Abstract
There is general consensus that spinal cord injuries (SCI) above T6 result in altered sympathetic control of the heart, which negatively influences cardiac structure and function. To by-pass disrupted circuitry and investigate cardiac responses under enhanced sympathetic activity we utilized dobutamine (DOB) stress echocardiography. Animals were divided into a T2, 25 g-cm contusive SCI (SCI) or an uninjured control (CON) group. Echocardiography was performed pre-SCI and at 1, 2 and 6 weeks post-SCI. Increasing doses of DOB (5, 10 & 20 µg/min/kg) were infused intravenously pre-SCI and at 1 and 6 weeks post-SCI. Parasternal-short axis images were used to compare group differences in systolic function and track changes in response to SCI and DOB over time. One week post-SCI, stroke volume (SV), end diastolic volume (EDV), cardiac output (CO) and ejection fraction (EF) were all reduced compared to CON and these deficits persisted to 6weeks. We also found an increase in collagen deposition at 6 weeks post SCI. Pre-SCI, DOB elicited a decrease in EDV and increases in CO, EF and HR but not SV. At 6 weeks following SCI, in addition to increases in CO, EF and HR, DOB also induced increases in SV. This is the first report, to our knowledge, of DOB responses in a contusive SCI model with persistent cardiac impairments. The return of CO to pre-SCI levels and the substantial increase in SV at low DOB dosages shows that impaired descending control of the heart is directly contributing to reduced resting SV after SCI.
Keywords: Echocardiography, Cardiac, Spinal cord injury
1. Introduction
Cardiovascular (CV) dysfunction is the leading cause of morbidity and mortality in the chronic SCI population (Garshick et al., 2005). Following high thoracic and cervical injuries descending sympathetic control of the vasculature and heart is disrupted leading to a dysregulation of blood pressure and heart rate (HR) and ultimately to cardiovascular decline. Cardiac dysfunction is further compromised by a reduction in demand due to immediate immobility and prolonged inactivity. In individuals with chronic tetraplegia, for example, cardiac atrophy (Kessler et al., 1986) is prevalent but can be partially reversed with appropriate training (Nash et al., 1991).
Despite the prevalence of cardiac dysfunction in spinal cord injured individuals (Hopman et al., 1992; Kessler et al., 1986), little is known about cardiac systolic function independent of the disrupted spinal circuitry. West et al. demonstrated that animals with a complete spinal transection at T3 have a blunted ability to develop LV pressure during periods of increased filling (West et al., 2014) and Lujan et al. demonstrated that following a T5 transection there is increased sympathetic support of HR and rates of contraction (Lujan et al., 2012). It is important to note that while the transection model disrupts all descending and ascending circuitry this model does not reflect the majority of clinical injuries which are anatomically incomplete even when deemed functionally complete (according to the National Spinal Cord Injury Statistical Center).
Dobutamine stress echocardiography (DSE) is a clinical technique used to investigate how the heart responds to drug-induced increases in sympathetic activation (Krahwinkel et al., 1997). Since Dobutamine is a sympathomimetic drug that primarily targets β-1 receptors, it stimulates chronotropy and inotropy (Ruffolo, 1987). DSE is classically used as a means to investigate cardiac regional wall motion for detection of coronary artery blockage, ischemia, and myocardial viability (Leite et al., 2015; Lualdi and Douglas, 1997; Pellikka et al., 1995; Wu et al., 2004). This clinical technique has recently been applied to the rodent (Plante et al., 2005) and is being implemented in experimental models to evaluate cardiac dysfunction in a variety of diseases/pathologies (Leite et al., 2015; Schneider et al., 2010). Moreover, the rodent responses to DOB infusion strongly resemble those in humans (Plante et al., 2005), most likely because the sympatho-excitatory pathways that innervate the heart are similar across rodents and humans making DSE a highly translatable technique.
The primary objective of this study was to investigate cardiac function and functional reserve following high-thoracic SCI by increasing sympathetic activation with a sympathetic agonist (Dobutamine).
2. Methods
Experiments were conducted on 14 female Sprague-Dawley (SD) rats (age = 15 wks, weight 250–260 g). Group size was determined from power analysis calculations. Based on previous work we would expect a group difference in SV (SD=27.62–44.46), the power to detect a true significant difference equals 80.4–85% with a sample size of approximately 6–8. All procedures were approved by the University of Louisville Animal Care and Use Committee. Animals were randomly assigned to one of two groups: uninjured control (CON n = 6) or T2 25 g-cm SCI (SCI n = 8). Cardiac function was assessed prior to SCI and at 1, 2 and 6 weeks post-SCI. Dobutamine stress echocardiography (DSE) was conducted pre-SCI and at post-SCI weeks 1 and 6. Hindlimb function during overground locomotion was assessed weekly using the BBB Open Field Locomotor Scale as described previously (Basso et al., 1995; Magnuson et al., 2009).
For SCI surgery, animals were anesthetized with a ketamine/xylazine/acepromazine cocktail (50/0.024/0.005 mg/kg i.p.) and a dorsalmid-line incision was made through the skin and musculature overlying the C8 to T4 spinal segments. A laminectomy was performed at T1 and T2 to expose the underlying T2 spinal cord and moderately-severe injuries (25 g–cm) were delivered with a MASCIS Impactor (Rutgers University, NJ). All animals were given Buprenorphine (0.1 mg/kg, SC) twice a day for three days, gentamycin (Gentamicin sulfate 15 mg/kg SC) once a day for seven days, and 5 ml of Lactated Ringers for five days and as needed for hydration. Bladders were expressed manually for five days or until their bladders emptied spontaneously. After recovery, animals were housed socially, two per cage, on the same 12-h light/dark cycle.
2.1. Dobutamine stress echocardiography (DSE) assessments
During DSE assessments, animals were maintained at surgical levels of isofluorane anesthesia (1.5–2%). Core body temperature was maintained at 36–37 °C and ventilation was monitored with inductance plethysmography. The tail vein was cannulated with a 25-gauge butterfly needle for drug administration. Images were captured along the parasternal short-axis (SAX) at the midventricular level with a high-resolution ultrasound imaging system (VisualSonics VEVO 2100) and probe (24 MHz) secured in a stereotactic stand (VisualSonics). Before drug administration, a pre-DOB image was captured for baseline systolic and diastolic measures. DOB was infused at progressively increasing dosages (5, 10 and 20 µg/kg/min; rates of: 2.10, 4.25 and 8.55 ml/h) for 4 min each, using an automated perfusion pump (KD Scientific, Holliston, MA). Four minutes of continuous drug infusion have previously been shown to elicit a maximal response at each dose (Plante et al., 2005). M-mode images were captured at the end of each four-minute administration. Anesthesia was then discontinued and the animals were monitored until fully recovered. Results for 10 cardiac cycles during expiration along the SAX were averaged for between group and dose response comparisons (West et al., 2014).
2.2. Histology
For histological analyses, animals were perfused with phosphate buffered saline via the ascending aorta to preserve cardiac tissue. The heart was then cleaned of excess fat and vessels and was weighed before being placed in 4% PFA. After 24 h in fixative, hearts were cryoprotected in 30% sucrose with sodium azide for two days and then blocked in cryoprotective media. The entire spinal column was also extracted and placed in 4% PFA for three days. The SC was then removed from the column and cryoprotected in 30% sucrose for at least 48 h.
Hearts were sectioned at 10 µm and processed for collagen deposition with conventional Masson's Trichrome stain. Images were captured at 20× magnification from the left ventricle free wall from five different sections at least 70 µm apart using consistent camera settings. Collagen deposition was quantified as a percent of the total area (40 µm2) of the image. Specifically, images were set to a threshold to identify collagen-positive tissue and the area of collagen-positive tissue was divided by the total area of the image (in pixels). The percent of collagen was averaged across the five images captured per animals to obtain one data point per animal.
Spinal Cords (SC) were sectioned through the epicenter at 30 µm. Sections were allowed to air dry for 30 min before storing at 4 °C overnight. The following day, SC sections were warmed for 20 min and processed for spared white matter using Eriochrome Cyanin (EC) stain as described previously (Smith et al., 2006).
2.3. Statistical analysis
Repeated measures analyses of variance (RM ANOVAs) were preformed to determine significant main effects and significant interactions between the main effects. For all analyses, parametric ANOVA assumptions were tested (normality and Mauchly's sphericity test). The Greenhouse-Geisser correction was used to adjust degrees of freedom and correct the p value when the variance test was significant revealing unequal variance. Following significant main effects, Tukey HSD post hoc t-tests for multiple comparisons were performed on the relevant comparisons of interest to decrease the occurrence of type 1 errors.
Between group comparisons (CON vs. SCI) for echocardiography assessments without Dobutamine were analyzed with RM ANOVA with one factor for time post-SCI (repeated) and one factor for group (independent). Dobutamine stress echocardiography responses were analyzed using RM ANOVA with repeated factors for time post-SCI (i.e. pre-SCI, 1 and 6 weeks post-SCI) and dose.
Anatomical parameters were analyzed with Independent t-tests between means with equal or unequal variance, as appropriate. Statistical analyses were performed with SPSS (v22). Data are displayed as means ± standard deviation (SD). Significance was set at P ≤ 0.05.
3. Results
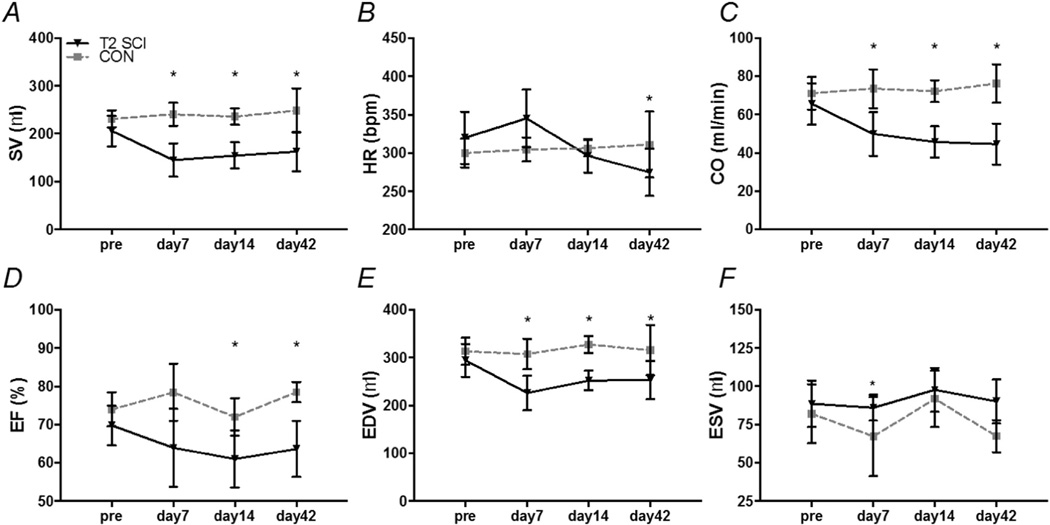
Our between group analysis revealed that stroke volume (SV), end diastolic volume (EDV) and cardiac output (CO) were all reduced at one week post-SCI compared to age matched CON (Fig. 1A, C & E; all P < 0.05). At two weeks post-SCI ejection fraction (EF) was also reduced compared to CON (Fig. 1D; P < 0.05). By six weeks post-SCI, cardiac flow indices (SV, CO & EF) and HR were all diminished compared to CON levels (Fig. 1A–D; all P < 0.05). End diastolic volume was reduced compared to CON (Fig. 1E; P < 0.05) although end systolic volume was no different. Relative wall thickness (RWT) and posterior wall thickness (PWT) remained unchanged after SCI.
Fig. 1.
Cardiac function of T2 25 g-cm spinal cord injured (SCI) female SD rats and age matched uninjured controls (CON) followed for six weeks post-SCI. A, stroke volume (SV). B, heart rate (HR). C, cardiac output (CO). D, ejection fraction (EF). E, end diastolic volume (EDV). F, end systolic volume (ESV). Data are represented as means ± standard deviations. * P ≤ 0.05 SCI vs. CON.
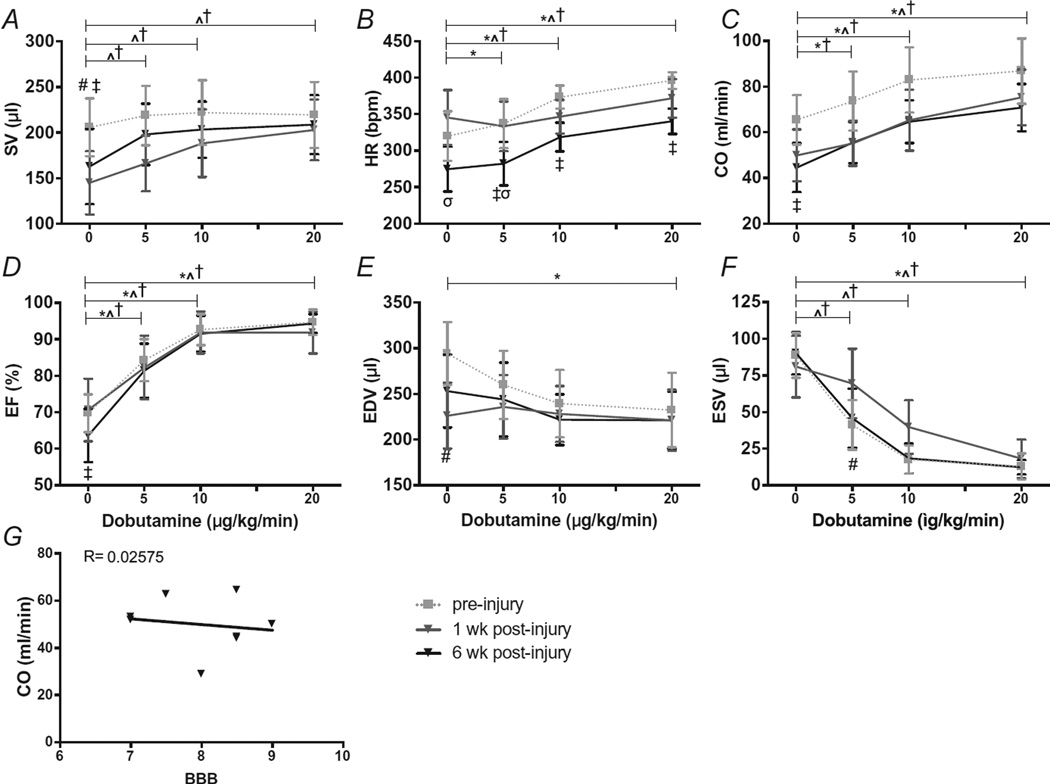
Our within group analysis revealed that with DOB administration pre-SCI there was a dose-dependent increase in HR and CO (Fig. 2B, C; P < 0.05 0 vs. 5, 10 & 20 µg) but not in SV (Fig. 2A; P = 1.0). End systolic and diastolic volumes (ESV, EDV) decreased with increasing concentrations of DOB (Fig. 2E, F; P < 0.01 0 vs. 20 µg), which resulted in an increase in the overall EF (Fig. 2D; P < 0.05 0 vs. 5, 10& 20 µg). Responses to DOB in the CON group were synonymous to those at pre-SCI and did not differ over time.
Fig. 2.
(A–F) Responses to increasing doses of Dobutamine pre-SCI and at one and six weeks post-SCI. A, stroke volume (SV). B, heart rate (HR). C, cardiac output (CO). D, ejection fraction (EF). E, end diastolic volume (EDV). F, end systolic volume (ESV).G, correlation of locomotor function with CO six weeks post-SCI (Pearson correlation R = 0.02575). There was a significant main effect for dose and time in all outcome measures (P < 0.05). There was a significant interaction effect for time and dose in all outcome measures (P < 0.05). Data are represented as means ± standard deviations. * P < 0.05 pre-SCI 0 µg vs. all other doses, ^ P < 0.051week post-SCI0 µg vs. all other doses, † P < 0.05 6week SCI 0 µg vs. all other doses, # P < 0.05 pre-SCI vs. 1 week post-SCI, ‡ P < 0.05 pre-SCI vs. 6 weeks post-SCI, σ P < 0.05 1 week post-SCI vs. 6 weeks post-SCI.
At one and six weeks post-SCI, DOB administration elicited a dose-dependent increase in HR and CO (Fig. 2B, HR P < 0.01 0 vs. 10 and 20 µg; C, CO P < 0.01 0 vs. 10 & 20 µg) as seen pre-SCI. Ejection fraction also increased with DOB at one and six weeks post-SCI (Fig. 2D; P < 0.05 0 vs. 5 µg; P < 0.01 0 vs. 10 and 20 µg). End systolic volume decreased with increasing DOB concentrations (Fig. 2. F; all P < 0.01 0 vs. 5, 10 & 20 µg) and while there appeared to be a decrease in EDV, the changes were not statistically significant. Unlike pre-SCI responses, DOB induced a substantial increase in SV at one and six weeks post-SCI (Fig. 2A; P < 0.05 0 vs. 5; P < 0.01 0 vs. 10 & 20 µg). In fact, SV and CO were no longer different from pre-SCI baseline (0 µg) with 5 µg/kg dose of DOB (P = 0.058 and 0.056 respectively). We also found an interaction effect between group and dose whereby the magnitude of change in CO from baseline (0 µg) to 5 µg was much larger 6 weeks post-SCI group pre-SCI (Fig. 2A&C; P < 0.005 interaction effect between time and dose).
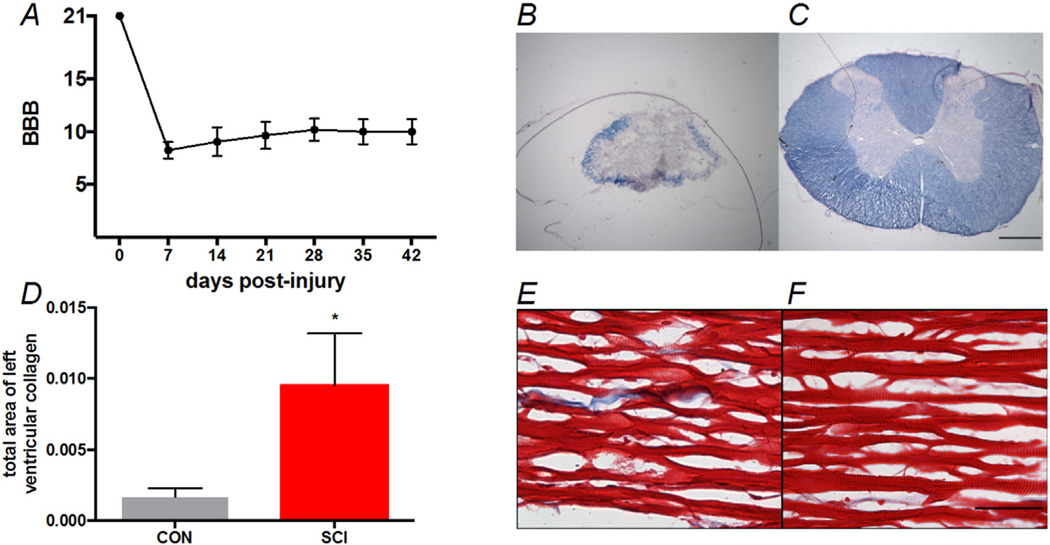
Histological assessments demonstrated an increase in collagen deposition following SCI compared to uninjured, age-matched controls (Fig. 3 E & F P < 0.005). The heart wet mass was also diminished compared to controls (Table 1, P < 0.05). The 25 g-cm injury resulted in 6.25 ± 1.9% spared white matter at the epicenter (Fig. 3B).
Fig. 3.
The BBB scores of the SCI group followed for six weeks after SCI and the histological comparisons of SCI groups to the CON group. A, Locomotor recovery from injury to six-weeks post-SCI. B&C, Representative images of white matter spared at the injury epicenter from and SCI and CON animal, respectively. Scale bar is 500 µm. E&F, Representative images of collagen in the left ventricle free wall of CON and SCI animals, respectively. Scale bar is 50 µm.
Table 1.
Anatomical and echocardiographic data for control and SCI rats at 42 days post-SCI.
| T2 SCI (n = 8) | CON (n = 6) | |
|---|---|---|
| Anatomical data | ||
| Body mass (g) | 259.13 ± 14.49 | 272.17 ± 7.86 |
| Heart mass (g) | 1.013 ± 0.18† | 1.08 ± 0.11 |
| Heart mass/femur | 1.22 ± 0.22 | 1.42 ± 0.27 |
| Collagen content | 0.0095 ± 0.0013†† | 0.0016 ± 0.0003 |
| Echocardiographic data | ||
| Dimensions | ||
| LVIDd (mm) | 6.95 ± 0.49††† | 7.67 ± 0.56 |
| LVIDs (mm) | 4.44 ± 0.30†† | 3.92 ± 0.26 |
| EDV (µl) | 253.03 ± 40.0††† | 315.9 ± 52.57 |
| ESV (µl) | 90.13 ± 14.45†† | 67.42 ± 10.47 |
| Systolic function | ||
| SV (µl) | 162.89 ± 41.27††† | 248.48 ± 46.44 |
| EF (%) | 63.64 ± 7.34† | 78.49 ± 2.68 |
| CO (ml/min) | 44.56 ± 10.73† | 76.25 ± 10.06 |
| Diastolic function | ||
| E (cm s−1) | 63.19 ±8.621††† | 81.23 ± 16.82 |
| A (cm s−1) | 46.011 ± 9.42 | 36.58 ± 23.7 |
| E/A | 1.4 ± 0.17† | 2.22 ± 0.71 |
LVIDd, left ventricular internal diameter at end-diastole; LVIDs, left ventricular internal diameter at end-systole; EDV, end-diastolic volume; ESV, end-systolic volume; SV, stroke volume; EF, ejection fraction; CȮ, cardiac output; E, peak transmitral filling velocity during early diastole; A, peak transmitral filling velocity during late diastole; CON, uninjured control; SCI, spinal cord injury; Data are displayed as mean ± SD
P < 0.001 vs. CON;
P < 0.01 vs. CON;
P < 0.05 vs. CON.
By 6weeks post-SCI, hindlimb function had recovered sufficiently to allow occasional weight-supported stepping without forelimb-hindlimb coordination (Fig. 3; BBB = 9.9 ± 1.2) (Basso et al., 1995). While we did not stratify our animal groups based on locomotor recovery, we could find no significant correlations between the BBB scores and indices of CO (Fig. 1 R2 = 0.02575, P = 0.7042).
4. Discussion
The main finding of this study was that cardiac systolic responses to beta-adrenergic stimulation (examined using DSE) are exaggerated at six weeks following a contusive SCI and that DOB administration elicited an increase in SV at six weeks post-SCI that did not occur pre-SCI. In addition, we extend observations of attenuated systolic function and cardiac fibrosis made in the complete T3 transection SCI model in adult male Wistar rats (West et al., 2014) to adult female Sprague-Dawley rats with incomplete contusion injuries that allowed the recovery of occasional weight-supported hindlimb stepping (BBB=9.9). We utilized T2, 25 g-cm contusions that spared >6% white matter cross-sectional area at the epicenter. Interestingly, we also demonstrate with a 5 µg/kg dose of DOB that SV and CO are returned to pre-SCI levels.
Rodent models with T3 and T2 injures have previously shown clinically relevant cardiovascular impairments such as reduced systolic function and altered hemodynamics (West et al., 2014; Squair et al., 2016). Here, we found that reduced systolic function (CO, SV and EF) was present at 1 week post-SCI and persisted at this low level at 6 weeks post-SCI. Such a rapid and sustained response with the T2 25 g-cm injury is in agreement with these previously defined models and mimics the clinical scenario (Eysmann et al., 1995; Kessler et al., 1986). Reduced left ventricle internal diameter (LVID; Table 1.) is most likely due to the diminished hind-limb function and subsequent cardiac unloading. This is in concert with human studies that have shown prolonged episodes of bed rest (i.e. cardiac unloading) result in decreased LVID, EDV and SV (Levine et al., 1997; Perhonen et al., 2001a,b). Furthermore, we believe the sustained reduction in HR six weeks post-SCI (Fig. 1) is a direct indication of altered autonomic balance in favor of overriding vagal tone (West et al., 2013). This is in agreement with the clinical population where individuals with tetraplegia present with lower resting HRs compared to able bodied and paraplegic individuals (Currie et al., 2016; Zhu et al., 2013). The administration of DOB, which bypasses the disrupted afferent circuitry, corrected these deficits.
In uninjured male rats, DOB elicits an increase in CO (lead by HR) and EF along with decreases in EDV and ESV (Plante et al., 2005) which mirrors the DOB responses in our uninjured female rats and in the able bodied clinical population (Pierard et al., 1989; Ruffolo, 1987). In the current study, we demonstrated that uninjured females (pre-SCI) respond in a similar pattern (Figs. 2 & 3A–F). We then demonstrated that female rats with contusive spinal cord injuries respond to DOB administration with an increase in CO due to increases in both HR and SV (Fig. 3A, B), suggesting that reduced SV at rest is likely a direct consequence of reduced descending sympathetic control. Arguably one of the most important findings in the present study was that animals with chronic SCI (i.e. 6 week data) exhibited an exaggerated response to low doses of DOB (5 µg/kg) compared to the pre-SCI and one-week data. While the mechanism underlying this increased responsiveness is not clear, it is possible that there are chronic changes in the number/sensitivity of cardiac beta-receptors that are not present at one week post-SCI but manifest by six weeks. Although no studies have directly investigated this hypothesis in the heart, similar findings have been noted in the alpha-adrenergic system where enhanced reactivity to norepinephrine and other alpha-mimetics were observed post-SCI (Krum et al., 1992;Mathias et al., 1976; Yeoh et al., 2004). An important consideration in the present study is that our DSE protocol failed to achieve a plateau in HR, SV or CO (maximum work) most likely due to an inability of the 20 µg/kg/min dosage to reach the upper limits of the Starling curve. Thus, we were unable to observe the full impact of the consequences of the measured myocardial fibrosis (i.e., increased collagen deposition). We had expected, based on previous work (Conrad et al., 1995), that cardiac fibrosis would increase the stiffness of the left ventricle and subsequently reduce contractile function/responsiveness. We also cannot speak to the comprehensive hemodynamic responses to DOB, because we did not assess blood pressure responses during drug administration. However, it is well known that DOB elicits an increase in systolic pressure which quickly stabilizes (Plante et al., 2005) and does not effect overall peripheral resistance (Badea et al., 2011; Segreti et al., 2008).
This is the first study, to our knowledge, to demonstrate persistent impairments in cardiac function following a contusive spinal cord injury (T2 25 g-cm) that are similar to those seen after a T3 complete transection. We observed increases in cardiac fibrosis and attenuated systolic function six weeks post-SCI, confirming previous findings (West et al., 2014). Moreover, using DSE we observed an increased responsiveness of the heart to low doses of a beta-agonist post-SCI as compared to pre-SCI. Specifically, we found that a low-dose administration of DOB resulted in an increase (and normalization) of SV, confirming for the first time that impaired descending control of the heart is directly contributing to reduced resting SV after SCI. Due to the similar neuroanatomical innervation of the heart between human and rodent (Dampney, 1994; Strack et al., 1988), we believe these findings are easily translatable and applicable to the clinical scenario.
Acknowledgments
The authors would like to acknowledge Darlene Burke for statistical analyses, Dr. William J Kowalski for intellectual input and Christine Yarberry for expert surgical and animal care assistance.
Grants
This research was funded by the Kentucky Spinal Cord and Head Injury Research Trust (15-7), the NIH (P30 RR031159), and the Kosair Charities Pediatric Heart Research Program.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- Badea CT, Hedlund LW, Cook J, Berridge BR, Johnson GA. Micro-CT imaging assessment of dobutamine-induced cardiac stress in rats. J. Pharmacol. Toxicol. Methods. 2011;63:24–29. doi: 10.1016/j.vascn.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation. 1995;91:161–170. doi: 10.1161/01.cir.91.1.161. [DOI] [PubMed] [Google Scholar]
- Currie KD, West CR, Krassioukov AV. Differences in left ventricular global function and mechanics in paralympic athletes with cervical and thoracic spinal cord injuries. Front. Physiol. 2016;7:110. doi: 10.3389/fphys.2016.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Eysmann SB, Douglas PS, Katz SE, Sarkarati M, Wei JY. Left ventricular mass and diastolic filling patterns in quadriplegia and implications for effects of normal aging on the heart. Am. J. Cardiol. 1995;75:201–203. doi: 10.1016/s0002-9149(00)80082-x. [DOI] [PubMed] [Google Scholar]
- Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopman MT, Oeseburg B, Binkhorst RA. Cardiovascular responses in paraplegic subjects during arm exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1992;65:73–78. doi: 10.1007/BF01466277. [DOI] [PubMed] [Google Scholar]
- Kessler KM, Pina I, Green B, Burnett B, Laighold M, Bilsker M, Palomo AR, Myerburg RJ. Cardiovascular findings in quadriplegic and paraplegic patients and in normal subjects. Am. J. Cardiol. 1986;58:525–530. doi: 10.1016/0002-9149(86)90027-5. [DOI] [PubMed] [Google Scholar]
- Krahwinkel W, Ketteler T, Godke J, Wolfertz J, Ulbricht LJ, Krakau I, Gulker H. Dobutamine stress echocardiography. Eur. Heart J. 1997;18(Suppl D):D9–D15. doi: 10.1093/eurheartj/18.suppl_d.9. [DOI] [PubMed] [Google Scholar]
- Krum H, Louis WJ, Brown DJ, Howes LG. Pressor dose responses and baroreflex sensitivity in quadriplegic spinal cord injury patients. J. Hypertens. 1992;10:245–250. doi: 10.1097/00004872-199203000-00007. [DOI] [PubMed] [Google Scholar]
- Leite S, Oliveira-Pinto J, Tavares-Silva M, Abdellatif M, Fontoura D, Falcao-Pires I, Leite-Moreira AF, Lourenco AP. Echocardiography and invasive hemodynamics during stress testing for diagnosis of heart failure with preserved ejection fraction: an experimental study. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H1556–H1563. doi: 10.1152/ajpheart.00076.2015. [DOI] [PubMed] [Google Scholar]
- Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. doi: 10.1161/01.cir.96.2.517. [DOI] [PubMed] [Google Scholar]
- Lualdi JC, Douglas PS. Echocardiography for the assessment of myocardial viability. J. Am. Soc. Echocardiogr. 1997;10:772–780. doi: 10.1016/s0894-7317(97)70125-1. [DOI] [PubMed] [Google Scholar]
- Lujan HL, Janbaih H, DiCarlo SE. Dynamic interaction between the heart and its sympathetic innervation following T5 spinal cord transection. J. Appl. Physiol. 2012;113:1332–1341. doi: 10.1152/japplphysiol.00522.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson DS, Smith RR, Brown EH, Enzmann G, Angeli C, Quesada PM, Burke D. Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabil. Neural Repair. 2009;23:535–545. doi: 10.1177/1545968308331147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias CJ, Frankel HL, Christensen NJ, Spalding JM. Enhanced pressor response to noradrenaline in patients with cervical spinal cord transection. Brain. 1976;99:757–770. doi: 10.1093/brain/99.4.757. [DOI] [PubMed] [Google Scholar]
- Nash MS, Bilsker S, Marcillo AE, Isaac SM, Botelho LA, Klose KJ, Green BA, Rountree MT, Shea JD. Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia. 1991;29:590–599. doi: 10.1038/sc.1991.87. [DOI] [PubMed] [Google Scholar]
- Pellikka PA, Roger VL, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part II. Dobutamine stress echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clin. Proc. 1995;70:16–27. doi: 10.1016/S0025-6196(11)64660-0. [DOI] [PubMed] [Google Scholar]
- Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and space-flight. J. Appl. Physiol. 2001a;91:645–653. doi: 10.1152/jappl.2001.91.2.645. [DOI] [PubMed] [Google Scholar]
- Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest: “cardiovascular deconditioning” or hypovolemia? Circulation. 2001b;103:1851–1857. doi: 10.1161/01.cir.103.14.1851. [DOI] [PubMed] [Google Scholar]
- Pierard LA, Berthe C, Albert A, Carlier J, Kulbertus HE. Haemodynamic alterations during ischaemia induced by dobutamine stress testing. Eur. Heart J. 1989;10:783–790. doi: 10.1093/oxfordjournals.eurheartj.a059571. [DOI] [PubMed] [Google Scholar]
- Plante E, Lachance D, Drolet MC, Roussel E, Couet J, Arsenault M. Dobutamine stress echocardiography in healthy adult male rats. Cardiovasc. Ultrasound. 2005;3:34. doi: 10.1186/1476-7120-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo RR., Jr The pharmacology of dobutamine. Am. J. Med. Sci. 1987;294:244–248. doi: 10.1097/00000441-198710000-00005. [DOI] [PubMed] [Google Scholar]
- Schneider C, Jaquet K, Geidel S, Malisius R, Boczor S, Rau T, Zienkiewicz T, Hennig D, Kuck KH, Krause K. Regional diastolic and systolic function by strain rate imaging for the detection of intramural viability during dobutamine stress echocardiography in a porcine model of myocardial infarction. Echocardiography. 2010;27:552–562. doi: 10.1111/j.1540-8175.2009.01066.x. [DOI] [PubMed] [Google Scholar]
- Segreti JA, Marsh KC, Polakowski JS, Fryer RM. Evoked changes in cardiovascular function in rats by infusion of levosimendan, OR-1896 [(R)-N-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)acetamide], OR-1855 [(R)-6-(4-aminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one], dobutamine, and milrinone: comparative effects on peripheral resistance, cardiac output, dP/dt, pulse rate, and blood pressure. J. Pharmacol. Exp. Ther. 2008;325:331–340. doi: 10.1124/jpet.107.132530. [DOI] [PubMed] [Google Scholar]
- Smith RR, Burke DA, Baldini AD, Shum-Siu A, Baltzley R, Bunger M, Magnuson DS. The Louisville swim scale: a novel assessment of hindlimb function following spinal cord injury in adult rats. J. Neurotrauma. 2006;23:1654–1670. doi: 10.1089/neu.2006.23.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squair JW, West CR, Popok D, Assinck P, Liu J, Tetzlaff W, Krassioukov AV. High thoracic contusion model for the investigation of cardiovascular function after spinal cord injury. J. Neurotrauma. 2016 doi: 10.1089/neu.2016.4518. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988;455:187–191. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- West CR, Romer LM, Krassioukov A. Autonomic function and exercise performance in elite athletes with cervical spinal cord injury. Med. Sci. Sports Exerc. 2013;45:261–267. doi: 10.1249/MSS.0b013e31826f5099. [DOI] [PubMed] [Google Scholar]
- West CR, Crawford MA, Poormasjedi-Meibod MS, Currie KD, Fallavollita A, Yuen V, McNeill JH, Krassioukov AV. Passive hind-limb cycling improves cardiac function and reduces cardiovascular disease risk in experimental spinal cord injury. J. Physiol. 2014;592:1771–1783. doi: 10.1113/jphysiol.2013.268367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Ireland LA, Sadaniantz A. Evaluation of aortic valve disorders using stress echocardiography. Echocardiography. 2004;21:459–466. doi: 10.1111/j.0742-2822.2004.t01-1-03082.x. [DOI] [PubMed] [Google Scholar]
- Yeoh M, McLachlan EM, Brock JA. Tail arteries from chronically spinalized rats have potentiated responses to nerve stimulation in vitro. J. Physiol. 2004;556:545–555. doi: 10.1113/jphysiol.2003.056424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Galea M, Livote E, Signor D, Wecht JM. A retrospective chart review of heart rate and blood pressure abnormalities in veterans with spinal cord injury. J. Spinal Cord Med. 2013;36:463–475. doi: 10.1179/2045772313Y.0000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]