechanism for the cancer cells. Our studies provide insight into uncovered changes in the metabolic program of hypoxic cancer cells that could represent a potential therapeutic target in the fight against cancer.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the Americas Hepato-Pancreato-Biliary Association Research Award (S.T.), the National Cancer Institute Grant Number T32CA113263 (S.T.), National Institute of Health R01-GM95566 (A.T.) and 1S10OD019973-01 (Center of Biologic Imaging).
List of Abbreviations
- PGC-1α
peroxisome proliferator-activated receptor gamma
- HCC
hepatocellular carcinoma
- HMGB1
High Mobility Group Box-1
- DEN
diethylnitrosamine
- TLR
Toll-like receptor
- ATP
adenosine triphosphate
- DNA
deoxyribonucleic acid
- DAMP
damage associated molecular pattern
- Alb-HMGB1−/−
hepatocyte specific HMGB1 knockout
- siRNA
small interfering ribonucleic acid
- ROS
reactive oxygen species
- OCR
oxygen consumption rate
- mtDNA
mitochondrial DNA
- NRF1
nuclear respiratory factor-1
- Tfam
transcription factor A, mitochondrial
- RT PCR
real time polymerase chain reaction
- TOM20
translocase of outer membrane 20
- 4-HNE
4-hydroxynonenal
- MAPK
mitogen-activated protein kinase
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Sherman M. Epidemiology of hepatocellular carcinoma. Oncology. 2010 Jul;78( Suppl 1):7–10. doi: 10.1159/000315223. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016 Jan;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007 Jun;132(7):2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008 Jul 24;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 5.Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. Journal of cellular biochemistry. 2009 Aug 15;107(6):1053–62. doi: 10.1002/jcb.22214. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Yan W, Tohme S, Chen M, Fu Y, Tian D, et al. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J Hepatol. 2015 Jul;63(1):114–21. doi: 10.1016/j.jhep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer metastasis reviews. 2010 Jun;29(2):285–93. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009 May 22;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warburg O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 10.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer cell. 2012 Mar 20;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salem AF, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Mitochondrial biogenesis in epithelial cancer cells promotes breast cancer tumor growth and confers autophagy resistance. Cell cycle. 2012 Nov 15;11(22):4174–80. doi: 10.4161/cc.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vellinga TT, Borovski T, de Boer VC, Fatrai S, van Schelven S, Trumpi K, et al. SIRT1/PGC1alpha-Dependent Increase in Oxidative Phosphorylation Supports Chemotherapy Resistance of Colon Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 Jun 15;21(12):2870–9. doi: 10.1158/1078-0432.CCR-14-2290. [DOI] [PubMed] [Google Scholar]
- 13.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nature cell biology. 2014 Oct;16(10):992–1003. 1–15. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsung A, Tohme S, Billiar TR. High-mobility group box-1 in sterile inflammation. Journal of internal medicine. 2014 Nov;276(5):425–43. doi: 10.1111/joim.12276. [DOI] [PubMed] [Google Scholar]
- 15.Chan DC. Mitochondrial fusion and fission in mammals. Annual review of cell and developmental biology. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 16.Yan W, Chang Y, Liang X, Cardinal JS, Huang H, Thorne SH, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012 Jun;55(6):1863–75. doi: 10.1002/hep.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006 Feb 24;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 19.Carchman EH, Whelan S, Loughran P, Mollen K, Stratamirovic S, Shiva S, et al. Experimental sepsis-induced mitochondrial biogenesis is dependent on autophagy, TLR4, and TLR9 signaling in liver. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013 Dec;27(12):4703–11. doi: 10.1096/fj.13-229476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H, Nace GW, McDonald KA, Tai S, Klune JR, Rosborough BR, et al. Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection. Hepatology. 2014 May;59(5):1984–97. doi: 10.1002/hep.26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardenes N, Corey C, Geary L, Jain S, Zharikov S, Barge S, et al. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014 May 1;123(18):2864–72. doi: 10.1182/blood-2013-09-529420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovascular research. 2008 Jul 15;79(2):208–17. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 23.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature reviews Drug discovery. 2009 Jul;8(7):579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 24.Chen R, Alvero AB, Silasi DA, Mor G. Inflammation, cancer and chemoresistance: taking advantage of the toll-like receptor signaling pathway. American journal of reproductive immunology. 2007 Feb;57(2):93–107. doi: 10.1111/j.1600-0897.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 25.Akira S, Takeda K. Toll-like receptor signalling. Nature reviews Immunology. 2004 Jul;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 26.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Lougharn P, Mowen K, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer research. 2016 Jan 12; doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007 Jun 21;447(7147):1012–6. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 28.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jul 17;104(29):12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Molecular cell. 2001 Nov;8(5):971–82. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 30.Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016 Mar 9; doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu XZ, Xie GR, Chen D. Hypoxia and hepatocellular carcinoma: The therapeutic target for hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2007 Aug;22(8):1178–82. doi: 10.1111/j.1440-1746.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu B, Zhang R, Tao G, Lehwald NC, Liu B, Koh Y, et al. Augmented Wnt signaling as a therapeutic tool to prevent ischemia/reperfusion injury in liver: Preclinical studies in a mouse model. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2015 Dec;21(12):1533–42. doi: 10.1002/lt.24331. [DOI] [PubMed] [Google Scholar]
- 33.Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J. Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke; a journal of cerebral circulation. 2008 Nov;39(11):3057–63. doi: 10.1161/STROKEAHA.108.520114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nature reviews Cancer. 2011 May;11(5):325–37. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 35.McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016 Mar 25;351(6280):1463–9. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang R, Zhang Q, Zeh HJ, 3rd, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 Aug 1;19(15):4046–57. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahbari NN, Wente MN, Schemmer P, Diener MK, Hoffmann K, Motschall E, et al. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. The British journal of surgery. 2008 Apr;95(4):424–32. doi: 10.1002/bjs.6141. [DOI] [PubMed] [Google Scholar]
- 38.Tohme S, Geller DA, Cardinal JS, Chen HW, Packiam V, Reddy S, et al. Radiofrequency ablation compared to resection in early-stage hepatocellular carcinoma. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2013 Mar;15(3):210–7. doi: 10.1111/j.1477-2574.2012.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.