Abstract
Pathogenic bacteria use the bloodstream as a highway for getting around the body, and thus have to find ways to enter and exit through the endothelium. Many bacteria approach this problem by producing toxins that can breach the endothelial barrier through diverse creative mechanisms, including directly killing endothelial cells (ECs), weakening the cytoskeleton within ECs, and breaking the junctions between ECs. Toxins can also modulate the immune response by influencing endothelial biology, and can modulate endothelial function by influencing the response of leukocytes. Understanding these interactions, in both the in vitro and in vivo contexts, is of critical importance for designing new therapies for sepsis and other severe bacterial diseases.
Introduction
Every successful bacterial pathogen must at some point interact with the body’s largest organ –the endothelium. This interaction can take many forms (Fig 1). Bacteria can disrupt the barrier function of the endothelium to gain access to the bloodstream and then again to gain access to new organs. Many bacteria have evolved toxins which can increase vascular permeability by either killing ECs or by interfering with the cytoskeleton or cell-cell junctions. This review will focus on some recent findings elucidating the activities of toxins and other secreted effectors that bacteria use to attack the endothelium (Table 1).
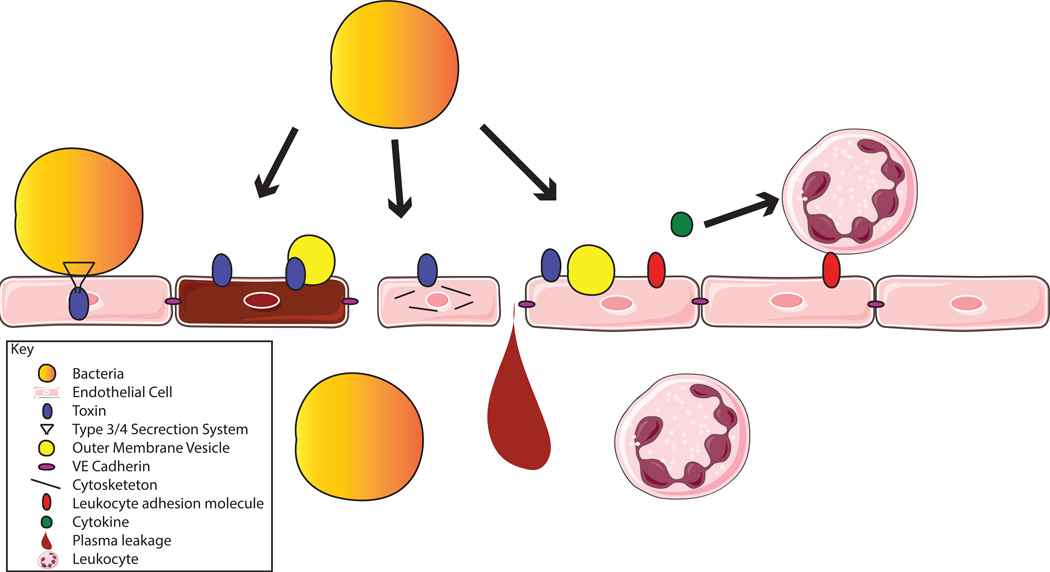
Figure 1.
The many ways bacteria can interact with ECs. Bacteria can adhere to ECs, and use type 3 or 4 secretion systems to inject toxins. Bacteria can secrete toxins into the bloodstream which then bind to ECs (in some cases through a receptor) and kill the cells. OMVs can also be used to deliver toxins to ECs. Toxins can also manipulate cells to disrupt their cytoskeleton and their contacts with other ECs. Toxins and OMVs can modulate the inflammatory state of ECs, affecting leukocyte adhesion molecules and cytokines, to increase or decrease leukocyte migration to the area. The end results of these actions can include bacterial breach of the endothelium, leakage of plasma and edema, and increased or decreased leukocyte migration and leukocyte-mediated damage in infected tissues.
Table 1.
Some Recent Findings in Bacterial Toxin/ Endothelial Cell Interactions
| Bacterium | Toxin | Models studied | Recent finding | Ref |
|---|---|---|---|---|
| Gram-positive | ||||
|
Streptococcus Pneumoniae |
Pneumolysin | Human umbilical vein ECs (HUVECs) |
Induces apoptosis through p38 MAPK activation |
[3] |
| Human lung microvascular ECs (HL-MVECs), intratracheal intoxication of mice |
Disrupts barrier function through PKCα |
[5] | ||
|
Staphylococcus aureus |
α-toxin | Intravenous and intradermal mouse intoxication, Human pulmonary artery ECs (HPAECs) |
Induces loss of endothelial barrier function |
[7] |
| Haploid human cells (HAP1), intranasal and skin mouse infection |
Requires PLEKHA7 for epithelial cell killing |
[9] | ||
| Bacilus cereus | Nhe, HBL | HUVECs | Kill ECs, particularly Nhe | [10] |
| Gram-negative | ||||
|
Pseudomonas aeruginosa |
ExoU | Human microvascular endothelial cell line-1 (HMEC-1) |
Induces a pro-thrombotic state | [13] |
| HMEC-1 | Cleaves ICAM-1 from the membrane |
[25] | ||
| ExoS, ExoT | HUVECs, Intranasal mouse infection |
Breaks down actin filaments through cofillin |
[15] | |
| ExoY | Pulmonary microvascular ECs (PMVECs) |
Impairs EC repair | [17] | |
| LasB | HUVECs, Intranasal neutropenic mouse infection |
Cleaves VE-Cadherin | [18] | |
| Exolysin | HUVECs, Intranasal mouse infection |
Kills ECs | [19] | |
| Shiga-toxigenic Escherichia coli |
SubAB | HBMECs | Decreases cytokine secretion | [23] |
| Shiga toxin 2 | HBMECs | Increases cytokine and chemokine expression |
[23] |
Toxins that disrupt endothelial barrier function directly
Toxins from gram-positive bacteria
Many gram-positive bacteria produce toxins that directly kill ECs, often by forming pores in the plasma membrane of host cells. Pneumolysin (Ply), a cholesterol-dependent cytolysin secreted by Streptococcus pneumoniae, is an early-discovered example of a toxin that targets ECs [1,2]. Ply kills ECs through apoptosis in a manner dependent on the activation of p38 MAPK [3]. Beyond simply killing ECs, Ply can also manipulate their biology in other ways. The pores formed by Ply in the plasma membrane of ECs cause calcium flux activating phospholipase A2, which has the potential to produce inflammatory mediators [4]. Further, through the activation of protein kinase C-α, Ply can increase endothelial permeability by perturbing the microtubule cytoskeleton, decreasing vascular endothelial (VE)-cadherin expression, and increasing production of arginase, which further impairs endothelial barrier function by reducing the production of NO [5].
Staphylococcus aureus also targets ECs, via α-toxin (also known as Hla) [6]. As with Ply, α-toxin can also have effects beyond direct killing, as it can impair the endothelial barrier by causing its receptor, ADAM10 [7] to cleave VE-Cadherin [8], disrupting cell-cell junctions and contributing to sepsis severity [8]. Junctional tropism appears to be key to α-toxin action, as PLEKHA7, a component of adherens junctions, is necessary for α-toxin to be able to kill epithelial cells [9]. Since ECs also express PLEKHA7, this host factor likely plays a similar role in their susceptibility to S. aureus α-toxin.
Lastly, Bacilus cereus produces several enterotoxins, including the three-component toxins non-hemolytic enterotoxin (Nhe) and hemolysin BL (HBL) that can also injure ECs. Based on in vitro experiments, ECs are highly susceptible to Nhe, and to a lesser extent to HBL. Further, while HBL kills rapidly by pore formation, Nhe kills more slowly, through mitochondrial damage [10]. This study investigated the cytotoxicity of these three toxins on different cell types, and found marked differences in susceptibilities, strongly suggesting the existence of specific receptors for these toxins.
Toxins from gram-negative bacteria
Many gram-negative bacteria are also adept at secreting toxins that disrupt endothelial integrity, through both cytolytic and non-cytolytic mechanisms. One of the best examples is Pseudomonas aeruginosa, which produces both type 2 and type 3 secretions systems (T2SS and T3SS). These systems secrete effectors that disrupt endothelial barrier integrity, causing devastating pathology in the lung. The T3SS can secrete exoenzymes S, T, U and Y. ExoU directly lyses ECs through its phospholipase activity, and this is partly achieved through the induction of oxidative stress [11,12]. ExoU can also induce a pro-thrombotic state, by causing ECs to release von Willebrand Factor (vWF) and vWF-expressing microparticles, resulting in increased platelet adhesion and disrupting vascular flow [13].
ExoS and ExoT are highly related toxins that cause leakage across the lung endothelium by manipulating the activities of Rho GTPases, leading to the formation of actin stress fibers [14]. The inhibition of Rho GTPases eventually leads to the dephosphorylation of Lim kinase, inducing the actin-severing activity of cofilin, finally leading to cell retraction [15]. Other studies, however, have found that the endothelial disruption of the P. aeruginosa T3SS is primarily mediated through ExoY, an adenylyl cyclase which causes microtubule breakdown, resulting in gaps between ECs and tissue edema [16]. Further, ExoY prevents repair of the endothelium by decreasing the replication of ECs, even several days after exposure. This potentially occurs because of prolonged phosphorylation of tau and the formation of tau aggregates [17]. To further complicate this picture, LasB, secreted through the T2SS, was recently found to cleave VE-Cadherin, further facilitating the disruption of endothelial monolayers. Epithelial-cadherin, however, was not affected, indicating the specificity of this toxin’s action [18]. Altogether, these findings demonstrate that P. aeruginosa is most certainly adept at destroying the barrier integrity of the endothelium.
There are distantly related isolates of P. aeruginosa that lack the T3SS, and yet are highly virulent. One representative of such isolates is strain CLJ1, which was isolated from a patient with hemorrhagic pneumonia [19]. This strain kills ECs by producing a novel toxin known as Exolysin (ExlA) [19]. ExlA is highly cytotoxic to ECs and increases lethality in a mouse pneumonia model [19]. Other isolates producing ExlA have been identified, and are toxic to ECs as well [20].
Toxins that affect the immune functions of endothelial cells
The endothelium also performs important immune functions. ECs sense microbes through toll like receptor (TLR) stimulation and other innate sensing mechanisms, becoming activated to secrete cytokines and chemokines and upregulate leukocyte adhesion molecules to direct leukocyte traffic to the infected area [21]. Many bacteria manipulate this response. For instance, Shiga-toxigenic Escherichia coli produces subtilase cytotoxin (SubAB), an AB5 cytotoxin, that cleaves BiP, an ER chaperone [22].
In several cells lines including ECs, this decreases the secreted levels of IL-8, MCP-1 and IL-6 post-translationally, seemingly by inducing ER stress and the unfolded protein response [23]. In contrast, Shiga toxin 2 (Stx2), another AB5 toxin, upregulates cytokine and chemokine expression [23]. This study found that the combination of SubAB and Stx2 inhibited the secretion of IL-8 from monocytic U937 cells, but did not test the combination in ECs. Thus, these two toxins together may either strengthen or dampen the secretion of cytokines from ECs, or potentially both in different situations.
P. aeruginosa ExoU can also modulate the leukocyte-directing functions of ECs. Through its phospholipase activity, ExoU causes a release of eicosanoids from ECs, which causes inflammation in mouse footpad and lung infection models [24]. ExoU also cleaves intracellular adhesion molecule-1 (ICAM-1), releasing it from the membrane. Soluble ICAM-1 increases inflammation by activating leukocytes, contributing to pathogenesis [25].
Autophagy: Endothelial cells fight back
There are two recent examples in the literature which illustrate that ECs are not completely defenseless against the toxins that bacteria attack them with, both involving autophagy. Maurer et. al. made the surprising observation that mice defective in autophagy are more susceptible to intravenous and lung infection with S. aureus strains that produce high amounts of α-toxin. Further, deficiency of autophagy in ECs is sufficient to render mice more susceptible to α-toxin. Autophagy protects ECs from α-toxin by decreasing the levels of α-toxin’s receptor, ADAM10, in a post-transcriptional manner [26]. This study illustrates how much we can learn from studying host-pathogen interactions in vivo and argues that in vivo studies are required to complement in vitro cell biology to fully uncover how pathogens interact with ECs and other host cells and organs.
In another example, intracellular Group B Streptococcus β-hemolysin/cytolysin (β-h/c) triggers autophagy in ECs, which in turn kills the majority of the internalized bacteria [27]. Thus, in both of these examples ECs are able to fight back with autophagy, but not effectively enough to completely prevent disease. Presumably there are examples of less successful pathogens that ECs are able to withstand through autophagy and other yet to be described resilience mechanisms. However, it is fair to assume that pathogens have developed clever ways to subverts ECs and their resilience mechanisms to promote infection and/or their survival within the mammalian host.
Outer Membrane Vesicles
Gram-negative bacteria release outer membrane vesicles (OMVs), another type of secreted effector. OMVs turn endotoxin and other surface-associated virulence factors into exotoxins, so that the inflammatory stimulus of localized bacteria is felt systemically. OMVs from E. coli cause increased ICAM-1, E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and IL-6 expression in ECs in vitro and in vivo. These effects are dependent on TLR4 and NFκB and are more profound than stimulation by free LPS [28,29]. Further, E. coli can use OMVs as another mechanism to secrete toxins. For instance, enterohemorrhagic E. coli (EHEC) produces EHEC-hemolysin (EHEC-Hly), a member of the repeats-in toxin family, which it can secrete free or OMV-associated. Free EHEC-Hly lyses ECs. OMV-associated EHEC-Hly, however, is endocytosed by ECs, escapes into the cytoplasm, and form pores in mitochondria, which ultimately leads to apoptosis [30].
Gram-positive bacteria can also release membrane vesicles (MVs) which can contribute to pathogenesis. S. pneumoniae, for example, releases MVs which are high in lipoproteins and contain pneumolysin [31]. The impact of OMVs and MVs on the endothelium is an interesting area for future research.
Effects on the vasculature: beyond endothelial cells
Bacterial toxins can also manipulate components of the vasculature other than ECs. Bacillus anthracis produces lethal toxin (LT), a metalloproteinase, and edema toxin (ET), an adenylate cyclase. LT and ET can both induce vascular shock in mice, which was thought to be mediated by their effects on the endothelium [32,33]. However, analysis of mice with various conditional deletions of capillary morphogenesis protein-2 (CMG2), a critical anthrax toxin receptor [34,35], clearly showed that LT kills by targeting cardiomyocytes and vascular smooth muscle, while ET kills by targeting hepatocytes, but endothelial targeting is not required for either process [36].
Vascular leakage can also be induced indirectly. S. aureus phenol-soluble modulin α4 (PSMα4) has recently been shown to stimulate heparin-binding protein release from neutrophils, which subsequently increases endothelial permeability [37]. Remarkably, an isogenic S. aureus mutant for PSMα4 caused less vascular leakage in mouse lung tissue than WT in an intravenous model of infection, even though this mutant still had α-toxin, which directly kills murine ECs.
These two examples illustrate the many ways that bacterial toxins can manipulate the vasculature without directly targeting ECs. Moreover, they demonstrate that the ability of toxins to mediate phenotypes in vitro does not guarantee that these phenotypes are important for pathogenesis of a given type of infection in vivo.
Conclusion
Pathogenic bacteria are clearly very adept at damaging and manipulating the endothelium in order to disseminate and cause severe disease. In response, we must be adept at developing ways to protect the endothelium from these attacks. While bacteria and toxin specific therapies are important, we should not lose sight of more generalized methods for protecting the vasculature. For instance, recently it has become increasingly clear that statins and angiotensin receptor blockers exert protective effects on the vasculature during infection [38–40]. Though several studies have proposed mechanisms of how these drugs protect ECs by strengthening them in a general manner [41–43], they may in some cases also act by protecting ECs from the actions of toxins, as has been shown for Ply in a mouse model of sickle cell disease [44]. Additional research into the effects of these and other drugs on the endothelium, bacteria, and bacterial toxins could be important as they could uncover novel strategies to protect the endothelium from infectious insults.
Highlights.
Endothelial targeting is an important part of the pathogenesis of many bacteria.
Many gram-positive bacteria such as S. pneumoniae, S. aureus, and B. cereus kill endothelial cells by releasing pore-forming toxins.
Gram-negative bacteria such as P. auruginosa compromise the barrier function of the endothelium by secreting toxins that disrupt the cytoskeleton and cell-cell interactions. There are examples of cytolytic toxins that gram-negative bacteria secrete as well.
Bacterial toxins can also influence the immune response by modulating the endothelial recruitment of immune cells.
Membrane vesicles are another example of a secreted effector that bacteria can use to target endothelial and other host cells.
Acknowledgments
We apologize to authors whose relevant work was not included in this review owing to space constraints. This work was supported in part by the US National Institutes of Health under award numbers GM007308, AI007180 and AI124606 to AL, and AI105129, AI099394, and AI121244 to VJT. VJT is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Rubins JB, Duane PG, Charboneau D, Janoff EN. Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect Immun. 1992;60:1740–1746. doi: 10.1128/iai.60.5.1740-1746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zysk G, Schneider-Wald BK, Hwang JH, Bejo L, Kim KS, Mitchell TJ, Hakenbeck R, Heinz HP. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect Immun. 2001;69:845–852. doi: 10.1128/IAI.69.2.845-852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou A, Wang H, Lan K, Zhang X, Xu W, Yin Y, Li D, Yuan J, He Y. Apoptosis induced by pneumolysin in human endothelial cells involves mitogen-activated protein kinase phosphorylation. Int J Mol Med. 2012;29:1025–1030. doi: 10.3892/ijmm.2012.946. [DOI] [PubMed] [Google Scholar]
- 4.Rubins JB, Mitchell TJ, Andrew PW, Niewoehner DE. Pneumolysin activates phospholipase A in pulmonary artery endothelial cells. Infect Immun. 1994;62:3829–3836. doi: 10.1128/iai.62.9.3829-3836.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas R, Yang G, Gorshkov BA, Zemskov EA, Sridhar S, Umapathy NS, Jezierska-Drutel A, Alieva IB, Leustik M, Hossain H, et al. Protein kinase C-alpha and arginase I mediate pneumolysin-induced pulmonary endothelial hyperpermeability. Am J Respir Cell Mol Biol. 2012;47:445–453. doi: 10.1165/rcmb.2011-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A. 2010;107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis. 2012;206:352–356. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Popov LM, Marceau CD, Starkl PM, Lumb JH, Shah J, Guerrera D, Cooper RL, Merakou C, Bouley DM, Meng W, et al. The adherens junctions control susceptibility to Staphylococcus aureus alpha-toxin. Proc Natl Acad Sci U S A. 2015;112:14337–14342. doi: 10.1073/pnas.1510265112. This study found that PLEKHA7, a component of adherens junctions, facilitates susceptibility to S. aureus α-toxin. Interestingly, α-toxin was still able to form pores in PLEKHA7 deficient cells, but these cells were able to recover. Importantly, PLEKHA7 deficient mice were also able to recover better from S. aureus infection.
- 10. Jessberger N, Dietrich R, Bock S, Didier A, Martlbauer E. Bacillus cereus enterotoxins act as major virulence factors and exhibit distinct cytotoxicity to different human cell lines. Toxicon. 2014;77:49–57. doi: 10.1016/j.toxicon.2013.10.028. The authors systematically study the cytotoxicity of B. cerueus toxins to multiple cell lines, and demonstrate differential targeting. This strongly argues that these toxins have receptors, and opens up the field to the search for these receptors.
- 11.Phillips RM, Six DA, Dennis EA, Ghosh P. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J Biol Chem. 2003;278:41326–41332. doi: 10.1074/jbc.M302472200. [DOI] [PubMed] [Google Scholar]
- 12.Saliba AM, de Assis MC, Nishi R, Raymond B, Marques Ede A, Lopes UG, Touqui L, Plotkowski MC. Implications of oxidative stress in the cytotoxicity of Pseudomonas aeruginosa ExoU. Microbes Infect. 2006;8:450–459. doi: 10.1016/j.micinf.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Freitas C, Assis MC, Saliba AM, Morandi VM, Figueiredo CC, Pereira M, Plotkowski MC. The infection of microvascular endothelial cells with ExoU-producing Pseudomonas aeruginosa triggers the release of von Willebrand factor and platelet adhesion. Mem Inst Oswaldo Cruz. 2012;107:728–734. doi: 10.1590/s0074-02762012000600004. [DOI] [PubMed] [Google Scholar]
- 14.Ganter MT, Roux J, Su G, Lynch SV, Deutschman CS, Weiss YG, Christiaans SC, Myazawa B, Kipnis E, Wiener-Kronish JP, et al. Role of small GTPases and alphavbeta5 integrin in Pseudomonas aeruginosa-induced increase in lung endothelial permeability. Am J Respir Cell Mol Biol. 2009;40:108–118. doi: 10.1165/rcmb.2007-0454OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber P, Bouillot S, Elsen S, Attree I. Sequential inactivation of Rho GTPases and Lim kinase by Pseudomonas aeruginosa toxins ExoS and ExoT leads to endothelial monolayer breakdown. Cell Mol Life Sci. 2014;71:1927–1941. doi: 10.1007/s00018-013-1451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res. 2004;95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- 17.Stevens TC, Ochoa CD, Morrow KA, Robson MJ, Prasain N, Zhou C, Alvarez DF, Frank DW, Balczon R, Stevens T. The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L915–L924. doi: 10.1152/ajplung.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golovkine G, Faudry E, Bouillot S, Voulhoux R, Attree I, Huber P. VE-cadherin cleavage by LasB protease from Pseudomonas aeruginosa facilitates type III secretion system toxicity in endothelial cells. PLoS Pathog. 2014;10:e1003939. doi: 10.1371/journal.ppat.1003939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elsen S, Huber P, Bouillot S, Coute Y, Fournier P, Dubois Y, Timsit JF, Maurin M, Attree I. A type III secretion negative clinical strain of Pseudomonas aeruginosa employs a two-partner secreted exolysin to induce hemorrhagic pneumonia. Cell Host Microbe. 2014;15:164–176. doi: 10.1016/j.chom.2014.01.003. This study characterizes a new strain of P. aeruginosa and identifies exolysin, a new, cytolytic toxin produced by this strain. This work represents a new direction for the study of P. aeruginosa virulence, which previously was thought to be exclusively mediated through the T3SS and T2SS secreted toxins.
- 20.Reboud E, Elsen S, Bouillot S, Golovkine G, Basso P, Jeannot K, Attree I, Huber P. Phenotype and toxicity of the recently discovered exlA-positive Pseudomonas aeruginosa strains collected worldwide. Environ Microbiol. 2016 doi: 10.1111/1462-2920.13262. [DOI] [PubMed] [Google Scholar]
- 21.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells--conditional innate immune cells. J Hematol Oncol. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MC, Rossjohn J, Talbot UM, Paton JC. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature. 2006;443:548–552. doi: 10.1038/nature05124. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Rogers TJ, Paton JC, Paton AW. Differential effects of Escherichia coli subtilase cytotoxin and Shiga toxin 2 on chemokine and proinflammatory cytokine expression in human macrophage, colonic epithelial, and brain microvascular endothelial cell lines. Infect Immun. 2014;82:3567–3579. doi: 10.1128/IAI.02120-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saliba AM, Nascimento DO, Silva MC, Assis MC, Gayer CR, Raymond B, Coelho MG, Marques EA, Touqui L, Albano RM, et al. Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell Microbiol. 2005;7:1811–1822. doi: 10.1111/j.1462-5822.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 25.Lins RX, de Assis MC, Mallet de Lima CD, Freitas C, Maciel Plotkowski MC, Saliba AM. ExoU modulates soluble and membrane-bound ICAM-1 in Pseudomonas aeruginosa-infected endothelial cells. Microbes Infect. 2010;12:154–161. doi: 10.1016/j.micinf.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 26. Maurer K, Reyes-Robles T, Alonzo F, 3rd, Durbin J, Torres VJ, Cadwell K. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host Microbe. 2015;17:429–440. doi: 10.1016/j.chom.2015.03.001. Using an autophagy deficient mouse model the authors discovered that autophagy protects ECs from S. aureus α-toxin by decreasing the amount of its receptor, ADAM-10, on the surface of ECs. This study is an excellent example of how much can be learned from studying host-pathogen interactions in vivo.
- 27.Cutting AS, Del Rosario Y, Mu R, Rodriguez A, Till A, Subramani S, Gottlieb RA, Doran KS. The role of autophagy during group B Streptococcus infection of blood-brain barrier endothelium. J Biol Chem. 2014;289:35711–35723. doi: 10.1074/jbc.M114.588657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Yoon YJ, Lee J, Choi EJ, Yi N, Park KS, Park J, Lotvall J, Kim YK, Gho YS. Outer membrane vesicles derived from Escherichia coli up-regulate expression of endothelial cell adhesion molecules in vitro and in vivo. PLoS One. 2013;8:e59276. doi: 10.1371/journal.pone.0059276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soult MC, Lonergan NE, Shah B, Kim WK, Britt LD, Sullivan CJ. Outer membrane vesicles from pathogenic bacteria initiate an inflammatory response in human endothelial cells. J Surg Res. 2013;184:458–466. doi: 10.1016/j.jss.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 30. Bielaszewska M, Ruter C, Kunsmann L, Greune L, Bauwens A, Zhang W, Kuczius T, Kim KS, Mellmann A, Schmidt MA, et al. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 2013;9:e1003797. doi: 10.1371/journal.ppat.1003797. This study found that OMVs from E. coli contain hemolysin, and that cytotoxicity from OMV-asscociated hemolysin is mediated through apoptosis, instead of through lysis, as it is through free hemolysin. This demonstrates the importance of studying toxins in the context in which they encounter host cells.
- 31.Olaya-Abril A, Prados-Rosales R, McConnell MJ, Martin-Pena R, Gonzalez-Reyes JA, Jimenez-Munguia I, Gomez-Gascon L, Fernandez J, Luque-Garcia JL, Garcia-Lidon C, et al. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J Proteomics. 2014;106:46–60. doi: 10.1016/j.jprot.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Warfel JM, Steele AD, D'Agnillo F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am J Pathol. 2005;166:1871–1881. doi: 10.1016/S0002-9440(10)62496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maddugoda MP, Stefani C, Gonzalez-Rodriguez D, Saarikangas J, Torrino S, Janel S, Munro P, Doye A, Prodon F, Aurrand-Lions M, et al. cAMP signaling by anthrax edema toxin induces transendothelial cell tunnels, which are resealed by MIM via Arp2/3-driven actin polymerization. Cell Host Microbe. 2011;10:464–474. doi: 10.1016/j.chom.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Crown D, Miller-Randolph S, Moayeri M, Wang H, Hu H, Morley T, Leppla SH. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc Natl Acad Sci U S A. 2009;106:12424–12429. doi: 10.1073/pnas.0905409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu S, Zhang Y, Moayeri M, Liu J, Crown D, Fattah RJ, Wein AN, Yu ZX, Finkel T, Leppla SH. Key tissue targets responsible for anthrax-toxin-induced lethality. Nature. 2013;501:63–68. doi: 10.1038/nature12510. Through the generation and analysis of many conditional knockout and knockin mouse strains the authors were able to demonstrate that B. anthracis LT amd ET cause lethality to mice through mechanisms that do not involve the targeting of endothelial cells. LT is lethal by targeting cardiomyocytes and vascular smooth muscle cells, while ET is lethal by targeting hepatocytes.
- 37. Li L, Pian Y, Chen S, Hao H, Zheng Y, Zhu L, Xu B, Liu K, Li M, Jiang H, et al. Phenol-soluble modulin alpha4 mediates Staphylococcus aureus-associated vascular leakage by stimulating heparin-binding protein release from neutrophils. Sci Rep. 2016;6:29373. doi: 10.1038/srep29373. This study demonstrates that S. aureus PSMα4 causes neutrophils to release heparin binding protein, which results in greater endothelial permeability. Importantly, this study finds that PSMα4 is functional in the presence of serum lipoproteins, as opposed to the other PSMα's, which clarifies how it can have a role in vivo.
- 38.Mortensen EM, Nakashima B, Cornell J, Copeland LA, Pugh MJ, Anzueto A, Good C, Restrepo MI, Downs JR, Frei CR, et al. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis. 2012;55:1466–1473. doi: 10.1093/cid/cis733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandermeer ML, Thomas AR, Kamimoto L, Reingold A, Gershman K, Meek J, Farley MM, Ryan P, Lynfield R, Baumbach J, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205:13–19. doi: 10.1093/infdis/jir695. [DOI] [PubMed] [Google Scholar]
- 40.Patel JM, Snaith C, Thickett DR, Linhartova L, Melody T, Hawkey P, Barnett AH, Jones A, Hong T, Cooke MW, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial) Crit Care. 2012;16:R231. doi: 10.1186/cc11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol. 2008;295:L575–L583. doi: 10.1152/ajplung.00428.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao H, Qin X, Ping D, Zuo K. Inhibition of Rho and Rac geranylgeranylation by atorvastatin is critical for preservation of endothelial junction integrity. PLoS One. 2013;8:e59233. doi: 10.1371/journal.pone.0059233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodor C, Nagy JP, Vegh B, Nemeth A, Jenei A, MirzaHosseini S, Sebe A, Rosivall L. Angiotensin II increases the permeability and PV-1 expression of endothelial cells. Am J Physiol Cell Physiol. 2012;302:C267–C276. doi: 10.1152/ajpcell.00138.2011. [DOI] [PubMed] [Google Scholar]
- 44.Rosch JW, Boyd AR, Hinojosa E, Pestina T, Hu Y, Persons DA, Orihuela CJ, Tuomanen EI. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest. 2010;120:627–635. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]