Abstract
Introduction
The purpose of this analysis was to evaluate the effects of an advanced practice nurse-delivered telehealth intervention on health care utilization by children with medical complexity (CMC). Because CMC account for a large share of health care utilization costs, finding effective ways to care for them is an important challenge requiring exploration.
Method
This was a secondary analysis of data from a randomized clinical trial with a control group and two intervention groups. The focus of the analysis was planned and unplanned clinical and therapy visits by CMC over a 30-month data collection period. Non-parametric tests were used to compare visit counts between and within the three groups.
Results
The number of unplanned visits decreased over time across all groups, with the greatest decrease in the video telehealth intervention group. Planned visits were higher in the video telehealth group across all time periods.
Discussion
APRN-delivered telehealth care coordination may support a shift from unplanned to planned health care service use among CMC.
Keywords: telehealth, care coordination, medical complexity
Children with medical complexity (CMC) are an important clinical population to study given their high health care utilization patterns. Common conditions affecting CMC include congenital or acquired multisystem conditions, cancer or cancer in remission with ongoing disability in multiple areas, and severe neurologic conditions with marked functional impairment (Cohen et al., 2011). Children with certain chronic conditions have been shown to incur medical care costs 2.5 to 20 times higher than children in general in the U.S., and in 2009 CMC accounted for $9.2 billion of U.S. hospital charges (Ireys, Anderson, Shaffer, & Neff, 1997; Berry et al., 2013). In fact, 20% of all U.S. children who use medical services have been shown to incur about 80% of all children’s health care expenditures (Simon, Berry, Feudtner, & Stone, 2010). CMC tend to have the most intensive health care needs and to be the most medically fragile (Hudson, 2013). Advances in health care have led to an increasing number of CMC surviving longer, so the relative medical complexity of hospitalized pediatric patients has increased over the past 15 years (Burns et al., 2010). Therefore, finding efficient ways to deliver the highest quality care to this high-need population is an important challenge in health care today.
The pediatric health care home model of care is advocated for children and youth with special health care needs, of which CMC are a subset; in this model, each family has an ongoing relationship with a primary health care provider and care is coordinated using a team-based model (Turchi, et al., 2014). While there are no current standards for the educational preparation or core functions of the care coordinator in the health care home (McAllister, et al., 2007; Wise, et al., 2007), improved outcomes for children have been demonstrated in studies of advanced practice registered nurse (APRN) delivered care coordination within the health care home (Cady et al., 2015; Looman, et al., 2015; National Association of Pediatric Nurse Practitioners, 2015). TeleFamilies examined the effectiveness of an APRN in an established health care home setting coordinating the care of CMC using telehealth technology compared to usual care and telephone triage. The primary goal for this analysis was to determine whether the intervention decreased the number of unplanned clinical visits and whether the availability of video telehealth technology was more effective than telephone-only telehealth technology.
Methods
Design
The focus of this study was a subset of data from the TeleFamilies Project. TeleFamilies Project, funded by NIH grant R01NR01883 from the National Institute of Nursing Research, was a three-armed randomized control trial with a baseline study period of six months after enrollment followed by the intervention period of two years. The control group receiving traditional health care home coordination was compared to two APRN telehealth care coordination intervention groups. One intervention group used telephone communication with the APRN (telephone group) and the other used telephone plus video communication with the APRN (video group).
Sample
The sample was identified using the children with special healthcare needs (CSHCN) screener (Bethell et al., 2002) applied to patients receiving care at the special needs clinic (SNC) of a large, urban, general pediatrics clinic affiliated with a nonprofit children’s hospital. Eligibility for the TeleFamilies Project was defined as meeting four of the five CSHCN screener criteria: need for prescription medication, need for medical care, functional limitation, need for special therapies for at least 12 months. The need or use of mental health counseling was the optional fifth criterion. This was the most commonly used CMC identification method at the time of study initiation; all TeleFamilies subjects also meet current CMC criteria (Cohen et al., 2012). The minimum age at enrollment was two years old to exclude infants who outgrow their conditions; the maximum was fifteen years of age at enrollment to ensure eligibility for pediatric care through 30 months of enrollment in the study.
All subjects were randomly assigned with three age stratifications (2-5, 6-12, and 13-15 years old) to one of the three groups, and at the conclusion of the six month baseline period they began the two year randomized control trial (RCT) period. There were 163 subjects enrolled in the TeleFamilies Project, with 55 subjects randomized into the control group, 54 in the telephone group, and 54 in the video group. Those agreeing to participate provided written informed consent, following the guidelines of the Institutional Review Boards. The subjects who did not complete the study due to voluntary withdrawal or death during the study were not included in the analysis, leaving a total of 148 subjects with 47 in the control group, 50 in the telephone group, and 51 in the video group.
Setting
For a six month baseline period, all subjects received the traditional health care home model primary care provider (PCP) coordinated care. Within this model the PCP manages overall care, delegating follow-up and coordination tasks to the care coordination team as needed. In the clinic where TeleFamilies was conducted, this team included a half-time medical assistant care coordinator and telephone triage provided by registered nurses. After-hours/weekend telephone triage was handled by an offsite service. All control group subjects continued to receive this model of care coordination throughout the 2-year intervention period.
APRN Telehealth Care Coordination Intervention
For intervention group subjects, the PCP continued to direct overall care. What changed was the addition of a single full-time APRN care coordinator who managed follow-up and coordination of the child’s care during and between clinic visits. The APRN was an experienced certified pediatric nurse practitioner who provided relationship-based care coordination, increasing the “nurse dose” available to intervention families (Looman et al., 2013) via telehealth.
As each family was randomized to an intervention group, the APRN initiated relationship-based care coordination via telehealth. The APRN explained her role and began developing a plan of care in partnership with the child’s family caregiver. For families in the telephone group, this occurred only by telephone. For families in the video group, a video visit was conducted between the family caregiver, APRN and child, when possible. While both intervention groups engaged with the APRN using telehealth, the video group experienced immediate ‘face-to-face’ contact with the APRN. A time-motion study conducted at the onset of the TeleFamilies project period showed no significant difference in duration of encounters between telephone telehealth and video telehealth interactions (Cady & Finkelstein, 2014), with an average duration of five minutes each. In both intervention groups, communication was initiated by either the child’s caregiver, which in all cases was the parent, or APRN using telephone telehealth. For subjects in the telephone group, this was the only telehealth mode available for communication with the APRN. Subjects in the video group had the option of ‘switching’ from telephone to video telehealth if either the caregiver or APRN felt it would improve assessment and/or communication.
While the APRN did not provide clinic visit care, she would check-in with families during scheduled PCP clinic visits to facilitate the care coordination process. The APRN’s expanded scope of practice facilitated assessment of the appropriate setting for illness management: telehealth, office visit or emergency department visit. The APRN’s knowledge of the patient through relationship-based care coordination facilitated communication of information to support handoffs during transitions of care. The APRN recommended office visits when an unmet need for routine (primary or specialty) follow-up care was identified or an emergent change in health required in-person assessment.
Data Collection
Data was collected using the Healthcare Service Utilization (HCSU) tool shown in Figure 1, completed monthly by a family caregiver. A planned visit was defined as being scheduled in advance for regular health maintenance (e.g. pre-operative physical, well-child check, hospitalization or ED follow-up visit) , while an unplanned visit was defined as being due to an acute change in the child’s health (e.g. acute illness, complication, or injury). Planned and unplanned visits could have occurred at any of the five health care service providers (primary care clinic visit, specialty care clinic visit, urgent care, emergency department, hospitalization) listed in Figure 1. For this analysis, all visit types/durations were treated equally, e.g. a planned clinic visit and a planned three day hospitalization each counted as one planned visit. Missed or cancelled visits were not tracked as they were not the focus of this study. The therapy visits included in the HCSU tool only include sessions that the child received outside of the school setting. CMC receive therapies in school, as those are required by federal law to be offered to aid in developing skills needed in the educational setting (Individuals with Disabilities Education Improvement Act of 2004, 2004). Once a child reaches school age, insurance often stops reimbursing therapies outside of those offered in school (Benedict, 2006). Most caregivers still elect to bring the child to additional therapy visits to support the family in caring for the child at home or the child’s ability to participate in community activities (Benedict, 2006), and these therapy visits are included in the HCSU tool. The HCSU tool was collected using multiple flexible methods—mail, e-mail, telephone, and in person at the clinic—to maximize the response rate (Finkelstein, Celebrezze, Cady, Lunos, & Looman, 2015). When data collection using these methods was unsuccessful, the electronic medical record (EMR) was reviewed for documented visits within the health care system.
Figure 1.
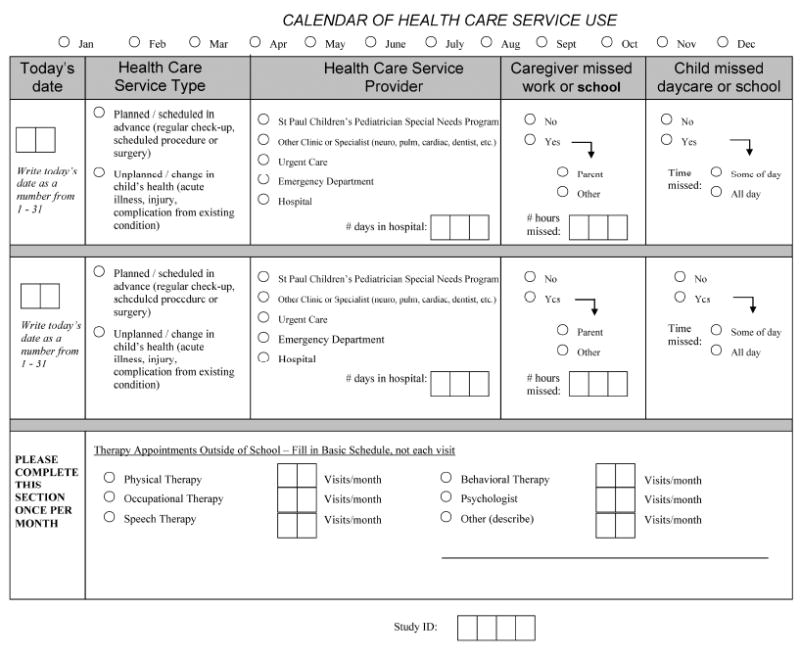
Analysis of Data
Data from the 30 months of each subject’s enrollment was grouped into a series of five six-month time periods for analysis: the six-month baseline, and four six-month periods during the two-year RCT. Means and standard deviations for planned visits and unplanned visits were calculated for each of the three intervention groups in each six-month period. Wilcoxon signed-rank tests were performed to compare differences within a group over time. Kruskal-Wallis H tests were performed to compare differences between the three groups in each six-month period, and a Mann-Whitney U Test was used to compare two groups when significant differences were found in the Kruskal-Wallis H test. The total, mean, and standard deviation for the number of visits of each type of therapy were calculated for both the baseline and the whole RCT period to provide a broader description of the subject population.
Results
The population statistics and Wilcoxon signed-rank test results are shown in Table 1. For unplanned visits, there were significant within-group differences between baseline and one or more of the six month RCT time periods for all three groups, with more visits in baseline. For planned visits, there were significant within-group differences between baseline and one of the six month RCT periods for the control group only. The video group had more planned visits in total number and average than the other two groups for all time periods. The standard deviation was larger for the video group than telephone or control groups as well. During baseline one subject in the video group was three standard deviations from the mean and two subjects were two standard deviations from the mean. In the telephone group, zero subjects were three standard deviations from the mean and one subject was two standard deviations from the mean. In the control group, one subject was three standard deviations from the mean and zero subjects were two standard deviations from the mean.
Table 1.
Summary of Unplanned and Planned Visit Data by Group, across Five Study Periods
| Control Group | Baseline | Months 1-6 RCT | Months 7-12 RCT | Months 13-18 RCT | Months 19-24 RCT |
|---|---|---|---|---|---|
| Total Number of Unplanned Visits | 115 | 88 | 67* | 67* | 74* |
| Total Number of Planned Visits | 254 | 219 | 215 | 220 | 196* |
| Range of Unplanned Visits | 0-12 | 0-7 | 0-10 | 0-7 | 0-10 |
| Range of Planned Visits | 0-19 | 0-14 | 0-19 | 0-14 | 0-18 |
| Average Number of Unplanned Visits per Subject±SD | 2.45±2.58 | 1.87±1.98 | 1.43±2.17 | 1.43±1.96 | 1.57±2.62 |
| Average Number of Planned Visits per Subject±SD | 5.40±3.81 | 4.66±4.08 | 4.57±3.92 | 4.68±4.09 | 4.17±3.90 |
|
| |||||
| Telephone Group | Baseline | Months 1-6 RCT | Months 7-12 RCT | Months 13-18 RCT | Months 19-24 RCT |
|
| |||||
| Total Number of Unplanned Visits | 115 | 100 | 83* | 71* | 75* |
| Total Number of Planned Visits | 293 | 271 | 276 | 288 | 285 |
| Range of Unplanned Visits | 0-8 | 0-9 | 0-10 | 0-10 | 0-6 |
| Range of Planned Visits | 0-13 | 0-14 | 0-14 | 0-22 | 0-23 |
| Average Number of Unplanned Visits per Subject±SD | 2.30±2.38 | 2.00±2.13 | 1.66±2.13 | 1.42±2.02 | 1.50±1.56 |
| Average Number of Planned Visits per Subject±SD | 5.86±3.36 | 5.42±3.85 | 5.52±4.04 | 5.76±5.09 | 5.70±5.05 |
|
| |||||
| Video Group | Baseline | Months 1-6 RCT | Months 7-12 RCT | Months 13-18 RCT | Months 19-24 RCT |
|
| |||||
| Total Number of Unplanned Visits | 124 | 125 | 100 | 88 | 69* |
| Total Number of Planned Visits | 322 | 302 | 356 | 381 | 328 |
| Range of Unplanned Visits | 0-7 | 0-7 | 0-12 | 0-8 | 0-5 |
| Range of Planned Visits | 0-23 | 0-18 | 0-23 | 0-29 | 0-35 |
| Average Number of Unplanned Visits per Subject±SD | 2.43±1.89 | 2.45±1.93 | 1.96±2.53 | 1.73±1.98 | 1.35±1.52 |
| Average Number of Planned Visits per Subject±SD | 6.31±4.97 | 5.92±4.03 | 6.98±4.91 | 7.47±6.14 | 6.43±6.65 |
Indicates a significant difference from baseline at p < 0.05, based on a Wilcoxon signed rank test
As shown in Table 2, the only comparisons that were found to be statistically significant with the Kruskal-Wallis H test were planned visits for all RCT months combined, planned visits for RCT months 7-12, and planned visits for RCT months 13-18. The Mann-Whitney U Test that compared these situations in groups of two to identify the source of the differences found for all cases that the statistical significance came from comparing the control group to the video group, and the video group always had more planned visits than the control group did for these three time periods.
Table 2.
Significance of Group Differences in Unplanned and Planned Visits across Five Study Periods
| Baseline | Months 1-6 RCT | Months 7-12 RCT | Months 13-18 RCT | Months 19-24 RCT | RCT All Months | |
|---|---|---|---|---|---|---|
| Unplanned visits (p-values) | 0.634 | 0.176 | 0.347 | 0.410 | 0.410 | 0.112 |
| Planned visits (p-values) | 0.622 | 0.177 | 0.028* | 0.049* | 0.132 | 0.029* |
Indicates the difference between groups is significant based on a Kruskal-Wallis H test; in each case, the video group was significantly higher than the control group.
The therapy data shown in Table 3 summarizes the utilization of the various types of therapy by each of the groups throughout the study. For the control group, occupational therapy showed the highest number of visits in both the baseline and RCT periods. For the telephone group, speech therapy showed the highest number of visits in baseline and RCT. For the video group, physical therapy showed the highest number of visits in baseline and RCT.
Table 3.
Number of Therapy Visits Reported, by Group, during Baseline and RCT Periods
| Total Visits | PT | OT | Speech | Behavioral | Psych | Other | All Types |
|---|---|---|---|---|---|---|---|
| Baseline (6 months) | |||||||
| Control Group | 156 | 196 | 135 | 2 | 7 | 32 | 528 |
| Telephone Group | 121 | 161 | 322 | 72 | 19 | 64 | 759 |
| Video Group | 249 | 221 | 152 | 13 | 12 | 54 | 701 |
|
| |||||||
| RCT (24 months) | |||||||
|
| |||||||
| Control Group | 285 | 460 | 402 | 7 | 41 | 125 | 1320 |
| Telephone Group | 277 | 320 | 599 | 414 | 85 | 416 | 2111 |
| Video Group | 745 | 697 | 700 | 96 | 99 | 243 | 2580 |
Note. Numbers indicate the total cumulative number of visits reported by all participants in each group during the specified time period. RCT: randomized controlled trial; PT: physical therapy; OT: occupational therapy.
Discussion
The findings from this analysis are important to help understand the possible impact and limitations of APRN telehealth care coordination on the number and type clinical visits for children with medical complexity. The number of unplanned visits was generally higher in the baseline period than in subsequent months for all groups (with the exception of the first 6 months of RCT for the video group). The largest decrease in the total number of unplanned visits was seen in the video group from baseline to the last period of the RCT. By the end of the study, the video group had the lowest mean and total number of unplanned visits. The data showed a significantly increased number of planned visits for the video group compared to other groups over the entire RCT period. From baseline to the end of the RCT, the control group saw a significant decrease in planned visits.
The care coordination intervention may have had a greater effect on unplanned visits for the video group in part due to their lower physical functioning at baseline, as measured on the PedsQL measure (Varni, Seid & Kurtin, 2001). Mean physical function scores among children in the video group (M=41.5) were lower than those in the control group (M=51.2) and significantly lower than those in the telephone group (M=58.5, p=.041); these scores did not change significantly from baseline to Year 2. Subjects in the video group were otherwise similar in terms of their demographic and baseline condition characteristics.
Another reason for a different effect of the intervention on the unplanned and planned visits in the telephone and video groups may be the perceived benefit and potential for connection with the APRN care coordinator by families in the video group. While the number of video visits was relatively small in this group (7% of video group encounters used video telehealth), the protocol for setting up the video technology included a video visit between family and APRN early in the intervention period. This visit may have helped to establish a relationship between the family caregiver and APRN earlier in the study for the video group than for telephone group. This increased engagement may have facilitated more effective coordination of care and a subsequent shift from unplanned to planned visits over time.
Reducing unplanned visits is a positive indicator because is suggests a reduction in instability of the condition, time spent in the emergency department, and unexpected work/school days missed for the family. Increasing planned visits is a positive indicator because it suggests better monitoring and control of the condition and more stability, enabling the family to be proactive about the child’s health. Planned visits may also signal better coordination of care with specialists and increased adherence to recommended well child care. While cost was not measured in this study, the shift from unplanned to planned visits may also represent a potential cost savings (de Stampa, Vedel, Buyck, et al., 2014). A study by Casey et al. (2011) found that the number of outpatient visits rose even as cost savings became apparent from reduced hospitalizations for CMC. This supports from an economic standpoint the goal of increasing planned visits while decreasing unplanned visits.
The APRN and telehealth interventions were not the only factors influencing the evolution of care over the RCT period. Reductions in unplanned visits could be related to other factors such as the systematic quality improvements efforts within the organization (concurrent with ACO incentives), changes in treatment protocol, the child aging, the season for some of the children in the group, or other variables. It is possible that the condition of some patients became more stable as the study went on, resulting in fewer clinical visits. Another potential factor impacting the APRN telehealth care coordination model was relationship building. While the APRN was an employee of the children’s hospital, she was new to the primary care clinic where TeleFamilies was conducted. There could have been difficulty adjusting to the new telehealth and APRN care model for the intervention groups at the start of the RCT period. For each group, there were more unplanned visits in total and average for months 1-6 of RCT than there were for the later three RCT periods.
Unplanned visits can be difficult to impact, even with a high intensity intervention such as APRN telehealth care coordination. Unplanned hospitalizations for CMC are likely less amenable to change through interventions given the underlying complexity of the conditions for these children (Berry, Agrawal, Cohen, & Kuo, 2013). 85% of children in the TeleFamilies Project had multiple complex chronic conditions, and approximately half required life-sustaining technology assistance. Previous studies of health care service use in this population have shown a correlation between increasing positive responses on the CSHCN screener and the number of hospital visits (Cohen et al., 2012; Kuo et al., 2014).
Limitations
A limitation of the study is that response rates for the HCSU tool differed between groups. The video group had a 94% response rate, the telephone group had an 88% response rate, and the control group had an 84% response rate (Finkelstein et al., 2015). The higher response rate for the video group could in part be contributing to the higher number of planned visits compared to the other groups. The significant decrease in the number of unplanned visits over time in the control, telephone, and video groups could be explained by failure of a respondent to report visits in at an out-of-system clinic or hospital, which would not have been detected in EMR audits. Families living at a greater distance from the primary clinic may have chosen to utilize a local hospital or emergency department in the event of an acute change in the child’s condition. This choice seems more likely for an unplanned visit than a planned visit. If these visits were not reported, then the data could show a lower number of unplanned visits, including in the control group. The unknown degree of effect of quality improvements at the clinic poses an additional study limitation. The study did not collect missed or cancelled visits. This creates a potential study limitation since a missed post-ED or post-hospitalization follow-up visit limits identification of emergent issues, and increases potential for a future unplanned visit.
Conclusion
This study was useful in quantifying the changes in health care utilization by CMC after an intervention with APRN mediated telehealth care coordination within a mature health care home. The interventions in this study could be useful in decreasing the number of unplanned clinical visits and increasing the number of planned clinical visits. This could lead to more effective and efficient care for the growing population of CMC.
Supplementary Material
Acknowledgments
Supported in part by funding from the National Institute of Nursing Research, National Institutes of Health, grant R01 NR010883
Footnotes
Disclosures: None of the authors have any conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Holly D. McKissick, Department of Biomedical Engineering, University of Minnesota.
Rhonda G. Cady, Nursing Research Specialist, Gillette Children’s Specialty Healthcare, St. Paul, MN.
Wendy S. Looman, School of Nursing, University of Minnesota.
Stanley M. Finkelstein, Department of Laboratory Medicine and Pathology/Health Informatics, University of Minnesota.
References
- American Academy of Pediatrics. National Center for Medical Home Implementation: Care Coordination. 2016 Retrieved April 11, 2016, from https://medicalhomeinfo.aap.org/tools-resources/Pages/Care-Coordination.aspx.
- Benedict RE. Disparities in use of and unmet need for therapeutic and supportive services among school-age children with functional limitations: A comparison across settings. Health Services Research. 2006;41(1):103–124. doi: 10.1111/j.1475-6773.2005.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JG, Agrawal RK, Cohen E, Kuo DZ. The landscape of medical care for children with medical complexity. Lenexa, KS: Children’s Hospital Association; 2013. Retrieved from https://www.childrenshospitals.org/issues-and-advocacy/children-with-medical-complexity/issue-briefs-and-reports/the-landscape-of-medical-care-for-children-with-medical-complexity. [Google Scholar]
- Bethell CD, Read D, Stein RE, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambulatory Pediatrics. 2002;2(1):38–48. doi: 10.1367/1539-4409(2002)002<0038:icwshc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Burns KH, Casey PH, Lyle RE, Bird TM, Fussell JJ, Robbins JM. Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126(4):638–646. doi: 10.1542/peds.2009-1658. [DOI] [PubMed] [Google Scholar]
- Cady RG, Erickson M, Lunos S, Finkelstein SM, Looman W, Celebrezze M, Garwick A. Meeting the needs of children with medical complexity using a telehealth advanced practice registered nurse care coordination model. Maternal and Child Health Journal. 2015;19(7):1497–506. doi: 10.1007/s10995-014-1654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady RG, Finkelstein SM. Task-technology fit of video telehealth for nurses in an outpatient clinic setting. Telemedicine Journal and e-Health: The Official Journal of the American Telemedicine Association. 2014;20(7):633–639. doi: 10.1089/tmj.2013.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey PH, Lyle RE, Bird TM, Robbins JM, Kuo DZ, Brown C, Burns K, et al. Effect of hospital-based comprehensive care clinic on health costs for Medicaid-insured medically complex children. Archives of Pediatrics and Adolescent Medicine. 2011;165(5):392–398. doi: 10.1001/archpediatrics.2011.5. [DOI] [PubMed] [Google Scholar]
- Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463–70. doi: 10.1542/peds.2012-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SKM, Simon TD, Srivastava R. Children with medical complexity: An emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stampa M, Vedel I, Buyck JF, Lapointe L, Bergman H, Beland F, Ankri J. Impact on hospital admissions of an integrated primary care model for very frail elderly patients. Archives of gerontology and geriatrics. 2014;58(3):350–355. doi: 10.1016/j.archger.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Finkelstein SM, Celebrezze M, Cady R, Lunos S, Looman WS. Strategies to maximize data collection response rates in a randomized control trial focused on children with medical complexity. Telemedicine and e-Health. 2015;22(4) doi: 10.1089/tmj.2015.0069. tmj.2015.0069-tmj.2015.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SM. Hospital readmissions and repeat emergency department visits among children with medical complexity: An integrative review. Journal of Pediatric Nursing. 2013;28(4):316–339. doi: 10.1016/j.pedn.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Individuals with Disabilities Education Improvement Act of 2004. Public Law 108-446 - 108th Congress. 2004 Dec 3; [Google Scholar]
- Ireys HT, Anderson GF, Shaffer TJ, Neff JM. Expenditures for care of children with chronic illnesses enrolled in the Washington State Medicaid program, fiscal year 1993. Pediatrics. 1997;100(2):197–204. doi: 10.1542/peds.100.2.197. [DOI] [PubMed] [Google Scholar]
- Kuo DZ, Melguizo-Castro M, Goudie A, Nick TG, Robbins JM, Casey PH. Variation in child health care utilization by medical complexity. Maternal and Child Health Journal. 2014:40–48. doi: 10.1007/s10995-014-1493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looman WS, Antolick M, Cady RG, Lunos Sa, Garwick AE, Finkelstein SM. Effects of a telehealth care coordination intervention on perceptions of health care by caregivers of children with medical complexity: A randomized controlled trial. Journal of Pediatric Health Care: Official Publication of National Association of Pediatric Nurse Associates & Practitioners. 2015;29(4):352–363. doi: 10.1016/j.pedhc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looman WS, Presler E, Erickson MM, Garwick AW, Cady RG, Kelly AM, Finkelstein SM. Care coordination for children with complex special health care needs: The value of the advanced practice Nurse’s enhanced scope of knowledge and practice. Journal of Pediatric Health Care. 2013;27(4):293–303. doi: 10.1016/j.pedhc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister JW, Presler E, Cooley WC. Practice-based care coordination: A medical home essential. Pediatrics. 2007;120(3):e723–e733. doi: 10.1542/peds.2006-1684. [DOI] [PubMed] [Google Scholar]
- National Association of Pediatric Nurse Practitioners. Position Statement on Pediatric Health Care/Medical Home: Key Issues on Care Coordination, Transitions, and Leadership. 2015 https://www.napnap.org/sites/default/files/userfiles/about/2015_NAPNAP_Pediatric_Health_Care_Home_PS_Final.pdf. [PubMed]
- Simon TD, Berry J, Feudtner C, Stone BL. Children with complex chronic conditions in inpatient hospital settings in the united states. Pediatrics. 2010;126(4):1–13. doi: 10.1542/peds.2009-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi RM, Antonelli RC, Norwood KW, Adams RC, Brei TJ, Burke RT, Levy SE, et al. Patient-and family-centered care coordination: A framework for integrating care for children and youth across multiple systems. Pediatrics. 2014;133(5):e1451–e1460. doi: 10.1542/peds.2014-0318. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: Reliability and validity of the pediatric quality of life inventory™ version 4.0 generic core scales in healthy and patient populations. Medical Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Wise PH, Huffman LC, Brat G. A critical analysis of care coordination strategies for children with special health care needs (Technical review No 14, AHRQ Publication No 07–0054) Rockville, MD: Agency for Healthcare Research and Quality; 2007. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


