Abstract
The BOLD signal reflects hemodynamic events within the brain, which in turn are driven by metabolic changes and neural activity. However, the link between BOLD changes and neural activity is indirect and can be influenced by a number of non-neuronal processes. Motion and physiological cycles have long been known to affect the BOLD signal and are present in both humans and animal models. Differences in physiological baseline can also contribute to intra- and inter-subject variability. The use of anesthesia, common in animal studies, alters neural activity, vascular tone, and neurovascular coupling. Most intriguing, perhaps, are the contributions from other processes that do not appear to be neural in origin but which may provide information about other aspects of neurophysiology. This review discusses different types of noise and non-neuronal contributors to the BOLD signal, sources of variability for animal studies, and insights to be gained from animal models.
Keywords: fMRI, rs-fMRI, functional connectivity, functional MRI, noise, non-neuronal contributions, animal studies
Introduction
Functional MRI (fMRI) and resting state fMRI (rs-fMRI) studies in humans and in animal models have long been intertwined. The blood oxygenation level dependent (BOLD) contrast that forms the basis for both methods was first demonstrated in rats (Ogawa et al., 1990). Functional MRI and rs-fMRI were then quickly developed in humans (Bandettini et al., 1992; Biswal et al., 1995; Kwong et al., 1992; Ogawa et al., 1992). Both techniques were rapidly translated to rodents and nonhuman primates to investigate the neurophysiology underlying the signals detected with MRI. As neurovascular coupling and the properties of the BOLD fluctuations became better understood, fMRI and rs-fMRI became widely used in studies of human brain function and dysfunction, largely due to their noninvasive nature and whole brain coverage. More recently, fMRI and rs-fMRI have been increasingly applied in animal studies as translational measures of altered brain activity in a variety of conditions. While many of the sources of noise are common across humans and animals, their relative contribution and manifestation can differ. Even more intriguing are contributions that are distinct from traditional noise sources that may prove to arise from other types of neurophysiological processes. This review describes different types of noise that are common in non-human fMRI and rs-fMRI scans, along with potential mitigations and future outlooks.
Functional MRI and rs-fMRI in animal models
Functional MRI and rs-fMRI in animal models are performed in much the same way as in humans (Huang et al., 1996; Pawela et al., 2008; Silva and Koretsky, 2002; Spenger et al., 2000; Zhao et al., 2008). For fMRI, a sensory stimulus or task is presented at known time points while a series of images are acquired. Areas of the brain where the BOLD signal changes during stimulation can be identified using correlation analysis or generalized linear model (GLM) methods. For rs-fMRI, a similar series of images are acquired but without any external stimuli. Whereas task-based MRI is the explicit examination of the changes in brain activation related to particular tasks, rs-fMRI provides a toolset to identify and compare functional network organization within the brain (Biswal et al., 1995). Functional networks can be identified with seed-based correlation analysis, ICA, or other techniques (Kalthoff et al., 2013; Keilholz et al., 2010; Pawela et al., 2008; Williams et al., 2010; Zhao et al., 2008).
Despite the range of brain volumes (from ~415 mm3 to mice (Kovacević et al., 2005) to ~1400 cm3 in humans (Lüders et al., 2002)) and the differences in gyrification, many areas of the brain are widely preserved across mammalian species, including sensory cortices, multimodal association areas, and motor cortices (Kaas, 2007; Krubitzer, 1995). The lack of local energy stores in the brain requires that the vasculature respond dynamically to changes in neuronal activity, and it appears that this neurovascular coupling is also relatively conserved across species, although the timing and shape of the hemodynamic response vary (de Zwart et al., 2005). As expected based on these similarities, fMRI studies in cats, rodents, and monkeys have demonstrated patterns of activation in response to sensory stimuli similar to those detected in humans (Aksenov et al., 2015; Huang et al., 1996; Keilholz et al., 2004; Kim and Uğurbil, 1997; Lee et al., 1999; Logothetis et al., 2001; Schroeter et al., 2014; Yang et al., 1996; Yen et al., 2011). Likewise, using rs-fMRI researchers have shown that numerous networks (including somatosensory, motor, visual, and even “default mode”) are present across species (Belcher et al., 2016, 2013; Ghahremani et al., 2016; Lu et al., 2012; Mantini et al., 2011; Pawela et al., 2008; Schroeder et al., 2016; Vincent et al., 2007; Williams et al., 2010; Zhao et al., 2008).
While many brain characteristics are common across species, certain species are better suited for particular types of studies than others. Rodents are commonly used in basic studies of neurophysiology or sensory pathways (Duong et al., 2000; Keilholz et al., 2006; Magnuson et al., 2010; Nasrallah et al., 2014; Pawela et al., 2010; Shih et al., 2013; Silva and Koretsky, 2002). Their brains are small and unconvoluted, and their controlled genetic backgrounds result in high similarity across individual animals. Cats have more complex brains and a visual system that more closely approximates that of a human, and are widely used in visual studies (Moon et al., 2013; Yen et al., 2011). Nonhuman primates have the most complex brains and are the most costly to work with, but provide the closest approximation to the human brain and have provided key insights into the neurophysiology underlying the BOLD response (Hutchison and Everling, 2012; J. V Liu et al., 2013; Logothetis et al., 2001; Mantini et al., 2011; Shmuel and Leopold, 2008; Vincent et al., 2007). Because of the necessity for restraint, most animals (rats, mice, cats) are typically anesthetized during the imaging session. This limits the type of study that can be performed and introduces potential confounds from the effects of anesthesia on both neurovascular coupling and the shape of the hemodynamic response function (J. V Liu et al., 2013; Silva et al., 2011). Rabbits, nonhuman primates, and dogs are more commonly examined without anesthesia, as they can be trained to remain still in the scanner and even to perform a task. Thus there is a spectrum of types of animal experiments, ranging from anesthetized mice at one end to awake nonhuman primates on the other. This review will primarily focus on anesthetized small animals, as the animal experiments at the other end of the spectra are in some ways more similar to human fMRI and rs-fmRI studies. Most other animals fall somewhere in between and the most important sources of variability will depend on the experimental setup.
Among rodents, rats have historically been the most popular subjects for fMRI and rs-fMRI, playing an important role in elucidating the origin and properties of the BOLD signal (Duong et al., 2000; Lee et al., 1999; Lu et al., 2007; Pan et al., 2010, 2011; Silva et al., 1999; Silva and Koretsky, 2002; Spenger et al., 2000). A prominent drawback of fMRI studies in rodents has been that they are limited to fairly simple sensory studies, because cognitive tasks cannot be performed by anesthetized animals in the scanner. However, rs-fMRI is in many ways more flexible than task or stimulus-related fMRI, allowing brain regions that are difficult to target with traditional sensory stimulation to be examined, including areas involved in cognition. Robust fMRI and rs-fMRI methodologies have more recently been developed for mice as well, unlocking the potential of using genetic manipulations to investigate brain function (Grandjean et al., 2014; Guilfoyle et al., 2013; Jonckers et al., 2014; Sforazzini et al., 2014; Stafford et al., 2014; Zerbi et al., 2015). The spatial resolution required for comparable coverage of the smaller mouse brain is higher, but high field scanners and sophisticated imaging techniques make the experiments feasible. Another recent development is the growing use of the awake rabbit model (Aksenov et al., 2015; Schroeder et al., 2016). Unlike rats or mice, rabbits freeze when uneasy and take well to restraint, which may minimize stress in the scanner environment and eliminate the need for anesthesia (Miller et al., 2003; Wyrwicz et al., 2000).
Types and effects of noise
For this review, we adopt a broad definition of noise as anything that interferes with the detection of the signal of interest (Figure 1). Functional MRI aims to detect changes in neural activity that occur in response to a task or stimulus; rs-fMRI ideally detects coordinated changes in neural activity over time across areas. A number of factors can reduce our ability to detect the desired changes. At the most basic level, there is image noise. This can include distortion, dropout, displacement, spatial modulation of image intensity, high frequency background fluctuations, thermal noise, etc. While the image signal to noise ratio (SNR) is usually defined as the average intensity in an area divided by the standard deviation of the background, artifacts such as signal dropout can change very slightly from image to image, affecting the time course of the functional data and reducing the sensitivity to the functional changes of interest in ways that are not readily captured with traditional measures of SNR. For this reason, we have included these issues along with more standard types of image noise. Image noise is typically related to choices made during image acquisition (spatial resolution, echo time, RF coil) and is commonly found in any neuroimaging study, whether in animals or in humans.
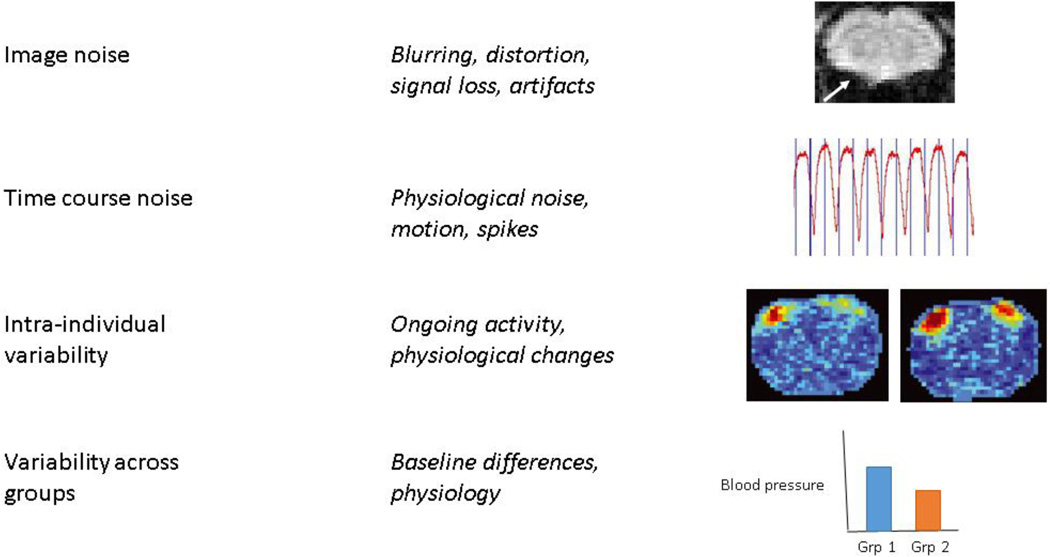
Figure 1. Types of noise.
Image noise refers inaccuracies in the image due to acquisition strategies, including loss of contrast, distortion, and artifacts. The arrow in the image at top indicates distortion in a coronal gradient-echo EPI image of a rat brain acquired at 9.4 T due to the susceptibility mismatch at the air-tissue interface near the ear canals. Time course noise refers to noise that affects images differently at different time points, giving it a temporal structure. Common sources are respiration, cardiac pulsation, and motion. An example respiratory time course is shown. Intra-individual variability may result from changes in baseline physiology, ongoing activity in the brain, or other factors that can change the state of an individual over time. Two representative activation maps from a single rat acquired at different time points in one scanning session are shown at right (adapted from (Matthew Evan Magnuson et al., 2014)). Considerable variability can be seen in experimentally-identical acquisitions. Variability across groups is an important consideration for studies comparing functional neuroimaging data across groups of animals and can result from baseline differences, particularly in factors that affect vascular tone or the relative contribution of physiological noise. A hypothetical difference in baseline blood pressure across two groups is shown for illustration purposes.
A second type of noise affects the time course of the fMRI or rs-fMRI scan. Examples include motion, cardiac pulsation and the respiratory cycle. This noise can introduce alterations in the time course ranging from slow, almost periodic modulations to abrupt spike-like signal changes. Time course noise can contribute to the signal from all voxels in the brain or be localized to distinct subsets of voxels. This type of noise is a major confound for both human and animal fMRI and rs-fMRI studies, and extensive work on how to best mitigate its effects can be found in the literature.
Two other perhaps more controversial types of noise will also be considered: noise that interferes with detection of activation or functional connectivity in an animal and noise that affects comparisons across groups. The first might arise from ongoing background activity (fluctuations comparable in amplitude to the response to a stimulus) or changes in physiological condition (increasing blood flow in response to intubation-induced hypercapnia) that overwhelm the signals of interest. The second can occur due to differences across groups (baseline cerebral blood flow, hypertension, changes in respiration or cardiac pulsation) that can be mistaken for changes in activation or connectivity, or that can obscure true changes. Both can be minimized though not neglected with careful evaluation and maintenance of animal physiology, as discussed in more detail later. Nevertheless, it should be noted that a portion of this “noise” is not really noise at all, but rather reflects ongoing processes in the brain that modulate the response to stimuli or the correlation between different areas. As part of this review, we discuss some contributions that may arise from these processes.
Image and time course noise
Acquisition-related image noise
As in human fMRI and rs-fMRI studies, rodent studies usually employ gradient echo (GE) echo planar imaging (EPI) to provide good temporal resolution and sensitivity to the BOLD signal. All GE-EPI sequences are vulnerable to susceptibility-based artifacts, blurring, distortion and signal dropout (Chung et al., 2011; Hoge et al., 2010; Tang and Huang, 2011). Some of these effects can be exacerbated in animal studies due to the relatively high field strength of many small animal magnets (7T or higher). Susceptibility interfaces, particularly around the ear canals, cause distortion that is often worst near the base of the brain. The strong magnetic field increases the frequency dispersion at susceptibility interfaces, making the artifact larger. In humans, the resulting signal dropout and distortion are sometimes mitigated by corrections based on phase mapping or on active shimming strategies (Gu et al., 2002; Tang and Huang, 2011). Some of these techniques are beginning to be applied to animal studies as well (Hong et al., 2015) but they are not yet widely available. Strong higher order shims are particularly helpful to minimize distortion when whole brain imaging is needed (Keilholz et al., 2006, 2004). In animal models, it is also sometimes possible to reduce susceptibility mismatches by filling air spaces with matching agents (Adamczak et al., 2010; R. Li et al., 2015).
T2 and T2* are shorter at high magnetic fields, with a correspondingly shorter TE for optimal BOLD sensitivity. For GE-EPI studies at fields of 7T or higher, echo times are typically 10–20 ms, with higher signal and less distortion at shorter TEs but more sensitivity to the BOLD signal at longer TEs (Adamczak et al., 2010; Ciobanu et al., 2015; J. V Liu et al., 2013; Williams et al., 2010). For comparison, TE values for human studies at 3T are typically around 30–40 ms (Jann et al., 2015; Küblböck et al., 2014; Magnuson et al., 2015; Y.-F. Wang et al., 2016). In addition, coil technology and advanced imaging sequences are usually less available for small animals, unless the researchers are willing and able to build their own. However, some types of technology are designed specifically for small animal imaging (cryoprobes, microcoils) and provide substantial improvement (Grandjean et al., 2014; Schroeter et al., 2014).
Time course noise: motion
In human fMRI and rs-fMRI studies, slight motions of the head are common and can lead to false patterns of activation (particularly if the motion is time-locked with the stimulus) or widespread correlation. Even small changes in position can alter the relative amounts of different tissues within a voxel, change the position of the slices, or give rise to spin-history effects (Friston et al., 1996; Muresan et al., 2005). Rigid-body realignment is typically applied to mitigate these effects, sometimes followed by “scrubbing” of scan time points with high levels of motion (Power et al., 2012). Rejection criteria are commonly based on voxel size, and in animal studies, voxels are much smaller than in humans (by approximately an order of magnitude in each direction for rats). Thus an acceptable level of motion for a human study may be < 1.5 mm but an acceptable level in a rodent study might be < 150 microns. This small tolerance for motion is made achievable by the fact that rodents are usually restrained in a stereotaxic device that uses ear bars and a tooth bar to immobilize the head. Motion can be further reduced with the use of anesthesia and, in some cases, paralytic agents. Therefore, the level of motion in rodent fMRI or rs-fMRI experiments is usually acceptable even with the stricter criteria imposed by the higher spatial resolution (Figure 2). Because the level of motion is typically low and motion correction may introduce artifacts itself (Grootoonk et al., 2000), many animal researchers take the approach of inspecting motion time courses for each scan and discarding any scans where significant motion occurs.
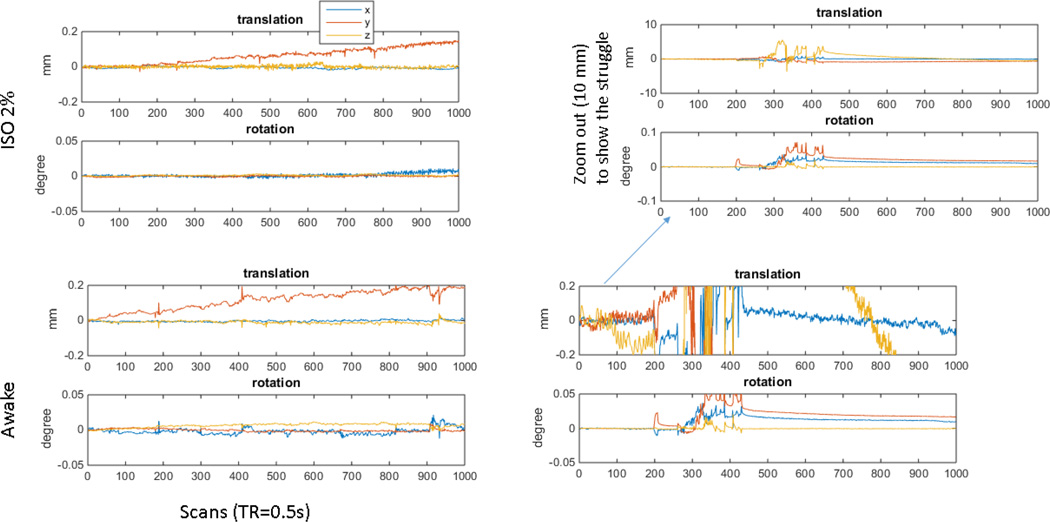
Figure 2. Motion time course from an anesthetized rat.
At top left are the translation and rotation time courses for a rs-fMRI scan (GE-EPI; TE 15 ms; TR 500 ms; 9.4 T) from a rat anesthetized with 1.2% isoflurane. Motion in x and y is well less than size of a voxel (0.4 mm). The linear drift in the y direction is likely due to a slow frequency drift that occurred as the temperature of the gradient rose, a problem that has been mitigated but not completely solved by the installation of a new gradient/shim insert (RRI, Billerica, MA) with better cooling and more stable temperature characteristics. The drift was corrected during postprocessing. For comparison, the time courses from a very successful study in an awake animal are shown at the bottom left. Motion is greater than in the anesthetized animal but remains below the size of a voxel throughout the scan. For contrast, a more typical scan from an awake rat is shown at the right. Sections of the scan have acceptable levels of motion, but a period of struggling beginning at ~ image 250 results in high amplitude motion that must be discarded prior to analysis.
In the rodent, respiration can cause apparent motion of the brain due to the movement of the thorax within the magnetic field. This results in frequency offsets and the apparent shifting of the brain in the phase encode direction (Kalthoff, Seehafer, Po, Wiedermann, & Hoehn, 2011). Similarly, apparent motion in the phase encode or slice directions can arise from a slow frequency drift, possibly associated with gradient heating (Figure 2). Careful application of motion correction and regression of motion and respiratory parameters can minimize the effect on further analysis.
Time course noise: physiological cycles
Physiological noise commonly arises from the respiratory and cardiac cycles and manifests as spatially structured noise that can introduce spurious correlations or activations (Murphy et al., 2013). In addition to contributions from the cycles themselves, variations in heart rate or breathing pattern can also add structured noise to the data (Birn et al., 2006; Chang et al., 2013). The effects of physiological cycles are particularly troublesome for rs-fMRI. In fMRI, activation maps are typically created from the combined responses to many stimuli. This averaging effectively suppresses noise that is not phase-locked to the stimulus. In rs-fMRI, however, the spontaneous neural activity that would ideally be detected is not predictable or time-locked. Any process that contributes to the BOLD signal in a spatially-structured manner can alter network connectivity or even introduce spurious “networks” unrelated to brain activity.
In many human studies, retrospective methods of physiological noise correction are applied, including regression of white matter (WM) and cerebrospinal fluid (CSF) signals. In rodents, however, the volume of white matter and CSF is relatively small and, though this method has been used successfully by some groups (Liska et al., 2015; Sforazzini et al., 2014; Sierakowiak et al., 2015), it can be difficult to obtain clean estimates of the WM and CSF signals, particularly in scans with relatively low spatial resolution. Ideally, the cardiac and respiratory cycles themselves be recorded during all animal experiments, particularly in anesthetized animals where physiological variables tend to change over time unless strict stability of anesthesia level, temperature, and oxygenation are maintained (Pan et al., 2015). Their contributions to the signal time course can then be minimized by regression on a voxel-by-voxel basis (Glover et al., 2000; Hu et al., 1995).
Sometimes constant recording of physiological cycles is not feasible due to scanner interference or other experimental constraints. Respiration is fairly easily captured with a small pressure balloon placed beneath the animal, but electrocardiography can be tricky in small animals placed in high field scanners. Blood oxygenation should be measured with a pulse oximeter as part of routine animal monitoring, and this measurement can also give the heart rate. While this strategy does not allow a full characterization of the cardiac cycle, it at least provides information about the stability of the animal’s condition over the course of the scan. If physiological noise correction based on cardiac and respiratory cycles can not be corrected directly due to the challenges in recording, they must at minimum be monitored, recorded and reported to demonstrate that the animals are in a stable physiological state during the study and that no baseline differences exist across groups.
In mechanically ventilated animals, the challenges are slightly different. The respiratory cycle is extremely stable, as shown by the narrow peak in Figure 3, and there is little variation during the course of a scan. However, it is critical that researchers measure arterial blood gases in ventilated animals, as they can quickly become hyper- or hypocapnic, with resulting changes in blood flow, activation patterns, and connectivity maps.
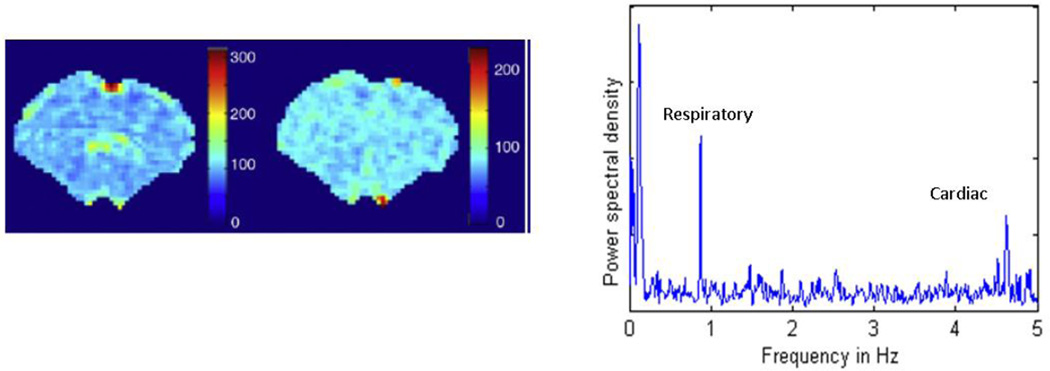
Figure 3. Distribution of cardiac and respiratory noise in the anesthetized rat.
(Left) The relative power for the respiration peak, plotted for each voxel in the brain. Most of the power is localized to medial surface areas near the sagittal sinus. (Center) The relative power for the cardiac peak, plotted for each voxel in the brain. Strong contributions are localized to a few spots near the surface and base of the brain. All data were obtained from a rs-fMRI scan in a rat anesthetized with alpha-chloralose and a TR of 100 ms (adapted from (Williams et al., 2010)). (Right) The power spectrum for one pixel demonstrating low frequency BOLD oscillations, respiration, and cardiac pulsation. Note that the animals in this study were anesthetized with alpha-chloralose and mechanically ventilated, which accounts for the sharpness of the respiratory peak.
Sometimes the primary contribution of respiration and cardiac pulsation can be removed directly based upon frequency. The rate of cardiac pulsation in rats depends on the age of the animal and the anesthetic used, but is around 5 Hz. Respiratory rates are closer to 1 Hz. With most small animal scanners, a single slice can be sampled rapidly enough to resolve the primary spectral peaks from these processes. Using a 100 ms TR, Williams et al. found that respiratory contributions are strongest near the midline of the brain and along the brain surface, while cardiac noise is highest near the base of the brain (2010; Figure 3). However, the sampling rates required to resolve the physiological cycles typically preclude the whole brain coverage needed for many studies and do not correct for contributions from aliased higher frequency harmonics.
Time course noise and appropriate choices of filters
Human rs-fMRI studies almost universally employ a lowpass filter cutoff of 0.08 or 0.1 Hz based on early studies that found that higher frequencies had stronger noise contributions (Cordes et al., 2001), though recent studies with higher sampling rates have demonstrated functional connectivity at substantially higher frequencies (Chen and Glover, 2014; Lee et al., 2013). Filtering out the higher frequency components can reduce many types of noise while improving sensitivity to the BOLD fluctuations, which are predominantly low in frequency. The 0.08 or 0.1 Hz cutoffs are routinely applied to animal rs-fMRI studies as well. While this may be appropriate for some anesthetics (isoflurane), for others the frequency range should be much broader. Pan et al. examined the coherence between infraslow electrical activity (< 1 Hz) and the BOLD fluctuations and found that significant coherence was limited to < 0.1 Hz under isoflurane but extended to about 0.25 Hz for dexmedetomidine (Pan et al., 2013). This finding should motivate a careful examination of the frequency spectrum of the BOLD signal across groups of animals and under different anesthetic conditions. For example, it is possible that changes in connectivity across groups of rats could reflect a change in vascular tone or other neurophysiological parameters that in turn results in a shift in the BOLD power spectrum, moving the main range of coherence beyond the high frequency cutoff.
Physiological condition
To ensure reproducible experimental results, groups of animals should be consistent in strain, gender, age, weight, housing condition, food and lighting cycle, etc (Hildebrandt et al., 2008). Anesthetics in particular can affect different strains/sexes differently (Hu et al., 2012; Petrinovic et al., 2016), and therefore can contribute to group differences. Physiological parameters should be monitored and controlled as much as possible during every experiment. Relevant parameters include anesthetic level, body temperature, oxygenation level, and respiratory and cardiac rhythms. Fluctuations in body temperature can contribute to drifts in the BOLD signal baseline, even when the temperature changes are within physiological ranges (Vanhoutte et al., 2006). Respiratory and cardiac contributions can be minimized during postprocessing if they are recorded and time-locked to image acquisition (Glover et al., 2000; Hu et al., 1995). Note that cardiac and respiratory rates vary substantially across species and, for small animals, are typically much faster than for humans. For example, rats typically have cardiac rates of 300–400 beats per minute and respiratory rates of 85–110 breaths per minute, although this depends on strain, sex, size, and time of day, and is often altered by anesthesia (Azar et al., 2011; Pass and Freeth, 1993). Physiological processes are even faster for mice, with cardiac rates of 500–700 beats per minute and respiratory rates of 100–160 breaths per minute, though again the rates are highly variable across conditions and strains (Groeben et al., 2003; Ho et al., 2011). In contrast, typical rates for human respiratory and cardiac cycles are 12–18 breaths per minute and 50–90 beats per minute (Barrett and Ganong, 2010; Spodick, 1996). For studies that compare different groups of animals, demonstrating that the heart rate and blood oxygenation levels are comparable across groups and stable over the course of the experiment greatly increases confidence that any effects are not due to alterations in basic physiology.
Data quality assessment is critical prior to further data analysis. Reviewing animal physiological recordings over the course of each scan and ensuring that the animal is in a stable and normal range is extremely important to ensure reproducible results. The quality of the imaging data should also be assessed. If any ‘spikes’ or short-lived high amplitude changes are detected in a time course, they might indicate noise from motion or other uncontrolled technical issues.
To maintain stable physiology, some monitoring choices are dependent upon the anesthetic agent used. α-chloralose depresses respiration, making artificial ventilation critical and making the administration of paralytic agents desirable. Invasive blood gas monitoring is required to ensure that levels are maintained at stable and physiological levels to minimize variability in the baseline cerebral blood flow (CBF). Rodents anesthetized with isoflurane or dexmedetomidine are generally not intubated and it is typically assumed that freely-breathing rodents self-regulate, but it is critical to maintain very stable body temperature and physiological parameters to minimize differences across scans and subjects. The effects of temperature on the BOLD signal are not completely understood, but in our experience, when body temperature drops functional activation is often lost completely, so the effects of temperature cannot easily be removed by regression. There is also considerable evidence that physiology changes over time during the application of certain anesthetics, and so the amount of time spent under anesthesia may need to be considered during analysis (Magnuson et al., 2014; Pawela et al., 2009). Issues associated with the choice of anesthesia are further discussed in a separate section.
Neurophysiological processes that could be considered noise
Neurometabolic and neurovascular coupling
A rich existing literature explores the relationship between neural activity, metabolism, and hemodynamics, particularly in animal (Devonshire et al., 2012; Duong et al., 2000; Hewson-Stoate et al., 2005; Kim and Uğurbil, 1997; Logothetis et al., 2001; Lu et al., 2007; Maandag et al., 2007; Martin et al., 2006a; Pan et al., 2011, 2013; Shmuel and Leopold, 2008; Silva et al., 2000; Yang et al., 1997). Briefly, an increase in localized neural activity leads to a demand for metabolic substrates in the activated area. Because the brain does not store large amounts of energy (Gatfield et al., 1966), fluctuations in brain activity are tightly coupled to energy delivery through the blood (Hyder et al., 2002; Roy and Sherrington, 1890). Some findings even suggest that the hemodynamic response can anticipate neural activity(Sirotin and Das, 2009). It is the coupling between neural activity, metabolism, and hemodynamics that forms the basis of fMRI and rs-fMRI. The increased flow of blood results in a decrease in the concentration of deoxyhemoglobin in the area and therefore an increased BOLD response (Ogawa et al., 1990). Because CBF, the cerebral metabolic rate for oxygen (CMRO2) and cerebral blood volume (CBV) all contribute to the BOLD signal, an accurate interpretation of changes observed in BOLD (whether across conditions or across species) relies on understanding the quantitative relationship between the underlying changes in CBF, CMRO2, and CBV. For example, the baseline level of CBF influences the magnitude of the response to stimulation (Cohen et al., 2002). Thus it may be necessary to measure each component of the BOLD signal to understand the source of the change. Recent work by Buxton et al. even suggests that CBF and CMRO2 may affect the BOLD signal in parallel rather than in series, with each having different sensitivities to the overall state of the brain and properties of the stimulus (Buxton et al., 2014).
Despite the substantial body of work exploring mechanisms of neurovascular coupling, the phenomenon remains poorly understood. The hemodynamic response to sensory stimuli in primary sensory cortex is the most well characterized (Berwick et al., 2008; Brinker et al., 1999; Martin et al., 2006). Robust signal increases can be observed during stimulation, even at the level of a single whisker barrel (Yang et al., 1996). Experiments linking fMRI and electrophysiology found that the BOLD increases most closely resemble the change in local field potentials (LFPs), though they also matched fairly well with multiple unit activity (MUA) (Logothetis et al., 2001). Outide of sensory cortex, the picture is less clear. Areas such as the thalamus do not always show activation during stimulation despite their clear involvement in the sensory pathway (Keilholz et al., 2006, 2004). It is not yet known whether the lack of activation reflects the effect of relatively poor spatial resolution on the small nuclei, or vagaries of neurovascular coupling that make the BOLD signal less sensitive to neural activity in these areas (Devonshire et al., 2012; Mishra et al., 2011). The negative BOLD signal is even more difficult to interpret, with different experiments supporting sources ranging from vascular steal to neural inhibition (Boorman et al., 2015; Huber et al., 2014). In some areas, increased neural activity has been observed without corresponding changes in hemodynamics (Huo et al., 2014; Mishra et al., 2011).
For rs-fMRI, it is generally assumed that the correlated BOLD fluctuations used to map functional connectivity measurements reflect coordinated neuronal communication filtered through neurometabolic and neurovascular coupling. However, the baseline activity of the brain dwarfs activity that occurs in response to a stimulus and the BOLD fluctuations may reflect many processes in addition to the time-varying neural activity that is generally of interest (Hyder et al., 2002). Recent work using simultaneous rs-fMRI and electrophysiological recording to examine the neural basis of spontaneous BOLD fluctuations found that high frequency LFPs, particularly in the gamma range, were tightly coupled to the BOLD signal from the same area and also to the global signal (Pan et al., 2011; Scholvinck et al., 2010; Shmuel and Leopold, 2008). On the other hand, lower frequencies (delta and theta) seem to be more closely related to the BOLD correlation between areas (Lu et al., 2007; Pan et al., 2011; Thompson et al., 2015). At the extreme end of the scale, infraslow electrical activity contributes strongly to BOLD fluctuations and interhemispheric correlation (Pan, Thompson et al. 2013). These infraslow oscillations can be observed in spike trains and are linked to changes in CBF (Huang et al., 2014). Taken together, these studies suggest that a combination of different neurophysiological processes may underlie the spontaneous BOLD fluctuations used to map functional connectivity. For reviews of the relationship between rs-fMRI and neural activity, see (Keilholz, 2014) and (Scholvinck et al., 2013).
While most previous work has focused on the relationship between neural activity and the hemodynamic response, glial cells outnumber neurons in the brain 10 to 50 fold (Herculano-Houzel, 2013) and their contributions to brain activity are barely beginning to be elucidated. The majority of glia in grey matter are protoplasmic astrocytes (Hansson and Rönnbäck, 2003). Morphologically, protoplasmic astrocytes are similar to neurons. They contain receptors for neurotransmitters, and respond to glutamatergic signaling with local increases in Ca2+ concentration and, at times, waves of Ca2+ signaling that propagating through gap junctions between adjacent astrocytes. Their many processes physically connect the vasculature with neurons to convey nutrients and waste across blood brain barrier. Importantly, this interface actively regulates neurovascular coupling. Astrocytic end-feet often surround the synapse, regulating the interstitial concentration of neurotransmitters through both reuptake and de novo release. The story of the direct and active involvement of glia in neuronal signaling is still evolving. The evidence thus far indicates that the glia are much more than a kind of support network and may contribute to fMRI and rs-fMRI in their own way.
From these studies it is clear that anything that changes neurovascular coupling can contribute to intra- and inter-individual variability in fMRI and rs-MRI. Anesthesia is probably the single biggest factor affecting neurovascular coupling in animal studies and will be discussed in depth in a later section. However, less-localized neural, glial and vascular processes can also contribute to the BOLD signal and increase variability. These include the global signal, vasomotion, and quasiperiodic patterns.
Global signal: noise or not?
The global signal is by definition not related to localized responses to stimuli or to the time-varying interactions that would ideally define functional networks. Global signal regression is sometimes employed to reduce noise on the assumption that any signal common to the whole brain is unlikely to be of interest. Its use is controversial, particularly for rs-fMRI, where global signal regression promotes more focal correlation patterns (Fox et al., 2009) but can also introduce artifactual anticorrelations (Murphy et al., 2009). Even for fMRI, there is evidence that global changes occur during tasks and can be detected with sufficient averaging (Gonzalez-Castillo et al., 2012). Studies have shown that the BOLD global signal in humans is linked to changes detectable by MEG or EEG (Tal et al., 2013; Wong et al., 2016, 2013, 2012). Caffeine affects both neural activity and global BOLD amplitude (Tal et al., 2013; Wong et al., 2012), and the global signal amplitude is related to changes in vigilance measured by EEG (Wong et al., 2016, 2013). In monkeys, gamma power from one electrode was correlated to changes in cerebral blood volume (CBV) from almost the entire brain, with varying delays (Scholvinck et al., 2010). The global signal has also been shown to be useful in distinguishing across patient groups (Hahamy et al., 2014; Yang et al., 2014), supporting a tie between global signal and brain function.
On the other hand, global signal regression has been used to reduce the effects of varying doses of isoflurane in rodents by minimizing nonspecific changes (Liu et al., 2013b). Similarly, Figure 5 shows that global signal regression reduces the differences in functional connectivity maps acquired from awake and anesthetized rats (discussed further in the section on anesthesia). Global signal regression also improves the correspondence between bandlimited power correlation of LFPs and BOLD correlation (Thompson et al., 2013b). These findings suggest that if the global BOLD signal does have a neural origin, it arises from a type of nonlocalized activity that differs from the coordination of localized responses or functional networks and may provide distinct information about brain function. Until the sources of the global BOLD signal are better understood, it is probably advisable to examine results of rs-fMRI in particular with and without global signal regression.
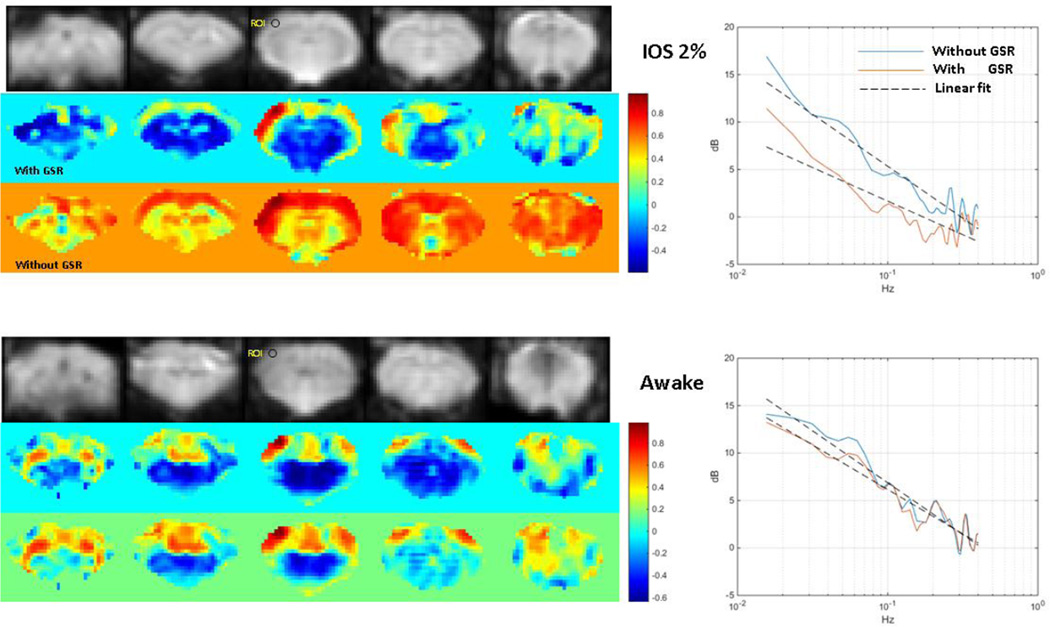
Figure 5. Functional connectivity and the effect of global signal regression in awake and anesthetized rats.
Resting state MRI data was acquired from one rat that had been acclimated to the scanner. The rat was originally anesthetized with isoflurane, then allowed to wake up inside the MRI scanner as data was acquired. Correlation with a seed in somatosensory cortex is widespread and strong when the rat is anesthetized (left top) but localized to the somatomotor network when the rat is awake (left bottom). The awake rat also exhibits anticorrelation between cortical and subcortical regions. Interestingly, global signal regression increases the specificity of the connectivity map in the anesthetized rat and introduces anticorrelations similar to those observed in the awake animal. Global signal regression has little effect in the awake animal. The power spectra from the seed region in somatosensory cortex show a similar effect, with global signal regression reducing power in the data acquired under isoflurane (right top) but having relatively small effects on the data from the awake animal (right bottom).
Vasomotion
The vasculature itself exhibits low frequency oscillatory behavior. Vasomotion is the spontaneous oscillation of the vasculature, particularly the small arteries, at approximately 0.1 Hz (Hudetz et al., 1998; Mayhew et al., 1996). These oscillations can be tied to neural activity and metabolism (Golanov et al., 1994; Vern et al., 1997) but can also exist independently, as vasomotion has been observed in isolated arteries (Osol W., 1988). Mayer waves, related to a systemic oscillation of arterial blood pressure, are predominant in larger vessels. The Mayer wave frequency varies across species, from ~0.1 Hz in humans to ~0.4 Hz in rats (Julien, 2006). Because the frequencies of vascular oscillations overlap at least partially with those of the BOLD fluctuations used to map functional connectivity (Colantuoni et al., 1994; Haxhiu et al., 1989; Hudetz et al., 1998; Julien, 2006; Mayhew et al., 1996; Obrig et al., 2000; Osol W., 1988; Vern et al., 1997), the accompanying changes in CBF and CBV (Colantuoni et al., 1994; Mayhew et al., 1996; Vern et al., 1997) almost certainly affect the BOLD signal.
Since vasomotion may be generated from within the vascular wall, synchronized neural events in spatially-distant areas, i.e. functional connectivity, could elevate the amplitude of vasomotion in a time-locked manner and possibly induce phase synchronization across areas. Phase synchrony in particular would be likely to contribute to the correlation typically measured in functional connectivity analysis. In addition to classical neural event-driven model of the hemodynamic response, neural metabolism might also facilitate vasomotion. The metabolite nitric oxide (NO), produced by either the endothelium (vascular origin) or neurons and astrocytes (neural origin), plays an important role in mediating vasomotion (Dirnagl et al., 1993; Morita-Tsuzuki et al., 1993). Thus, we speculate that the resting-state BOLD signal may be modulated by slow hemodynamic oscillations due to vasomotion, or that the resting brain neurovascular coupling partially results from neurally-modulated vasomotion.
In support of a contribution from vasomotion to functional connectivity, we observed a narrow peak (~0.15 Hz) in the power spectrum of the BOLD fluctuations from primary somatosensory cortex in rats imaged with a short TR (100 ms), single slice protocol (Majeed et al., 2009; Williams et al., 2010). We hypothesized that the peak was related to the inflow sensitivity imparted by the short TR, which increased the sensitivity to CBF. To further examine this peak, we performed rs-fMRI studies with BOLD and CBV contrast in the same rats. The CBV-weighted studies exhibited a prominent peak between 0.15 and 0.2 Hz as well, suggesting that there is a specific contribution from the vasculature at these frequencies (Magnuson et al., 2010). In contrast, when we simultaneously acquired LFPs and rs-fMRI from the same site under the same conditions, the coherence between LFPs and BOLD was smooth and broad, ranging from 0.01 to 0.25 Hz (Pan et al., 2013). By comparison, we infer that the narrow-band peak is related to a ‘resonance’ of the vasculature, correlated across hemispheres, which could enhance or even introduce correlation to the BOLD time courses of two areas, therefore affecting rs-fMRI studies. These oscillations may also impact variability in the responses observed across scans in fMRI experiments. The contribution of vasomotion is likely to be strongly dependent upon the physiological state of the animal, as it has been shown that hypotensive conditions enhance vasomotor oscillations while hypercapnia reduces them (Hudetz et al., 1992).
Quasiperiodic patterns
A portion of the enormous ongoing “background” activity of the brain manifests as large-scale quasiperiodic, spatiotemporal patterns of activation. Evidence of quasiperiodicity in brain function comes from multiple groups (Chow et al., 2013; Grigg and Grady, 2010; Ko et al., 2011; J. M. Li et al., 2015; Nikulin et al., 2014) and shows that quasiperiodic activity persists across a number of states, including sleep, wakefulness, and anesthesia. Quasiperiodic patterns (QPPs) in the BOLD signal were first observed as bilateral waves of signal propagating from lateral to medial areas in anesthetized rats (Majeed et al., 2009). The implementation of a pattern-finding algorithm allowed the detection of similar patterns in human subjects, this time involving alternation between a default mode-like network (DMN) and a task positive-like network (TPN), with propagation along the cortex between network nodes (Majeed et al., 2011).
In the rat, QPPs are similar in spatial extent and timing to the correlation between infraslow activity and the BOLD signal (Pan et al., 2013; Thompson et al., 2014), suggesting that they reflect infraslow electrical activity. These findings are consistent with other observations of quasiperiodicity in brain function, all of which fall into the infraslow regime (Chow et al., 2013; Ko et al., 2011; J. M. Li et al., 2015; Nikulin et al., 2014). The relatively long time scale and repetitive nature of the patterns is evidence that they are not directly tied to cognitive processing. QPPs can be observed in CBV-weighted images as well as BOLD-weighted images (Magnuson et al., 2010), and callosotomy disrupts their interhemispheric coordination but not their intrahemispheric organization (Magnuson et al., 2014).
What is the source of these patterns and what is their effect on functional imaging? Both are currently unknown but there are intriguing hints. It has long been hypothesized that functional connectivity is at least partially mediated by a subcortical driver like the rostral ventrolateral medulla (RVLM) (Drew et al., 2008). Many subcortical nuclei like the RVLM, locus coeruleus, the pedunculopontine nucleus, and the raphe nucleus have widespread cortical projections and rhythmic output that could theoretically drive spatially structured activity like that seen in the QPPs. In rats, QPPs appear to reflect a process tied to infraslow oscillations that is not strongly coupled to higher frequency activity (Pan et al., 2013; Thompson et al., 2013; Thompson et al., 2015, 2014). In humans, the phase of the QPP has been linked to performance on a vigilance task, with the DMN dominant phase tied to greater variability in reaction time (Abbas et al., 2016). This is not surprising given the that two primary networks involved in the QPP in humans have been linked to performance on many tasks (Fox et al., 2007, 2006; Kelly et al., 2008; Magnuson et al., 2015; Thompson et al., 2013a). Given these findings, it is possible that the QPP influences the response to an individual stimulus, at least for some types of stimuli. The QPPs certainly influence rs-fMRI, particularly in the DMN. Recent work shows that the QPPs account for up to 25% of the variance in that network and contribute significantly to connectivity there (K. Wang et al., 2016).
Noise and the dynamic brain
Using the simplest analysis, fMRI data are typically presented as activation maps for a particular type of task, or as a comparison of these maps across groups or conditions. Resting state fMRI scans are more commonly presented as maps of the networks, along with correlation values for nodes within networks or between networks. These data are merely the tip of an entire iceberg of information that can be obtained from the BOLD signal. A number of analysis techniques are under development to obtain more information about the timing of the activity from the existing BOLD data. These methods are vulnerable to additional types of noise compared to the standard steady state methodology.
Causality
There has been substantial interest in the use of Granger causality to determine whether one area drives or is driven by another area (Deshpande et al., 2011; Jiao et al., 2011; Krueger et al., 2011). There are a number of implementations for functional neuroimaging studies, but at the heart they determine whether the addition of information from one area improves the prediction of signal in another area. In terms of noise, the most serious confound for Granger analysis is that neurovascular coupling varies across different areas of the brain (Devonshire et al., 2012; Huber et al., 2014; Huo et al., 2014), and so causality may detect differences in hemodynamic response latencies rather than actual directed neural influences. Experiments that examine causality must be very carefully designed and interpreted in light of these effects. Animal studies that use simultaneous measurements of BOLD and neural activity may help to determine in what cases BOLD-based Granger causality can accurately reflect neural information flow.
Spontaneous BOLD fluctuations
Traditional analysis of rs-fMRI examines relationships that persist for the course of an entire scan. Theoretically, at least part of the variability in the BOLD signal over time arises from spontaneous neural activity. If this is the case, dynamic analysis of rs-fMRI data may provide insight into the spontaneous reconfiguration of brain networks. The most common method of dynamic analysis involves calculating correlation between areas during a series of windowed time segments of the scan. Sliding window correlation (SWC) is intuitive and easy to implement, yet a number of studies have shown that it performs poorly at detecting actual changes in correlation (Hindriks et al., 2015; Shakil et al., 2016). The inherent properties of the BOLD signal result in variability in correlation over time even in mismatched data from different scans or different animals (Handwerker et al., 2012; Keilholz et al., 2013). This is a type of noise that makes it difficult to identify the signals of interest. However, evidence links connectivity measured in short time windows in humans to behavioral performance (Thompson et al., 2013a), and sliding window correlation at least partially reflects time-varying connectivity based on electrophysiological measurements in rodents (Thompson et al., 2013b). Moreover, SWC can be used along with other analysis methods to distinguish dynamic transitions that occur in patients from those that occur in healthy controls (Li et al., 2014; Rashid et al., 2014; Yu et al., 2014). It appears that despite its poor estimation of actual correlation values, SWC preserves some of the underlying structure in the data. How much structure is likely to depend on the signal to noise ratio, the size of the networks examined, and the parameters used (window length, overlap, etc). The search for better dynamic analysis techniques is currently being pursued with much vigor and may improve sensitivity to transitions that are of interest. Again, multimodal animal experiments are likely to play a key role in the development of better dynamic imaging methods by providing a gold standard for comparison so that unwanted sources of variability can be optimally minimized.
Ongoing activity and variability
It is well established that both the neural response to a stimulus and the BOLD response vary substantially from one instance of the stimulus to another. Individual responses to stimuli can vary considerably from scan to scan, even in very stable anesthetized animals (as shown in Figure 1). The variability in response is at least partially explained by variability in ongoing ‘background’ brain activity and has been observed at multiple scales. For example, at the single cell level, spiking variability in a single neuron in visual cortex during stimulation is affected by global fluctuations in neural activity in cats (Scholvinck et al., 2015). At a larger scale, researchers found that the phase of ongoing EEG activity in human visual cortex influences visual perception (Busch et al., 2009). Another study using MEG found that the prestimulus power rather than phase was linked to visual response time (Bompas et al., 2015).
In anesthetized animals, Maandag et al. showed that in a state of high baseline energy as measured by global glucose consumption (1% halothane), more energy was devoted to fast signaling (firing rates of 13–40 Hz) than in a lower baseline energy state (α-chloralose), where most firing rates fell into the 1–13 Hz range (35). When the forepaw was stimulated, activation measured with fMRI was localized to contralateral somatosensory cortex in the low energy state, but spread to secondary somatosensory areas and ipsilateral cortex in the high energy state. These studies suggest that some of the variability across individual instances of stimulation can be accounted for by the background activity of the brain and should not necessarily be considered noise. If not of interest, the contribution from background activity can be minimized by averaging across multiple trials.
The effects of anesthesia
Because even small amounts of motion can ruin a fMRI or rs-fMRI scan, and because most animals experience substantial stress when immobilized, anesthesia is usually used in functional studies in animals to minimize both motion and stress (which can itself affect the BOLD signal through changes in baseline physiology). The agents used range from mild sedatives to general anesthetics, and there are considerable differences in their effects on the baseline metabolism of the brain (Maandag et al., 2007). Responses to sensory stimuli can typically be detected in anesthetized animals, and the general structure of functional networks is preserved during anesthesia (H Lu et al., 2012; Vincent et al., 2007). However, these general similarities overlay numerous differences that should not be overlooked.
Both fMRI and rs-fMRI rely on consistent neurovascular coupling to represent neural activity. However, anesthetic agents often have side effects on animal physiology that alter vascular resistance and therefore neurovascular coupling. The processes that mediate neurovascular coupling are not completely understood, but are likely to involve modulation of dilation and constriction of adjacent arterioles by neural metabolites, such as CO2, K+ and NO. Because neurovascular coupling is altered, the hemodynamic response to neural activity also varies across anesthetics. After the start of the stimulation, there is an anesthesia-dependent time lag before the BOLD signal increases, and the signal reaches its peak well after the stimulus has begun (de Zwart et al., 2005; Schroeter, Schlegel, Seuwen, Grandjean, & Rudin, 2014; Silva, Lee, Iadecola, & Kim, 2000). The overall shape of the hemodynamic response is also affected by anesthesia, with awake animals exhibiting a more complex response than anesthetized animals (Martin et al., 2006b). Both the optimal frequency of stimulation for fMRI and the range of frequency coherence for rs-fMRI can vary substantially across anesthetics (Aksenov et al., 2015; Guilfoyle et al., 2013; Jonckers et al., 2014; Keilholz et al., 2004; X Liu et al., 2012; X Liu et al., 2012; Masamoto et al., 2006; Pan et al., 2013; Williams et al., 2010; Zhao et al., 2008).
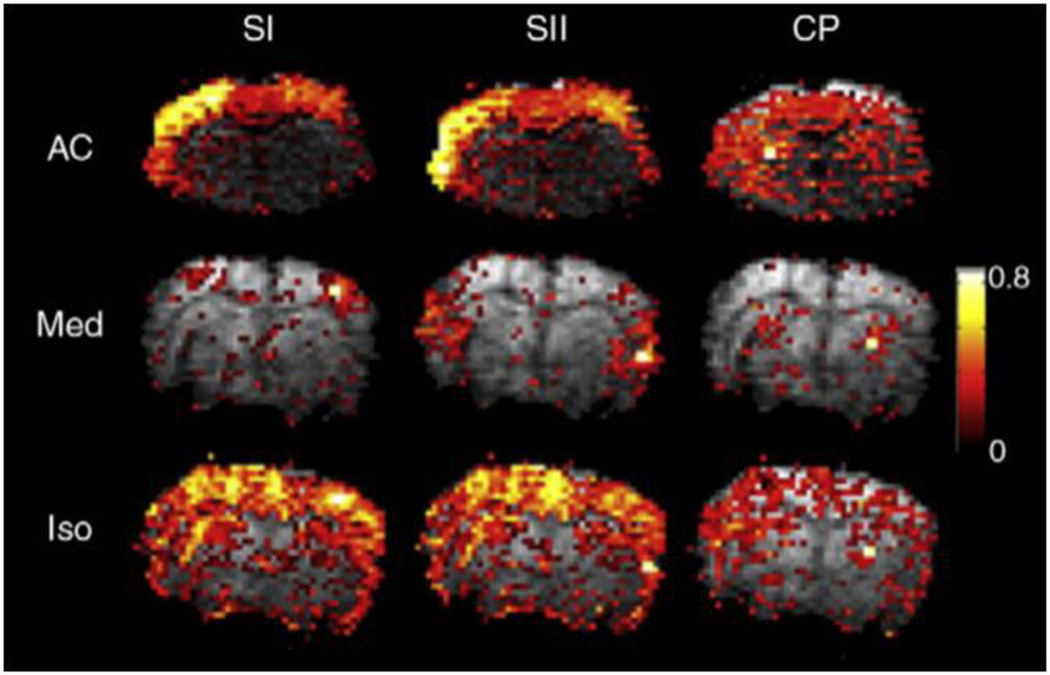
Different anesthetics can affect neurovascular coupling in different ways (Franceschini, Radhakrishnan et al. 2010), which in turn affects the fMRI response and functional connectivity measured with rs-fMRI (Grandjean et al., 2014; Pan et al., 2013; Williams et al., 2010). A comparison of functional connectivity in three different brain areas under three different anesthetics from (Williams et al., 2010) is shown in Figure 4. The extent, magnitude, and specificity of the correlation with a seed region varies across anesthetics and networks.
Figure 4. Differences in functional connectivity under different anesthetics.
Connectivity based on seed regions in primary somatosensory cortex, secondary somatosensory cortex, and the caudate putamen are shown for individual rats under three anesthetics (Williams et al., 2010). The strength of the correlation with the seed, the spatial extent, and the specificity vary by anesthetic and by network. Similar effects were observed during group analysis.
Many of the earliest fMRI and rs-fMRI studies used α-chloralose, a GABAergic anesthetic which has relatively minor effects on neurovascular coupling and neural activity. Ueki et al. demonstrated localized metabolic activation under α-chloralose after electrical stimulation in rats that could not be observed under halothane (Butler, 1964; Ueki et al., 1992). Further work found that regional CBF responses were stable and greater under α-chloralose than in halothane-anesthetized rats (Lindauer, Villringer et al. 1993). However, the shape of the response to stimulation was similar under both anesthetic agents, suggesting that coupling between neural activity and blood flow changes can be relatively preserved despite disrupted neurometabolic coupling. This emphasizes the need for careful consideration of the effects of anesthesia on both blood flow and metabolism in order to interpret the BOLD response.
The volatile GABAergic agent isoflurane is convenient, widely available and commonly used for functional studies, though the associated vasodilation and the suppression of neural activity (especially during deep anesthesia) complicate interpretation (Fukuda, Vazquez et al. 2013). Volatile anesthetics tend to increase cerebral blood flow by inducing vasodilation via ATP-sensitive K+ channels of arteriolar smooth muscle cells (Iida, Ohata et al. 1998). However, these effects can vary by region, with localized CBF decreases observed in areas such as the thalamus, probably due to the predominance of neural inhibition (Ramani and Wardhan 2008). The amount of vasodilation and neural suppression is dependent upon the dose of isoflurane, and the extent of the functional networks detected under isoflurane exhibit a similar dose-dependence (X. Liu et al., 2013). Isoflurane typically produces periodic burst-suppression rhythms across the whole brain, which can obscure lower amplitude fluctuations between regions known to be functionally connected (Kalthoff, Po et al. 2013; Magnuson, Thompson et al. 2014). Reduction in functional connectivity can be observed in humans as well under the analogous anesthetic sevoflurane (Peltier, Kerssens et al. 2005).
Sedation rather than general anesthesia is often preferable for fMRI and rs-fMRI in rodents. Medetomidine and its active enantiomer, dexmedetomidine, are α2-adrenergic agonists that induce sedation (Kalthoff et al., 2013; Weber et al., 2006). It appears to act through endogenous sleep pathways via the locus coeruleus (which has the highest presynaptic α2-adrenergic receptor concentration) that in turn decrease the afferent input to the thalamus and thalamic activity (Correa-Sales, Rabin et al. 1992; Nelson, Lu et al. 2003; Bonhomme, Maquet et al. 2008). Dexmedetomidine administration causes a marked decrease in global CBF (Zornow, Fleischer et al. 1990; Prielipp, Wall et al. 2002; Drummond, Dao et al. 2008; Fukuda, Vazquez et al. 2013), which may arise from cerebral vasoconstriction (Prielipp et al., 2002; Zornow et al., 1990). It has become popular for fMRi and rs-fMRI studies as it lacks the dose-dependent vasodilation and neural suppression that occur under isoflurane. It should be noted that the coherent range for LFP and BOLD fluctuations under dexmedetomidine is wider than for isoflurane (~0.25 Hz compared to ~ 0.1 Hz; Pan et al., 2013). One potential problem is the stability of the preparation for long studies. Pawela et al. suggested that the initial dose of medetomidine be tripled after the first couple of hours to maintain sedation (Pawela, Biswal et al. 2009). Magnuson et al. also found that the characteristics of the spontaneous BOLD fluctuations and functional connectivity changed over time while rats were sedated with medetomidine (Matthew Evan Magnuson et al., 2014). Clearly there is a need for further studies on the exact effects of prolonged administration of (dex-) medetomine in functional imaging studies.
The variability of neurovascular coupling and metabolic condition under different anesthetics is a potential confound for functional neuroimaging studies, but fortunately the influence of anesthesia appears to modulate neurovascular coupling instead of abolishing it (Pan, Thompson et al. 2013). Nonetheless, especial caution should be used during transitions between drugs or dose levels because transient instabilities in hemodynamics can occur. It is often wise to implement a waiting period to ensure that a stable state is reached.
Although most fMRI and rs-fMRI experiments in animals utilize anesthesia is to ensure that animals remain motionless, awake rodents have been imaged by a few groups (Aksenov et al., 2015; King et al., 2005; Wyrwicz et al., 2000; Zhang et al., 2010). Hemodynamic responses have shorter latencies and narrower response functions in awake rats (Martin, Martindale et al. 2006). Although awake animal models are closer to human studies and can potentially examine more complex responses to stimuli, major challenges remain. Motion artifacts can occur due to struggling, changes in leg position, head motion, chewing and vocalizations (Febo, 2011). Examples of motion from a very successful rs-fMRI scan acquired in an awake rat and another from a more typical scan are shown in Figure 2. While there are fairly long time periods in the typical scan where motion in the awake animal is comparable to that observed in the anesthetized animal, there are also periods with large levels of motion that generally must be discarded. Unanesthetized rodents are typically acclimated to the stress of restraint over several days in an effort to minimize the acute stress induced by the scanning session (Febo 2011). However, the acclimation process itself might affect brain function as it could be considered a chronically stressful situation; indeed, some research groups have used restraint to develop a depression model in rodents (Willner, D'Aquila et al. 1995; Stepanichev, Dygalo et al. 2014).
The general network structure observed in the awake rat is preserved under anesthesia, though a detailed examination readily identifies differences (Liang et al., 2015; J. V Liu et al., 2013). Figure 5 shows functional connectivity before and after global signal regression in one rat that was acclimated to the scanner environment, imaged under isoflurane, then imaged again after being allowed to wake up in the scanner. Prior to global signal regression, the correlation between the seed region in somatosensory cortex and the rest of the brain is strong and widespread. After global signal regression, the strength and extent of the correlation is similar to that observed in an awake rat, and anticorrelation between cortical and subcortical areas can be seen. In contrast, the functional connectivity map for the awake rat exhibits anticorrelation even prior to global signal regression, and little change is observed after regression is performed. This is somewhat reminiscent of the findings that caffeine administration (increasing alertness) can have effects similar to global signal regression in humans (Wong et al., 2012), and that global signal regression can minimize dose-dependent changes in connectivity in rats anesthetized with isoflurane (X. Liu et al., 2013). A similar change can be seen in the power spectrum from the seed region. A loss of low frequency power is observed after global signal regression in the data acquired under anesthesia, while little change is seen in the power spectrum obtained while the animal was awake. In Figure 6, power spectra for the BOLD signal are shown for two rats that were acclimated to the scanner and initially anesthetized with isoflurane. Resting state fMRI data was acquired while they were anesthetized, as they were allowed to wake up, and as they were reanesthetized. The power is low during the initial period of anesthesia, increases as the rats wake, and then decreases again as they are reanesthetized. Future work should further explore the global signal as a marker for levels of arousal, and the potential for mitigating anesthetic effects using global signal regression.
Figure 6. BOLD power spectra from awake and anesthetized rats.
Resting state MRI data (9.4T; TR 1s; TE 14ms; 20 slices; 0.5 × 0.5 × 1 mm voxels; 1000 repetitions) was acquired from two rats that had been acclimated to the scanner. Each was originally anesthetized with isoflurane, then allowed to wake up inside the MRI scanner as data was acquired. Power spectra were calculated for the whole brain BOLD signal by Welch's method with 8 segments and 50% overlap. In both animals, the power during the initial scan acquired under anesthesia is low but increases as the rat is allowed to wake up. Maximal power is observed in the second scan acquired with 0% isoflurane after the anesthetic has completely worn off. As the animals are re-anesthetized, the power decreases to approximately the original level.
Most of the research discussed above was performed in rats. Anesthetics such as α-chloralose that required intubation and careful monitoring of arterial blood gases to maintain normal physiology are more demanding to use in mice due to the increased difficulty of arterial cannulation and their relatively small blood volumes, which limits the size and number of blood gas samples that can be obtained. Most fMRI and rs-fMRI studies in mice are instead performed under anesthetics like isoflurane, dexmedetomidine, or urethane. It is tempting to directly translate practices developed in rats to mice, but more and more work is showing that the two species have distinct properties important to fMRI and rs-fMRI. The hemodynamic response in mice shows more transient changes than in rats, and the spatial extent of the response depends strongly on the anesthetic agent used, partly due to changes in systemic physiology (Schlegel et al., 2015; Schroeter et al., 2014). Despite these changes, very similar resting state networks are observed in the mouse (Sforazzini et al., 2014).
The requirement for restraint is a major challenge for fMRI and rs-MRI in small animals. When anesthesia is used, its impacts on baseline physiology and neural activity should be carefully considered during the design of the experiment and the interpretation of the findings. For example, different stimulation frequencies are optimal for somatosensory studies under different anesthetic agents (Keilholz et al., 2004; Zhao et al., 2008), and the frequency range of the BOLD fluctuations used to map functional connectivity also varies (Thompson et al., 2013b). If not carefully controlled for, slight variations in the level of anesthesia over time can impact the results of neuroimaging studies within individuals. Systemic effects can also be present across groups, when one group has a greater or lesser susceptibility to the anesthetic agent. However, the problem is not entirely mitigated by moving to unanesthetized animals, as some individuals or groups may be more susceptible to stress experienced during training and/or imaging. One approach that has proven successful for our group and others is to perform experiments under at least two very different anesthetic conditions, so that common findings across conditions can be determined.
Noise as a signal
When the desired signal is that of neural changes in response to stimulation or of coordinated variations in activity over time, the number of potential noise sources can be disheartening. However, unlike image noise or time series noise, many of the processes that contribute to inter- or intra-subject variability have the potential to be of interest themselves. For example, the global BOLD signal can easily be obtained from any rs-fMRI experiment and may reflect fluctuations in overall levels of excitability across the brain. The relative amplitude of vasomotion across the brain might provide information similar to that of cardiovascular reactivity measurements (Lythgoe et al., 1999), and its inherently bandlimited nature suggests that it may be possible to obtain vasomotor contrast with a bandpass filter. The quasiperiodic patterns can be isolated using a pattern-finding algorithm (Majeed et al., 2011) and are linked to patterns of infraslow electrical activity. Infraslow activity is still understudied, although it appears to play a role in attention and attention-related disorders (Helps et al., 2010; Monto et al., 2008). If infraslow activity can be mapped with the BOLD signal and a pattern-finding algorithm, it will be possible to study it with a spatial and temporal resolution unobtainable with other techniques.
Network dynamics and dynamic responses to stimuli have proven challenging to characterize well to date, but separate consideration of different aspects of the BOLD signal (CBF, CBV, CMRO2) may improve sensitivity. The effect of ongoing variability in brain activity on the response to stimulation is responsible for a sizable portion of the trial-to-trial variability observed in fMRI. A better understanding of the background activity and its relationship to evoked activity would be a leap forward in our knowledge of the brain. Animal models are the ideal platform for the necessary experiments, as well-maintained anesthesia provides very stable conditions, minimal genetic variation can be achieved, and multimodal methods can be employed to better understand the BOLD signal fluctuations that are the basis for so many studies.
Conclusions
Numerous factors related to image acquisition, animal condition, neurophysiological processes, and neural activity contribute to the variability observed in the BOLD signal in animal experiments. As in human studies, it is important to minimize and account for undesirable variability whenever possible, particularly motion and physiological noise. Most of these methods are similar for animals and humans but there are some notable differences. The frequency range of the BOLD signals contributing to functional connectivity, in particular, is generally wider in animals and depends heavily on the type of anesthesia used. The use of anesthesia itself is an important difference that can have major impacts on the results of fMRI and rs-fMRI studies. It is essential to maintain anesthetized animals in stable, physiological conditions and to continuously monitor (and ideally record) physiological cycles. Other potentially confounding factors are less easy to address. Global signal regression, for example, discards neurally-relevant information but appears to improve sensitivity to time-varying interactions between areas. Until its role in brain function is better understood, the most conservative course is to analyze data with and without regression of the global BOLD signal. Quasiperiodic patterns appear to form part of the background activity of the brain and may influence the response to incoming stimuli. Vasomotion may or may not be linked to neural activity but clearly can contribute to the BOLD signal fluctuations. Until these processes are better understood, at a minimum they should be considered during the interpretation of functional neuroimaging data. For example, increased functional connectivity in hypotensive rats could arise from increased vasomotion rather than increased coordination of neural activity. Ultimately, multimodal studies in animal models may help us to identify, separate and interpret different components of the BOLD signal, improving sensitivity to the neural variability that is typically of interest while at the same time providing new information about the functional organization of the brain.
Highlights.
Image noise, time course noise, intra-animal variability and inter-group variability all affect sensitivity to changes in neural activity using MRI
Sources of variability in animal studies differ from those in human studies, primarily due to the use of anesthesia
Multimodal experiments in animal studies might identify neurophysiological sources for nonneuronal contributions to the BOLD signal
Acknowledgments
The authors would like to thank Anzar Abbas, Hyun Koo Chung, and Amrit Kashyap for helpful discussions. The research behind this review paper was supported by NIH R01 NS078095, R21 NS072810, R21NS057718, NSF BCS INSPIRE 1533260, Air Force Center of Excellence on Bio-nano-enabled Inorganic/Organic Nanostructures and Improved Cognition (BIONIC), and the Center for Systems Imaging at Emory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas A, Majeed W, Thompson G, Keilholz SD. Phase of quasi-periodic patterns in the brain predicts performance on psychomotor vigilance task in humans. Proc Int Soc Magn Reson Med. 2016:1192. [Google Scholar]
- Adamczak JM, Farr TD, Seehafer JU, Kalthoff D, Hoehn M. High field BOLD response to forepaw stimulation in the mouse. Neuroimage. 2010;51:704–712. doi: 10.1016/j.neuroimage.2010.02.083. [DOI] [PubMed] [Google Scholar]
- Aksenov DP, Li L, Miller MJ, Iordanescu G, Wyrwicz AM. Effects of anesthesia on BOLD signal and neuronal activity in the somatosensory cortex. J. Cereb. Blood Flow Metab. 2015;35:1819–1826. doi: 10.1038/jcbfm.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar T, Sharp J, Lawson D. Heart rates of male and female Sprague-Dawley and spontaneously hypertensive rats housed singly or in groups. J. Am. Assoc. Lab. Anim. Sci. 2011;50:175–184. [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn. Reson. Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Barrett KE, Ganong WF. Ganong’s review of medical physiology. McGraw-Hill Medical; 2010. [Google Scholar]
- Belcher AM, Yen CC, Stepp H, Gu H, Lu H, Yang Y, Silva AC, Stein Ea. Large-scale brain networks in the awake, truly resting marmoset monkey. J. Neurosci. 2013;33:16796–16804. doi: 10.1523/JNEUROSCI.3146-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, Yen CC-C, Notardonato L, Ross TJ, Volkow ND, Yang Y, Stein EA, Silva AC, Tomasi D. Functional Connectivity Hubs and Networks in the Awake Marmoset Brain. Front. Integr. Neurosci. 2016;10:9. doi: 10.3389/fnint.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick J, Johnston D, Jones M, Martindale J, Martin C, Kennerley AJ, Redgrave P, Mayhew JEW. Fine detail of neurovascular coupling revealed by spatiotemporal analysis of the hemodynamic response to single whisker stimulation in rat barrel cortex. J. Neurophysiol. 2008;99:787–798. doi: 10.1152/jn.00658.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RMM, Diamond JBB, Smith MaA, Bandettini PaA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bompas A, Sumner P, Muthumumaraswamy SD, Singh KD, Gilchrist ID. The contribution of pre-stimulus neural oscillatory activity to spontaneous response time variability. Neuroimage. 2015;107:34–45. doi: 10.1016/j.neuroimage.2014.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman L, Harris S, Bruyns-Haylett M, Kennerley A, Zheng Y, Martin C, Jones M, Redgrave P, Berwick J. Long-Latency Reductions in Gamma Power Predict Hemodynamic Changes That Underlie the Negative BOLD Signal. J. Neurosci. 2015;35:4641–4656. doi: 10.1523/JNEUROSCI.2339-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker G, Bock C, Busch E, Krep H, Hossmann KA, Hoehn-Berlage M. Simultaneous recording of evoked potentials and T2*-weighted MR images during somatosensory stimulation of rat. Magn Reson Med. 1999;41:469–473. doi: 10.1002/(sici)1522-2594(199903)41:3<469::aid-mrm7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J. Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R. Pharmacokinetics of Halothane and Ether. Brit J Anaesth. 1964;36:193–199. doi: 10.1093/bja/36.3.193. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Griffeth VEM, Simon AB, Moradi F. Variability of the coupling of blood flow and oxygen metabolism responses in the brain: a problem for interpreting BOLD studies but potentially a new window on the underlying neural activity. Front. Neurosci. 2014;8:139. doi: 10.3389/fnins.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze H-J, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Glover GH. BOLD fractional contribution to resting-state functional connectivity above 0.1Hz. Neuroimage. 2014;107:207–218. doi: 10.1016/j.neuroimage.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HM, Horovitz SG, Carr WS, Picchioni D, Coddington N, Fukunaga M, Xu Y, Balkin TJ, Duyn JH, Braun AR. Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proc Natl Acad Sci U S A. 2013;110:10300–10305. doi: 10.1073/pnas.1217691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J-Y, In M-H, Oh S-H, Zaitsev M, Speck O, Cho Z-H. An improved PSF mapping method for EPI distortion correction in human brain at ultra high field (7T) MAGMA. 2011;24:179–190. doi: 10.1007/s10334-011-0251-1. [DOI] [PubMed] [Google Scholar]
- Ciobanu L, Solomon E, Pyatigorskaya N, Roussel T, Le Bihan D, Frydman L. fMRI contrast at high and ultrahigh magnetic fields: Insight from complementary methods. Neuroimage. 2015;113:37–43. doi: 10.1016/j.neuroimage.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim S-G. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J. Cereb. Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Colantuoni A, Bertuglia S, Intaglietta M. Microvascular vasomotion: origin of laser Doppler flux motion. Int J Microcirc Clin Exp. 1994;14:151–158. doi: 10.1159/000178823. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies Contributing to Functional Connectivity in the Cerebral Cortex in “Resting-state” Data. Am. J. Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- de Zwart JA, Silva AC, van Gelderen P, Kellman P, Fukunaga M, Chu R, Koretsky AP, Frank JA, Duyn JH. Temporal dynamics of the BOLD fMRI impulse response. Neuroimage. 2005;24:667–677. doi: 10.1016/j.neuroimage.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Santhanam P, Hu X. Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage. 2011;54:1043–1052. doi: 10.1016/j.neuroimage.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire IM, Papadakis NG, Port M, Berwick J, Kennerley AJ, Mayhew JEW, Overton PG. Neurovascular coupling is brain region-dependent. Neuroimage. 2012;59:1997–2006. doi: 10.1016/j.neuroimage.2011.09.050. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Lindauer U, Villringer A. Nitric oxide synthase blockade enhances vasomotion in the cerebral microcirculation of anesthetized rats. Microvasc. Res. 1993;45:318–323. doi: 10.1006/mvre.1993.1028. [DOI] [PubMed] [Google Scholar]
- Drew PJJ, Duyn JHH, Golanov E, Kleinfeld D. Finding coherence in spontaneous oscillations. Nat Neurosci. 2008;11:991–993. doi: 10.1038/nn0908-991. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Silva AC, Lee SP, Kim SG. Functional MRI of calcium-dependent synaptic activity: cross correlation with CBF and BOLD measurements. Magn Reson Med. 2000;43:383–392. doi: 10.1002/(sici)1522-2594(200003)43:3<383::aid-mrm10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Febo M. Technical and conceptual considerations for performing and interpreting functional MRI studies in awake rats. Front. psychiatry. 2011;2:43. doi: 10.3389/fpsyt.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gatfield PD, Lowry OH, Schulz DW, Passonneau JV. Regional Energy Reserves in Mouse Brain and Changes With Ischaemia and Anaesthesia*. J. Neurochem. 1966;13:185–195. doi: 10.1111/j.1471-4159.1966.tb07512.x. [DOI] [PubMed] [Google Scholar]
- Ghahremani M, Hutchison RM, Menon RS, Everling S. Frontoparietal Functional Connectivity in the Common Marmoset. Cereb. Cortex. 2016 doi: 10.1093/cercor/bhw198. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D, Glover Gary H, Ress David, L T-Q. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Yamamoto S, Reis DJ. Spontaneous waves of cerebral blood flow associated with a pattern of electrocortical activity. Am J Physiol Regul Integr Comp Physiol. 1994;266:R204–R214. doi: 10.1152/ajpregu.1994.266.1.R204. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Saad ZS, Handwerker DA, Inati SJ, Brenowitz N, Bandettini PA. Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc Natl Acad Sci U S A. 2012;109:5487–5492. doi: 10.1073/pnas.1121049109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean J, Schroeter A, Batata I, Rudin M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage. 2014;102:838–847. doi: 10.1016/j.neuroimage.2014.08.043. [DOI] [PubMed] [Google Scholar]
- Grigg O, Grady CLL. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One. 2010;5:e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeben H, Meier S, Tankersley CG, Mitzner W, Brown RH. Heritable differences in respiratory drive and breathing pattern in mice during anaesthesia and emergence. Br. J. Anaesth. 2003;91:541–545. doi: 10.1093/bja/aeg222. [DOI] [PubMed] [Google Scholar]
- Grootoonk S, Hutton C, Ashburner J, Howseman AM, Josephs O, Rees G, Friston KJ, Turner R. Characterization and correction of interpolation effects in the realignment of fMRI time series. Neuroimage. 2000;11:49–57. doi: 10.1006/nimg.1999.0515. [DOI] [PubMed] [Google Scholar]
- Gu H, Feng H, Zhan W, Xu S, Silbersweig DA, Stern E, Yang Y. Single-shot interleaved zshim EPI with optimized compensation for signal losses due to susceptibility-induced field inhomogeneity at 3 T. Neuroimage. 2002;17:1358–1364. doi: 10.1006/nimg.2002.1274. [DOI] [PubMed] [Google Scholar]
- Guilfoyle DN, Gerum SV, Sanchez JL, Balla A, Sershen H, Javitt DC, Hoptman MJ. Functional connectivity fMRI in mouse brain at 7T using isoflurane. J. Neurosci. Methods. 2013;214:144–148. doi: 10.1016/j.jneumeth.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A, Calhoun V, Pearlson G, Harel M, Stern N, Attar F, Malach R, Salomon R. Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect. 2014;4:395–403. doi: 10.1089/brain.2014.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DaA, Roopchansingh V, Gonzalez-Castillo J, Bandettini Pa A. Periodic changes in fMRI connectivity. Neuroimage. 2012;63:1712–1719. doi: 10.1016/j.neuroimage.2012.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E, Rönnbäck L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, van Lunteren E, Deal EC, Cherniack NS. Role of the ventral surface of medulla in the generation of Mayer waves. Am J Physiol Regul. Integr. Comp Physiol. 1989;257:R804–R809. doi: 10.1152/ajpregu.1989.257.4.R804. [DOI] [PubMed] [Google Scholar]
- Helps SKK, Broyd SJJ, James CJJ, Karl A, Chen W, Sonuga-Barke EJSJ. Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Res. 2010;1322:134–143. doi: 10.1016/j.brainres.2010.01.057. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc. Natl. Acad. Sci. U.S.A. 2012 doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson-Stoate N, Jones M, Martindale J, Berwick J, Mayhew J. Further nonlinearities in neurovascular coupling in rodent barrel cortex. Neuroimage. 2005;24:565–574. doi: 10.1016/j.neuroimage.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Hildebrandt IJ, Su H, Weber WA. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J. 2008;49:17–26. doi: 10.1093/ilar.49.1.17. [DOI] [PubMed] [Google Scholar]
- Hindriks R, Adhikari MH, Murayama Y, Ganzetti M, Mantini D, Logothetis NK, Deco G. Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuroimage. 2015;127:242–256. doi: 10.1016/j.neuroimage.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D, Zhao X, Gao S, Hong C, Vatner DE, Vatner SF. Heart Rate and Electrocardiography Monitoring in Mice. Curr. Protoc. Mouse Biol. 2011;1:123–139. doi: 10.1002/9780470942390.mo100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge WS, Tan H, Kraft RA. Robust EPI Nyquist ghost elimination via spatial and temporal encoding. Magn. Reson. Med. 2010;64:1781–1791. doi: 10.1002/mrm.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, To XV, Teh I, Soh JR, Chuang K-H. Evaluation of EPI distortion correction methods for quantitative MRI of the brain at high magnetic field. Magn. Reson. Imaging. 2015;33:1098–1105. doi: 10.1016/j.mri.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Hu FY, Hanna GM, Han W, Mardini F, Thomas SA, Wyner AJ, Kelz MB. Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine β-hydroxylase knockout mice. Anesthesiology. 2012;117:1006–1017. doi: 10.1097/ALN.0b013e3182700ab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med. 1995;34:201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- Huang L, Liu Y, Li M, Hu D. Hemodynamic and electrophysiological spontaneous low-frequency oscillations in the cortex: Directional influences revealed by Granger causality. Neuroimage. 2014;85(Part 2):810–822. doi: 10.1016/j.neuroimage.2013.07.061. doi: http://dx.doi.org/10.1016/j.neuroimage.2013.07.061. [DOI] [PubMed] [Google Scholar]
- Huang W, Plyka I, Li H, Eisenstein EM, Volkow ND, Springer CS., Jr Magnetic resonance imaging (MRI) detection of the murine brain response to light: temporal differentiation and negative functional MRI changes. Proc Natl Acad Sci U S A. 1996;93:6037–6042. doi: 10.1073/pnas.93.12.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber L, Goense J, Kennerley AJ, Ivanov D, Krieger SN, Lepsien J, Trampel R, Turner R, Möller HE. Investigation of the neurovascular coupling in positive and negative BOLD responses in human brain at 7 T. Neuroimage. 2014;97:349–362. doi: 10.1016/j.neuroimage.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Biswal BB, Shen H, Lauer KK, Kampine JP. Spontaneous fluctuations in cerebral oxygen supply. An introduction. Adv Exp Med Biol. 1998;454:551–559. doi: 10.1007/978-1-4615-4863-8_66. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Roman RJ, Harder DR. Spontaneous flow oscillations in the cerebral cortex during acute changes in mean arterial pressure. J Cereb Blood Flow Metab. 1992;12:491–499. doi: 10.1038/jcbfm.1992.67. [DOI] [PubMed] [Google Scholar]
- Huo B-XB-X, Smith JB, Drew PJ. Neurovascular coupling and decoupling in the cortex during voluntary locomotion. J. Neurosci. 2014;34:10975–10981. doi: 10.1523/JNEUROSCI.1369-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Everling S. Monkey in the middle: why non-human primates are needed to bridge the gap in resting-state investigations. Front. Neuroanat. 2012;6:29. doi: 10.3389/fnana.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc Natl Acad Sci U S A. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K, Gee DG, Kilroy E, Schwab S, Smith RX, Cannon TD, Wang DJJJ. Functional connectivity in BOLD and CBF data: Similarity and reliability of resting brain networks. Neuroimage. 2015;106:111–122. doi: 10.1016/j.neuroimage.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Q, Lu G, Zhang Z, Zhong Y, Wang Z, Guo Y, Li K, Ding M, Liu Y. Granger causal influence predicts BOLD activity levels in the default mode network. Hum Brain Mapp. 2011;32:154–161. doi: 10.1002/hbm.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonckers E, Delgado Y, Palacios R, Shah D, Guglielmetti C, Verhoye M, Van der Linden A. Different anesthesia regimes modulate the functional connectivity outcome in mice. Magn. Reson. Med. 72, spcone-spcone. 2014 doi: 10.1002/mrm.24990. [DOI] [PubMed] [Google Scholar]
- Julien C. The enigma of Mayer waves: Facts and models. Cardiovasc. Res. 2006;70:12–21. doi: 10.1016/j.cardiores.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Evolution of Nervous Systems. Oxford: Academic Press; 2007. pp. 27–48. [Google Scholar]
- Kalthoff D, Po C, Wiedermann D, Hoehn M. Reliability and spatial specificity of rat brain sensorimotor functional connectivity networks are superior under sedation compared with general anesthesia. NMR Biomed. 2013;26:638–650. doi: 10.1002/nbm.2908. [DOI] [PubMed] [Google Scholar]
- Keilholz SD. Review Article: The Neural Basis of Time-Varying Resting State Functional Connectivity. Brain Connect. 2014;4:1–32. doi: 10.1089/brain.2014.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz SD, Magnuson M, Thompson G. Evaluation of data-driven network analysis approaches for functional connectivity MRI. Brain Struct Funct. 2010;215:129–140. doi: 10.1007/s00429-010-0276-7. [DOI] [PubMed] [Google Scholar]
- Keilholz SD, Magnuson ME, Pan WJ, Willis M, Thompson GJ. Dynamic properties of functional connectivity in the rodent. Brain Connect. 2013;3:31–40. doi: 10.1089/brain.2012.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP. Functional MRI of the rodent somatosensory pathway using multislice echo planar imaging. Magn Reson Med. 2004;52:89–99. doi: 10.1002/mrm.20114. [DOI] [PubMed] [Google Scholar]
- Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP, Keilholz Shella D, Raman Mira, Merkle Hellmut, Koretsky Alan P, S AC. BOLD and CBV-weighted functional magnetic resonance imaging of the rat somatosensory system. Magn. Reson. Med. 2006;55:316–324. doi: 10.1002/mrm.20744. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim SG, Uğurbil K. Comparison of blood oxygenation and cerebral blood flow effects in fMRI: estimation of relative oxygen consumption change. Magn. Reson. Med. 1997;38:59–65. doi: 10.1002/mrm.1910380110. [DOI] [PubMed] [Google Scholar]
- King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko AL, Darvas F, Poliakov A, Ojemann J, Sorensen LB. Quasi-periodic fluctuations in default mode network electrophysiology. J Neurosci. 2011;31:11728–11732. doi: 10.1523/JNEUROSCI.5730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacević N, Henderson JT, Chan E, Lifshitz N, Bishop J, Evans AC, Henkelman RM, Chen XJ. A three-dimensional MRI atlas of the mouse brain with estimates of the average and variability. Cereb. Cortex. 2005;15:639–645. doi: 10.1093/cercor/bhh165. [DOI] [PubMed] [Google Scholar]
- Krubitzer L. The organization of neocortex in mammals: are species differences really so different? Trends Neurosci. 1995;18:408–417. doi: 10.1016/0166-2236(95)93938-t. [DOI] [PubMed] [Google Scholar]
- Krueger F, Landgraf S, van der Meer E, Deshpande G, Hu X. Effective connectivity of the multiplication network: a functional MRI and multivariate Granger Causality Mapping study. Hum Brain Mapp. 2011;32:1419–1431. doi: 10.1002/hbm.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küblböck M, Woletz M, Höflich A, Sladky R, Kranz GS, Hoffmann A, Lanzenberger R, Windischberger C. Stability of low-frequency fluctuation amplitudes in prolonged resting-state fMRI. Neuroimage. 2014;103:249–257. doi: 10.1016/j.neuroimage.2014.09.038. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HL, Zahneisen B, Hugger T, LeVan P, Hennig J. Tracking dynamic resting-state networks at higher frequencies using MR-encephalography. Neuroimage. 2013;65:216–222. doi: 10.1016/j.neuroimage.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Lee SP, Silva AC, Ugurbil K, Kim SG. Diffusion-weighted spin-echo fMRI at 9.4 T: microvascular/tissue contribution to BOLD signal changes. Magn Reson Med. 1999;42:919–928. doi: 10.1002/(sici)1522-2594(199911)42:5<919::aid-mrm12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Li JM, Bentley WJ, Snyder AZ, Raichle ME, Snyder LH. Functional connectivity arises from a slow rhythmic mechanism. Proc. Natl. Acad. Sci. U.S.A. 2015;112 doi: 10.1073/pnas.1419837112. 1419837112- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Liu X, Sidabras JW, Paulson ES, Jesmanowicz A, Nencka AS, Hudetz AG, Hyde JS. Restoring susceptibility induced MRI signal loss in rat brain at 9.4 T: A step towards whole brain functional connectivity imaging. PLoS One. 2015;10:e0119450. doi: 10.1371/journal.pone.0119450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu D, Jiang X, Jin C, Zhang X, Guo L, Zhang J, Hu X, Li L, Liu T. Dynamic functional connectomics signatures for characterization and differentiation of PTSD patients. Hum Brain Mapp. 2014;35:1761–1778. doi: 10.1002/hbm.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Liu X, Zhang N. Dynamic resting state functional connectivity in awake and anesthetized rodents. Neuroimage. 2015;104:89–99. doi: 10.1016/j.neuroimage.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska A, Galbusera A, Schwarz AJ, Gozzi A. Functional connectivity hubs of the mouse brain. Neuroimage. 2015;115:281–291. doi: 10.1016/j.neuroimage.2015.04.033. [DOI] [PubMed] [Google Scholar]
- Liu X, Li R, Yang Z, Hudetz AG, Li SJ. Differential effect of isoflurane, medetomidine, and urethane on BOLD responses to acute levo-tetrahydropalmatine in the rat. Magn Reson Med. 2012;68:552–559. doi: 10.1002/mrm.23243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu X-H, Zhang Y, Chen W. The change of functional connectivity specificity in rats under various anesthesia levels and its neural origin. Brain Topogr. 2013;26:1–15. doi: 10.1007/s10548-012-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu X-H, Zhang Y, Chen W. Neural Origin of Spontaneous Hemodynamic Fluctuations in Rats under Burst-Suppression Anesthesia Condition. Cereb. Cortex. 2012;21:374–384. doi: 10.1093/cercor/bhq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JV, Hirano Y, Nascimento GC, Stefanovic B, Leopold DA, Silva AC. fMRI in the awake marmoset: Somatosensory-evoked responses, functional connectivity, and comparison with propofol anesthesia. Neuroimage. 2013;78:186–195. doi: 10.1016/j.neuroimage.2013.03.038. doi: http://dx.doi.org/10.1016/j.neuroimage.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci U S A. 2012;109:3979–3984. doi: 10.1073/pnas.1200506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders E, Steinmetz H, Jäncke L. Brain size and grey matter volume in the healthy human brain. Neuroreport. 2002;13:2371–2374. doi: 10.1097/01.wnr.0000049603.85580.da. [DOI] [PubMed] [Google Scholar]
- Lythgoe DJ, Williams SC, Cullinane M, Markus HS. Mapping of cerebrovascular reactivity using BOLD magnetic resonance imaging. Magn. Reson. Imaging. 1999;17:495–502. doi: 10.1016/s0730-725x(98)00211-2. [DOI] [PubMed] [Google Scholar]
- Maandag NJ, Coman D, Sanganahalli BG, Herman P, Smith AJ, Blumenfeld H, Shulman RG, Hyder F. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci U S A. 2007;104:20546–20551. doi: 10.1073/pnas.0709515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson M, Majeed W, Keilholz SD. Functional connectivity in blood oxygenation level-dependent and cerebral blood volume-weighted resting state functional magnetic resonance imaging in the rat brain. J Magn Reson Imaging. 2010;32:584–592. doi: 10.1002/jmri.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Pan WJ, Keilholz SD. Time-dependent effects of isoflurane and dexmedetomidine on functional connectivity, spectral characteristics, and spatial distribution of spontaneous BOLD fluctuations. NMR Biomed. 2014;27:291–303. doi: 10.1002/nbm.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Pan W-J, Keilholz SD. Effects of severing the corpus callosum on electrical and BOLD functional connectivity and spontaneous dynamic activity in the rat brain. Brain Connect. 2014;4:15–29. doi: 10.1089/brain.2013.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson ME, Thompson GJ, Schwarb H, Pan W-J, McKinley A, Schumacher EH, Keilholz SD. Errors on interrupter tasks presented during spatial and verbal working memory performance are linearly linked to large-scale functional network connectivity in high temporal resolution resting state fMRI. Brain Imaging Behav. 2015 doi: 10.1007/s11682-014-9347-3. [DOI] [PubMed] [Google Scholar]
- Majeed W, Magnuson M, Hasenkamp W, Schwarb H, Schumacher EHH, Barsalou L, Keilholz SDD. Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. Neuroimage. 2011;54:1140–1150. doi: 10.1016/j.neuroimage.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed W, Magnuson M, Keilholz SD, Majeed M, Keilholz S, WM Spatiotemporal Dynamics of Low Frequency Fluctuations in BOLD fMRI of the Rat. J Magn Reson Imag. 2009;30:384–393. doi: 10.1002/jmri.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand J-B, Joly O, Simone L, Sawamura H, Wardak C, Orban GA, Buckner RL, Vanduffel W. Default mode of brain function in monkeys. J Neurosci. 2011;31:12954–12962. doi: 10.1523/JNEUROSCI.2318-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Jones M, Martindale J, Mayhew J. Haemodynamic and neural responses to hypercapnia in the awake rat. Eur. J. Neurosci. 2006a;24:2601–2610. doi: 10.1111/j.1460-9568.2006.05135.x. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006b;32:33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2006;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Mayhew JEW, Askew S, Zheng Y, Porrill J, Westby GWM, Redgrave P, Rector DM, Harper RM. Cerebral Vasomotion: A 0.1-Hz Oscillation in Reflected Light Imaging of Neural Activity. Neuroimage. 1996;4:183–193. doi: 10.1006/nimg.1996.0069. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Chen N, Li L, Tom B, Weiss C, Disterhoft JF, Wyrwicz AM. fMRI of the conscious rabbit during unilateral classical eyeblink conditioning reveals bilateral cerebellar activation. J. Neurosci. 2003;23:11753–11758. doi: 10.1523/JNEUROSCI.23-37-11753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, DeSalvo MN, Enev M, Sanganahalli BG, Hyder F, Blumenfeld H. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci. 2011;31:15053–15064. doi: 10.1523/JNEUROSCI.0101-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto S, Palva S, Voipio J, Palva JM. Very Slow EEG Fluctuations Predict the Dynamics of Stimulus Detection and Oscillation Amplitudes in Humans. J. Neurosci. 2008;28:8268–8272. doi: 10.1523/JNEUROSCI.1910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon CH, Fukuda M, Kim S-G. Spatiotemporal characteristics and vascular sources of neural-specific and -nonspecific fMRI signals at submillimeter columnar resolution. Neuroimage. 2013;64:91–103. doi: 10.1016/j.neuroimage.2012.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita-Tsuzuki Y, Bouskela E, Hardebo JE. Effects of nitric oxide synthesis blockade and angiotensin II on blood flow and spontaneous vasomotion in the rat cerebral microcirculation. Acta Physiol. Scand. 1993;148:449–454. doi: 10.1111/j.1748-1716.1993.tb09581.x. [DOI] [PubMed] [Google Scholar]
- Muresan L, Renken R, Roerdink JBTM, Duifhuis H. Automated correction of spin-history related motion artefacts in fMRI: simulated and phantom data. IEEE Trans. Biomed. Eng. 2005;52:1450–1460. doi: 10.1109/TBME.2005.851484. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA. Resting-state fMRI confounds and cleanup. Neuroimage. 2013;80:349–359. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah FA, Low S-MA, Lew SK, Chen K, Chuang K-H. Pharmacological insight into neurotransmission origins of resting-state functional connectivity: α2-adrenergic agonist vs antagonist. Neuroimage. 2014;103:364–373. doi: 10.1016/j.neuroimage.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Fedele T, Mehnert J, Lipp A, Noack C, Steinbrink J, Curio G. Monochromatic ultra-slow (~0.1 Hz) oscillations in the human electroencephalogram and their relation to hemodynamics. Neuroimage. 2014;97:71–80. doi: 10.1016/j.neuroimage.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, Villringer A. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage. 2000;12:623–639. doi: 10.1006/nimg.2000.0657. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osol W, G H. Spontaneous vasomotion in pressurized cerebral arteries from genetically hypertensive rats. Am J Physiol. 1988;254:H28–H33. doi: 10.1152/ajpheart.1988.254.1.H28. [DOI] [PubMed] [Google Scholar]
- Pan W, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. Simultaneous FMRI and electrophysiology in the rodent brain. J. Vis. Exp. 2010;42:2–5. doi: 10.3791/1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W-J, Billings JCW, Grooms JK, Shakil S, Keilholz SD. Considerations for resting state functional MRI and functional connectivity studies in rodents. Front. Neurosci. 2015;9:269. doi: 10.3389/fnins.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W-J, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. Broadband Local Field Potentials Correlate with Spontaneous Fluctuations in Functional Magnetic Resonance Imaging Signals in the Rat Somatosensory Cortex Under Isoflurane Anesthesia. Brain Connect. 2011;1:119–131. doi: 10.1089/brain.2011.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W-JJ, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S. Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage. 2013;74:288–297. doi: 10.1016/j.neuroimage.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass D, Freeth G. The Rat. ANZCCART News. 1993;6:1–4. [Google Scholar]
- Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, Schulte ML, Matloub HS, Hudetz AG, Hyde JS. Resting-state functional connectivity of the rat brain. Magn Reson Med. 2008;59:1021–1029. doi: 10.1002/mrm.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Li R, Jones SR, Cho YR, Matloub HS, Hyde JS. Interhemispheric neuroplasticity following limb deafferentation detected by resting-state functional connectivity magnetic resonance imaging (fcMRI) and functional magnetic resonance imaging (fMRI) Neuroimage. 2010;49:2467–2478. doi: 10.1016/j.neuroimage.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawela CP, Biswal BB, Hudetz AG, Schulte ML, Li R, Jones SR, Cho YR, Matloub HS, Hyde JS. A protocol for use of medetomidine anesthesia in rats for extended studies using taskinduced BOLD contrast and resting-state functional connectivity. Neuroimage. 2009;46:1137–1147. doi: 10.1016/j.neuroimage.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrinovic MM, Hankov G, Schroeter A, Bruns A, Rudin M, von Kienlin M, Künnecke B, Mueggler T. A novel anesthesia regime enables neurofunctional studies and imaging genetics across mouse strains. Sci. Rep. 2016;6:24523. doi: 10.1038/srep24523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prielipp RC, Wall MH, Tobin JR, Groban L, Cannon MA, Fahey FH, Gage HD, Stump DA, James RL, Bennett J, Butterworth J. Dexmedetomidine-induced sedation in volunteers decreases regional and global cerebral blood flow. Anesth. Analg. 2002;95:1052–1059. doi: 10.1097/00000539-200210000-00048. table of contents. [DOI] [PubMed] [Google Scholar]
- Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front. Hum. Neurosci. 2014;8:897. doi: 10.3389/fnhum.2014.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. J. Physiol. 1890;11:85–158.17. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel F, Schroeter A, Rudin M. The hemodynamic response to somatosensory stimulation in mice depends on the anesthetic used: Implications on analysis of mouse fMRI data. Neuroimage. 2015;116:40–49. doi: 10.1016/j.neuroimage.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Leopold DA, Brookes MJ, Khader PH. The contribution of electrophysiology to functional connectivity mapping. Neuroimage. 2013;80:297–306. doi: 10.1016/j.neuroimage.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA, Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA, Scholvinck ML. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Saleem AB, Benucci A, Harris KD, Carandini M. Cortical State Determines Global Variability and Correlations in Visual Cortex. J. Neurosci. 2015;35:170–178. doi: 10.1523/JNEUROSCI.4994-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MP, Weiss C, Procissi D, Disterhoft JF, Wang L. Intrinsic connectivity of neural networks in the awake rabbit. Neuroimage. 2016;129:260–267. doi: 10.1016/j.neuroimage.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter A, Schlegel F, Seuwen A, Grandjean J, Rudin M. Specificity of stimulus-evoked fMRI responses in the mouse: the influence of systemic physiological changes associated with innocuous stimulation under four different anesthetics. Neuroimage. 2014;94:372–384. doi: 10.1016/j.neuroimage.2014.01.046. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforazzini F, Schwarz AJ, Galbusera A, Bifone A, Gozzi A. Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. Neuroimage. 2014;87:403–415. doi: 10.1016/j.neuroimage.2013.09.050. [DOI] [PubMed] [Google Scholar]
- Shakil S, Lee CH, Keilholz SD. Evaluation of sliding window correlation performance for characterizing dynamic functional connectivity and brain states. Neuroimage. 2016;133:111–128. doi: 10.1016/j.neuroimage.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih Y-YI, Chen Y-Y, Lai H-Y, Kao Y-CJ, Shyu B-C, Duong TQ. Ultra high-resolution fMRI and electrophysiology of the rat primary somatosensory cortex. Neuroimage. 2013;73:113–120. doi: 10.1016/j.neuroimage.2013.01.062. doi: http://dx.doi.org/10.1016/j.neuroimage.2013.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierakowiak A, Monnot C, Aski SN, Uppman M, Li T-Q, Damberg P, Brené S. Default Mode Network, Motor Network, Dorsal and Ventral Basal Ganglia Networks in the Rat Brain: Comparison to Human Networks Using Resting State-fMRI. PLoS One. 2015;10:e0120345. doi: 10.1371/journal.pone.0120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siero JCW, Hendrikse J, Hoogduin H, Petridou N, Luijten P, Donahue MJ. Cortical depth dependence of the BOLD initial dip and poststimulus undershoot in human visual cortex at 7 Tesla. Magn. Reson. Med. 2015;73:2283–2295. doi: 10.1002/mrm.25349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci U S A. 2002;99:15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Iadecola C, Kim SG. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J Cereb Blood Flow Metab. 2000;20:201–206. doi: 10.1097/00004647-200001000-00025. [DOI] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Silva AC, Liu JV, Hirano Y, Leoni RF, Merkle H, Mackel JB, Zhang XF, Nascimento GC, Stefanovic B. Longitudinal functional magnetic resonance imaging in animal models. Methods Mol. Biol. 2011;711:281–302. doi: 10.1007/978-1-61737-992-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotin YB, Das A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature. 2009;457:475–479. doi: 10.1038/nature07664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spenger C, Josephson A, Klason T, Hoehn M, Schwindt W, Ingvar M, Olson L. Functional MRI at 4.7 tesla of the rat brain during electric stimulation of forepaw, hindpaw, or tail in single-and multislice experiments. Exp Neurol. 2000;166:246–253. doi: 10.1006/exnr.2000.7524. [DOI] [PubMed] [Google Scholar]
- Spodick DH. Normal sinus heart rate: appropriate rate thresholds for sinus tachycardia and bradycardia. South. Med. J. 1996;89:666–667. doi: 10.1097/00007611-199607000-00003. [DOI] [PubMed] [Google Scholar]
- Stafford JM, Jarrett BR, Miranda-Dominguez O, Mills BD, Cain N, Mihalas S, Lahvis GP, Lattal KM, Mitchell SH, David SV, Fryer JD, Nigg JT, Fair Da. Large-scale topology and the default mode network in the mouse connectome. Proc. Natl. Acad. Sci. 2014;111:201404346. doi: 10.1073/pnas.1404346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal O, Diwakar M, Wong C-W, Olafsson V, Lee R, Huang M-X, Liu TT. Caffeine-Induced Global Reductions in Resting-State BOLD Connectivity Reflect Widespread Decreases in MEG Connectivity. Front. Hum. Neurosci. 2013;7:63. doi: 10.3389/fnhum.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-W, Huang T-Y. Real-time feedback optimization of z-shim gradient for automatic compensation of susceptibility-induced signal loss in EPI. Neuroimage. 2011;55:1587–1592. doi: 10.1016/j.neuroimage.2011.01.045. [DOI] [PubMed] [Google Scholar]
- Thompson GJ, Magnuson ME, Merritt MD, Schwarb H, Pan WJ, Mckinley A, Tripp LD, Schumacher EH, Keilholz SD. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Hum. Brain Mapp. 2013a;34:3280–3298. doi: 10.1002/hbm.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Merritt MD, Pan WJ, Magnuson ME, Grooms JK, Jaeger D, Keilholz SD. Neural correlates of time-varying functional connectivity in the rat. Neuroimage. 2013b;83:826–836. doi: 10.1016/j.neuroimage.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan W-J, Billings JCW, Grooms JKK, Shakil S, Jaeger D, Keilholz SD. Phase-amplitude coupling and infraslow (<1 Hz) frequencies in the rat brain: relationship to resting state fMRI. Front Integr Neurosci. 2014;8:41. doi: 10.3389/fnint.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan W-J, Keilholz SD. Different dynamic resting state fMRI patterns are linked to different frequencies of neural activity. J. Neurophysiol. 2015;114:114–124. doi: 10.1152/jn.00235.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan WJ, Magnuson ME, Jaeger D, Keilholz SD. Quasi-periodic patterns (QPP): Large-scale dynamics in resting state fMRI that correlate with local infraslow electrical activity. Neuroimage. 2014;84:1018–1031. doi: 10.1016/j.neuroimage.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki M, Mies G, Hossmann KA. Effect of alpha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand. 1992;36:318–322. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte G, Verhoye M, Van der Linden A. Changing body temperature affects the T2* signal in the rat brain and reveals hypothalamic activity. Magn. Reson. Med. 2006;55:1006–1012. doi: 10.1002/mrm.20861. [DOI] [PubMed] [Google Scholar]
- Vern BA, Leheta BJ, Juel VC, LaGuardia J, Graupe P, Schuette WH. Interhemispheric synchrony of slow oscillations of cortical blood volume and cytochrome aa3 redox state in unanesthetized rabbits. Brain Res. 1997;775:233–239. doi: 10.1016/s0006-8993(97)01028-7. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Wang K, Majeed W, Thompson GJ, Ying K, Zhu Y, Keilholz SD. Quasi-periodic pattern of fMRI contributes to functional connectivity and explores differences between Major Depressive disorder and control. Proc Int Soc Magn Reson Med. 2016:1683. [Google Scholar]
- Wang Y-F, Long Z, Cui Q, Liu F, Jing X-J, Chen H, Guo X-N, Yan JH, Chen H-F. Low frequency steady-state brain responses modulate large scale functional networks in a frequency-specific means. Hum. Brain Mapp. 2016;37:381–394. doi: 10.1002/hbm.23037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Ramos-Cabrer P, Wiedermann D, van Camp N, Hoehn M. A fully noninvasive and robust experimental protocol for longitudinal fMRI studies in the rat. Neuroimage. 2006;29:1303–1310. doi: 10.1016/j.neuroimage.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Williams KA, Magnuson M, Majeed W, LaConte SM, Peltier SJ, Hu X, Keilholz SD. Comparison of α-chloralose, medetomidine and isoflurane anesthesia for functional connectivity mapping in the rat. Magn. Reson. Imaging. 2010;28:995–1003. doi: 10.1016/j.mri.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, DeYoung PN, Liu TT. Differences in the resting-state fMRI global signal amplitude between the eyes open and eyes closed states are related to changes in EEG vigilance. Neuroimage. 2016;124:24–31. doi: 10.1016/j.neuroimage.2015.08.053. [DOI] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage. 2013;83:983–990. doi: 10.1016/j.neuroimage.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT. Anti-correlated networks, global signal regression, and the effects of caffeine in resting-state functional MRI. Neuroimage. 2012;63:356–364. doi: 10.1016/j.neuroimage.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrwicz AM, Chen N, Li L, Weiss C, Disterhoft JF. fMRI of visual system activation in the conscious rabbit. Magn. Reson. Med. 2000;44:474–478. doi: 10.1002/1522-2594(200009)44:3<474::aid-mrm19>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Yang GJ, Murray JD, Repovs G, Cole MW, Savic A, Glasser MF, Pittenger C, Krystal JH, Wang X-J, Pearlson GD, Glahn DC, Anticevic A. Altered global brain signal in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 2014;111:7438–7443. doi: 10.1073/pnas.1405289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hyder F, Shulman RG. Functional MRI BOLD signal coincides with electrical activity in the rat whisker barrels. Magn Reson Med. 1997;38:874–877. doi: 10.1002/mrm.1910380604. [DOI] [PubMed] [Google Scholar]
- Yang X, Hyder F, Shulman RG. Activation of single whisker barrel in rat brain localized by functional magnetic resonance imaging10.1073/pnas.93.1.475. PNAS. 1996;93:475–478. doi: 10.1073/pnas.93.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CC-C, Fukuda M, Kim S-G. BOLD responses to different temporal frequency stimuli in the lateral geniculate nucleus and visual cortex: insights into the neural basis of fMRI. Neuroimage. 2011;58:82–90. doi: 10.1016/j.neuroimage.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Erhardt EB, Sui J, Du Y, He H, Hjelm D, Cetin MS, Rachakonda S, Miller RL, Pearlson G, Calhoun VD. Assessing dynamic brain graphs of time-varying connectivity in fMRI data: Application to healthy controls and patients with schizophrenia. Neuroimage. 2014 doi: 10.1016/j.neuroimage.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbi V, Grandjean J, Rudin M, Wenderoth N. Mapping the mouse brain with rs-fMRI: An optimized pipeline for functional network identification. Neuroimage. 2015;123:11–21. doi: 10.1016/j.neuroimage.2015.07.090. [DOI] [PubMed] [Google Scholar]
- Zhang N, Rane P, Huang W, Liang Z, Kennedy D, Frazier JA, King J. Mapping resting-state brain networks in conscious animals. J. Neurosci. Methods. 2010;189:186–196. doi: 10.1016/j.jneumeth.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Zhao T, Zhou L, Wu Q, Hu X. BOLD study of stimulation-induced neural activity and resting-state connectivity in medetomidine-sedated rat. Neuroimage. 2008;39:248–260. doi: 10.1016/j.neuroimage.2007.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornow MH, Fleischer JE, Scheller MS, Nakakimura K, Drummond JC. Dexmedetomidine, an alpha 2-adrenergic agonist, decreases cerebral blood flow in the isoflurane-anesthetized dog. Anesth. Analg. 1990;70:624–630. doi: 10.1213/00000539-199006000-00008. [DOI] [PubMed] [Google Scholar]