Abstract
In 1960, Dr. Bayard Clarkson described a woman experiencing sporadic, recurrent episodes of shock and anasarca. Plasma from an acute attack induced a “shock”-like syndrome when injected into rats. The enigmatic “Systemic Capillary Leak Syndrome” (SCLS) named for Dr. Clarkson is characterized by transient and severe, but reversible, hemoconcentration and hypoalbuminemia due to leakage of fluids and macromolecules into tissues. Although < 500 cases of SCLS have been reported in the literature since 1960, the condition is probably under-diagnosed due to lack of awareness and a high mortality without treatment. Allergists should be vigilant of this diagnosis since its presentation can resemble more common plasma leakage syndromes including angioedema or systemic anaphylaxis. Although the precise molecular etiology of SCLS remains unknown, substantial advances over the last five years have increased our understanding of SCLS pathogenesis.
INTRODUCTION
Dr. Clarkson’s index patient exemplifies SCLS: an otherwise healthy 34 year-old woman with sudden onset low-grade fevers, progressively declining blood pressure, rapid swelling of the face, arms, and legs, and rising hematocrit despite intravenous fluid resuscitation. The unexplained shock and anasarca were followed several days later by a period of massive diuresis and diminution of peripheral edema. The patient eventually died of pulmonary edema and cardiac failure during the diuresis phase of an attack.
Dr. Clarkson demonstrated that the albumin-binding dye T-1824 (Evans blue) was quickly cleared from the intravascular compartment in a patient suffering an acute attack, suggesting a primary role for vascular endothelial hyper-permeability (VEH).1 Although studies of thyroid, gonadal, and adrenal steroid function and tests of immune and metabolic function available at the time were unremarkable, an anomalous gamma globulin (“paraprotein”) was identified in the serum of the index patient. We now know that this monoclonal gammopathy of unknown significance (MGUS) is present in 68 to 85% of patients with SCLS.2
In this review, we discuss the diagnosis of this rare and often fatal disease; clinical management of acute SCLS; prophylactic considerations; and new mechanistic concepts gleaned from recent studies. Increased recognition of SCLS and an improved understanding of pathophysiology are vital to improving outcomes.
INCIDENCE OF IDIOPATHIC SCLS
In our previous survey of the SCLS literature, we found 126 cases that were reported between 1960 and 2010.3 Over the last six years, 134 new cases of SCLS have been identified,4–49 including a European registry of 28 cases50 and our NIH cohort of 58 patients.51 The demographics of these patients are described in Table 1. SCLS occurs sporadically and has been described most commonly in middle-aged, Caucasian adults (median age at diagnosis 48; age range newborn to 85 years). Since our cohort and the published literature now includes patients of Asian, African-American, Hispanic, and Middle Eastern ancestry, the purported Caucasian predominance may simply reflect unawareness of this rare condition in some parts of the world. There is no sex predominance (52% were female). SCLS has also been reported in fourteen children during the past six years.5, 17, 21, 25, 28, 35, 50, 52
Table 1.
Characteristics of 134 new cases of SCLS reported between 2010–2016
| Age at diagnosis* | 48.3 years (newborn-85 years)± |
| Sex (female/male) | 71/63 (52.2%) |
| Identifiable precipitating trigger for attacks | 44% |
| MGUS+ | 68% (adults only) |
| M-spike concentration (g/L)*# | 3.5 (1–19) |
| Breakthrough episodes on theophylline-based regimen | 84% (47/56) |
| Median annual attack frequency on theophylline-based regimen | 2.25 (0–20) |
| Breakthrough episodes on IVIG/SCIG | 25% (18/73) |
| Median annual attack frequency on IVIG/SCIG | 0 (0–3.3) |
| Overall mortality rate | 14%& |
75th percentile = 49.8 years; 25th percentile = 16 years;
Median (range) of monoclonal paraprotein (M-spike) concentration by immunofixation electrophoresis;
(based on 41 cases excluding European registry [concentrations not reported]);
includes two non-SCLS related deaths.
CLINICAL PRESENTATION
Allergy per se is not thought to incite SCLS flares. Typical allergic triggers have not been reported; allergic symptoms such as urticaria, focal angioedema (e.g. tongue or perioral), wheezing, or stridor are not observed upon presentation; and allergy therapies including corticosteroids, epinephrine, or antihistamines have proven largely ineffective in SCLS.53, 54 Nonetheless, inflammation is intimately linked to disease flares. In the European case registry, ~3/4 of subjects experienced a viral-like prodrome immediately prior to attacks.50 In our review of individual cases published over the last six years, the NIH cohort, and the European registry, 44% had a readily identifiable trigger for flares, which included infections (typically upper respiratory) such as influenza, respiratory syncytial virus, and West Nile virus (39%, 52/134). Other potential triggers included intense physical exertion—particularly when coupled with heat exposure—or extended travel, which occurred in 5% of the cases reported (7/134).
Patients with the classical acute form of SCLS rapidly develop shock and anasarca due to plasma extravasation (up to 70% of total plasma volume). We have termed this the “leak” phase, which usually lasts for several days. Because there are no uniform criteria to fully define recovery from flares (e.g. hemodynamic stabilization, resolution of edema, normalization of renal function), it is difficult to assess episode duration precisely from case reports. However, based on our analysis of 30 of the cases published over the last six years in which such information was provided, the median duration of SCLS episodes is 3.8 days (range, 1–27 days). Visceral spaces including CNS or lungs do not contain edema in classical SCLS although pleural and pericardial effusions, ascites, and even edema of the GI tract wall have been detected during disease flares.
Severity and frequency of SCLS flares vary considerably across patients and may change over time. In the NIH cohort, the frequency of attacks ranges from once every ten years to once every 3–5 days. Similarly, in the European registry, the median annual attack frequency was 1.23 (range, 0.13 to 21.18)50 Gousseff et al. classified SCLS episodes as “severe” based on the presence of one or more of the following: 1) systolic blood pressure < 60 mm Hg; 2) mean blood pressure < 65 mm Hg; 3) loss of consciousness; 4) admission to the ICU.50 Some patients we have cared for have experienced milder events characterized by a smaller drop in blood pressure, fatigue, weakness, and/or dizziness; increased thirst; decreased urinary output; and mild edema of the extremities that may only manifest as muscle tightness or discomfort. A third subset of patients may experience unexplained, non-cyclical peripheral edema but not acute hypotensive episodes, which we have designated “chronic” SCLS. The “post-leak” phase usually begins 48 hours to one week after the onset of shock, in which fluids are mobilized from peripheral tissues into the intravascular space followed by normalization of blood pressures and diuresis. Death from SCLS typically occurs during the post-leak phase due to pulmonary edema arising from therapeutic intravenous fluid administration during the earlier leak phase.
Complications of acute SCLS crises relate to hypoperfusion-related multiorgan dysfunction (MODS), hypercoagulability due to hemoconcentration and increased serum viscosity, and anasarca. In our cohort, these phenomena led to acute renal failure (14% of patients), thrombosis or pulmonary embolism (14%), and pericardial effusions/cardiac tamponade (9%).55 Commonly, the massive peripheral edema associated with SCLS episodes results in compartment syndromes of the extremities, necessitating fasciotomies (50%) to treat rhabdomyolysis. Nearly one third of our patients have experienced permanent disability as a result of these muscle complications, including sensorimotor neuropathy, foot drop, and even limb amputations.
DIAGNOSTIC CONSIDERATIONS
The SCLS diagnostic triad is composed of the “3 H’s”: 1) hypotension (typically systolic blood pressure < 90 mm Hg); 2) hemoconcentration (hematocrit > 49–50% in men, 43–45% in women), and hypoalbuminemia (< 3.0 g/dL) in the absence of secondary causes for such abnormalities. Fluid resuscitation is typically followed by rapidly developing symmetrical edema of the face, trunk, and extremities. As hemoconcentration worsens due to vascular leak, hemoglobin values continue to rise, often > 20 g/dL, even after administration of saline intravenously. In several of our patients, this striking paradox has led to a mistaken diagnosis of polycythemia vera and unnecessary phlebotomy. Serum albumin and total protein levels may be within the normal range initially, but decline rapidly as protein extravasates. The initial evaluation includes routine blood and urine cultures to exclude sepsis, serum tryptase to rule out anaphylaxis, and a serum immunofixation electrophoresis to determine whether a paraprotein is present. In the 134 cases we identified over the last six years, 68% overall had MGUS, most commonly of the IgG kappa isotype. This was present exclusively in adults and not in any children with SCLS. Because there are no validated biomarkers and MGUS is not specific to this syndrome, SCLS remains a clinical diagnosis.
Additional tests may help evaluate other potential causes of hypotension and/or edema. Urinalysis is necessary to exclude proteinuria as a primary cause of hypoalbuminemia and edema (e.g. nephrotic syndrome), particularly in patients with chronic SCLS. Neuroendocrine tumors such as carcinoid or pheochromocytoma or inferior vena cava syndrome resulting from obstructing tumor or coagulopathy may be associated with acute hypotension, but they are not typically associated with high hemoglobin or low serum albumin. Complement studies such as C3, C4, and CH50 measured during an acute SCLS event may not be useful to exclude hereditary forms of angioedema. Serum levels may be low due to leakage of complement proteins rather than pathway activation. Nonetheless, we routinely assess C1 esterase inhibitor levels and function to exclude types I and II hereditary angioedema.
Chest radiograph, electrocardiogram, pro-brain naturietic peptide (pro-BNP), and echocardiogram help exclude primary congestive heart failure. Although overt heart failure is not a feature of SCLS, edema may occur in the heart muscle itself and contribute to contractile dysfunction. Myocardial edema without inflammatory infiltrates was seen in three out of four biopsies reported in the literature, including a post-mortem examination in a patient in the NIH cohort who died of acute SCLS.13, 14, 33, 56 We have obtained evidence of myocardial edema in active SCLS using cardiovascular MRI (CMRI).56, 57 Dynamic measurement of extracellular volume (ECV) of heart tissue by CMRI imaging of gadolinium contrast agents is a novel means to detect active vascular leak in vivo. Calculation of the ECV is based on measurement of the longitudinal relaxation time constant (T1) of tissue before and after administration of a gadolinium-based contrast agent, which is exclusively present in the extracellular space.58, 59 Myocardial ECV was significantly higher in patients with active symptoms of SCLS (Figure 4), suggesting myocardial vascular leak. Finally, we have observed a transient drop in left ventricular ejection fraction (to ~10–15%) during an episode in several young men without prior cardiac history. We speculate that this could result from Takotsubo syndrome, or stress-induced cardiomyopathy, which may relate to the high doses of vasopressors needed in these patients.60
Figure 4. Cardiovascular MRI suggests myocardial vascular leak in SCLS.
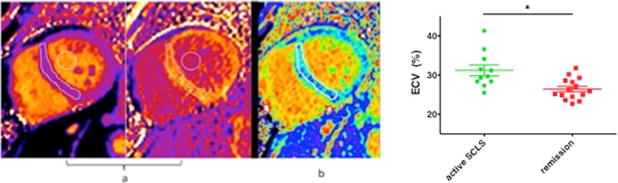
(A) Pre-and post-contrast myocardial T1 maps in a patient with SCLS. Regions of interest drawn within the septum and blood pool are used to calculate the extracellular volume fraction (ECV). (B) Mid-ventricular short axis ECV map in the same patient. The color scale for displaying ECV values was chosen so that green colors represent the mean ± 3 s.d. of normal myocardium from age-matched controls. (C) CMRI demonstrates increased myocardial ECV in patients with active VEH compared to asymptomatic group. **p=0.003, Mann-Whitney. Adapted from References56, 57.
PATHOPHYSIOLOGY OF SCLS: THE ROLE OF VASCULAR ENDOTHELIAL HYPER-PERMEABILITY (VEH)
A definitive mechanism for acute SCLS remains lacking; thus, treatments have been developed by trial-and-error. Although lymphatic abnormalities have not formally been excluded, albumin turnover studies suggest that vascular barrier dysfunction leading to VEH could account for the clinical phenotype.1 Histopathology of the peripheral microvasculature from patient biopsies has not shown gross anomalies, evidenced of disrupted angiogenesis, or the presence of inflammatory cells, complement, or immunoglobulin to suggest a vasculitis.3, 26, 49 The absence of persistent structural abnormalities is consistent with a defective, but reversible, barrier dysfunction response of the blood vessels. Several results suggest the presence of humoral factors promoting VEH during disease flares: 1) transient spikes in monocyte/macrophage-associated inflammatory mediators during flares; 2) transient elevation in circulating angiogenic proteins during flares;51, 61 and 3) impairment of in vitro microvascular endothelial cell (EC) barrier function provoked by acute but not convalescent SCLS sera.61, 62
The proinflammatory mediators CXCL10, CCL2, and IL-6 were elevated in the acute sera of 36 SCLS cases compared to similar numbers of either convalescent sera or healthy control sera. Monocytes from patients with SCLS produced more CXCL10 than those from healthy controls.51 Since none of these factors is specific to SCLS, these abnormalities may reflect a cytokine/chemokine upregulation induced by common infections that are suspected triggers for SCLS crises (e.g. viral URI). Known angiogenic permeability factors including vascular endothelial growth factor (VEGF) and angiopoietin-2 (Angpt-2) also appear to be transiently elevated around disease flares. In the NIH study of 36 cases, VEGF and Angpt-2 levels were significantly elevated in acute sera relative to convalescent sera. Serial measurements in several patients revealed that VEGF spiked at the very onset of symptoms and decreased rapidly whereas Angpt-2 levels peaked later and declined more slowly.61 The ephemeral, cyclical pattern of SCLS flares and the absence of chronic edema during remission periods suggests that endothelial barrier function returns to normal between crises.
Circulating factors induced during acute SCLS flares provoke EC barrier dysfunction through canonical mechanisms. For example, vascular endothelial (VE)-cadherin is an EC-specific adherens junction protein whose homotypic interactions between adjacent cells are necessary for intact barriers.63 Virtually all of the major signaling pathways that modulate barrier function ultimately reduce junctional localization of VE-cadherin. Acute but not basal SCLS sera attenuated membrane VE-cadherin in microvascular EC monolayers and also induced actin stress fiber formation (Figure 1). EC contraction and temporary attenuation of adherens junctions may permit leakage of solutes and proteins into the extravascular space during acute episodes.
Figure 1. Acute SCLS sera induce junctional destabilization and contraction of endothelial cells.
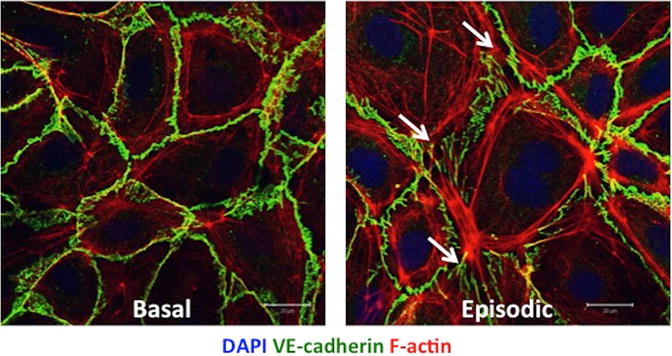
Application of same-subject SCLS patient sera from non-symptomatic period (Basal, left) v. that obtained during an acute attack (Episodic, right) to primary human dermal microvascular endothelial cells demonstrates disruption of junctional VE-cadherin (green), increased actin stress fibers (red), and development of gaps between adjacent cells (white arrows).
Blockade of Angpt-2 with a neutralizing peptide attenuated acute SCLS sera-evoked EC barrier dysfunction in vitro, suggesting a pathogenic role for the Angpt-2 pathway.61 Angpt-2 and Angpt-1 are ligands of the receptor Tie2 (Figure 2). Angpt-1 counteracts barrier dysfunction and VEH signaled by diverse mediators, spanning inflammatory molecules, bacterial products, and angiogenic factors.64 Angpt-2 is considered a context-dependent antagonist of Tie2. Angpt-1 signals through Tie2 to stimulate NADPH oxidase and nearby Rac1. Active Rac1 promotes barrier defense by stabilizing VE-cadherin and inducing cell spreading. Active Rac1 is stabilized by IQGAP1, and it also signals through p190RhoGAP to inhibit RhoA, a related small-molecule GTPase that favors cell contraction, junction destabilization, and loss of barrier integrity. Hypomorphic genetic manipulations of p47phox, IQGAP1, or p190RhoGAP induce an EC phenotype similar to what we might predict in SCLS: minimal basal abnormalities, yet unusual susceptibility to otherwise ordinary inflammatory stressors.65,66, 67
Figure 2. Angpt-Tie2 pathway mediates endothelial barrier function in response to inflammatory stressors.
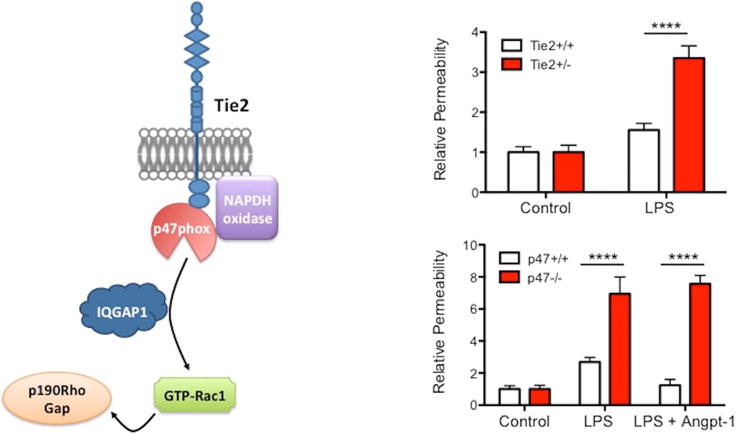
(A) Schematic of Tie2-dependent barrier defense pathway in endothelium. (B–C) Vascular permeability measurements from lungs of mice treated with LPS with and without Angpt-1 demonstrating enhanced susceptibility to vascular leakage arising from incomplete loss of expression of either Tie2 (receptor for Angpt-1) (B) or p47Phox (C). Results are normalized to respective genetic controls to illuminate differences in responses to LPS. ****p<0.0001, 2-way ANOVA. Adapted from References65, 66, 84.
To test the possible contribution of the Angpt-Tie2 pathway and/or other permeability inducers to SCLS, we have expanded blood-outgrowth ECs (BOECs) from circulating precursors obtained by venipuncture. These cells possess structural and molecular characteristics of mature ECs.68 Preliminary results suggest abnormal gene expression patterns in SCLS BOECs compared to those from healthy controls.69 RNA sequencing of SCLS- and healthy BOECs highlighted several gene products, including Angpt-1 and its receptor, Tie2, and pathways that were markedly dysregulated in the SCLS BOECs. Among the most notably induced pathways were cellular movement—migration requiring loss of the stable intercellular contacts and cytoskeletal shape required for barrier defense—and the inflammatory response itself (Figure 3, see highlighted box). The RNAseq results are consistent with our published observations that the physical mechanism of VEH in SCLS is related to structural rearrangements between adjacent microvascular ECs resulting in paracellular gaps. In the near future, SCLS ECs derived from induced pluripotent stem cells (iPSC-ECs) will also be tested for their responses to inflammatory mediators to determine if the primary defect in SCLS lies in the endothelial compartment.
Figure 3. RNAseq of SCLS BOECs implicates persistent pro-migratory and pro-inflammatory responses.
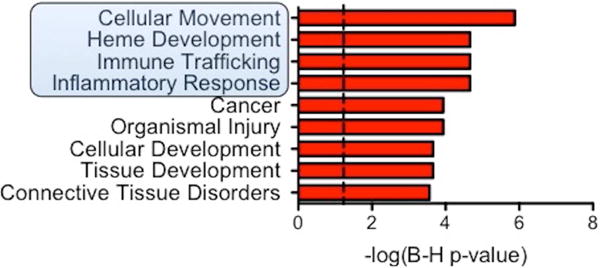
Ingenuity pathway analysis of RNAseq results from 6 SCLS BOECs v. 6 control BOECs organized by Benjamini-Hochberg corrected p-value. Dash p=0.05. Highlighted box indicates categories with particular functional relevance to SCLS.
The role of specific gene defects in SCLS, if any, is also unknown; e.g. whether the endothelium is genetically programmed for hyper-responsiveness to routine stimuli. There are no consistent familial aggregations in SCLS.3, 54 In one reported case of familial SCLS, the index case was correctly diagnosed, but the diagnosis of SCLS was not firmly established in the relatives.70 The sporadic occurrence of SCLS and lack of pedigrees could be consistent with rare genetic events such as compound heterozygosity (both alleles mutated and defective but at different locations)71 or two or more rare hemizygous mutations in different genes, autosomal recessive inheritance with incomplete penetrance, or somatic mutations confined to one or more cell lineages. Such abnormalities may be difficult to detect using standard whole exome sequencing. To determine the contribution of genetic abnormalities to the SCLS phenotype, we have performed a genome-wide association study of 12 patients and 18 controls (which included several asymptomatic relatives with no history of the disease).72 From unbiased high-density mapping of single-nucleotide polymorphisms (SNPs), a small genetic interval, 3p25.3, was identified as the highest-ranking candidate susceptibility locus (p ~10−6) with an odds ratio of ~41. Odds ratios (7–41) and p values (8×10−4 and 4×10−6) for the top SCLS-associated variants were outsized for such a small sample size. These results imply high penetrance for a rare disease allele that remains to be identified.
The role of the paraprotein present in most SCLS sera, if any, is unknown. Although the monoclonal Ig could act as an autoantibody to promote VEH during acute episodes, limited prior studies have failed to identify a common target antigen or cell. In fact, treatment of healthy ECs with purified SCLS Ig in vitro failed to induce cytotoxicity, activation, or morphological abnormalities.61, 73, 74 We speculate that if the paraprotein contributes to disease pathogenesis, a co-factor confined to disease flares may be required to unmask its activity since the Ig is also present during convalescent periods.
TREATMENT OF ACUTE SCLS
All treatment strategies are based on observational data rather than controlled trials because of disease infrequency. Patients presenting with hypotension and hemoconcentration consistent with acute SCLS should be treated in an intensive care setting with intravenous fluids sufficient to counteract intravascular volume depletion, maintain organ perfusion, and avoid severe metabolic acidosis (Figure 5). It is unclear whether continuous crystalloid infusion is superior to boluses of saline or protein-containing solutions such as albumin, but theoretically, albumin may have a longer half-life in the bloodstream and promote intravascular volume expansion because of its reverse oncotic effects. The benefits of maintaining normotension and normal central venous pressure (4–8 mm Hg) may outweigh the risk of excessive fluids leading to compartment syndromes. Interestingly, a recent case report found elevated levels of methemoglobin in an SCLS patient during a severe episode, suggestive of increased nitric oxide (NO) production contributing to hypotension.19 The authors reported that a single intravenous bolus of methylene blue, which neutralizes NO, sustainably reversed severe hypotension in their patient. Subsequently, we found that methylene blue administration had no pressor effect in a patient of ours (unpublished data).
Figure 5. Proposed treatment algorithms for active SCLS (left) and prophylactic management (right).
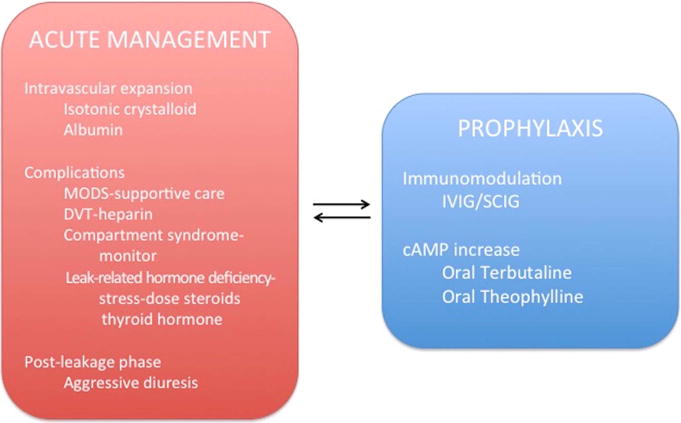
MODS-multi-organ dysfunction syndrome; DVT-deep venous thrombosis; IVIG-intravenous immunoglobulins; SCIG-subcutaneous immunoglobulin; cAMP-cyclic adenosine monophosphate.
Metabolic abnormalities include mild lactic acidosis, elevated creatinine phosphokinase (CPK) and/or liver transaminases. Short-lived oliguria is common and does not necessarily require invasive renal replacement therapy in the absence of traditional indications. Deep venous thrombosis (DVT) may occur due to severe hemoconcentration, increased serum viscosity, and stasis resulting from hypovolemia and compartment syndrome. Chronic anticoagulation should be considered in patients who experience thrombotic events during episodes. Monitoring of extremity compartment pressures by an orthopedic specialist is highly recommended in order to assess the need for fasciotomy and muscle debridement to prevent muscle and nerve necrosis. Studies suggest that the risk for such outcomes is higher when intracompartmental pressures are greater than 40 mm Hg.75 During the post-leak phase, aggressive diuresis is arguably the most important intervention in order to prevent flash pulmonary edema.
Both mild and severe acute SCLS episodes are self-limited by nature. Because vascular leak associated with SCLS nearly always remits spontaneously after several days, it is inherently difficult to assess the efficacy of acute interventions. We have found no conclusive evidence that anti-inflammatory agents including corticosteroids can abort or delay the progression of an acute SCLS attack. Similarly, although therapeutic targeting of factors linked to acute SCLS flares (e.g. VEGF, TNFα) have been used in isolated cases,74, 76 compelling evidence for use of these strategies is lacking. However, a recent SCLS case report suggested the possibility of adrenal and thyroid deficiency arising from extravasational loss of hormones and their serum binding proteins,15 providing a rationale for the use of stress-dose steroids (and perhaps even thyroid replacement) in the initial management of an episode. Theophylline has been reported to be effective in the treatment of acute SCLS.76, 77 Based on published cases, our own experience, and its theoretical ability to counteract VEH (see below), we currently recommend administration of aminophylline intravenously for severe flares. Finally, Lambert et al. reported that administration of high dose intravenous immunoglobulin (IVIG, 1–2 g/kg) rapidly corrected hypotension and promoted diuresis in three patients suffering acute flares.78 While we have not observed comparable rapid effects of IVIG in two hospitalized patients (unpublished data), quantitative studies of in vivo vascular barrier function before and after such acute treatments may inform IVIG’s application as a rescue therapy.
PROPHYLACTIC THERAPY AND PROGNOSIS
Although the utility of potential disease-modifying agents in acute SCLS remains uncertain, it has become abundantly clear over the last several years that prophylactic therapy is highly beneficial. Gousseff et al. reported a 5-year mortality rate of 15% in those receiving maintenance therapies v. 80% in those who had not.50 A Mayo clinic study of more than 25 SCLS patients showed that terbutaline and theophylline given orally significantly reduced the frequency and severity of SCLS episodes.54, 79 The rationale for use of these drugs is their ability to increase intracellular cyclic AMP (cAMP) levels, which may counteract inflammatory signaling pathways that induce endothelial permeability.80 Intracellular cAMP is thought to exert its actions on EC barrier function through two principal effectors: protein kinase A (PKA) and the guanine nucleotide exchange factor for Rap GTPases, Epac. Rho-family GTPases such as RhoA promote myosin light chain kinase (MLCK) phosphorylation, which in turn induces actomyosin bundling and cell contraction, a principal mechanism of VEH. By increasing Rap and PKA activity, cyclic cAMP inhibits RhoA and attenuates cortical actin remodeling and destabilizing VE-cadherin junctional interactions to fortify the vascular barrier.81 Although several of our patients have responded well to this regimen, our experience and a review of case reports published over the last six years suggest that nearly 84% of patients broke through this treatment with disease flares (Table 1). Although some of the episodes were associated with “sub-optimal” serum theophylline levels (based on ranges that have been used for asthma), it should be noted that therapeutic ranges for SCLS have not been established. In addition, these drugs were poorly tolerated due to side effects such as tremor, irritability, palpitations, and insomnia.55.
Based on its potential immunomodulatory and anti-cytokine properties, IVIG has been widely used for the treatment of autoimmune and MGUS-associated syndromes.82, 83 The mechanism of action of IVIG in SCLS is unknown, but we speculate that anti-idiotypic effects on the putative monoclonal autoantibody or neutralization of proinflammatory cytokines by antibodies present in IVIG preparations are two possible mechanisms by which IVIG exerts its prophylactic effects in SCLS. Over the last 8–10 years, monthly prophylaxis with IVIG has become the most commonly applied therapy in the NIH cohort. In the European registry, 8/18 patients (44%) experienced no new attacks while on IVIG, some for prolonged periods of follow up.50 The dosing of IVIG ranged greatly in this study—from 0.4–2 g/kg/month. We recently performed a questionnaire-based longitudinal study of IVIG therapy in 29 patients in our cohort.55 Eighteen out of 22 patients who responded had been treated with IVIG, with a median interval of 32 months (range 10–59 months). Strikingly, median attack frequency declined from 2.6 episodes/patient/yr to zero after commencing IVIG prophylaxis (p=0.0001). Fifteen of those 18 patients did not experience new SCLS episodes while on IVIG prophylaxis, and the side effect profile was favorable. Based on our analysis of the NIH cohort and recent published case reports in which a precise treatment history was provided, the median annual attack frequency in subjects treated with theophylline and terbutaline was 2.25 (range 0–20, 27 patients). By contrast, median annual attack frequency in patients treated with IVIG or SCIG was zero (range 0–3.3, 40 patients). In light of the impracticality of prospective, randomized clinical trials, we now highly recommend empiric prophylaxis with IVIG as front-line therapy for newly diagnosed cases of SCLS and a history of severe episodes. Although the optimal dose and duration of IVIG therapy for SCLS remain to be established, most of our patients receive 2 g/kg/month initially. In some cases, doses have been tapered to 1 g/kg/month after a prolonged initial remission period. We have not yet withdrawn IVIG therapy in any patient who has responded favorably. In the past, some patients were treated simultaneously with theophylline, terbutaline, and IVIG. However, we do not currently recommend this approach since in our experience treatment with theophylline and terbutaline frequently adds burdensome side effects without augmenting the efficacy of IVIG alone.
CONCLUSIONS
Treatment of acute SCLS remains primarily supportive. Prophylaxis with IVIG appears promising, but this therapy is nonspecific and expensive. Mechanistic understanding of SCLS is in its infancy. As a result, clinicians today cannot predict when or how badly SCLS will flare; targeted therapies do not yet exist, and prolonged remission or cure remains elusive. Our working hypothesis invokes exaggerated microvascular endothelial responses to surges of otherwise routinely encountered inflammatory mediators. This emerging disease model lends itself to innovative patient-centered translational research in the ways highlighted above. It is our hope that detailed and personalized investigation of intra-endothelial responses among individual SCLS sufferers may illuminate novel genetic and molecular control mechanisms. In turn such advances could deliver the diagnostic, prognostic, and therapeutic tools sorely needed to combat this devastating disease.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clarkson B, Thompson D, Horwith M, Luckey EH. Cyclical edema and shock due to increased capillary permeability. Am J Med. 1960;29:193–216. doi: 10.1016/0002-9343(60)90018-8. [DOI] [PubMed] [Google Scholar]
- 2.Amoura Z, Papo T, Ninet J, Hatron PY, Guillaumie J, Piette AM, et al. Systemic capillary leak syndrome: report on 13 patients with special focus on course and treatment. Am J Med. 1997;103:514–9. doi: 10.1016/s0002-9343(97)00272-6. [DOI] [PubMed] [Google Scholar]
- 3.Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med. 2010;153:90–8. doi: 10.1059/0003-4819-153-2-201007200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yardimci B, Kazancioglu R. Idiopathic Systemic Capillary Leak Syndrome: A Case Report. Iran Red Crescent Med J. 2016;18:e29249. doi: 10.5812/ircmj.29249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulihova K, Prochazkova M, Semberova J, Janota J. Fatal Primary Capillary Leak Syndrome in a Late Preterm Newborn. Indian J Pediatr. 2016;83:1197–9. doi: 10.1007/s12098-016-2134-y. [DOI] [PubMed] [Google Scholar]
- 6.Gunes AR, Berlit P, Weber R. Severe cerebral involvement due to idiopathic systemic capillary leak syndrome. Clin Case Rep. 2016;4:429–31. doi: 10.1002/ccr3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durand Bechu M, Rouget A, Recher C, Azoulay E, Bounes V. A Systemic Capillary Leak Syndrome (Clarkson Syndrome) in a Patient with Chronic Lymphocytic Leukemia: A Case Report in an Out-of-Hospital Setting. Case Rep Emerg Med. 2016;2016:5347039. doi: 10.1155/2016/5347039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scanvion Q, Lefevre G, Hachulla E, Hatron PY, Lambert M. Subcutaneous Immunoglobulin Therapy Prevents Systemic Capillary Leak Syndrome Attack. Am J Med. 2016;129:e77–8. doi: 10.1016/j.amjmed.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 9.Garnes M, Boen J. A man in his sixties with severe hypotension and oedema. Tidsskr Nor Laegeforen. 2015;135:2073–6. doi: 10.4045/tidsskr.13.1692. [DOI] [PubMed] [Google Scholar]
- 10.Ledochowski S, Freichet M, Prieur C, Friggeri A, Lega JC. An uncommon cause of distributive shock: Lessons from two consecutive cases of idiopathic systemic capillary leak syndrome (Clarkson’s disease) Anaesth Crit Care Pain Med. 2015;34:251–3. doi: 10.1016/j.accpm.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Aroney N, Ure S, White H, Sane S. Recurrent undifferentiated shock: Idiopathic Systemic Capillary Leak Syndrome. Clin Case Rep. 2015;3:527–30. doi: 10.1002/ccr3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning TE, Manning AE, Manning PJ. Systemic capillary leak syndrome: a case-report. N Z Med J. 2015;128:51–3. [PubMed] [Google Scholar]
- 13.Hirosaki Y, Hayashidani S, Ouchi S, Ohshima T, Nakano R, Yamamoto H. A fatal case of acute progression of generalized edema and simultaneous flash pulmonary edema in a patient with idiopathic systemic capillary leak syndrome: a case report. J Med Case Rep. 2015;9:90. doi: 10.1186/s13256-015-0544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zancanaro A, Serafini F, Fantin G, Murer B, Cicardi M, Bonanni L, et al. Clinical and pathological findings of a fatal systemic capillary leak syndrome (Clarkson disease): a case report. Medicine (Baltimore) 2015;94:e591. doi: 10.1097/MD.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Then C, Ritzel K, Seibold C, Mann JF, Reincke M. Multiglandular hormone deficiency in a patient with systemic capillary leak syndrome. Case Rep Med. 2015;2015:958283. doi: 10.1155/2015/958283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozawa T, Yamaguchi H, Kiyomatsu T, Saito S, Ishihara S, Sunami E, et al. A case report of idiopathic systemic capillary leak syndrome that occurred during the postoperative period of abdominoperineal resection for colorectal cancer. Int Surg. 2015;100:58–62. doi: 10.9738/INTSURG-D-13-00206.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerketta J, Lodh M, Mandal K. Clarkson disease – systemic capillary leak syndrome in a 6-year-old girl: case report. Paediatr Int Child Health. 2015;35:160–3. doi: 10.1179/2046905514Y.0000000161. [DOI] [PubMed] [Google Scholar]
- 18.Val-Flores LS, Fior A, Santos A, Reis L, Bento L. Is this septic shock? A rare case of distributive shock. Rev Bras Ter Intensiva. 2014;26:416–20. doi: 10.5935/0103-507X.20140064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umbrello M, Gardinali M, Ottolina D, Zanforlin G, Iapichino G. Systemic capillary leak syndrome: is methylene blue the silver bullet? Case Rep Crit Care. 2014;2014:141670. doi: 10.1155/2014/141670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamou H, Grassmann JP, Betsch M, Wild M, Hakimi M, Windolf J, et al. Systemic capillary leak syndrome associated with a rare abdominal and four-limb compartment syndrome: a case report. J Med Case Rep. 2014;8:196. doi: 10.1186/1752-1947-8-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasa T, Ohashi H, Kihira K, Koike Y, Otake K, Inoue M, et al. 10-year-old girl with life-threatening idiopathic systemic capillary leak syndrome: a case report. BMC Pediatr. 2014;14:137. doi: 10.1186/1471-2431-14-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayama M, Shime N, Mio T. Invasive pulmonary aspergillosis in a patient presenting with idiopathic systemic capillary leak syndrome. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-203764. pii: bcr2014203764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marra AM, Gigante A, Rosato E. Intravenous immunoglobulin in systemic capillary leak syndrome: a case report and review of literature. Expert Rev Clin Immunol. 2014;10:349–52. doi: 10.1586/1744666X.2014.882771. [DOI] [PubMed] [Google Scholar]
- 24.Choi KM, Park CS, Kim MH. Systemic capillary leak syndrome (Clarkson’s disease) during elective pylorus-preserving pancreaticoduodenectomy: case report. Korean J Hepatobiliary Pancreat Surg. 2014;18:38–41. doi: 10.14701/kjhbps.2014.18.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perme T, Pokorn M, Markelj G, Avcin T, Battelino T, Ursic T, et al. Two episodes of systemic capillary leak syndrome in an 8-year-old boy, following influenza A virus infection. Pediatr Infect Dis J. 2014;33:222–4. doi: 10.1097/INF.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 26.Bouchet JL, Vital C, Ferrer X, Vital A. Systemic capillary leak syndrome in an 85-year-old man (Clarkson’s syndrome) Rev Neurol (Paris) 2014;170:713–4. doi: 10.1016/j.neurol.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Munoz-Guillen NM, Leon-Lopez R, de la Cal-Ramirez MA, Duenas-Jurado JM. Systemic capillary leak syndrome: hypoalbuminemia, hemoconcentration and shock. Presentation of a case. Semergen. 2014;40:e33–6. doi: 10.1016/j.semerg.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Aldemir-Kocabas B, Karbuz A, Ciftci E, Demir M, Ince E. An unusual cause of secondary capillary leak syndrome in a child: rotavirus diarrhea. Turk J Pediatr. 2013;55:90–3. [PubMed] [Google Scholar]
- 29.Sheehan JR, Keating L, Chan A, Walden A. Distributive shock due to systemic capillary leak syndrome treated with high-dose immunosuppression. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-009048. pii: bcr2013009048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn J, Odom SR, Saillant N, Ojeifo OA, Abramson Z, Gupta A, et al. Capillary leak syndrome and abdominal compartment syndrome from occult rectal malignancy. Am Surg. 2012;78:E443–5. [PubMed] [Google Scholar]
- 31.Miyata K, Mikami T, Mikuni N, Aisaka W, Irifune H, Narimatsu E. Malignant hemispheric cerebral infarction associated with idiopathic systemic capillary leak syndrome. Case Rep Neurol. 2013;5:175–82. doi: 10.1159/000355637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milani GP, Dellepiane RM, Castellazzi ML, Mazzoni MB, Bianchetti MG, Fossali EF. Episodic idiopathic systemic capillary leak syndrome in a girl. Pediatr Int. 2013;55:e81–2. doi: 10.1111/ped.12068. [DOI] [PubMed] [Google Scholar]
- 33.Juthier F, Ennezat PV, Fornes P, Hachulla E, Hatron PY, Robin E, et al. Myocardial involvement in systemic capillary leak syndrome: first demonstration by pathologic findings. Eur Heart J Acute Cardiovasc Care. 2012;1:248–52. doi: 10.1177/2048872612455142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez Martinez ML, Pico Brezmes S, Alvarez Arguello MJ, Alonso Marguello A. General anaesthesia in a patient with Clarkson syndrome. Rev Esp Anestesiol Reanim. 2012;59:107–8. doi: 10.1016/j.redar.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Piastra M, Pietrini D, Conti G, De Rosa G, Rigante D. Sudden shock from capillary leak. Lancet. 2012;379:976. doi: 10.1016/S0140-6736(11)61819-9. [DOI] [PubMed] [Google Scholar]
- 36.Almagro P, Marti JM, Garcia Pascual L, Rodriguez-Carballeira M. Successful treatment of systemic capillary leak syndrome with intravenous immunoglobulins. Rev Clin Esp. 2012;212:218–9. doi: 10.1016/j.rce.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Rahn T, Plehn A, Lemm H, Abromeit S, Dambeck S, Busanny F, et al. Shock, hemoconcentration, and generalized edema in a 47-year-old man. Internist (Berl) 2012;53:341–4. doi: 10.1007/s00108-011-2961-x. [DOI] [PubMed] [Google Scholar]
- 38.Teutonico A, Chimienti D, Antonelli M, Bruno A, Libutti P, Lisi P, et al. The systemic capillary leak syndrome: a scarcely known nephrological entity. J Nephrol. 2012;25:262–5. doi: 10.5301/jn.5000065. [DOI] [PubMed] [Google Scholar]
- 39.Berti de Marinis G, Bertozzi I, Allemand E, Tezza F, Randi ML, Naso A, et al. A 40-year-old man with recurrent fainting, hypotension, lower limb edema and oliguria with body weight gain and secondary erythrocytosis. Intern Emerg Med. 2012;7:453–6. doi: 10.1007/s11739-011-0692-6. [DOI] [PubMed] [Google Scholar]
- 40.Brown RH, Downey C, Izaddoost S. Compartment syndrome in all four extremities: a rare case associated with systemic capillary leak syndrome. Hand (N Y) 2011;6:110–4. doi: 10.1007/s11552-010-9305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa N, Ota H, Tanabe Y, Kabara M, Matsuki M, Chinda J, et al. A Case of Idiopathic Systemic Capillary Leak Syndrome with High Serum Levels of G-CSF on Exacerbation. Intern Med. 2011;50:597–600. doi: 10.2169/internalmedicine.50.4857. [DOI] [PubMed] [Google Scholar]
- 42.Merceron S, Lacave G, Henry-Lagarrigue M, Guezennec P, Troche G, Legriel S, et al. Fatal Clarkson syndrome mimicking a septic shock. Med Mal Infect. 2011;41:336–8. doi: 10.1016/j.medmal.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Bencsath KP, Reu F, Dietz J, Hsi ED, Heresi GA. Idiopathic systemic capillary leak syndrome preceding diagnosis of infiltrating lobular carcinoma of the breast with quiescence during neoadjuvant chemotherapy. Mayo Clin Proc. 2011;86:260–1. doi: 10.4065/mcp.2010.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zipponi M, Eugster R, Birrenbach T. High-dose intravenous immunoglobulins: a promising therapeutic approach for idiopathic systemic capillary leak syndrome. BMJ Case Rep. 2011 doi: 10.1136/bcr.12.2010.3599. pii: bcr1220103599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ansari A, Birendra KC, Marvin M, Kubat A, Fritz T. An unusual case of swelling–Clarkson’s syndrome. BMJ Case Rep. 2011 doi: 10.1136/bcr.06.2011.4405. pii: bcr0620114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagao Y, Harada H, Yamanaka H, Fukuda K. Possible mediators for systemic capillary leak syndrome. Am J Med. 2011;124:e7–9. doi: 10.1016/j.amjmed.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 47.Guillaume M, Tolsma V, Colombe B, Bosseray A, Massot C. Idiopathic systemic capillary leak syndrome: a case report with cardiac involvement. Rev Med Interne. 2011;32:e69–71. doi: 10.1016/j.revmed.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Simon DA, Taylor TL, Bayley G, Lalonde KA. Four-limb compartment syndrome associated with the systemic capillary leak syndrome. J Bone Joint Surg Br. 2010;92:1700–2. doi: 10.1302/0301-620X.92B12.25225. [DOI] [PubMed] [Google Scholar]
- 49.Saugel B, Umgelter A, Martin F, Phillip V, Schmid RM, Huber W. Systemic Capillary Leak Syndrome associated with hypovolemic shock and compartment syndrome. Use of transpulmonary thermodilution technique for volume management. Scand J Trauma Resusc Emerg Med. 2010;18:38. doi: 10.1186/1757-7241-18-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gousseff M, Arnaud L, Lambert M, Hot A, Hamidou M, Duhaut P, et al. The Systemic Capillary Leak Syndrome: A Case Series of 28 Patients From a European Registry. Ann Intern Med. 2011;154:464–71. doi: 10.7326/0003-4819-154-7-201104050-00004. [DOI] [PubMed] [Google Scholar]
- 51.Xie Z, Chan E, Yin Y, Ghosh CC, Wisch L, Nelson C, et al. Inflammatory Markers of the Systemic Capillary Leak Syndrome (Clarkson Disease) J Clin Cell Immunol. 2014;5:1000213. doi: 10.4172/2155-9899.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu P, Xie Z, Frith K, Wong M, Kakakios A, Stone KD, et al. Idiopathic systemic capillary leak syndrome in children. Pediatrics. 2015;135:e730–5. doi: 10.1542/peds.2014-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atkinson JP, Waldmann TA, Stein SF, Gelfand JA, Macdonald WJ, Heck LW, et al. Systemic capillary leak syndrome and monoclonal IgG gammopathy; studies in a sixth patient and a review of the literature. Medicine (Baltimore) 1977;56:225–39. doi: 10.1097/00005792-197705000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Kapoor P, Greipp PT, Schaefer EW, Mandrekar SJ, Kamal AH, Gonzalez-Paz NC, et al. Idiopathic systemic capillary leak syndrome (Clarkson’s disease): the Mayo clinic experience. Mayo Clin Proc. 2010;85:905–12. doi: 10.4065/mcp.2010.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie Z, Chan EC, Long LM, Nelson C, Druey KM. High-dose intravenous immunoglobulin therapy for systemic capillary leak syndrome (Clarkson disease) Am J Med. 2015;128:91–5. doi: 10.1016/j.amjmed.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ertel A, Pratt D, Kellman P, Leung S, Bandettini P, Long LM, et al. Increased myocardial extracellular volume in active idiopathic systemic capillary leak syndrome. J Cardiovasc Magn Reson. 2015;17:76. doi: 10.1186/s12968-015-0181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, et al. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012;14:64. doi: 10.1186/1532-429X-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, et al. Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology. 1999;211:698–708. doi: 10.1148/radiology.211.3.r99jn41698. [DOI] [PubMed] [Google Scholar]
- 59.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–44. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 60.Chlus N, Cavayero C, Kar P, Kar S. Takotsubo Cardiomyopathy: Case Series and Literature Review. Cureus. 2016;8:e649. doi: 10.7759/cureus.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie Z, Ghosh CC, Patel R, Iwaki S, Gaskins D, Nelson C, et al. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome) Blood. 2012;119:4321–32. doi: 10.1182/blood-2011-08-375816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie Z, Ghosh CC, Parikh SM, Druey KM. Mechanistic classification of the systemic capillary leak syndrome: Clarkson disease. Am J Respir Crit Care Med. 2014;189:1145–7. doi: 10.1164/rccm.201310-1746LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–20. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milam KE, Parikh SM. The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation. Tissue Barriers. 2015;3:e957508. doi: 10.4161/21688362.2014.957508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghosh CC, Mukherjee A, David S, Milam KE, Hunter JT, Parikh SM. Angiopoietin-1 requires oxidant signaling through p47phox to promote endothelial barrier defense. PLoS One. 2015;10:e0119577. doi: 10.1371/journal.pone.0119577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, et al. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282:23910–8. doi: 10.1074/jbc.M702169200. [DOI] [PubMed] [Google Scholar]
- 67.David S, Ghosh CC, Mukherjee A, Parikh SM. Angiopoietin-1 requires IQ domain GTPase-activating protein 1 to activate Rac1 and promote endothelial barrier defense. Arterioscler Thromb Vasc Biol. 2011;31:2643–52. doi: 10.1161/ATVBAHA.111.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dudek AZ, Bodempudi V, Welsh BW, Jasinski P, Griffin RJ, Milbauer L, et al. Systemic inhibition of tumour angiogenesis by endothelial cell-based gene therapy. Br J Cancer. 2007;97:513–22. doi: 10.1038/sj.bjc.6603883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sek AC, Xie Z, Terai K, Long LM, Nelson C, Dudek AZ, et al. Endothelial Expression of Endothelin Receptor A in the Systemic Capillary Leak Syndrome. PLoS One. 2015;10:e0133266. doi: 10.1371/journal.pone.0133266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sion-Sarid R, Lerman-Sagie T, Blumkin L, Ben-Ami D, Cohen I, Houri S. Neurologic Involvement in a Child With Systemic Capillary Leak Syndrome. Pediatrics. 2010;125:e687–92. doi: 10.1542/peds.2009-1691. [DOI] [PubMed] [Google Scholar]
- 71.Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. 2010;42:30–5. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie Z, Nagarajan V, Sturdevant DE, Iwaki S, Chan E, Wisch L, et al. Genome-wide SNP analysis of the Systemic Capillary Leak Syndrome (Clarkson disease) Rare Dis. 2013;1 doi: 10.4161/rdis.27445. pii: e27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang W, Ewan PW, Lachmann PJ. The paraproteins in systemic capillary leak syndrome. Clin Exp Immunol. 1993;93:424–9. doi: 10.1111/j.1365-2249.1993.tb08195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lesterhuis WJ, Rennings AJ, Leenders WP, Nooteboom A, Punt CJ, Sweep FC, et al. Vascular endothelial growth factor in systemic capillary leak syndrome. Am J Med. 2009;122:e5–7. doi: 10.1016/j.amjmed.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 75.Arato E, Kurthy M, Sinay L, Kasza G, Menyhei G, Masoud S, et al. Pathology and diagnostic options of lower limb compartment syndrome. Clin Hemorheol Microcirc. 2009;41:1–8. doi: 10.3233/CH-2009-1145. [DOI] [PubMed] [Google Scholar]
- 76.Dowden AM, Rullo OJ, Aziz N, Fasano MB, Chatila T, Ballas ZK. Idiopathic systemic capillary leak syndrome: Novel therapy for acute attacks. J Allergy Clin Immunol. 2009;124:1111–3. doi: 10.1016/j.jaci.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 77.Pratesi A, Valoti P, Baldasseroni S, Marchionni N, Tarantini F. Sudden cardiac arrest in a 73-year-old woman caused by systemic capillary leak syndrome. Intern Emerg Med. 2016;11:719–20. doi: 10.1007/s11739-016-1449-z. [DOI] [PubMed] [Google Scholar]
- 78.Lambert M, Launay D, Hachulla E, Morell-Dubois S, Soland V, Queyrel V, et al. High-dose intravenous immunoglobulins dramatically reverse systemic capillary leak syndrome. Crit Care Med. 2008;36:2184–7. doi: 10.1097/CCM.0b013e31817d7c71. [DOI] [PubMed] [Google Scholar]
- 79.Tahirkheli NK, Greipp PR. Treatment of the systemic capillary leak syndrome with terbutaline and theophylline. A case series. Ann Intern Med. 1999;130:905–9. doi: 10.7326/0003-4819-130-11-199906010-00015. [DOI] [PubMed] [Google Scholar]
- 80.Liu H, Yu X, Yu S, Kou J. Molecular mechanisms in lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Int Immunopharmacol. 2015;29:937–46. doi: 10.1016/j.intimp.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Roberts OL, Dart C. cAMP signalling in the vasculature: the role of Epac (exchange protein directly activated by cAMP) Biochem Soc Trans. 2014;42:89–97. doi: 10.1042/BST20130253. [DOI] [PubMed] [Google Scholar]
- 82.Dezsi L, Horvath Z, Vecsei L. Intravenous immunoglobulin: pharmacological properties and use in polyneuropathies. Expert Opin Drug Metab Toxicol. 2016 Aug 22;:1–16. doi: 10.1080/17425255.2016.1214715. [DOI] [PubMed] [Google Scholar]
- 83.Theaudin M, Lozeron P, Lacroix C, Chretien P, Ducot B, Denier C, et al. Short and long-term effect of IVIg in demyelinating neuropathy associated with MGUS, experience of a monocentric study. Rev Neurol (Paris) 2011;167:897–904. doi: 10.1016/j.neurol.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Ghosh CC, David S, Zhang R, Berghelli A, Milam K, Higgins SJ, et al. Gene control of tyrosine kinase TIE2 and vascular manifestations of infections. Proc Natl Acad Sci U S A. 2016;113:2472–7. doi: 10.1073/pnas.1519467113. [DOI] [PMC free article] [PubMed] [Google Scholar]


