Abstract
Age-related arterial inflammation is associated with dysfunction of the arteries and increased risk for cardiovascular disease. To determine if aging increases arterial immune cell infiltration as well as the populations of immune cells principally involved, we tested the hypothesis that large elastic and resistance arteries in old mice would exhibit increased immune cell infiltration compared to young controls. Additionally, we hypothesized that vasoprotective lifestyle interventions such as life-long caloric restriction or 8 weeks of voluntary wheel running would attenuate age-related arterial immune cell infiltration. The aorta and mesenteric vasculature with surrounding perivascular adipose was excised from young normal chow (YNC, 4–6 months, n = 10), old normal chow (ONC, 28–29 months, n = 11), old caloric restricted (OCR, 28–29 months, n = 9) and old voluntary running (OVR, 28–29 months, n = 5) mice and digested to a single cell suspension. The cells were then labeled with antibodies against CD45 (total leukocytes), CD3 (pan T cells), CD4 (T helper cells), CD8 (cytotoxic T cells), CD19 (B cells) CD11b and F4/80 (macrophages) and analyzed by flow cytometry. Total leukocytes, T cells (both CD4+ and CD8+ subsets), B cells and macrophages in both aorta and mesentery were all 5–6 fold greater in ONC compared to YNC. Age-related increases in T cell (both CD4+ and CD8+), B cell and macrophage infiltration in aorta were abolished in OCR mice. OVR mice exhibited 50% lower aortic T cell and normalized macrophage infiltration. B cell infiltration was not effected by VR. Age-related mesenteric CD8+ T cell and macrophage infiltration was normalized in OCR and OVR mice compared to young mice, whereas B cell infiltration was normalized by CR but not VR. Splenic CD4+ T cells from ONC mice exhibited a 3 fold increase in gene expression for the T helper (Th)1 transcription factor, Tbet, and a 4 fold increase in FoxP3, a T regulatory cell transcription factor, compared to YNC. Splenic B cells and mesenteric macrophages from old mice exhibited decreased proinflammatory cytokine gene expression regardless of treatment group. These results demonstrate that aging is associated with infiltration of immune cells around both the large-elastic and resistance arteries and that the vasoprotective lifestyle interventions, CR and VR, can ameliorate age-related arterial immune cell infiltration.
Keywords: inflammation, T cells, B cells, macrophages, aorta, mesentery
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in the industrialized world and aging is the primary and best predictive risk factor for future CVD diagnosis (Lakatta 2003). A majority of CVDs are diseases of the arteries including myocardial infarction, stroke, and peripheral artery disease. Vascular aging contributes to increased risk for CVD primarily through increases in large-artery stiffness and impairments in endothelium-dependent dilation (EDD) (Blackwell et al., 2004; Donato et al., 2007; Gerhard et al., 1996; Lakatta and Levy 2003). Associated with age-related arterial dysfunction is arterial inflammation which has been observed in both humans and animals (Belmin et al., 1995; Donato et al., 2008; Donato et al., 2007; Morgan et al., 2013; Song et al., 2012). Further, inhibition of inflammatory signaling can improve arterial function in older adults (Pierce et al., 2009; Walker et al., 2012). In the characterization of age-related arterial inflammation, the vast majority of investigations have focused on inflammatory signals originating from the blood vessel per se.
In the past decade, evidence has emerged that cells from both the innate and adaptive immune systems directly contribute to arterial dysfunction induced by acute experimental hypertension. Specifically, these investigations have found that T cells, B cells and macrophages accumulate in the perivascular tissue that surrounds large arteries and that these immune cells induce vascular dysfunction (Chan et al., 2015; Guzik et al., 2007; Wenzel et al., 2011). More recently, CD8+ T cells have been shown to be the T cell subset primarily responsible for increased arterial stiffness and impaired EDD associated with experimental hypertension (Trott et al., 2014; Wu et al., 2014). Whether a similar phenomenon occurs with advancing age and which immune cell subtypes might contribute to age-related arterial inflammation is unknown.
Certain lifestyle interventions can improve arterial function in older humans and rodents. Specifically, both our laboratory and others have contributed to extensive evidence demonstrating that caloric restriction (CR) (Csiszar et al., 2009; Donato et al., 2013; Fornieri et al., 1999; Lynch et al., 1999; Rippe et al., 2010; Walker et al., 2014) as well as exercise (DeSouza et al., 2000; Durrant et al., 2009; Fleenor et al., 2010; Jablonski et al., 2015; Lesniewski et al., 2011; Trott et al., 2009) improve vascular function in large elastic and resistance arteries in old rodents and humans while also reducing markers of arterial inflammation. Using a histological approach, our laboratory has found increases in total T cells and macrophages in aortas from old mice and that voluntary running (VR) reduces macrophage infiltration in old mice (Lesniewski et al., 2011). However, such histological staining was semi-quantitative and only allowed for the analysis of a small section of artery. In this investigation, we employed flow cytometry to assess infiltration of T cells (both CD4+ and CD8+ subtypes), B cells and macrophages in the whole aorta (large elastic artery) and mesenteric vascular arcade (resistance arteries) with age. We chose both artery types as large elastic arteries play an important role in dampening pulse pressure whereas resistance arteries regulate systemic vascular resistance and local tissue perfusion. Further, we sought to determine whether CR or VR would influence arterial immune cell infiltration and immune cell gene expression in old mice.
For the present study, we hypothesized that aging would result in increased infiltration of both CD4+ and CD8+ T cells, B cells and macrophages in both the aorta and mesenteric vascular arcade. We also hypothesized that CR or VR would attenuate age-related immune cell infiltration. Lastly, we hypothesized that aging would promote proinflammatory immune cell gene expression and that this would be ameliorated by CR or VR.
2. MATERIALS AND METHODS
2.1 Mice
All animal experiments conformed to the Guide and Use of Laboratory Animals and were approved by the University of Utah and Veteran’s Affairs Medical Center-Salt Lake City (VAMC-SLC) Animal Care and Use Committees. Young male (4–6 month) B6D2F1 mice were obtained from Charles River Inc. and old (28–29 months) male B6D2F1 mice were obtained from the National Institute of Aging (NIA) colony maintained by Charles River Inc. All mice were housed in standard mouse cages on a 12:12 light:dark cycle in the animal facility at the VAMC-SLC. Four groups of mice were employed for this study: Young normal chow (YNC, n = 10), old normal chow (ONC, n = 11), old caloric restriction (OCR, n = 9) and old voluntary running (OVR, n =5). YNC, ONC and OVR mice were allowed access to normal mouse chow and water ad libitum. OCR mice were obtained from the NIA colony where caloric restriction is initiated at 14 weeks of age at approximately 10% below ad libitum intake, this is increased to approximately 25% restriction at 15 weeks and to approximately 40% restriction at 16 weeks, which is maintained throughout the life of the animal. ONC and OCR mice were randomized at the NIA colony at 14 weeks of age and singly housed. Out of all normal chow fed old mice, we randomized mice to either stay in their home cage (ONC) or to be singly housed in a cage containing a running wheel for eight weeks prior to sacrifice (OVR). Running distance for each mouse was monitored daily.
2.2 Flow cytometry and cell sorting
To remove circulating leukocytes from arteries, following sacrifice, the chest cavity was opened and the right atrium was nicked. A cannula was placed in the left ventricle and the animals were perfused at physiological pressure until the effluent was cleared of blood. Aortas were dissected from the aortic arch to the renal artery bifurcation, skeletal muscle and lymph nodes were removed but perivascular fat and connective tissue was left intact. Blood vessels and perivascular tissue of the mesenteric vascular arcade was dissected away from the intestinal wall with care to avoid puncturing the wall and to avoid mesenteric lymph nodes. Perivascular tissue around both the aorta and mesenteric vascular arcade was included as this is where arterial immune cells accumulate (Guzik et al., 2007; Lesniewski et al., 2011).
Following dissection, spleens, aortas, and mesenteric vascular arcades were digested using collagenase type XI (125 U/ml), collagenase type I-S (450 U/ml), and hyaluronidase I-S (60 U/ml) dissolved in DPBS buffer containing calcium and magnesium for 30 min at 37°C. The tissues were further dispersed using repeated pipetting and the resultant homogenate was passed through a 70-μm sterile filter, yielding single-cell suspensions. Single cell suspensions were labeled with the following anti-mouse antibodies at a 1:100 concentration: Brilliant Violet-CD45 (total leukocytes), APC-CD3- (pan T cells), FITC-CD4 (T helper cells), PE-CD8 (cytotoxic T cells), APC Cy7-CD19 (B Cells), PE Cy7-CD11b and PerCP Cy5.5-F4/80 (macrophages). All antibodies were obtained from Tonbo Biosciences. Dead cells were labeled with Ghost Dye (Tonbo) and excluded from analysis. Cell subpopulations were assessed on a BD FACSAria and the following cell types were sorted into individual tubes: CD4+ T cells, CD8+ T cells, B cells and macrophages. The “flow minus one” technique was used to establish gating, as described previously (Trott et al., 2014).
2.3 qPCR
Following sorting, total RNA from the cell samples was extracted using the RNeasy kit (QIAGEN) following manufacturer’s directions. In preliminary experiments, we found that T and B cells sorted from aorta and mesentery did not yield sufficient quantity and/or quality RNA so we elected to perform qPCR on splenic T and B cells. We used mesenteric macrophages as the mesentery contains a relatively large pool of tissue resident macrophages and because the spleen contains few fully differentiated macrophages. cDNA was synthesized using the Quantitect reverse transcription kit (QIAGEN). qPCR was performed on a BioRad CFX 96 Real Time system with RT2 SYBR green master mix (QIAGEN) with primers for the following genes: tnfa fwd: CTGAACTTCGGGGTGATCGG, rev: GGCTTGTCACTCGAATTTTGAGA; ifng fwd: CCTGCGGCCTAGCTCTGA, rev: GCCATGAGGAAGAGCTGCA; tbet fwd: ACCAGAGCGGCAAGTGGG, rev: TGGACATATAAGCGGTTCCCTG; gata3 fwd: CTACGCTCCTTGCTACTCAGG, rev: GGAGGGAGAGAGGAATCCGA; prf1 fwd: TCTTGGTGGGACTTCAGCTTT, rev: TCCATACACCTGGCACGAAC foxp3 fwd: GGCCCTTCTCCAGGACAGA, rev: GCTGATCATGGCTGGGTTGT; il10 fwd: GCTCTTACTGACTGGCAT, rev: CGCAGCTCTAGGAGCATGTG 18s fwd: TAGAGGGACAAGTGGCGTTC, rev: CGCTGAGCCAGTCAGTGT. 18s mRNA was used as the reference gene. Target gene expression fold change compared to YNC was determined using the ΔΔCt method.
2.4 Statistics
One way-ANOVA was used to determine group differences. Least squares differences post hoc tests were used when appropriate. ANCOVA was employed to determine whether arterial immune cell infiltration was influenced by body, visceral adipose or soleus muscle mass. Data are mean ± SEM. Means were considered significantly different at p≤0.05.
3. RESULTS
3.1 Animal and splenic leukocyte characteristics
Animal body mass, mesenteric vascular arcade, heart, soleus muscle and epididymal (visceral) fat pad weights are presented in Table 1. Visceral adipose tissue mass was smaller in ONC mice compared to YNC (p < 0.05, Table 1). The mass of the mesenteric vascular arcade and associated perivascular adipose followed a similar pattern (Table 1). Body, heart and spleen mass were smaller in OCR mice compared to ONC (p < 0.05, Table 1). Mice provided with voluntary running wheels (OVR) mice ran an average of 0.2 ± 0.04 km/day. Voluntary running normalized age-related decreases in soleus muscle and mesenteric vascular arcade mass (p < 0.05, Table 1).
Table 1.
Animal body and tissue mass
| Group | YNC | ONC | OCR | OVR |
|---|---|---|---|---|
| Body (g) | 33.7 ± 0.7 | 36.4 ± 1.0* | 29.2 ± 0.8† | 35.5 ± 1.4 |
| Mesentery (g) | 0.22 ± 0.01 | 0.16 ± 0.01* | 0.11 ± 0.01* | 0.24 ± 0.04† |
| Heart (g) | 0.18 ± 0.009 | 0.22 ± 0.007* | 0.16 ± 0.004† | 0.20 ± .008*† |
| Soleus (g) | 0.012 ± 0.0009 | 0.011 ± 0.0005 | 0.009 ± 0.0004* | 0.014 ± 0.0010† |
| Visceral adipose (g) | 0.62 ± 0.05 | 0.33 ± 0.03* | 0.24 ± 0.03* | 0.42 ± 0.09* |
YNC: young normal chow, ONC: old normal chow, OCR: old caloric restricted, OVR: old voluntary running. n = 5–11/group. Differences were assessed with one way ANOVA and LSD post hoc tests.
Different from YNC,
different from ONC, p < 0.05
Data are means ± SEM
We assessed the numbers and populations of splenic leukocytes to determine whether aging or aging combined with CR or VR alters the immune system on a systemic level. OCR mice exhibited a greater percentage of splenic monocytes compared to the other groups (p < 0.05, Table 2).
Table 2.
Splenic leukocyte characteristics
| Group | YNC | ONC | OCR | OVR |
|---|---|---|---|---|
| Spleen mass (g) | 0.097 ± 0.001 | 0.124 ± 0.042 | 0.045 ± 0.002† | 0.112 ± 0.024 |
| Total number of CD45+ cells (x107) | 4.1 ± 0.6 | 7.4 ± 1.2 | 4.2 ± 0.7 | 4.9 ± 0.5 |
| % CD3+ cells | 18.5 ± 1.0 | 14.1 ± 1.7 | 16.2 ± 2.5 | 17.1 ± 2.5 |
| CD4/CD8 ratio | 1.23 ± 0.06 | 1.00 ± 0.13 | 1.05 ± 0.29 | 1.04 ± 0.26 |
| % CD19+ cells | 60.8 ± 0.8 | 63.6 ± 5.4 | 61.9 ± 2.8 | 67.2 ± 3.5 |
| % CD11b+ cells | 7.3 ± 1.2 | 10.3 ± 1.2 | 13.4 ± 1.0* | 9.5 ± 1.3 |
YNC: young normal chow, ONC: old normal chow, OCR: old caloric restricted, OVR: old voluntary running. n = 5–11/group. Differences were assessed with one way ANOVA and LSD post hoc tests.
Different from YNC, p < 0.05
Data are means ± SEM.
3.2 Arterial leukocyte infiltration
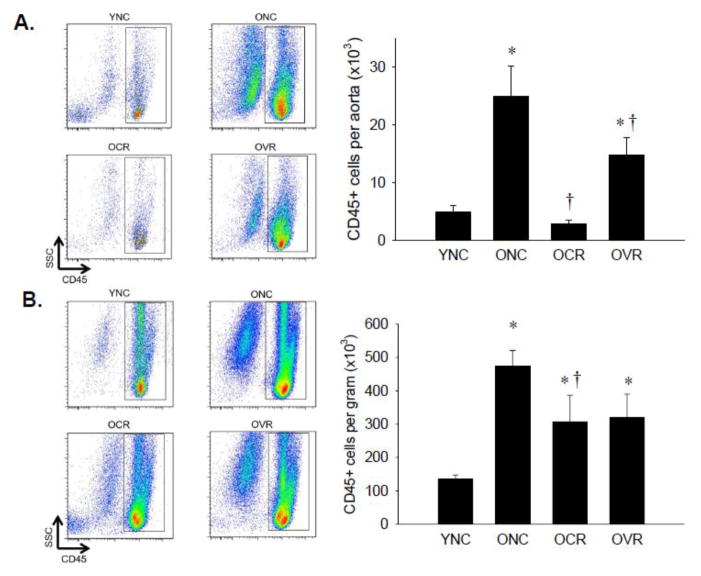
Both the aorta (Figure 1A) and mesentery (Figure 1B) of ONC mice exhibited 5-fold greater numbers of CD45+ total leukocytes compared to YNC (p < 0.05). In the aorta, this increase was abolished in OCR mice and attenuated by 41% in OVR mice (Figure 1A, p < 0.05 vs. ONC). In the mesentery, CR attenuated (p < 0.05, −36%) and VR attenuated (−33%) mesenteric leukocyte infiltration although this was not significantly different from ONC (p = 0.07, Figure 1B). To control for differences in tissue mass, mesenteric leukocyte infiltration data is expressed as cell number per gram of tissue throughout. Importantly, ONC mice also had higher raw numbers of leukocytes per mesentery compared to YNC (78,781 ± 9,848 vs. 30,430 ± 3,399, p < 0.05) despite smaller tissue mass. Group differences in both aortic and mesenteric CD45+ immune cell infiltration persisted when body mass, visceral adipose mass or soleus muscle mass were controlled for as covariates.
Figure 1. Leukocyte infiltration of aorta and mesenteric vascular arcade.
Aortas (A) and mesenteric vascular arcade (B) from young normal chow (YNC), old- (O) normal chow (NC), voluntary running (VR) and calorie restricted (CR) mice were digested to a single cell suspension and stained with antibodies against CD45 to assess total leukocytes. Representative flow cytometry plots are shown on the left of each panel, summary data is shown on the right. n = 5–11/group. Differences were assessed with one-way ANOVA with LSD post hoc tests. * different from YNC, † different from ONC, p≤0.05. Data are means ± SEM.
3.3 Arterial T cell infiltration
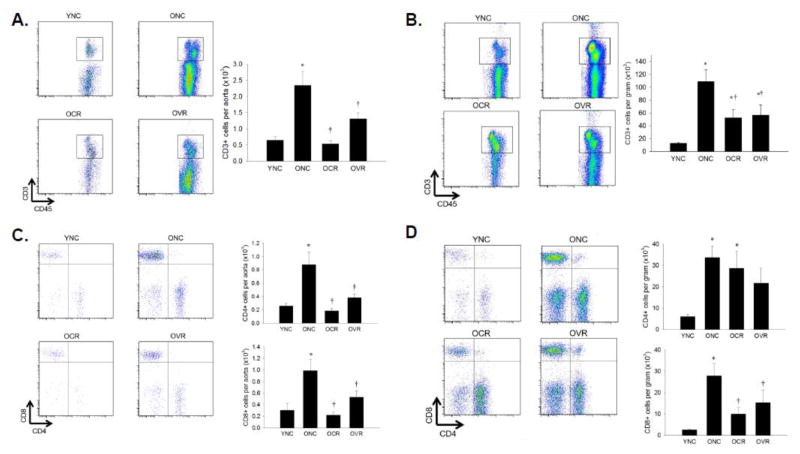
Aortas from ONC mice exhibited a 5-fold increase in CD3+ T cell infiltration (p < 0.05 vs. YNC) that was abolished by CR and 50% lower in VR (Figure 2A, both p < 0.05 vs. ONC), the mesenteric vasculature exhibited a 50% lower CD3+ immune cell infiltration in both OCR and OVR mice compared to ONC (Figure 2B, both p < 0.05). In the aorta, infiltration of both CD4+ (T helper) and CD8+ (T cytotoxic) subsets were attenuated by similar magnitudes to total T cells by either CR or VR (Figure 2C, both p < 0.05 vs. ONC. In the mesentery, both CD4+ and CD8+ subsets were increased 6-fold in ONC compared to YNC (p < 0.05). Caloric restriction and VR decreased CD8+ T cells by 50% (both p < 0.05 vs. ONC) but had no effect on CD4+ T cell infiltration (Figure 2D).
Figure 2. T cell infiltration of aorta and mesenteric vascular arcade.
Aortas (A) and mesenteric vascular arcade (B) from young normal chow (YNC), old- (O) normal chow (NC), voluntary running (VR) and calorie restricted (CR) mice were digested to a single cell suspension and incubated with antibodies against CD45 and CD3 to assess total leukocytes CD4+ and CD8+ T cell populations were assessed in the same groups by addition of antibodies against CD4 and CD8 in aorta (C) and mesentery (D). Representative flow cytometry plots are shown on the left of each panel, summary data is shown on the right. n = 5–11/group. Differences were assessed with one-way ANOVA with LSD post hoc tests. * different from YNC, † different from ONC, p≤0.05. Data are means ± SEM.
3.4 Arterial B cell infiltration
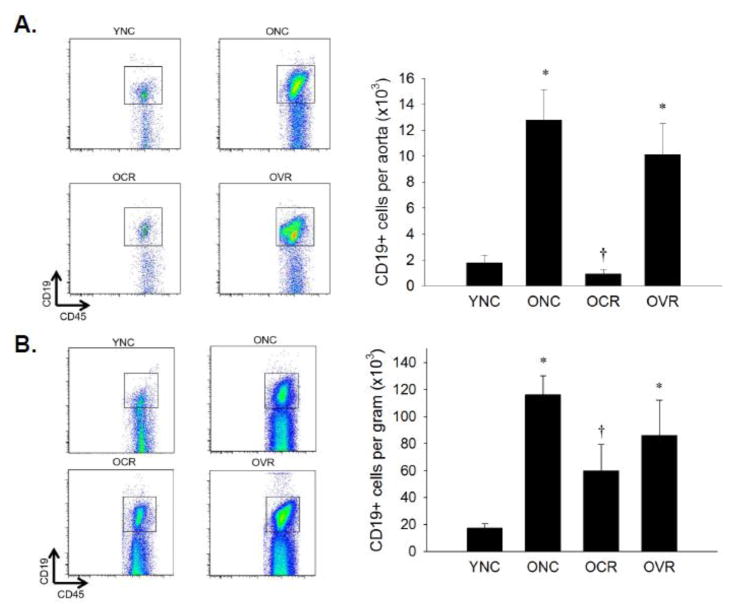
Aortas and mesenteries from ONC mice exhibited 6-fold greater B cell infiltration compared to YNC (Figure 3A & B, both p < 0.05). This increase was abolished in the aorta and decreased 50% in the mesentery by CR only (p < 0.05 vs. ONC).
Figure 3. B cell infiltration of aorta and mesenteric vascular arcade.
Aortas (A) and mesenteric vascular arcade (B) from young normal chow (YNC), old- (O) normal chow (NC), voluntary running (VR) and calorie restricted (CR) mice were digested to a single cell suspension and stained with antibodies against CD45 and CD19 to assess B cell infiltration. Representative flow cytometry plots are shown on the left of each panel, summary data is shown on the right. n = 5–11/group. Differences were assessed with one-way ANOVA with LSD post hoc tests. * different from YNC, † different from ONC, p≤0.05. Data are means ± SEM.
3.5 Arterial macrophage infiltration
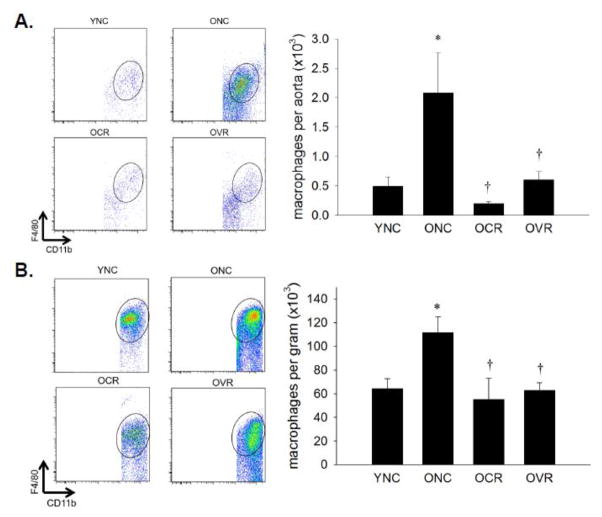
CD11b, F4/80 double positive macrophages were 4-fold greater in aorta and 2-fold greater in mesentery of ONC mice compared to YNC (p < 0.05, Figure 4A & B). Age-related increases in arterial macrophages were normalized by both CR and VR (Figure 4A & B, both p < 0.05 vs. ONC).
Figure 4. Macrophage infiltration of aorta and mesenteric vascular arcade.
Aortas (A) and mesenteric vascular arcade (B) from young normal chow (YNC), old- (O) normal chow (NC), voluntary running (VR) and calorie restricted (CR) mice were digested to a single cell suspension and stained with antibodies against CD45, CD11b and F4/80 to assess macrophage infiltration. Representative flow cytometry plots are shown on the left of each panel, summary data is shown on the right., n = 5–11/group. Differences were assessed with one-way ANOVA with LSD post hoc tests. * different from YNC, † different from ONC, p≤0.05. Data are means ± SEM.
3.6 Immune cell gene expression
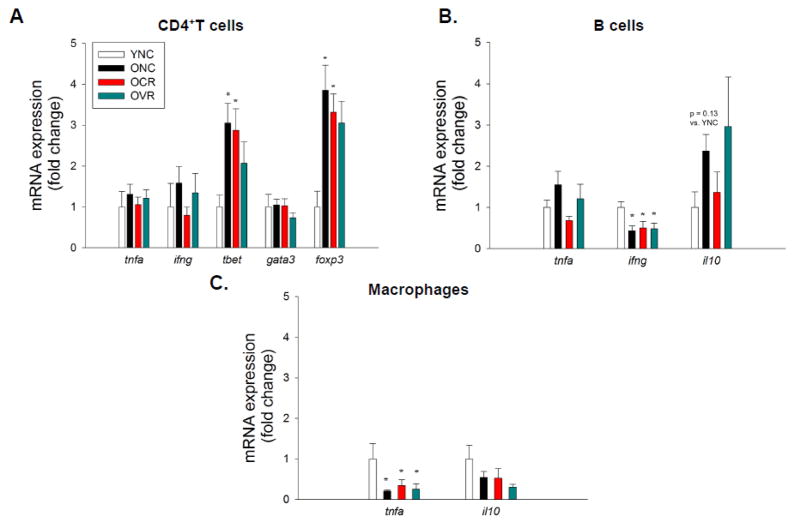
We examined gene expression of pro- and anti-inflammatory genes in splenic T and B cells and mesenteric infiltrating macrophages. In CD4+ T helper (Th) cells, we found no gene expression differences for the proinflammatory cytokines tumor necrosis factor (TNF)-α (tnfa) and interferon (IFN)-γ (ifng) (Figure 5A). We examined Th cell transcription factors and found that the Th1 transcription factor, Tbet (tbet), was increased 3-fold in CD4+ cells from ONC mice compared to YNC (p < 0.05), this increase was not altered in OCR or OVR mice (Figure 5A). In a similar manner, the T regulatory transcription factor, FoxP3 (foxp3), was increased 4-fold in CD4+ cells from ONC mice compared to YNC (p < 0.05) and not normalized by CR or VR (Figure 5A). There were no differences in gene expression of the Th2 transcription factor, Gata3 (gata3) (Figure 5A). We found no differences in gene expression for tnfa, ifng and the cytotoxic protein perforin-1 (prf1) in CD8+ (T cytotoxic) cells (data not shown). B cells exhibited no differences in tnfa gene expression; however, B cell ifng gene expression was decreased 50% in ONC, OCR and OVR compared to YNC mice (p < 0.05, Figure 5B). Although not statistically significant, (p = 0.13) B cells from ONC mice also exhibited greater anti-inflammatory cytokine Interleukin-10 (il10) gene expression compared to YNC (Figure 5B). Macrophages from ONC, OCR and OVR mice exhibited 50% lower tnfa gene expression compared to YNC (p < 0.05), il10 expression was unchanged (Figure 5C).
Figure 5. Immune cell pro- and anti-inflammatory gene expression. Splenic.
CD4+ T cells (A) and B cells (B) and mesenteric macrophages (C) were isolated using Flow Activated Cell Sorting (FACS). Following RNA isolation and cDNA synthesis qPCR was employed to assess gene expression for inflammatory cytokines TNF-α (tnfa), IFN-γ (ifng), anti-inflammatory cytokine Interleukin-10 (il10) and T cell transcription factors Tbet (tbet), GATA3 (gata3) and FoxP3 (foxp3). 18s RNA was used as a reference gene and mRNA fold change was calculated using the ΔΔCt method. n = 4–11/group. Differences were assessed with one-way ANOVA with LSD post hoc tests. * different from YNC, † different from ONC, p 0.05. Data are means ± SEM.
4. DISCUSSION
4.1 Summary of major findings
The major findings of this investigation are as follows: 1) Aging is associated with significant increases in total leukocytes, T cells (both CD4+ and CD8+ subtypes), B cells and macrophages in both aorta and mesenteric vasculature and their surrounding perivascular tissue. 2) Age-related CD4+ and CD8+ T cell, B cell and macrophage infiltration in aorta and CD8+ T cell, B cell and macrophage infiltration in mesentery is normalized by CR. 3) Voluntary running attenuates age-related CD4+ and CD8+ T cell and macrophage, but not B cell infiltration in aorta and also attenuates CD8+ T cell and macrophage infiltration of the mesenteric vasculature. 4) Aging results in alterations in CD4+ T cell transcription factor expression that is not normalized by CR or VR. 5) Aging results in decreased proinflammatory cytokine gene expression in B cells and macrophages that is not altered by CR or VR.
These results demonstrate that in addition to inflammatory signals emanating from the arterial wall, age-related arterial inflammation is also characterized by increased arterial infiltrating immune cells. In addition, these results demonstrate that vasoprotective lifestyle interventions result in decreased arterial immune cell burden. Lastly, aging is associated with increased CD4+ T cell proinflammatory transcription factor gene expression and decreased expression of proinflammatory cytokine gene expression in B cells and macrophages.
4.2 Aging, arterial inflammation and vascular function
The present investigation demonstrates increased CD4+ and CD8+ T cell, B cell and macrophage infiltration in the aorta and mesenteric vascular arcade with age. The concept that inflammation contributes to age-related arterial dysfunction is not new; however, the vast majority of investigations into age-related arterial inflammation focus on inflammatory mediators in the vascular smooth muscle and endothelium. (Belmin et al., 1995; Donato et al., 2008; Donato et al., 2007; Morgan et al., 2013; Song et al., 2012; Spinetti et al., 2004). The concept that immune cells per se contribute to arterial dysfunction was elucidated by an investigation by Guzik et al. where the authors found that T cells directly mediate arterial dysfunction in experimental hypertension (Guzik et al., 2007). More recently, macrophages, B cells, and the subset of CD8+ T cells specifically have been implicated in mediating arterial dysfunction using similar methods (Chan et al., 2015; Trott et al., 2014; Wenzel et al., 2011; Wu et al., 2014). In the present study we chose to focus on T cells, B cells and macrophages, as each has been shown to directly mediate arterial dysfunction. Wenzel et al. 2011, found that both macrophages and neutrophils infiltrated the aorta in experimental hypertension but that only macrophages directly induced endothelial dysfunction. Whether neutrophils or other innate immune cells infiltrate the vasculature with age and whether they play a role in age related arterial dysfunction is unknown and a topic for future study.
Our group was the first to report increased T cell and macrophage staining in the aortas of old (29–31 month) mice (Lesniewski et al., 2011). Other groups have found increases in arterial immune cells in middle aged genetic models of cardiovascular disease (Wu et al., 2015; Du et al., 2016). Importantly, in the present study we employed a model of healthy aging, old (28–29 month) B6D2F1 mice. In a similar manner to our observations in arteries and associated perivascular adipose, aging has been shown to increase T cells and macrophages in visceral adipose tissue (Lumeng et al., 2011). The mechanism of age-related arterial immune cell infiltration is unknown. There is evidence for arterial production of immune cell recruiting chemokines with age, whether these chemokines are produced by the artery per se or are adipokines originating from the surrounding perivascular adipose is as yet undetermined. Although we found the pattern of arterial immune cell infiltration was not dependent on changes in muscle or fat mass with age it is also possible that the sum production of adipo- and myokines might alter systemic immune cell migration and infiltration.
Whether immune cells directly contribute to age-related arterial dysfunction is unknown, however, there is evidence that cells from the aging immune system can contribute to arterial dysfunction in humans. Giant cell arteritis is an inflammatory disease of the arteries involving T cells and macrophages and the principal risk factor for development of this disease is advanced age (Weyand et al., 2012). With age, T cells develop a proinflammatory, end differentiated T effector memory phenotype (Fagnoni et al., 1996; Merino et al., 1998; Weyand et al., 2014). The proportion of end differentiated T effector memory cells are predictive of cardiovascular disease mortality in octogenarians and mortality after stroke (Nadareishvili et al., 2004; Spyridopoulos et al., 2015). Further, patients with rheumatoid arthritis, have a greater proportion of these cells and are at greater risk for cardiovascular disease even when controlling for traditional risk factors (del Rincon et al., 2001; Gerli et al., 2004). Depletion of B cells in older adults with rheumatoid arthritis improves EDD (Hsue et al., 2014). These data, combined with our observation of marked arterial immune cell infiltration support the concept that the immune system may be a contributor to age-related arterial dysfunction and risk for cardiovascular disease.
4.3 Vasoprotective lifestyle interventions and arterial immune cell infiltration
Data from this study demonstrate that the vasoprotective lifestyle interventions, CR and VR, can ameliorate age-related arterial immune cell infiltration. Caloric restriction increases lifespan in numerous mammalian species (Masoro 2005). Similar to previous observations (Chen et al., 1998; Donato et al., 2013), in this study, CR resulted in lower body, heart and spleen mass compared to old controls. Extensive evidence from our group and others show that CR promotes arterial health with age by preserving nitric oxide-bioavailability and EDD, preventing age-related large artery stiffening and, attenuating inflammatory signaling in the artery (Csiszar et al., 2009; Donato et al., 2013; Fornieri et al., 1999; Lynch et al., 1999; Rippe et al., 2010; Walker et al., 2014). This study is the first to demonstrate that CR also decreases arterial immune cell infiltration and suggests a decreased immune cell burden may contribute to preserved arterial function with CR. Notably, CR also normalizes age-related changes in the adaptive immune system in both rodents and non-human primates (Chen et al., 1998; Messaoudi et al., 2006) and decreases macrophage marker gene expression in visceral adipose of aged rats (Sierra Rojas et al., 2016). Combined, this supports the concept that CR may have synergistic effects on both the artery and the immune system that act to preserve vascular function with age.
Exercise has also been shown to improve both EDD, large artery stiffness and decrease proinflammatory markers in both older rodents and adults (DeSouza et al., 2000; Durrant et al., 2009; Fleenor et al., 2010; Jablonski et al., 2015; Lesniewski et al., 2011; Trott et al., 2009). We found that VR attenuated T cell and macrophage infiltration in both the aorta and mesenteric vasculature. In accord with our previous observations (Lesniewski et al., 2011) we found that aortic macrophage infiltration was attenuated with VR; however, in contrast, in the present study we found that VR also attenuated aortic T cell infiltation. This may be due to the differences between quantifying immune cell infiltration by histology of arterial sections vs. flow cytometry of the whole artery. In contrast to CR, VR did not attenuate age-related accumulation of B cells in the aorta and mesentery. Because both exercise and CR improve arterial function with age, T cells and macrophages might play a relatively greater role in age-related arterial inflammation and dysfunction than B cells. In addition, it is possible that lifelong CR prevents arterial accumulation of B cells whereas 8 weeks of VR is not sufficient to reverse already existing B cell infiltration. Notably, exercise slows T cell aging in rodents and aerobic fitness is inversely related to circulating end differentiated T effector memory cells in older adults (Spielmann et al., 2011; Woods et al., 2003). This study demonstrates that in addition to the well described mechanisms of vasoprotection by CR and VR, these interventions also reduce vascular immune cell infiltration. Interestingly, both CR and VR reduced infiltration of both T cell subsets in the aorta but only CD8+ T cells in the mesentery. The mechanism behind these differences and the respective roles of these T cell subtypes in the aged vasculature are unknown. Further, the mechanisms by which CR and exercise might modulate the interaction between the immune system and vasculature are important topics for future study.
The mice in the present study ran considerably less than old B6D2F1 mice in previous reports (Durrant et al., 2009; Lesniewski et al., 2011). Although voluntary wheel running does not allow us to control exercise duration or intensity we chose this modality as forced treadmill training in rodents has been shown to activate neuronal stress pathways that can also mediate arterial immune cell infiltration and activation (Marvar et al., 2010; Marvar et al., 2012; Yanagita et al., 2007). Although we do not know the relative intensity of exercise in the present study, this data suggests physical activity beyond that of cage control mice is sufficient to reduce arterial immune cell infiltration. Whether greater amounts of physical activity might further decrease age-related arterial immune cell infiltration is an interesting question. A limitation of the present study is that due to attrition typical of older mice (Forster et al., 2003) we did not achieve our target sample size of 8 per group in the OVR group as calculated based on data from Lesniewski et al., 2011. Despite this limitation, the major conclusions of this investigation remain. We found significant differences in total aortic immune cells, T cells and macrophages when the OVR group was compared to ONC. We also found differences in mesenteric T cells and macrophages when comparing these same groups.
4.4 Aging and immune cell gene expression
We hypothesized that aging would increase proinflammatory immune cell gene expression and that this would be ameliorated by CR or VR. We found that CD4+ T cells exhibited greater Tbet and FoxP3 expression with age and that this was not altered by CR or VR. Tbet is the primary transcription factor that leads naïve T cells down the proinflammatory Th1 linage and to produce IFN-γ. In stimulated, in vitro conditions CD4+ cells from old mice produce more IFN-γ compared to cells from young mice (Hobbs et al., 1993). Although we found no change in splenic T cell IFN-γ gene expression, it is possible that these cells are incompletely or undifferentiated in the spleen and become fully differentiated as they migrate to peripheral tissues where they induce inflammation and dysfunction. We also found that aging resulted in greater expression of the transcription factor FoxP3, which drives cells to a T regulatory phenotype. These cells orchestrate the resolution of inflammation and are typically considered anti-inflammatory. However, T regulatory cells have recently been shown to mediate age-related insulin resistance (Bapat et al., 2015), suggesting that aging may result in a shift to a pathogenic phenotype in these cells.
Contrary to our hypothesis, we found a no changes in CD8+ T cell gene expression for proinflammatory or cytotoxic genes. In B cells, we found a decrease in gene expression for the proinflammatory cytokine IFN-γ, no change for TNF-α and a trend for increased expression of the anti-inflammatory cytokine, IL-10. In mesenteric macrophages, TNF-α expression was decreased with age. In contrast to our findings, splenic B cells and visceral adipose resident macrophages from aged mice have been shown to secrete more TNF-α in vitro (Lumeng et al., 2011; Ratliff et al., 2013). This difference may be due differences in methodology, e.g. qPCR vs. cytokine secretion and/or unstimulated primary cells vs. stimulated cells in culture. It should be noted that despite exhibiting decreased or unchanged gene expression for proinflammatory cytokines, the magnitude of change in arterial infiltrating immune cells with age (~5-fold increase in both aorta and mesentery) could lead to increased local proinflammatory cytokine concentrations contributing to arterial dysfunction despite lower levels of cytokine gene expression from individual cells.
4.5 Conclusions
In summary, our data indicate that aging results in a profound infiltration of T cells, B cells and macrophages in both the aorta and mesenteric vasculature. Lifelong CR almost completely normalized age-related arterial immune cell infiltration and VR attenuated T cell and macrophage but not B cell infiltration. These are the first data to demonstrate that immune cells per se contribute to age-related arterial inflammation in both large elastic arteries and the resistance vasculature. We are also the first to report that CR can prevent and VR partially reverse age-related arterial immune cell infiltration. Important topics for future study include; whether and which subtype(s) of arterial infiltrating immune cells directly contribute to age-related arterial dysfunction, mechanisms by which these cells are recruited to the artery and, the mechanisms by which CR and VR attenuate arterial immune cell infiltration.
Highlights.
Aging is associated with increases in both large- and resistance-artery infiltrating leukocytes
Age-related arterial leukocyte infiltration is normalized by caloric restriction
Age-related arterial leukocyte infiltration is attenuated by voluntary running
Aging results in alterations in CD4+ T cell transcription factor expression
Aging results in decreased proinflammatory cytokine gene expression in B cells and macrophages
Acknowledgments
ACKNOWLEGEMENTS AND FUNDING SOURCES
This work was supported by National Institute of Aging award numbers K02AG045339, R01AG050238, R01AG040297, R01AG048366, and by Merit Review Award 1I01BX002151 from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. SAA was supported by National Heart Lung and Blood Institute award number R25HL108828-06. Flow Cytometry work was supported by the University of Utah Flow Cytometry Facility, National Cancer Institute award number 5P30CA042014-24 and National Center for Research Resources award number 1S10RR026802-01. The authors would like to thank James Marvin and Chris Leukel for their flow cytometry expertise.
Abbreviations
- YNC
young normal chow
- ONC
old normal chow
- OCR
old caloric restriction
- OVR
old voluntary running
- CD
cluster of differentiation
- CVD
cardiovascular disease
- EDD
endothelium dependent dilation
- VAMC-SLC
Veteran’s Affairs Medical Center-Salt Lake City
- TNF-α
Tumor necrosis factor-α
- IFN-γ
- IL
interleukin
- Th1
T helper 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, Zhou C, Liang Y, LeBlanc M, Liddle C, Atkins AR, Yu RT, Downes M, Evans RM, Zheng Y. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528:137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmin J, Bernard C, Corman B, Merval R, Esposito B, Tedgui A. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268:H2288–2293. doi: 10.1152/ajpheart.1995.268.6.H2288. [DOI] [PubMed] [Google Scholar]
- Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A, Drummond GR. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension. 2015;66:1023–1033. doi: 10.1161/HYPERTENSIONAHA.115.05779. [DOI] [PubMed] [Google Scholar]
- Chen J, Astle CM, Harrison DE. Delayed immune aging in diet-restricted B6CBAT6 F1 mice is associated with preservation of naive T cells. J Gerontol A Biol Sci Med Sci. 1998;53:B330–337. doi: 10.1093/gerona/53a.5.b330. discussion B338–339. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis and rheumatism. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NF kappa B, reduced I kappa B alpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12:772–783. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Henson GD, Hart CR, Layec G, Trinity JD, Bramwell RC, Enz RA, Morgan RG, Reihl KD, Hazra S, Walker AE, Richardson RS, Lesniewski LA. The impact of ageing on adipose structure, function and vasculature in the B6D2F1 mouse: evidence of significant multisystem dysfunction. J Physiol. 2014;592:4083–4096. doi: 10.1113/jphysiol.2014.274175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Wong C, Song Y, Shen H, Mori D, Rotllan N, Price N, Dobrian AD, Meng H, Kleinstein SH, Fernandez-Hernando C, Goldstein DR. Age-associated vascular inflammation promotes monocytosis during atherogenesis. Aging Cell. 2016;15:766–777. doi: 10.1111/acel.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P. Expansion of cytotoxic CD8+ CD28- T cells in healthy ageing people, including centenarians. Immunology. 1996;88:501–507. doi: 10.1046/j.1365-2567.1996.d01-689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornieri C, Taparelli F, Quaglino D, Jr, Contri MB, Davidson JM, Algeri S, Ronchetti IP. The effect of caloric restriction on the aortic tissue of aging rats. Connective Tissue Res. 1999;40:131–143. doi: 10.3109/03008209909029109. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. Faseb J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Gerli R, Schillaci G, Giordano A, Bocci EB, Bistoni O, Vaudo G, Marchesi S, Pirro M, Ragni F, Shoenfeld Y, Mannarino E. CD4+CD28− T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation. 2004;109:2744–2748. doi: 10.1161/01.CIR.0000131450.66017.B3. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs MV, Weigle WO, Noonan DJ, Torbett BE, McEvilly RJ, Koch RJ, Cardenas GJ, Ernst DN. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- Hsue PY, Scherzer R, Grunfeld C, Imboden J, Wu Y, Del Puerto G, Nitta E, Shigenaga J, Schnell Heringer A, Ganz P, Graf J. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. JAHA. 2014;3:e001267. doi: 10.1161/JAHA.114.001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski KL, Donato AJ, Fleenor BS, Nowlan MJ, Walker AE, Kaplon RE, Ballak DB, Seals DR. Reduced large elastic artery stiffness with regular aerobic exercise in middle-aged and older adults: potential role of suppressed nuclear factor kappa B signalling. J Hypertens. 2015;33:2477–2482. doi: 10.1097/HJH.0000000000000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2011;301:H1025–1032. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, Oatmen K, Martinez-Santibanez G, Julius A, Garg S, Yung RL. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, Cooney PT, Bennett SA, Thornton PL, Khan AS, Ingram RL, Sonntag WE. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging. 1999;20:191–200. doi: 10.1016/s0197-4580(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvar PJ, Vinh A, Thabet S, Lob HE, Geem D, Ressler KJ, Harrison DG. T lymphocytes and vascular inflammation contribute to stress-dependent hypertension. Biol Psychiatry. 2012;71:774–782. doi: 10.1016/j.biopsych.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Age Devel. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Merino J, Martinez-Gonzalez MA, Rubio M, Inoges S, Sanchez-Ibarrola A, Subira ML. Progressive decrease of CD8high+ CD28+ CD57− cells with ageing. Clin Exp Immunol. 1998;112:48–51. doi: 10.1046/j.1365-2249.1998.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, Douek DC, Mori M, Nikolich-Zugich J. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, Donato AJ. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am J Physiol Heart Circ Physiol. 2013;305:H251–258. doi: 10.1152/ajpheart.00197.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Lesniewski LA, Lawson BR, Beske SD, Seals DR. Nuclear factor-KappaB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation. 2009;119:1284–1292. doi: 10.1161/CIRCULATIONAHA.108.804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff M, Alter S, Frasca D, Blomberg BB, Riley RL. In senescence, age-associated B cells secrete TNFalpha and inhibit survival of B-cell precursors. Aging Cell. 2013;12:303–311. doi: 10.1111/acel.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra Rojas JX, Garcia-San Frutos M, Horrillo D, Lauzurica N, Oliveros E, Carrascosa JM, Fernandez-Agullo T, Ros M. Differential Development of Inflammation and Insulin Resistance in Different Adipose Tissue Depots Along Aging in Wistar Rats: Effects of Caloric Restriction. J Gerontol A Biol Sci Med Sci. 2016;71:310–322. doi: 10.1093/gerona/glv117. [DOI] [PubMed] [Google Scholar]
- Song Y, Shen H, Schenten D, Shan P, Lee PJ, Goldstein DR. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2012;32:103–109. doi: 10.1161/ATVBAHA.111.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann G, McFarlin BK, O’Connor DP, Smith PJ, Pircher H, Simpson RJ. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav Immun. 2011;25:1521–1529. doi: 10.1016/j.bbi.2011.07.226. [DOI] [PubMed] [Google Scholar]
- Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- Spyridopoulos I, Martin-Ruiz C, Hilkens C, Yadegarfar ME, Isaacs J, Jagger C, Kirkwood T, von Zglinicki T. CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell. 2015 doi: 10.1111/acel.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2009;106:1925–1934. doi: 10.1152/japplphysiol.91232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension. 2014;64:1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA, Donato AJ. Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age. 2014;36:559–569. doi: 10.1007/s11357-013-9585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension. 2012;60:1517–1523. doi: 10.1161/HYPERTENSIONAHA.112.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Liao YJ, Goronzy JJ. The immunopathology of giant cell arteritis: diagnostic and therapeutic implications. J Neuro Ophthalmol. 2012;32:259–265. doi: 10.1097/WNO.0b013e318268aa9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Current Opinion in Rheumatology. 2014;26:93–100. doi: 10.1097/BOR.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Woods JA, Ceddia MA, Zack MD, Lowder TW, Lu Q. Exercise training increases the naive to memory T cell ratio in old mice. Brain Behav Immun. 2003;17:384–392. doi: 10.1016/s0889-1591(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Wu J, Saleh MA, Kirabo A, Itani HA, Montaniel KR, Xiao L, Chen W, Mernaugh RL, Cai H, Bernstein KE, Goronzy JJ, Weyand CM, Curci JA, Barbaro NR, Moreno H, Davies SS, Roberts LJ, 2nd, Madhur MS, Harrison DG. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2015 doi: 10.1172/JCI87425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagita S, Amemiya S, Suzuki S, Kita I. Effects of spontaneous and forced running on activation of hypothalamic corticotropin-releasing hormone neurons in rats. Life Sciences. 2007;80:356–363. doi: 10.1016/j.lfs.2006.09.027. [DOI] [PubMed] [Google Scholar]