Abstract
Background
Avoiding donor specific antibody (DSA) is difficult for sensitized patients. Improved understanding of the risk of low level DSA is needed.
Methods
We retrospectively compared the outcomes of 954 patients transplanted with varied levels of baseline DSA detected by single antigen beads and B flow cytometric crossmatch (XM). Patients were grouped as follows: -DSA/-XM, +DSA/-XM, +DSA/low+XM, +DSA/high+XM, and −DSA/+XM and followed for a mean of 4.1±1.9 years (similar among groups, p=0.49).
Results
Death-censored allograft survival was similar in all groups except the +DSA/high+XM group, which was lower at 79.1% versus 96.2% in the −DSA/-XM group (p<0.01). The incidence of chronic antibody mediated rejection (CAMR) based on surveillance biopsy was higher with increasing DSA (8.2% -DSA/-XM, 17.0% +DSA/-XM, 30.6%+DSA/low + XM, and 51.2% +DSA/high+XM, p<0.01), but similar in groups without baseline DSA (8.1% -DSA/-XM vs. 15.4% -DSA/+XM, p=0.19). Having a calculated panel reactive antibody (cPRA) ≥80% was independently associated with CAMR (HR 5.2, p=0.03) even when DSA was undetected at baseline. By 2 years posttransplant, the incidence of CAMR was 19.4% in patients with cPRA ≥80% and undetected DSA and negative XM at baseline.
Conclusion
Kidney transplantation with low level DSA with or without a low positive XM is a reasonable option for highly sensitized patients and may be advantageous compared to waiting for a negative XM deceased donor. The risk for CAMR is low in patients with no DSA even if the XM is positive. Patients with cPRA≥80% are at risk for CAMR even if no DSA is detected.
INTRODUCTION
Performing a kidney transplant in a recipient with donor-specific alloantibody (DSA) is generally associated with an increased risk of antibody mediated rejection (AMR) and subsequent allograft loss 1-7, but the risk associated with DSA is variable 8-10. The histologic outcomes following transplantation with low level DSA that is only detected with sensitive testing remain unclear.
Prolonged waiting for a kidney just to avoid low level DSA may not always be in the best interest of the patient. Factors other than the presence of alloantibody impact allograft and patient survival such as preemptive kidney transplantation11,12, living versus deceased donor 13, delayed graft function14,15, and recurrent renal disease16,17 among others. Prolonged waiting on dialysis also carries risk 18,19. In fact, patients who receive transplants from HLA-incompatible donors have improved survival than matched controls who remain on the waiting list 20,21. Improved understanding of the risk of low level DSA is needed to improve donor selection and increase access to transplantation for sensitized patients.
The aim of this study was to examine the outcomes of patients with low level DSA and/or low positive XM in the current era of antibody characterization using single antigen beads (SAB) and B-flow cytometric crossmatch (XM). The outcomes assessed were allograft and patient survival, the incidence of early acute clinical AMR, and the prevalence of chronic AMR based on surveillance biopsies at 1,2 and 5 years posttransplant. We also assessed the factors associated with chronic AMR.
MATERIALS AND METHODS
We performed a retrospective cohort study of all adult solitary kidney transplant recipients transplanted since our center has routinely performed sensitive DSA testing with both SAB and B-flow cytometric crossmatch (XM) [October 2007 through May 2014]. We excluded all pediatric, ABO incompatible and dual organ transplant recipients. In patients who received more than 1 transplant during the study time period, the second transplant was excluded from analysis (n=5), and we excluded patients if baseline SAB results were not available (n=9). Data was collected by chart review.
We compared patient and allograft survival, cause of allograft loss, early acute clinical AMR, surveillance allograft histology, and predictors of chronic AMR among the following 4 groups with increasing DSA: no DSA with >1000 mean fluorescence intensity (MFI) and negative XM [-DSA/-XM (n= 795)]; DSA with >1000 MFI and negative XM [+DSA/-XM (n=53)]; DSA any MFI and positive XM up to mean channel shift of 199 [+DSA/low + XM (n=36)]; and DSA any MFI and positive XM mean channel shift of >200 [+DSA/high + XM (n=43)]. We also studied patients with positive XM without detectable DSA (any MFI) at baseline [-DSA/+XM (n=26)].
Donor Specific Antibody Assessment
A solid phase assay (LAB screen, One Lambda, Canoga Park, CA, USA) was used to identify baseline alloantibody specificities. An MFI of 1000 was considered positive in patients with negative XM to avoid false positivity from laboratory variability or background. The cPRA was calculated based on MFI >2000 because of center practice.
Flow Cytometric Crossmatch Testing
B flow cytometric crossmatch testing was used and reported because B cells express both class I and II HLA antigens, and thus this test can detect both anti class I or II alloantibodies. Specifically, a 3 color flow cytometric crossmatch was performed in this study. Fluorescein isothiocyanate (FITC)-conjugated F(ab)’2 goat antihuman IgG was used to assess alloantibody binding by way of indirect immunofluorescence and 2 other fluorescence parameters (CD3 PerCP, and CD19PE) for identifying T and B cells. Donor cells were treated with pronase (1ug/2ml) for 15 minutes. Next donor cell aliquots (300,000) were mixed with 20 μL of controls or patient serum and incubated for 30 minutes. After washing, fluorescent antibodies were added and incubated for an additional 15 minutes and washed and aliquots acquired on FACS Calibur (BD Biosciences) using a 1024 scale. The interpretation of the flow cytometric crossmatch was performed by directly comparing the fluorescence intensity of donor B lymphocytes after being treated with patient serum to the fluorescent intensity of donor cells after treatment with a negative control serum. The interpretation was based on the right or bright “shift” of the mean channel fluorescence noted from the negative control and donor sera. A mean channel fluorescence shift greater than 106 for was considered positive for B cells.
Auto-crossmatch testing was performed in some cases when the XM was higher than expected based on DSA results. This test was done by performing the routine T and B-flow cytometric crossmatch using recipient lymphocytes and serum. The most immediate pretransplant or predesensitization assessment was considered baseline.
Biopsy Assessment
Surveillance biopsies are standard of care for all patients transplanted at our center regardless of baseline DSA. Allograft histology from surveillance biopsies taken at 1, 2, and 5 years posttransplant was compared. To determine the overall incidence of acute cellular rejection and chronic AMR, both surveillance and indication biopsies were examined. Kidney biopsy tissue was processed for light microscopy and immunofluorescence for C4d (AbD Serotec).
Light microscopy features of biopsies were scored by Banff criteria 22-24 on all biopsies. Early acute clinical AMR was defined as an increase in serum creatinine >0.3mg/dl; increased DSA; and histologic findings of acute, active AMR. Acute, active AMR (Banff 2013) was specifically 1) ptc + g score ≥ 2 or 2) ptc >0 or g> 0 and C4d >1. The presence of cg score >0 signified chronic AMR. Electron microscopy was not routinely done and not used as criteria for chronic AMR. Acute cellular rejection was based on histological criteria only and included borderline acute cellular rejection. Transplant arteriopathy was not compared between groups because of the potential for sampling error and unclear distinguishing histopathologic features between arteriosclerosis from hypertension and transplant arteriopathy 25-27.
Immunosuppression and treatment protocols
Patients received ATG (Thymoglobulin Sangstadt, Menlo Park Ca, 1.5mg/kg/d for 4 doses; anti-CD25 receptor antibodies (Simulect, Novartis Pharmaceuticals, East Hanover, NJ); or alemtuzumab (Campath, Genzyme, Cambridge, MA) as induction per center protocol. ATG was predominantly used for induction, especially in patients with known DSA. Anti-CD25 receptor antibodies were predominantly used in patients greater than age 65 with negative XM. The standard maintenance immunosuppression consisted of prednisone, tacrolimus, and mycophenolate mofetil in patients who received induction with ATG or anti-CD25 antibodies. Tacrolimus and mycophenolate mofetil were used alone in patients induced with alemtuzumab.
Some patients were included in desensitization studies during this studied time period. Eculizumab was given for desensitization from June 2008 through October 2011 for patients with XM mean channel shift of >/= 200. The specific details of that desensitization protocol has been described previously28. Patients treated with eculizumab after October 2011 were enrolled in another clinical trial in which the criteria for enrollment was to have a XM positive at any level. Another desensitization trial with bortezomib was started in 2008. This was given to a subset of patients with cPRA>90% and a XM >300 against their original intended donor29.
Treatment for early acute clinical AMR included plasmapheresis, intravenous immunoglobulin, and occasional eculizumab. No patients received rituximab or splenectomy for acute clinical AMR. Because no therapy is proven effective for chronic AMR, we did not routinely provide therapy for patients with isolated AMR beyond 30 days posttransplant.
Laboratory monitoring
At yearly intervals, patients had assessment of their renal function that included iothalamate clearance and 24-hour urine protein testing.
Statistical Analysis
Statistical analysis was performed on JMPv10. (SAS, Cary, NC). For numerical data, groups were compared with the t-test or the Wilcoxon rank sum test as indicated. The reference group for statistical comparisons was the −DSA/-XM group. The Cochran Armitage Test for Trend was used to compare ordinal data when applicable. The Fisher’s exact test was used to compare counts and percentages. Cumulative incidences were described by Kaplan–Meier estimates. Time-to-event data were compared with the log-rank test. Both univariate and multivariate Cox Regression models were performed to examine the risk factors for chronic antibody mediated rejection. Factors that had a p-value <0.05 were included in the multivariate model. Hazard ratios (HRs) were described by their point estimate and corresponding 95% confidence intervals. Testing was two-sided at the 0.05 level.
RESULTS
Patient demographics
Patient demographics are shown in Table 1. Compared to −DSA/-XM recipients, patients with +DSA/ high +XM and −DSA/ + XM were younger (p<0.01) and more likely to be female (p<0.01). All patients were predominantly Caucasian and recipients of living donor grafts. They were also similar in respect to the etiology of their renal disease, HLA matching, and follow-up.
Table 1.
Patient Demographics
| -DSA-XM | +DSA-XM | +DSA Low + XM | +DSA High + XM | -DSA + XM | p-value | |
|---|---|---|---|---|---|---|
|
| ||||||
| N=795 | N=53 | N=36 | N=43 | N=26 | ||
|
| ||||||
| Age (Mean age±std) | 52.7±13.9 | 50.4±15.1 | 50.6±12.1 | 47.0±13.4 | 45.6±13.9 | p<0.01 |
|
| ||||||
| Female Gender n(%) | 297(37.4) | 25(47.2) | 25(69.4) | 30(69.7) | 12(57.7) | p<0.01 |
|
| ||||||
| Race n(%) | p=0.58 | |||||
| White | 718(90.3) | 52(98.1) | 34(94.4) | 40(93.0) | 22(84.6) | |
| Hispanic | 18(2.3) | 0(0) | 1(2.9) | 2(4.7) | 2(7.7) | |
| African American | 33 (4.2) | 0(0) | 1(2.9) | 0(0) | 1(3.9) | |
| Asian | 12(1.5) | 1(1.9) | 0(0) | 1(2.3) | 1(3.9) | |
| American Indian/Pacific Islander | 14(1.8) | 0(0) | 0(0) | 0(0) | 0(0) | |
|
| ||||||
| Donor type n(%) | ||||||
| LURD | 351(44.2) | 35(66.0) | 17(47.2) | 29(67.4) | 10(38.5) | |
| LRD | 305(38.3) | 14(26.4) | 11(30.6) | 12(27.9) | 11(42.3) | p<0.01 |
| Deceased Donor | 139(17.5) | 4(7.6) | 8(22.2) | 2(4.7) | 5(19.2) | |
|
| ||||||
| Etiology of ESRD n(%) | ||||||
| Glomerulonephritis | 282(35.5) | 21(39.6) | 20(55.6) | 19(44.2) | 10(38.5) | |
| Diabetes | 148(18.6) | 6(11.3) | 2(5.6) | 1(2.3) | 4(15.4) | |
| Hypertension | 48(6.0) | 1(1.9) | 0(0) | 2(4.7) | 0(0) | p=0.13 |
| Cystic kidney disease | 131(16.9) | 10(18.9) | 6(16.7) | 6(14.0) | 5(19.2) | |
| Other | 137(17.2) | 12(22.6) | 4(11.4) | 12(27.9) | 6(23.1) | |
| Unknown | 49(6.2) | 3(576) | 4(11.4) | 3(7.0) | 1(3.9) | |
|
| ||||||
| HLA mismatch (mean +/-std) | 3.4±1.9 | 4.2±1.4 | 3.9±1.5 | 3.7±1.3 | 3.0±1.7 | p=0.09 |
|
| ||||||
| Induction n(%) | ||||||
| Anti-Thymocyte Globulin | 402(50.6) | 42(77.4) | 34(94.4) | 42(97.7) | 20(76.9) | |
| Basiliximab | 241(30.3) | 5(9.4) | 1(2.98) | 1(2.3) | 3(11.5) | p<0.01 |
| Alemtuzumab | 151(19.0) | 7(13.2) | 1(2.8) | 0(0) | 3(11.5) | |
| None | 1(0.1) | 0(0) | 0(0) | 0(0) | 0(0.0) | |
|
| ||||||
| Prior solid organ transplant n(%) | 133(16.7) | 11(20.8) | 18(50.0) | 23(53.5) | 6(23.1) | p<0.01 |
|
| ||||||
| Dialysis prior to transplantation n(%) | ||||||
| Yes | 424(53.3) | 26(49.1) | 24(66.7) | 34(79.1) | 12(46.2) | P=0.04 |
| No | 288(36.2) | 21(39.6) | 11(30.6) | 7(16.3) | 12(46.2) | |
| Unknown | 83(10.4) | 6(11.3) | 1(2.8) | 2(4.6) | 2(7.8) | |
|
| ||||||
| Time on Dialysis* (mean +/-std) | 2.2±0.1 | 1.8±0.5 | 2.5±0.5 | 3.5±0.4 | 2.9±0.7 | P=0.03 |
|
| ||||||
| Percent cPRA** (mean +/-std) | 12.3±26.9 | 42.2±36.3 | 54.5±38.2 | 79.8±26.3 | 16.2±29.2 | p<0.01 |
|
| ||||||
| cPRA ≥80% n(%)** | 51(6.6) | 12(23.1) | 15(41.6) | 28(68.3) | 2(8.0) | p<0.01 |
|
| ||||||
| B flow XM (mean channel shift ±std) | NA | NA | 147.9±37.1 | 283.3±67.7 | 163.1± 56.2 | p<0.01 |
|
| ||||||
| Auto-B flow XM positivity n/N (%) | NA | NA | 1/7 (14.3) | 1/5 (20.0) | 2/8(25.0) | P=0.87 |
|
| ||||||
| Desensitization n(%) | ||||||
| Plasmapheresis/IVIG(alone) | 0(0%) | 0(0%) | 0(0%) | 3(7.0%) | 0(0%) | |
| Eculizumab† | 0 (0%) | 0(0%) | 2(5.6%) | 35(81.0%) | 0(0%) | p<0.01 |
| Bortezomib | 2(0.25%) | 1(1.9%) | 0(0%) | 7(14.3%) | 0(0%) | |
|
| ||||||
| Follow-up(Mean years±std) | 4.1±1.9 | 4.3±1.7 | 3.8±1.8 | 4.1±2.1 | 3.7±1.9 | p=0.49 |
Patients who received a preemptive transplant were excluded.
Calculated based on MFI 2000.
All patients who received eculizumab also received plasmapheresis and IVIG.
Induction and/or maintenance immunosuppression therapy was different among the groups (p<0.01). More patients in the −DSA/-XM group received basiliximab for induction (with tacrolimus, mycophenolate mofetil, and prednisone for maintenance) or alemtuzumab for induction (with tacrolimus and mycophenolate mofetil for maintenance) as compared to the other groups. Patients in the +DSA/high+XM group were more likely to have had a prior solid organ transplant [53.5% (23/43), p<0.01] and dialysis prior to transplant [79.1% (34/43), p=0.04].
The proportion of highly sensitized patients (cPRA ≥80) was progressively higher in the groups with increasingly DSA (p<0.01). The mean XM channel shift was 147.9±37.1 in the +DSA/low +XM group; 283 ± 67.7 in the + DSA/high +XM group, and 163.1±56.2 in the −DSA/+XM group. Few patients had an auto-B flow XM performed: 19.4% (7/36) in the +DSA/low + XM group,11.6% (5/43) in the DSA+/high +XM group, and 30.8% (8/26) in the −DSA/+XM group. Of the auto-B flow XM tests that were performed, few were positive: [14.3%,(1/7) in the +DSA/low+XM group; 20.0%, (1/5) in the +DSA/high+XM group; and 25.0% (2/8) in the −DSA/+XM group, p=0.87].
Most patients in the +DSA/high + XM group received desensitization, but it was uncommon in the other groups including the +DSA/low + XM group (Table 1). Specifically, in the +DSA/high + XM group: 7.0% (3/43) received plasmapheresis and intravenous immunoglobulin (IVIG) alone, 81.0% (35/43) received eculizumab, plasmapheresis, and IVIG as part of clinical trial, and 14.3% (7/43) received bortezomib pretransplant. In the +DSA/low+XM group, 5.6% (2/36) received eculizumab, plasmapheresis, and IVIG as part of clinical trial for desensitization. No other patients in the +DSA/low+XM group received desensitization therapy prior to or at the time of transplant (Table 1).
DSA characteristics
The proportion of patients with anti-class I DSA alone was similar among the groups with DSA detected at baseline (Figure 1). More patients in the +DSA/high+XM group had both anti class I and II DSA at baseline [27.9% (12/43) versus 3.7% (2/53) in the +DSA/-XM group and 11.1% (4/36) in the +DSA/low+XM group, p<0.01]. The mean sum MFI of anti-Class I, anti-Class II, and anti-Class I+II was also highest in the +DSA/high +XM group, p<0.01.
Figure 1. Donor Specific Antibody Characteristics.
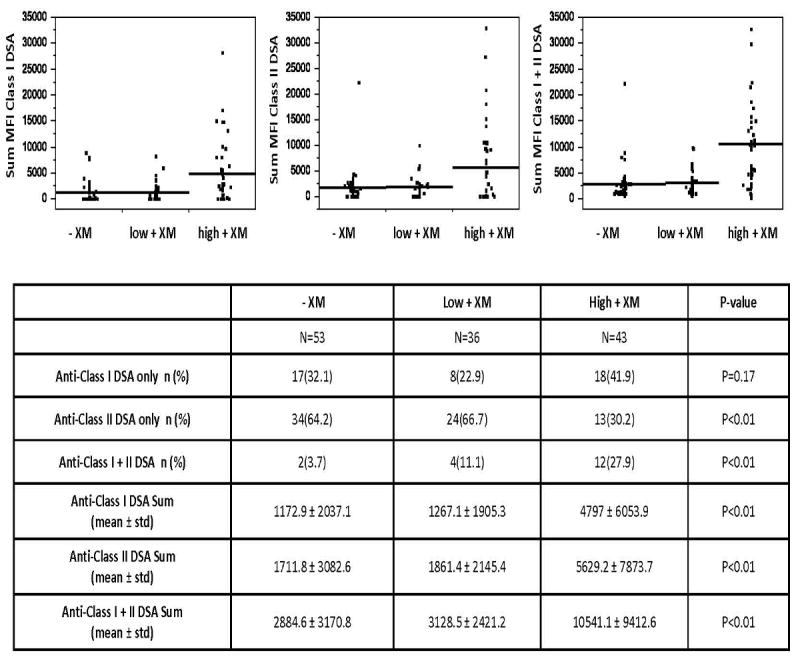
The characteristics of donor specific antibody present at baseline among the following transplant groups: +DSA/-XM (-XM), +DSA/low+XM (low+XM), and +DSA/high+XM (high+XM).
Patient and Allograft survival
Patient survival was similar in all groups over mean follow-up of 4.1±1.9 years (p=0.82) [actual patient survival: 93.4% -DSA/-XM; 96.3% +DSA/ -XM; 97.2% +DSA/low+ XM; 97.7% +DSA/high + XM and 92.3% -DSA/+XM] (Figure 2). Overall uncensored graft survival also was similar, p=0.06 [actual overall allograft survival: 90.2% -DSA/-XM; 83.0% +DSA/ -XM; 94.4% +DSA/low + XM; 76.7% +DSA/high +XM, and 88.5% -DSA/+XM] (Figure 2). However, death-censored allograft survival was lower in the +DSA/high+ BFXM group (79.1%; p<0.01), but similar in other groups [actual death censored allograft survival: 96.2% -DSA/-XM; 86.8% +DSA/-XM; 97.2% +DSA/low + XM; and 96.2% -DSA/+XM] (Figure 2).
Figure 2. Patient and Allograft Survival stratified by DSA and B-flow Crossmatch at Baseline.
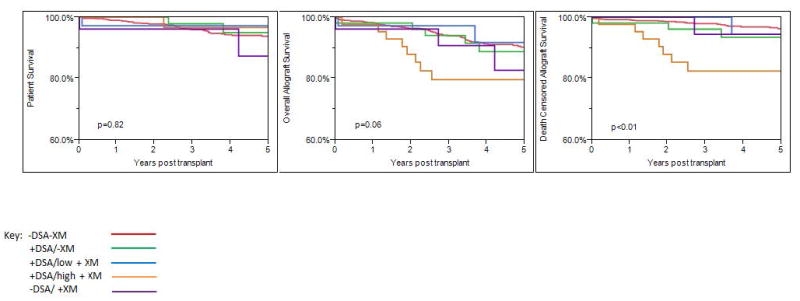
The mean follow-up was similar among groups and was 4.1±1.9 years, p=0.82. Patient and overall allograft survival were similar among groups, p=0.82 and p=0.06 respectively. Lower death-censored allograft survival was observed in the +DSA/high+XM group, p<0.01. Death-censored allograft survival was similar in other groups,
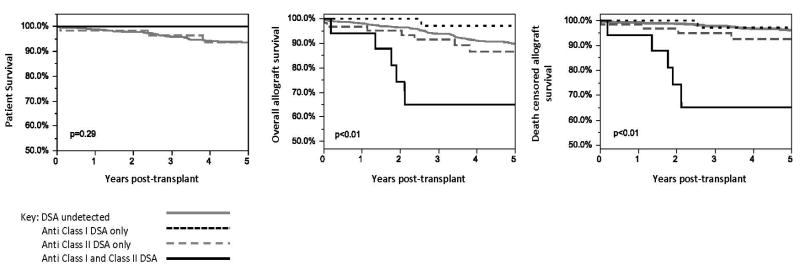
Patient survival was also similar when the patients were grouped by the class of DSA present at baseline, p=0.29, (Figure 3). Both overall and death-censored allograft survival were the lowest when both anti-class I and II were present at baseline, p<0.01. Also, patients with only anti-class II DSA at baseline had inferior death-censored allograft survival compared to patients with only anti-class I or undetected DSA at baseline (Figure 3). Death-censored allograft survival was 70.6% in patients with both anti Class I and II, 87.1% if only anti-Class II was present, 92.9% if only anti class I was present, and 96.2% if DSA was undetected, p<0.01. Death-censored allograft survival was similar among patients who received eculizumab and/or bortezomib in the +DSA/low+XM and +DSA/high+XM groups (See Supplemental Table 1).
Figure 3. Patient and Allograft Survival stratified by DSA Class at Baseline.
Patients with both class I + class II DSA at baseline had inferior overall and death-censored allograft (p<0.01).
Chronic AMR was identified prior to allograft loss in 75.0% (6/8) of the cases of allograft failure in the +DSA/high+XM group (Table 2). Chronic AMR was also identified prior to graft loss in 16.7% (5/30) of the cases of allograft failure with -DSA/-XM; 37% (3/8) of loses with +DSA/-XM, and 100.0% (1/1) of loses in the +DSA/-XM group. No patients in the +DSA/low+XM group had documented chronic AMR prior to allograft loss, and the 1 allograft failure in the +DSA/-XM group was in the setting of de novo DSA.
Table 2.
Causes of Allograft loss
| N=number of allograft failures | -DSA/-XM N=30 | + DSA/-XM N=8 | +DSA/ low+XM N=1 | +DSA/ high+XM N=8 | -DSA/ +XM N=1 | p-value |
|---|---|---|---|---|---|---|
| Chronic antibody mediated rejection n(%) | 5 (16.7) | 3(37.5) | 0(0) | 6(75.0) | 1/(100.0)* | P=0.22 |
| Documented nonadherence: acute rejection n(%) | 6(20.0) | 2(25.0) | 0(0) | 0(0) | 0(0) | P=0.24 |
| Recurrent/De novo Glomerular Disease n(%) | 6(20.0) | 1(12.5) | 1(100.0) | 1(12.5) | 0(0) | P=0.29 |
| Allograft thrombosis n(%) | 3(10.0) | 0(0) | 0(0) | 0(0) | 0(0) | P=0.27 |
| IFTA† n(%) | 3(10.0) | 0(0) | 0(0) | 0(0) | 0(0) | P=0.27 |
| Systemic Illness n(%) | 6/(20.0) | 2(25.0) | 0(0) | 1(12.5) | 0(0) | P=0.24 |
| Unknown n(%) | 1(3.3) | 0(0) | 0(0) | 0(0) | 0(0) | P=0.29 |
In setting of de novo DSA,
IFTA=interstitial fibrosis and tubular atrophy
Early acute clinical antibody mediated rejection
Early acute clinical AMR occurred in 16.3% (7/43) of patients in the +DSA/high +XM group, which was higher than the −DSA/-XM group [0.9 % (7/795), p<0.01] (Table 3). The incidence of early acute clinical AMR was 5.5% (2/36) in the +DSA/low+XM group, which was numerically higher than the −DSA/-XM group but this did not reach statistical significance, p=0.05. No patients in the +DSA/-XM group and 3.9% (1/26) of patients in the −DSA/+XM group had early acute clinical AMR, which was similar to the −DSA/-XM group (p=1.0 and p=0.22, respectively). No allografts were lost in the first year posttransplant from early acute clinical AMR.
Table 3.
Early Acute Clinical AMR based on Histologic Criteria
| -DSA/- XM n=795 | + DSA/ - XM n=53 | + DSA/ Low +XM n=36 | + DSA/ High +XM n=43 | -DSA/+XM n=26 | |
|---|---|---|---|---|---|
| Early acute clinical AMR n (%) | 7(0.9%) | 0(0%) | 2 (5.5%) | 7 (16.3%) | 1 (3.9%) |
| p-value | reference | p=1.0 | p=0.05 | p<0.01 | p=0.22 |
Antibody mediated rejection detected on surveillance biopsy
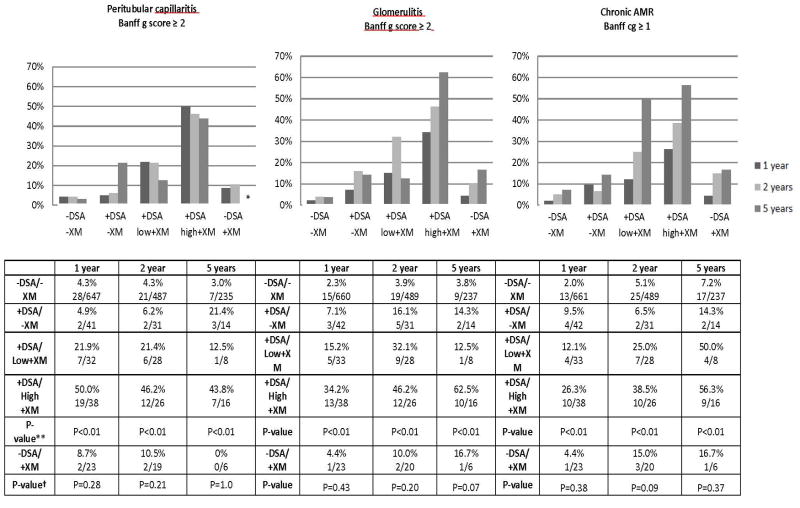
There was a clear pattern of increased microvascular inflammation and chronic AMR when a higher level of DSA was detected at baseline (Figure 4). This pattern was present at 1,2, and 5 years (p<0.01 at 1,2 and 5 years Cochran Test for Trend comparing −DSA/-XM, +DSA/+XM, +DSA/low+XM, and +DSA/high+XM groups). At 1 year, the prevalence of peritubular capillaritis was only 4.3% (28/647) in the −DSA/-XM group and 50.0% (19/38) in the +DSA/high+XM group. At 5 years, it was present in 3.0% (7/235) of -DSA/-XM group and 43.8% (7/16) in the +DSA/high+XM group. Chronic AMR was present in 2.0% (13/661) of the allografts in the -DSA/-XM group and 26.3% (10/38) of the allografts in the +DSA/high+XM group at 1 year. By 5 years, this increased to 7.2% (17/237) in the −DSA/-XM group and 56.3% (9/16) in the +DSA/high+XM group. In contrast, no difference in microvascular inflammation (peritubular capillaritis or glomerulitis) or chronic AMR was detected between the −DSA/-XM group and the – DSA/+XM group at 1, 2 or 5 years (p>0.05 at 1, 2 and 5 years for peritubular capillaritis, glomerulitis, and chronic AMR) (Figure 4).
Figure 4. Microvascular Inflammation and Chronic AMR detected on Surveillance Biopsy.
The prevalence of peritubular capillaritis, glomerulitis, and chronic AMR was higher in groups with higher DSA at all time points studied (p<0.01 *Cochran test for trend −DSA/-XM and +DSA groups). The prevalence of peritubular capillaritis, glomerulitis, and chronic AMR was similar in patients with undetected DSA and negative or positive XM. († -DSA/-XM and −DSA/+XM groups p=0.37,p=0.09, and p=0.38 respectively). ** No patients in the −DSA/+XM group had peritubular capillaritis at 5 years.
Acute cellular rejection
The rate of acute cellular rejection detected on surveillance biopsy was similar among all groups at 1 and 2 years (Table 4). At 5 years posttransplant, the rate of acute cellular rejection was similar among patients in the −DSA/-XM group and the +DSA/low+XM, +DSA/high+XM, and −DSA/+XM groups. The prevalence of acute cellular rejection on 5 year surveillance biopsies was higher in the +DSA/-XM group 21.4% (3/14) than the −DSA/-XM reference group at 2.1% (5/238), p< 0.01; but the overall number of patients in the +DSA/-XM group who reached the 5 year posttransplant biopsy time point was small.
Table 4.
Acute Cellular Rejection Detected on Surveillance Biopsy
| 1 year | p - value | 2 years | p-value | 5 years | p-value | |
|---|---|---|---|---|---|---|
| -DSA/-XM | 4.1% 27/661 | Ref | 5.4% 28/518 | Ref | 2.1% 5/238 | Ref |
| +DSA/-XM | 4.8% 2/42 | P=0.68 | 3.2% 1/31 | P=1.0 | 21.4% 3/14 | P<0.01 |
| +DSA/Low+XM | 6.1% 2/33 | P=0.64 | 0% 0/28 | P=0.39 | 0% 0/8 | P=1.0 |
| +DSA/High +XM | 2.6% 1/36 | P=1.0 | 7.6% 2/26 | P=0.68 | 0% 0/16 | P=1.0 |
| -DSA/+XM | 4.3% 1/23 | P=1.0 | 0% 0/20 | P=0.62 | 16.7% 1/6 | P=0.14 |
The overall incidence of acute cellular rejection (determined by examining all biopsies performed) during mean follow-up of 4.1±1.9 years was similar in all groups as compared to the −DSA/-XM group. The incidence was the following: 17.2% (137/795) -DSA/-XM reference, 15.1% (8/53) +DSA/-XM, p=0.85; 8.3% (3/36) +DSA/low + XM, p=0.25; 25.6% (11/43) +DSA/high+XM, p=0.16; and 23.1%(6/26) in the −DSA/+XM group, p=0.43.
Allograft function
Patients in the +DSA/high + XM group had a lower iothalamate GFR compared to the −DSA/-XM group at 1, 2, and 5 years as shown in (Figure 5) [46.9±16.7 vs. 58.7±17.7ml/min/BSA at 1 year, p<0.01; 47.7 ± 12.4 vs. 56.8±17.1 ml/min/BSA at 2 years, p<0.01; and 41.9±11.1 vs 54.7 ±17.2 ml/min/BSA at 5 years, p=0.02]. Proteinuria was also higher in the +DSA/high +XM group at 1 year compared to the −DSA/-BFXM group, [0.5±1.2mg/dl vs. 0.2±0.6, p<0.01], but similar at 2 and 5 years. Despite the differences between +DSA/high+XM and −DSA/-XM groups, the iothalamate GFR and proteinuria were similar among the other groups at 1,2 and 5 years posttransplant.
Figure 5. Allograft Function and Proteinuria.
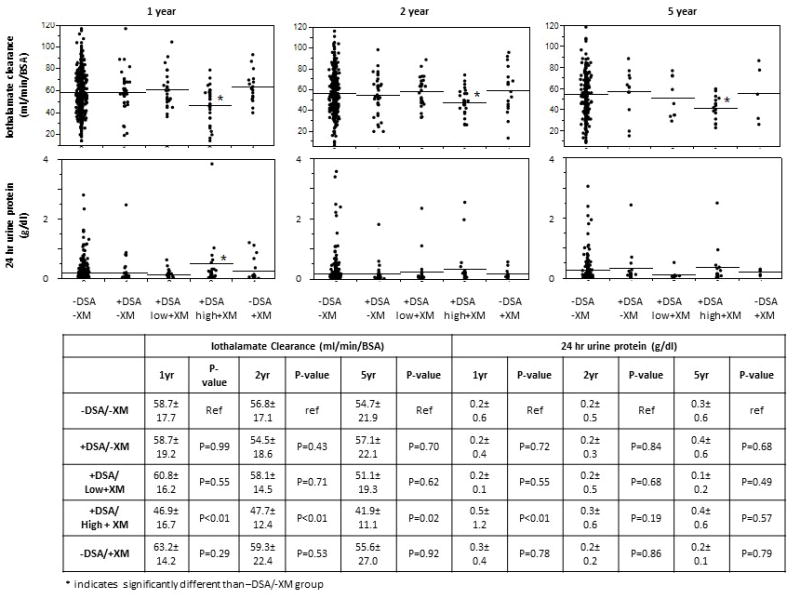
Patients in the +DSA/high+XM group had lower GFR at 1,2 and 5 years posttransplant than the −DSA/-XM group as measured by iothalamate clearance, p<0.01. GFR was between the −DSA/-XM and +DSA/-XM, +DSA/low+XM, and −DSA/+XM groups at 1,2 and 5 years. Proteinuria was also higher in the +DSA/high+XM group as compared to the −DSA/-XM group at 1 year posttransplant, p<0.01.
Risk Factors for Chronic Antibody Mediated Rejection
The overall incidence of chronic AMR was 11.7% (111/953) during follow-up (determined by examining all biopsies obtained). The incidence correlated with increasing DSA at baseline and was the following: 8.2% (65/795) -DSA/-XM, 17.0% (9/53) +DSA/-XM, 30.6% (11/36) +DSA/low + XM, and 51.2% (22/43) in the +DSA/high+XM group, p<0.01 (Cochran Test for Trend) (Figure 6). However, the incidence of chronic AMR was similar in the −DSA/-XM and −DSA/+XM groups (8.2% (65/795) vs. 15.4% (4/26), p=0.19). Chronic AMR was similar among patients who received eculizumab and/or bortezomib in the +DSA/low+XM and +DSA/high+XM groups (See Supplemental Table 1).
Figure 6. Incidence of Chronic AMR.
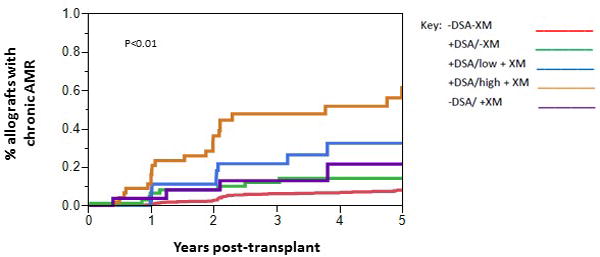
The overall incidence of chronic AMR was 11.7% and was determined by examining both surveillance and indication biopsies. The incidence correlated with increasing DSA at baseline and was the following: 8.2% -DSA/-XM, 17.0% +DSA/-XM, 30.6%+DSA/low + XM, and 51.2% in the +DSA/high+XM group, p<0.01 (Cochran Test for Trend). The incidence of chronic AMR was similar in the −DSA/-XM and −DSA/+XM groups (8.2% vs. 15.4%, p=0.19).
Early acute clinical AMR (HR 4.1,p<0.01), acute cellular rejection (HR 3.0, p<0.01), cPRA ≥ 80% (HR 3.8, p<0.01), class I DSA alone(HR 2.6, p<0.01), class II DSA alone (HR 3.5, p<0.01), class I and II DSA (HR 6.4, p<0.01), sum of Class I and II DSA (HR 1.8, p<0.01), +DSA/low + XM (HR 3.7, p<0.01) and +DSA high + XM at baseline (HR 7.3,p<0.01) were risk factors for chronic AMR in the univariate Cox proportional hazards model (Table 5). After adjustment in the multivariate model, the only independent risk factors for chronic AMR were acute cellular rejection (HR 2.3,p<0.01) or having DSA and a high + XM (HR 2.8, p=0.03) at baseline.
Table 5.
Cox Regression Models of Chronic Antibody Mediated Rejection in Different Patients Groups
| All Patients | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Variables | HR (CI) | p-value | HR (CI) | p-value |
| Early Acute clinical AMR | 4.1(1.6-8.6) | p<0.01 | 1.3(0.5-3.0) | p=0.6 |
| Acute cellular rejection | 3.0(2.1-4.4) | P<0.01 | 2.3(1.5-3.4) | P<0.01 |
| cPRA ≥80% | 3.8 (2.5-5.7) | p<0.01 | 1.6(0.9-2.7) | p=0.12 |
| Class I DSA only | 2.6(1.3-4.5) | p<0.01 | 0.9(0.4-2.3) | p=0.98 |
| Class II DSA only | 3.5(2.2-5.6) | p<0.01 | 1.5(0.7-3.5) | p=0.29 |
| Class I + II DSA | 6.4(2.9-12.3) | p<0.01 | 1.5(0.4-4.4) | p=0.50 |
| Sum all DSA* | 1.8(1.6-2.1) | p<0.01 | 1.1 (0.8-1.5) | p=0.43 |
| +DSA/-XM | 1.4 (0.7-2.6) | p=0.35 | ||
| +DSA/low+XM | 3.7 (1.8-6.5) | p<0.01 | 2.3(0.9-5.7) | p=0.07 |
| +DSA/high+XM | 7.3 (4.5-11.5) | p<0.01 | 2.8(1.1-7.4) | p=0.03 |
| -DSA/+XM | 1.5 (0.5-3.6) | p=0.44 | ||
| Full model | p<0.01 | |||
| Patients with DSA detected at the time of transplant (+DSA/-XM, +DSA/low+XM, +DSA/high+XM) | ||||
| Univariate | Multivariate | |||
| Variables | HR (CI) | p-value | HR (CI) | p-value |
| Early Acute clinical AMR | 1.7(0.4-4.8) | p=0.40 | ||
| Acute cellular rejection | 1.3(0.7-2.5) | P=0.40 | ||
| cPRA ≥80% | 1.7(0.9-3.2) | p=0.09 | ||
| Class I DSA only | 0.7(0.4-1.4) | p=0.33 | ||
| Class II DSA only | 1.0(0.6-1.9) | p=0.99 | ||
| Class I+II DSA only | 2.2(0.9-4.4) | p=0.07 | ||
| Sum MFI all DSA | 1.5(1.2-1.8) | p<0.001 | ||
| Patients with no DSA detected at time of transplant (-DSA/-XM and −DSA/+XM) | ||||
| Univariate | Multivariate | |||
| Variables | HR (CI) | p-value | HR (CI) | p-value |
| Early Acute clinical AMR | 4.8(1.2-12.9) | p=0.03 | 2.1(0.5-6.0) | p=0.26 |
| Acute cellular rejection | 3.9(2.4-6.3) | P<0.01 | 3.6(2.2-5.8) | P<0.01 |
| Number of HLA mismatches | 1.2(1.0-1.4) | P=0.01 | 1.3(1.1-1.5) | P<0.01 |
| cPRA ≥80% | 3.1(1.5-5.7) | p<0.01 | 4.5(2.1-8.7) | p<0.01 |
| Full model | p<0.01 | |||
Among the patients with identified DSA at baseline (groups +DSA/-XM, +DSA/low+XM, and +DSA/high+XM), the only factor associated with chronic AMR was the sum MFI of DSA (Table 5). We examined sum MFI of as an ordinal variable (MFI <5000, 5000 to <10,000, 10,000 to <15,000, 15,000 to <20,000, and > 20,000). Essentially, for every increase in the sum MFI of DSA by 5000 MFI, the incidence of chronic AMR increased by 50% (HR 1.5, p<0.01). This association was also present when the +DSA/high + XM group was examined alone (data not shown, HR 1.4, p<0.02).
In patients with undetected DSA at the time of transplant (-DSA/-XM and −DSA/+XM groups); acute cellular rejection, number of HLA mismatches, and cPRA ≥80% were independently associated with chronic AMR (HR 3.6, p<0.01; HR 1.3, p<0.01, and HR 4.5, p<0.01; respectively) (Table 5).
Outcomes of the highly sensitized (cPRA≥80%)
Because having a cPRA≥80% was identified as a risk factor for chronic AMR, we specifically examined the allograft survival and histologic outcomes in patients with cPRA ≥80%. Only patients with undetected DSA baseline were studied to avoid the confounding influence of known DSA. Patient, overall allograft, and death-censored allograft survival between patients with or without cPRA ≥80% was similar; but the histology was different.
As early as 1 year posttransplant, a higher proportion of allografts had microvascular inflammation in the cPRA ≥80% group [peritubular capillaritis 13.6% (6/44) vs. 3.9 % (24/610) p<0.01; glomerulitis 9.1 %(4/44) vs 1.9%(12/622) p<0.01]. By 2 years, more microvascular inflammation and chronic AMR was present in patients with cPRA ≥80% [peritubular capillaritis 16.1% (5/31) vs. 3.9 % (18/461) p<0.01; glomerulitis 16.1 % (5/31) vs. 3.5 % (16/464) p<0.01; chronic AMR 19.4% (6/31) vs. 4.7% (22/464), p<0.01]. The number of patients with baseline cPRA ≥80 that had a protocol 5-year biopsy was small, but we found numerically higher peritubular capillaritis, glomerulitis, and chronic AMR; but this did not reach statistical significance [peritubular capillaritis 13.3%(2/15) vs. 2.3%(5/218), p=0.07; glomerulitis 6.7%(1/15) vs 4.1%(7/210) p=0.49; and chronic AMR 20.0%(3/15) vs. 6.8% (15/220), p=0.10].
DISCUSSION
The current study provides new insight into the outcomes of kidney transplants in recipients with evidence of pretransplant DSA. In a cohort comprised mainly of Caucasian living donor transplant recipients who did not receive desensitization therapy, the death-censored actuarial allograft survival was similar among patients with no identified DSA and low level DSA (with or without a low positive B flow XM) at mean follow-up of 4.1 years. Histologic evidence of antibody mediated injury detected by surveillance biopsy was increased in patients with DSA, but renal function was preserved during follow-up. The increased histologic evidence of antibody mediated injury may lead to inferior allograft survival for some patients in the long-term, but the benefits of avoiding dialysis for select patients with limited transplant options likely outweighs the risk of transplantation with low level DSA.
The current kidney allocation system provides greater access to transplantation for sensitized patients. However, our data suggests that waiting for a negative crossmatch deceased donor kidney may not always be advantageous. In our cohort, highly sensitized patients (cPRA ≥80%) were at risk for antibody mediated injury within a year posttransplant even when no DSA was detected at baseline. The option of a kidney transplant in the setting of low level DSA might actually be as good or better than waiting on dialysis for a negative crossmatch cadaveric transplant, especially when considering the possibility of receiving a preemptive transplant from a living donor (either in a paired donor system or with the patient’s original donor). The overall half-life of a cadaveric transplant in the US is roughly 9 years as compared to 14 years for a living donor kidney, thus factors for graft loss beyond those immunologic should be considered 30,31. Further study of the outcomes of those highly sensitized patients who received deceased donor organs resulting from the new allocation system is needed to better understand the consequences of the policy change.
Another important finding from our study was that patients with undetected DSA and an isolated positive XM (pronase treated) had outcomes comparable to patients with no DSA and a negative B flow cytometric XM. The cause of the positive XM in these cases is unclear. Longer term follow-up and the study of potential non-HLA antibodies is needed in this group, but we believe that transplantation should be at least considered in these cases. Patients with identified DSA and high XM had the worst outcomes, but it is worth emphasizing that about half of these patients did not have chronic AMR at roughly 5 years posttransplant. Also, the incidence of early acute clinical AMR was only 16.3% with the use of eculizumab, plasmapheresis, and IVIG. Therefore, transplantation in the setting of DSA and high XM could be an option for a select subgroup of highly sensitized transplant candidates who have not yet benefited from paired donation programs or the new allocation system. In general, patients with anti-class II DSA had inferior allograft survival in our cohort and others 5,32, therefore the avoidance of anti-class II may be a reasonable approach if a +DSA/high + XM transplant is planned. Further study is needed to better understand the DSA characteristics associated with chronic AMR and allograft loss.
Although the role of desensitization was not directly studied in our patient population, our results suggest that the practice of routine desensitization in patients with low level DSA positivity with or without low level positive flow cytometric warrants further study. The incidence of early acute clinical AMR in patients with low positive XM who were not desensitized was comparable if not lower than reported in previous studies in which routine plasmapheresis, intravenous immunoglobulin, or rituximab was used 7,33,34. The impact of desensitization therapy on chronic AMR in the absence of early acute AMR is also unclear. We do not recommend that current desensitization strategies be completely abandoned. Instead, we recommend further study on this subject because of the potential risks associated with desensitization.
This study had limitations including its nonrandomized retrospective design, relatively short follow-up, and that many patients of low immunologic risk were not treated with maintenance prednisone. Longer follow up might show that the subclinical histological findings of AMR on surveillance biopsy years might translate into decreased graft survival at 10 years and beyond as has been shown in other studies35-37. Early single antigen bead kits had limited DPB1 and DQA1 antigen coverage, and so it is possible that DSA towards these antigens was missed in some patients which could have impacted results. However, since 2011 we have been routinely looking for DSA towards these antigens. We also do not have long term DSA data or detailed antibody information such as prozone, titer, C1q, or subclasses—all of which have been shown to risk stratify patients at risk for chronic AMR9,38-40. Another important limitation is that our cohort was mainly Caucasian living donor transplant recipients. It is not known whether our results can be extrapolated to a minority or deceased donor transplant population.
In conclusion, kidney transplantation with low level DSA (with or without a low + XM) and no specific desensitization therapy may be an acceptable option for sensitized transplant candidates. Allograft survival at approximately 5 years is similar to that for patients with undetected DSA at baseline. However, several questions remain. Long term follow-up of these patients is needed to better understand the DSA and patient characteristics associated with chronic AMR and allograft loss. We also need to study the histology and long term allograft survival of the highly sensitized transplant candidates who received kidneys as part of the new kidney allocation system. Answers to these important questions will help with donor selection for the highly sensitized transplant candidate.
Supplementary Material
Acknowledgments
This work is dedicated to Paul I. Terasaki. Thank you Dr. Terasaki for showing us all the power of 100 μL of serum.
Funding for work: This publication was made possible by CTSA Grant Number KL2 TR000136 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
Abbreviations
- AMR
Antibody mediated rejection,
- XM
B- flow cytometric crossmatch,
- cPRA
calculated panel reactive antibody,
- DSA
donor specific antibody,
- MFI
mean fluorescence intensity,
- SAB
single antigen beads
Footnotes
Carrie Schinstock MD
Participated in research design;
Participated in the writing of the paper
Participated in the data collection
Participated in data analysis
Schinstock declares conflict of interest: Alexion Pharmaceuticals
Manish Gandhi MD
Participated in research design
Participated in the writing of the paper
Participated in data analysis
Gandhi declares no conflict of interest
Wisit Cheungpasitporn MD
Participated in the writing of the paper
Participated in the data collection
Participated in data analysis
Cheungpasitporn declares no conflict of interest
Donald Mitema MD
Participated in the writing of the paper
Participated in the data collection
Participated in data analysis
Mitema declares no conflict of interest
Mikel Prieto MD
Participated in the writing of the paper
Participated in data analysis
Prieto declares no conflict of interest
Patrick Dean MD
Participated in the writing of the paper
Participated in data analysis
Dean declares no conflict of interest
Lynn Cornell MD
Participated in the writing of the paper
Participated in the data collection
Participated in data analysis
Cornell declares conflict of interest: Alexion Pharmaceuticals
Fernando Cosio MD
Participated in the writing of the paper
Participated in data analysis
Participated in design of research
Cosio declares no conflict of interest
Mark Stegall MD
Participated in the design of the research
Participated in data analysis
Participated in the writing of the paper
Stegall declares conflict of interest: Alexion Pharmaceuticals
References
- 1.Gloor JM, Cosio FG, Rea DJ, et al. Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant. 2006;6(8):1841–1847. doi: 10.1111/j.1600-6143.2006.01416.x. [DOI] [PubMed] [Google Scholar]
- 2.Loupy A, Suberbielle-Boissel C, Hill GS, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009;9(11):2561–2570. doi: 10.1111/j.1600-6143.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 3.Mohan S, Palanisamy A, Tsapepas D, et al. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012;23(12):2061–2071. doi: 10.1681/ASN.2012070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glotz D, Antoine C, Julia P, et al. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg) Am J Transplant. 2002;2(8):758–760. doi: 10.1034/j.1600-6143.2002.20809.x. [DOI] [PubMed] [Google Scholar]
- 5.Bentall A, Cornell LD, Gloor JM, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13(1):76–85. doi: 10.1111/j.1600-6143.2012.04291.x. [DOI] [PubMed] [Google Scholar]
- 6.Haririan A, Nogueira J, Kukuruga D, et al. Positive cross-match living donor kidney transplantation: longer-term outcomes. Am J Transplant. 2009;9(3):536–542. doi: 10.1111/j.1600-6143.2008.02524.x. [DOI] [PubMed] [Google Scholar]
- 7.Lefaucheur C, Suberbielle-Boissel C, Hill GS, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Contrib Nephrol. 2009;162:1–12. doi: 10.1159/000170788. [DOI] [PubMed] [Google Scholar]
- 8.Gloor JM, Winters JL, Cornell LD, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10(3):582–589. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 9.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–1226. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 10.Lefaucheur C, Viglietti D, Bentlejewski C, et al. IgG Donor-Specific Anti-Human HLA Antibody Subclasses and Kidney Allograft Antibody-Mediated Injury. J Am Soc Nephrol. 2016;27(1):293–304. doi: 10.1681/ASN.2014111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol. 2002;13(5):1358–1364. doi: 10.1097/01.asn.0000013295.11876.c9. [DOI] [PubMed] [Google Scholar]
- 12.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344(10):726–731. doi: 10.1056/NEJM200103083441004. [DOI] [PubMed] [Google Scholar]
- 13.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74(10):1377–1381. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 14.Moreso F, Seron D, Gil-Vernet S, et al. Donor age and delayed graft function as predictors of renal allograft survival in rejection-free patients. Nephrol Dial Transplant. 1999;14(4):930–935. doi: 10.1093/ndt/14.4.930. [DOI] [PubMed] [Google Scholar]
- 15.Yarlagadda SG, Klein CL, Jani A. Long-term renal outcomes after delayed graft function. Adv Chronic Kidney Dis. 2008;15(3):248–256. doi: 10.1053/j.ackd.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Hariharan S, Adams MB, Brennan DC, et al. Recurrent and de novo glomerular disease after renal transplantation: a report from Renal Allograft Disease Registry (RADR) Transplantation. 1999;68(5):635–641. doi: 10.1097/00007890-199909150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347(2):103–109. doi: 10.1056/NEJMoa013036. [DOI] [PubMed] [Google Scholar]
- 18.Schnuelle P, Lorenz D, Trede M, Van Der Woude FJ. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol. 1998;9(11):2135–2141. doi: 10.1681/ASN.V9112135. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 20.Orandi BJ, Luo X, Massie AB, et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. N Engl J Med. 2016;374(10):940–950. doi: 10.1056/NEJMoa1508380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 22.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Tranplant. 2008;8(4):753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 23.Solez K, Colvin RB, Racusen LC, et al. Banff ’05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7(3):518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 24.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 25.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 26.Batal I, Lunz JG, 3rd, Aggarwal N, et al. A critical appraisal of methods to grade transplant glomerulitis in renal allograft biopsies. Am J Transplant. 2010;10(11):2442–2452. doi: 10.1111/j.1600-6143.2010.03261.x. [DOI] [PubMed] [Google Scholar]
- 27.Hill GS, Nochy D, Loupy A. Accelerated arteriosclerosis: a form of transplant arteriopathy. Curr Opinion Organ Transplant. 2010;15(1):11–15. doi: 10.1097/MOT.0b013e3283342684. [DOI] [PubMed] [Google Scholar]
- 28.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11(11):2405–2413. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 29.Moreno Gonzales MA, Gandhi MJ, Schinstock CA, et al. 32 Doses of Bortezomib for Desensitization Is Not Well Tolerated and Is Associated With Only Modest Reductions in Anti-HLA Antibody. Transplantation. 2016 doi: 10.1097/TP.0000000000001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14(Suppl 1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 31.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11(3):450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 32.Issa N, Cosio FG, Gloor JM, et al. Transplant glomerulopathy: risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation. 2008;86(5):681–685. doi: 10.1097/TP.0b013e3181837626. [DOI] [PubMed] [Google Scholar]
- 33.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3):242–251. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 34.Kraus ES, Parekh RS, Oberai P, et al. Subclinical rejection in stable positive crossmatch kidney transplant patients: incidence and correlations. Am J Transplant. 2009;9(8):1826–1834. doi: 10.1111/j.1600-6143.2009.02701.x. [DOI] [PubMed] [Google Scholar]
- 35.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90(1):68–74. doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 36.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9(11):2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 37.Loupy A, Vernerey D, Tinel C, et al. Subclinical Rejection Phenotypes at 1 Year Post-Transplant and Outcome of Kidney Allografts. J Am Soc Nephrol. 2015;26(7):1721–1731. doi: 10.1681/ASN.2014040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tambur AR, Herrera ND, Haarberg KM, et al. Assessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am J Transplant. 2015 doi: 10.1111/ajt.13295. [DOI] [PubMed] [Google Scholar]
- 39.Lefaucheur C, Viglietti D, Bentlejewski C, et al. IgG Donor-Specific Anti-Human HLA Antibody Subclasses and Kidney Allograft Antibody-Mediated Injury. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation. 2011;91(3):342–347. doi: 10.1097/TP.0b013e318203fd26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.