Abstract
MRI is a valuable tool to assess myelin during development and demyelinating disease processes. While multiexponential T2 and quantitative magnetization transfer measures correlate with myelin content, neither provides the total myelin volume fraction. In many cases correlative measures are adequate; but to assess microstructure of myelin, (e.g. calculate the g-ratio using MRI), an accurate measure of myelin volume fraction is imperative. Using a volumetric model of white matter, we relate MRI measures of myelin to absolute measures of myelin volume fraction and compare them to quantitative histology. We assess our approach in control mice along with two models of hypomyelination and one model of hypermyelination and find strong agreement between MRI and histology amongst models. This work investigates the sensitivities of MRI myelin measures to changes in axon geometry and displays promise for estimating g-ratio from MRI.
Keywords: myelin, g-ratio, magnetic resonance imaging, MRI, histology, tuberous sclerosis
1. INTRODUCTION
There is a long-standing effort to develop MRI methods that are not just sensitive to myelin but report on changes in myelin with specificity. Recent interest in using MRI to measure the g-ratio (Stikov et al., 2015, 2010; West et al., 2016) has raised the aims of myelin imaging a step further, beyond specificity to accuracy. That is, an ideal method for g-ratio imaging includes more than just a correlative measure of myelin content, but an absolute measure of myelin volume fraction (MVF). To date, two myelin imaging techniques have been particularly well studied: myelin water imaging (MWI) via multi-exponential T2 (MET2) analysis (Mackay et al., 1994) and quantitative magnetization transfer (qMT) imaging (Sled and Pike, 2001). Both techniques have been shown to provide correlative measures of myelin content (Laule et al., 2006; Odrobina et al., 2005; Schmierer et al., 2007; Webb et al., 2003), but exactly how each relates to MVF remains unclear.
In the case of MWI, white matter is modeled as being comprised of two micro-anatomically separated water compartments with different transverse relaxation time constants (T2): 1) water trapped between the lipid bilayers of myelin (myelin water, T2 = 5 – 40 ms, depending on static field strength, B0), and 2) water in both the intra- and extra-axonal spaces (i/e water, T2 = 30–100 ms, depending on B0). Given sufficient signal-to-noise ratio (SNR), multiple spin-echo amplitudes can be fitted to a model that distinguishes these water pools based on T2, and the myelin water fraction (MWF) is typically reported as a measure of relative myelin content (Mackay et al., 1994; Menon et al., 1992; Whittall et al., 1997).
Measures of MWF have been shown to correlate with optical density in luxol fast blue stained sections of cadaver brain from MS patients (Laule et al., 2006) and with direct measures of myelin cross sectional area in electron microscopy of control and injured rat nerve (Odrobina et al., 2005; Webb et al., 2003). Also, Laule et al., used literature values of the composition of white matter to predict MWFs that were in close agreement with their observed values (Laule et al., 2004). However, none of these studies attempted to explicitly estimate and/or validate values of MVFs from MWF measures. The relationship between MWF and MVF depends on the relative water proton densities in the myelin and non-myelin compartments, but may also depend on the rate at which water exchanges between these compartments (Zimmerman and Brittin, 1957). Studies in rat spinal cord have indicated that variations in MWFs between different white matter tracts may be due to differences in water exchange rates, mediated by variations in axon diameter and myelin thickness (Dula et al., 2010; Harkins et al., 2012). This effect has been postulated to exist in brain (Russell-Schulz et al., 2013; Sled et al., 2004), but it remains unclear to what extent it effects observed MWF values.
Similar to MWI, the qMT method is based on a two-pool model of protons in white matter, but instead of two anatomically separated pools they are two pools of different molecular origins, water protons and protons bound to macromolecules. Although the bound proton signal is not typically measured directly, the exchange of magnetization between the bound and water protons results in contrast that depends on bound proton concentration (Henkelman et al., 1993; Wolff and Balaban, 1989). Thus, given an appropriate series of images with different MT contrast, the ratio of bound protons to total protons, or bound pool fraction (BPF), can be estimated. Note that, unlike the two-pool model used for MWI, this two-pool model: i) incorporates no anatomical information (both water and bound protons pools are assumed to be well mixed from one anatomical compartment, meaning that myelin is not explicitly part of the model), and ii) is predicated on the exchange of magnetization between the two pools (while the MWI model assumes no exchange of magnetization between the two water pools) (Gochberg and Gore, 2007; Sled and Pike, 2001). The lack of anatomy in the model presents a problem in relating BPF to MVF because bound protons will exist in both myelin and non-myelin regions of the tissue, and there is no reason to believe that all bound protons exchange magnetization with water at the same rate. As in MWI, this raises the question of whether geometric characteristics of axons/myelin contribute to the measured BPF.
Similar to literature on MWF, measures of BPF (or similar/related quantities) have been demonstrated to linearly correlate with MVF as measured by histology in both human cadaver brain (Schmierer et al., 2007) and rodent brain and nerve (Janve et al., 2013; Odrobina et al., 2005; Thiessen et al., 2013; Underhill et al., 2011). Stikov et al. have recently used such a linear correlation to estimate MVF from BPF (Stikov et al., 2015), but otherwise, there has been limited effort in explicitly estimating MVF from estimates from qMT measures.
Using literature information on the composition of white matter, this study proposes analytical expressions for computing estimates of MVF from MET2 and qMT data. These approaches are applied with high resolution 3D MRI protocols to excised and fixed mouse brains from control mice and three mouse models of abnormal myelination. MRI results are quantitatively evaluated with transmission electron microscopy.
2. THEORY
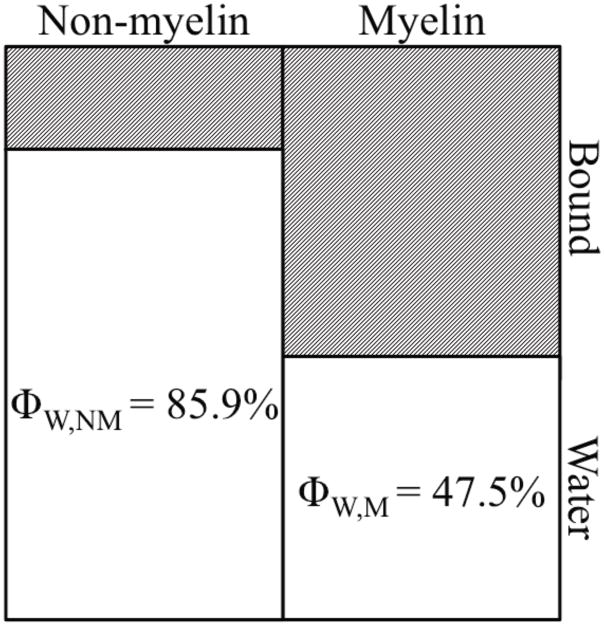
To derive myelin volume measures from MRI, a model of white matter tissue that uses volumes, not just populations, of the different proton pools is presented in Fig 1. The model includes four proton pools, with volumes of bound and water protons in the myelin (VB,M and VW,M, respectively) and non-myelin (VB,NM and VW,NM, respectively). The model assumes exchange of longitudinal magnetization between the bound and water protons, enabling qMT analysis, but no exchange of water or magnetization between myelin and non-myelin compartments. The MVF (fM) by definition is
Figure 1.
Volumetric model of white matter. Equations 8 and 12 are used to derive accurate myelin volume fractions (fM,T2 and fM,MT) from MWF and BPF, respectively.
| [1] |
Using the simplifying assumption that molar concentration of protons is equal in all four compartments (see Appendix), magnetization fractions are equal to volume fractions, which permits BPF measured by qMT to be expressed in terms of compartment volume fractions,
| [3] |
Similarly, the myelin water fraction (MWF) measured by MET2 is
| [4] |
From previous literature (see Appendix), the volume fraction of water in myelin (ΦW,M) is estimated as
| [5] |
and for non-myelin is
| [6] |
Combining Eqs 1, and 4–6, MWF can be written in terms of fM
| [7] |
which can then be solved to write fM as a function of MWF,
| [8] |
with the additional “T2” subscript indicating that this is myelin volume fraction as estimated by MET2 analysis.
For qMT analysis, there is an additional unknown: the volume fraction of the non-myelin bound proton pool, defined here as β.
| [9] |
Assuming that β were known, and from Eq 3,
| [10] |
then combining Eqs 5 and 10, results in
| [11] |
The sum of Eqs 10 and 11 is the myelin volume fraction,
| [12] |
with the “MT” subscript indicating that this is myelin volume fraction as estimated by qMT analysis.
3. MATERIALS AND METHODS
3.1 Tissue Preparation
The Vanderbilt University Institutional Animal Care and Use Committee approved animal studies. Fifteen adult mice were anesthetized with isoflurane and sacrificed via transcardial perfusion. The perfusion consisted of 1X phosphate-buffered saline (PBS) wash followed by 2.5% glutaraldehyde + 2% paraformaldehyde (modified Karnovsky solution). Following perfusion, brains were quickly removed from skull and immersed in the fixative solution for 1 week. Brains were then washed with 1X PBS + 0.01% sodium azide, changing wash 4–5 times over 1 week to remove excess fixative. In all cases, 1.0 mM Gd-DTPA (Magnevist; Berlex, Montville, NJ) was included in the perfusate, immersion and wash solutions, resulting in relatively uniform distribution of Gd-DTPA throughout the brain.
This study used control animals (n=6) along with two previously described models of hypomyelination and one of hypermyelination (n = 3 for each model). All models utilized the Olig2-Cre driver to conditionally target proteins involved in PI3K/Akt signaling in oligodendrocyte precursor cells. In the first model, the Tsc2 gene is deleted (Tsc2 CKO–conditional knockout) and exhibits extreme loss of myelin (Carson et al., 2015). The second model targets Rictor (Rictor CKO), a key component of the mTORC2 complex, and also displays hypomyelination, but less severe than the Tsc2 model and similar to the Rictor Emx1-Cre model shown previously (Carson et al., 2013). The third model results from the deletion of Pten (Pten CKO) leading to activation of the PI3K/Akt signaling pathway and subsequent hypermyelination (Harrington et al., 2010).
3.2 Magnetic Resonance Imaging
All imaging was performed on a 15.2-T 11-cm horizontal bore Bruker (Rheinstetten, Germany) BioSpec scanner, using a 35-mm diameter Bruker quadrature volume coil for transmission and reception. To provide a signal-free background and prevent tissue dehydration, brains were placed in an MR-compatible tube filled with perfluoropolyether liquid (Fomblin, Solvay Solexis, Thorofarem NJ, USA). Both MET2 and qMT scans were encoded with a matrix size of 128 × 96 × 72 over a 1.92 × 1.44 × 1.08 cm3 FOV, providing 150 μm isotropic resolution.
For MET2 imaging, a 3D multiple spin-echo sequence was used with non-selective excitation and refocusing pulses, 160 μs and 100 μs in duration, respectively. Each refocusing pulse was surrounded by 428 μs duration 6 G/cm amplitude crusher gradients, phase-encoding gradients were rewound after each echo, and a two-part (+X/-X) phase cycling scheme was used. With these constraints, secondary echoes that were not excited by the initial excitation pulse were removed, and the observed echo magnitudes could be computed with the extended phase graph (EPG) algorithm (Hennig, 1991; Lebel and Wilman, 2010; Prasloski et al., 2012). Scan parameters were: repetition time (TR) = 520 ms, echo time (TE) = 5.8 ms, number of echoes (NE) = 18, receiver bandwidth (BW) = 38.5 kHz, and number of excitations (NEX) = 6. Total scan time was ≈ 6 hr.
For qMT imaging, a 3D selective inversion-recovery prepared fast spin echo sequence (Gochberg and Gore, 2007) was used with 8 collected echoes, 5-ms echo spacing and centric phase encoding. To ensure that the longitudinal magnetization (Mz) recovered from Mz = 0 at the start of every pre-delay period, 8 additional refocusing pulses, also with 5-ms echo spacing, followed the 8th echo. A 1-ms hard pulse was used to selectively invert the free water magnetization, while macromolecular spins were mostly unaffected. The sequence was repeated NI = 15 times with inversion times (TI) log-spaced from 3.5 to 2000 ms. A constant predelay (Td) of 590 ms resulted in a scan time of ~ 3.5hr.
3.3 Data Analysis
All data analysis was performed using MATLAB R2015a (The Mathworks, Natick MA) and MET2 analyses were performed using the freely available Multi Exponential Relaxation Analysis (MERA) toolbox (Does, 2014). Prior to Fourier reconstruction, k-space data for all images were apodized using a 3D Tukey window with a 0.25 taper-to-window ratio and zero-padded 2×, resulting in 75 μm nominal isotropic resolution.
For each voxel, the MET2 analysis used a separable non-linear approach. The T2 spectrum was estimated by linear inverse (Whittall and MacKay, 1989) using a non-negative least-squares fit (Lawson and Hanson, 1974) of the NE echo magnitudes to the sum of 100 EPG-defined signals with T2 values logarithmically spaced between TE/2 as 500 ms. The linear model was augmented with minimum curvature constraint weighted at a constant and conservative level across all voxels (μ = 0.002). This linear inverse was repeated to find the refocusing pulse flip angle (θ) by non-linear regression, similar to previous work (Lebel and Wilman, 2010; Prasloski et al., 2012). From all spectra, the myelin water fraction (MWF) was defined as the fraction of signal with peak T2 < 17 ms and long T2 component was defined as the peak T2 from the largest fractional component >17ms. Since we utilize the fractional components defined by peak T2, the MWF cutoff was not as critical. For example, across all white matter voxels from all brains (segmented as voxels with MWF > 5%), <1% of signals had a peak T2 between 15.3ms and 19.9ms, so defining the cut-off between myelin and non-myelin water anywhere in this domain had little effect on the MWF estimates. See Supplementary Fig S1 displaying representative spectra from each mouse model.
For qMT analysis, the NI image magnitudes were fitted voxel-wise to the Bloch- McConnell equations describing longitudinal relaxation and magnetization transfer between water and macromolecular protons (Gochberg and Gore, 2007; Li et al., 2010). The five fitted model parameters were: M0f, M0b, kmf, R1f, and Sf, where M0f/b are the equilibrium magnetizations of the free and bound pools, respectively, kbf is the rate constant of magnetization transfer from the bound to free water pool, R1f is the longitudinal relaxation rate of the free water pool, and Sf is the efficiency of the inversion pulse on the free water pool. The corresponding R1b and Sb values were constrained to R1b = 1s and Sb = 0.83 in accord with prior studies (Gochberg and Gore, 2007). The bound pool fraction (BPF) was then defined as BPF = M0b/(M0b + M0f).
After MET2 and qMT analysis, all parameter maps of a given model were co-registered in order to define closely comparable regions of interest (ROI) in each brain. For each mouse model, the first spin echo image of one brain was arbitrarily defined as the reference and the corresponding image from each other brain was registered to the reference using a rigid affine registration followed by a non-rigid deformable demons registration (Thirion, 1998). The resulting deformation fields of this registration were then applied to parameter maps. Four ROIs were drawn corresponding to the four regions extracted for histology (below): 3 in the corpus callosum in the mid-sagittal slice (genu (GCC), mid-body (MidCC), and splenium (SCC)) and the other in the anterior commissure (AC). In addition, a cortical gray matter (GM) ROI was drawn in the sagittal slice for comparison. All ROIs are shown in Supplementary Fig S2. The white matter ROIs had an average size = 12.5 ± 1.9 voxels and the same ROIs were used to extract measures from each co-registered parameter map. Mean ROI values from different parameter maps and values derived from histology were compared using two-sample t-tests (α = 0.05) and Pearson’s linear correlation.
3.4 Microscopy
For each brain, after MRI, a 1–2 mm thick sagittal section of tissue was cut from the left hemisphere beginning at the mid-brain. Subsequently, 4 regions of white matter were cut from the slice: the genu, mid-body, and splenium of the corpus callosum and the anterior commissure. Tissue samples were then processed for Transmission Electron Microscopy (TEM) in the Vanderbilt Cell Imaging Shared Resource-Research Electron Microscopy facility. Samples were placed in 1% osmium tetroxide in cacodylate buffer for 12 hours and dehydrated in graded ethanol. Tissue was then embedded in epoxy resin and thick sections (0.5–1 μm) were collected and stained with 1% toluidine blue. Subsequently, ultra-thin section (~ 500 × 500 × 0.07 μm) were cut and then collected on 300-mesh copper grids. Copper grids were stained at room temperature with 2% uranyl acetate (aqueous) for 15 minutes and then with lead citrate for 10 minutes.
Ultra-thin sections were imaged on the Philips/FEI Tecnai T12 electron microscope (FEI Company, Hillsboro, OR) at various magnifications and pictures were acquired with a side-mounted AMT CCD camera. For quantification of myelinated axon microstructure, 6–12 15,000× images were collected (~300 axons) per ROI per animal. Each image was analyzed semi-automatically to derive myelin volume fraction (fM,HIST), axon volume fraction (fA,HIST), as well as per axon measures of diameter (di) and myelin thickness (Δi) (subscript “i” indicating the ith axon).
The histology pipeline was implemented using MATLAB 2015a (The Mathworks, Natick MA) and is shown in Fig 2. First, a local Otsu threshold was applied to each image, resulting in a binary image (myelin=1, non-myelin=0). In this binary image, the operator manually identified an intra-axonal point of each myelinated axon and corrected the labeling of pixels that were deemed to be erroneously identified as myelin. Beginning from each manually identified seed point, an active contour algorithm (Kass et al., 1988) was used to segment each individual axon. The resulting image (Fig 2d) provided per axon measures of area and diameter ( ai and , respectively, for the ith axon) and total axon volume fraction. (Note that only axons lying fully within the image frame were included in the per-axon measures, but all axon area contributed to the axon volume fraction.)
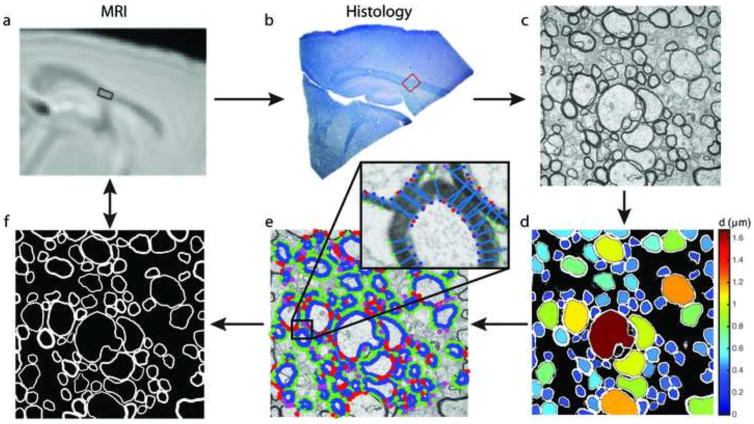
Figure 2.
Histology flow chart. a) MRI T2-weighted image with box drawn for ROI analysis. b) Histology thick slice where the red box signifies the location of ultra-thin sections. c) Transmission Electron Microscopy (TEM) 15,000X image. d) Output of region-growing algorithm with color denoting axon diameter (d), and the fraction of area from all axons = fA,HIST. e) For each axon, normal lines are grown from seeds from the region-growing axon edge (blue stars) until the image value is less than the mean image value of seeds to obtain a measures of myelin thickness (green stars). The measurement is disregarded if i) the end point lands in another axon space (red stars) or ii) if the line is > 3 times median absolute deviation (magenta stars). A median myelin thickness is calculated for each axon. f) Myelin is then grown using the median thickness from the region-growing axon boundary to obtain a segmented myelin image, with the fraction of area from all myelin = fM,HIST.
Because the Otsu threshold resulted in uneven segmentation of the myelin, the myelin of each myelinated axon was independently segmented on the original histology image using the final boundary of the axon segmentation as the starting contour. Each contour was grown outward along lines normal to its tangent (lines spaced ~0.06 μm apart along the starting contour) until reaching a pixel intensity greater than the average intensity of pixels from the initial contour (Fig 2e). In order to reject normal lines that grew into the myelin of adjacent axons, a line was discarded if 1) its terminal point matched the location of an axon boundary defined above, or 2) its length was more than 3 times the median absolute deviation (Leys et al., 2013). The ends of the remaining normal lines created a contour defining the outer myelin boundary, and the median length of these lines defined the myelin thickness for that axon, Δi. Finally, myelin area for each axon was determined by uniformly growing the initial contour of each axon to a uniform thickness = Δi, as shown in Fig 2f. The resulting binary image provided a measure of myelin volume fraction, fM,HIST, for each image. Across all images per mouse and brain region, mean values , d̄, and Δ̄ were calculated.
4. RESULTS
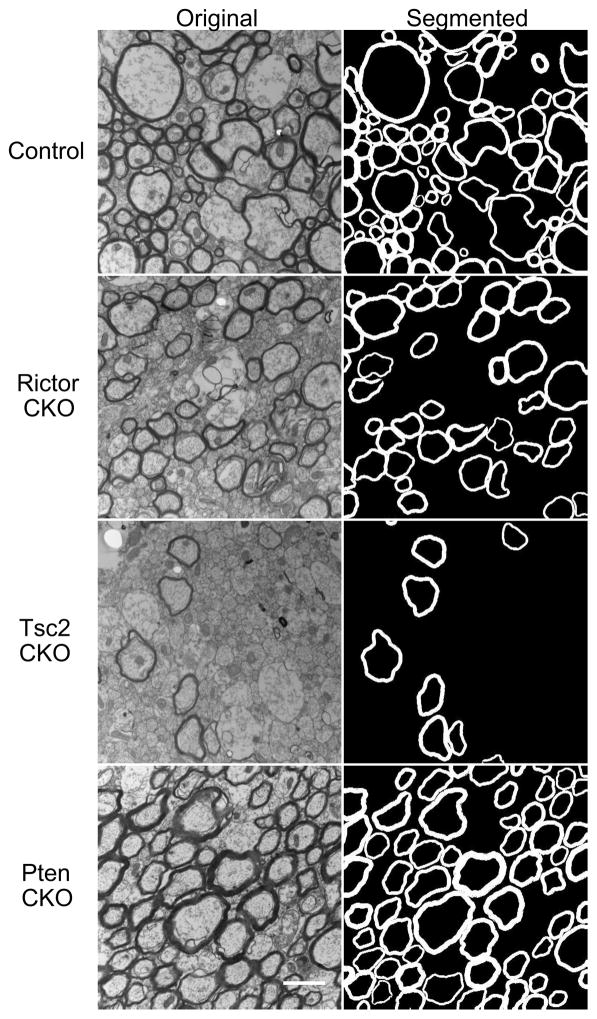
Representative TEM histology in Fig 3 demonstrates the abnormal myelination characteristics expected from these mouse models of tuberous sclerosis. The Rictor CKO and especially the Tsc2 CKO mice exhibited loss of myelinated axons, consistent with previous literature (Carson et al., 2015, 2013). Similarly consistent with literature (Harrington et al., 2010), the myelin in Pten CKO mice was noticeably thicker than in control mice. These variations in both myelin content and myelin thickness made these a useful combination of models for evaluating the specificity of MRI methods for reporting on myelin volume fraction.
Figure 3.
Representative 15,000X (left) TEM Histology images and (right) segmented myelin images from (top-bottom) control, Rictor CKO, Tsc2 CKO, and Pten CKO mice with scale bar = 1 μm.
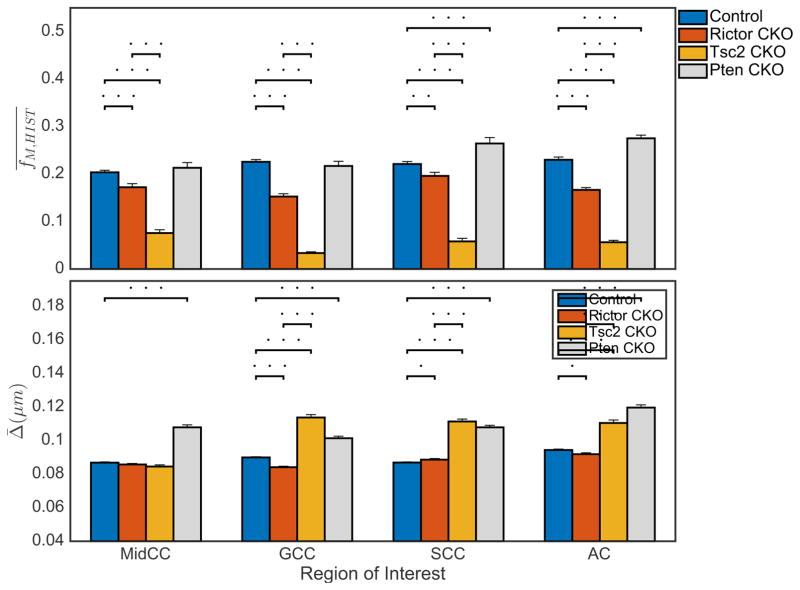
A summary of the detailed quantitative evaluation of the TEM histology is presented in Fig 4. The mean ± SD (across animals) of and Δ̄ are shown for each of the four white matter tracts and all four different mouse models. As expected from Fig 3, fM,HIST is significantly reduced in both Rictor and Tsc2 CKO compared to controls, and slightly higher in the SCC and AC regions of the Pten mice. The control and Rictor CKO mice showed similar myelin thickness, while Δ̄ was generally greater in Tsc2 CKO and Pten CKO mice. Note that because the quantitative histology relied on direct visualization of myelinated axons in cross section, it was not possible to extract measures of MVF or myelin thickness from regions of gray matter.
Figure 4.
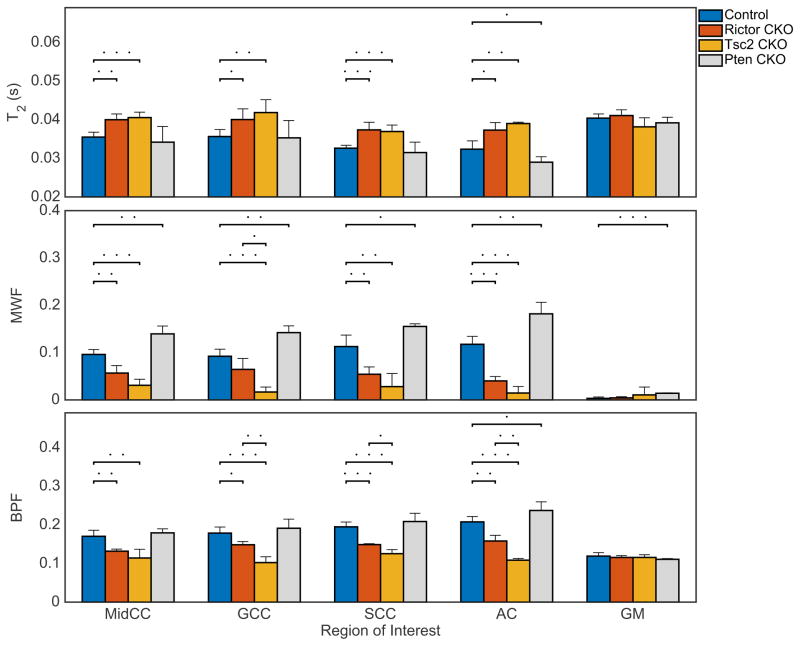
Region of interest (ROI) analysis of mean myelin volume fraction (fM,HIST) and mean myelin thickness (Δ̄) from (left-right) mid-body of corpus callosum (MidCC), genu of corpus callosum (GCC), splenium of corpus callosum (SCC), anterior commissure (AC) and cortical gray matter (GM) in control, Rictor CKO, Tsc2 CKO, and Pten CKO mice. Dots above bars represent significant differences of (.) = p < 0.05, (. .) = p < 0.01, (…) = p < 0.001.
Representative MRI data from control and the three mouse models are shown in Fig 5—sagittal slices from a T2-weighted image (TE = 5.8 ms), long T2 component value, MWF, and BPF parameter maps. As expected from histology (Fig 3 and 4), white matter regions are generally invisible in Tsc2 CKO mice, reduced in contrast in Rictor CKO mice, and enhanced in contrast in Pten CKO mice. A detailed summary of MRI measures is presented in Fig 6, which shows mean values (±SD across animals) of the long T2, MWF, and BPF for controls, Rictor, Tsc2, and Pten CKO mice in the 4 white matter ROIs and the cortical gray matter ROI. Statistically significant differences in T2, MWF, and BPF were found between controls and both models of hypomyelination (Rictor and Tsc2 CKO), consistent with the loss of myelin observed by histology. Between the Rictor and Tsc2 CKO mice, differences in all measures were consistent with less myelin in the Tsc2 CKO mice, but only reached statistical significance for BPF in 3 of 4 ROIs and in 1 for MWF. Similarly, the Pten mice, expected to exhibit hypermyelination, had generally increased MWF and BPF and decreased T2, although the BPF and T2 differences were only statistically significant in the AC. Additionally, Supplementary Fig S3 displays strong correlation between MWF and BPF, as expected.
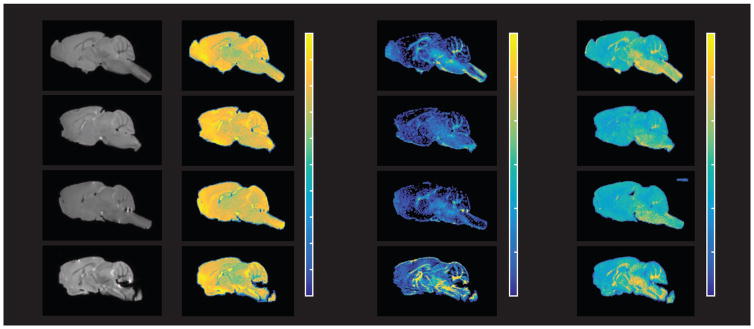
Figure 5.
Representative MRI images of (left-right) T2-weighted, long T2 component, myelin water fraction (MWF), and bound pool fraction (BPF) parameter maps from (top-bottom) control, Rictor CKO, Tsc2 CKO, and Pten CKO mice.
Figure 6.
Region of interest (ROI) analysis of (top-bottom) long T2 component, myelin water fraction (MWF), and bound pool fraction (BPF) from (left-right) mid-body of corpus callosum (MidCC), genu of corpus callosum (GCC), splenium of corpus callosum (SCC), anterior commissure (AC) and cortical gray matter (GM) in control, Rictor CKO, Tsc2 CKO, and Pten CKO mice. Dots above bars represent significant differences of (.) = p < 0.05, (. .) = p < 0.01, (…) = p < 0.001.
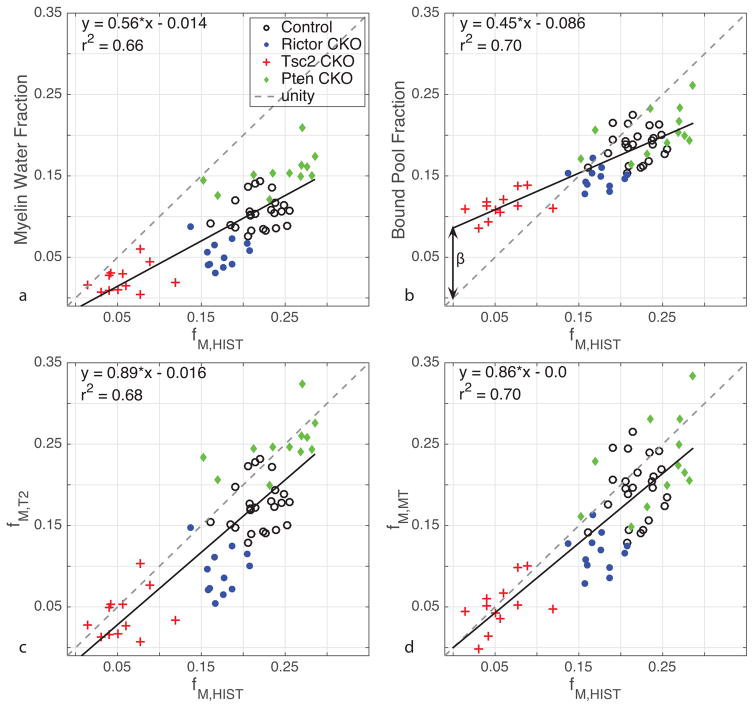
For the purpose of this study, more important than detecting differences in myelin between animals or brain regions, is the accuracy with which MRI can measure myelin content and how these measures are sensitive to myelin thickness. To this end, Fig 7 displays scatter plots of (top) MWF and BPF vs fM,HIST. As expected from previous literature (Laule et al., 2006; Odrobina et al., 2005; Schmierer et al., 2007; Thiessen et al., 2013; Underhill et al., 2011; Webb et al., 2003) both MWF and BPF show strong linear correlation with histological measures of MVF (r = 0.81, 0.84, respectively); however, neither measure is accurate (i.e., lines of best fit do not lie near line of unity).
Figure 7.
(a,b) MWF and BPF versus fM,HIST with line of best fit and equations shown and line of unity (gray, dashed). r = 0.81 and 0.84, respectively. Mean standard errors from MWF and BPF are 0.007 and 0.004, respectively. (c,d) fM,T2 and fM,MT versus fM,HIST with line of best fit and equations shown and line of unity (gray, dashed). r = 0.82 and 0.84, respectively. In all cases, linear fits were statistically significant, p ≪ 0.01.
Also shown in Fig 7 (bottom) are the two calibrated MRI measures of MVF, fM,T2 and fM,MT, vs fM,HIST, which demonstrate the potential for both MET2 and qMT to provide accurate, not just correlative (r = 0.82, 0.84 respectively), measures of MVF, with lines of best fit lying near the line of unity (slopes near 1). Overall, fM,T2 slightly underestimates fM,HIST and there is a noticeable variation between animal models, which may be due to the sensitivity of MET2 to variations in myelin thickness. In contrast, fM,MT appears to estimate fM,HIST more accurately, although this is a somewhat misleading observation because the fM,MT data were calibrated at fM,HIST = 0 (β = 0.086 from the intercept of the linear fit of BPF and fM,HIST; Fig 7b). The fM,T2 data would lie closer to the line of unity if it were also calibrated to histology; however, variations between animal models would remain. The extent to which variations in myelinated axon density alone might be driving the correlations between MRI and histology measures of myelin is presented in Supplementary Fig S4. This shows that with the exception of the Tsc2 CKO mice, which involves a substantial reduction in myelinated axons, there is no correlation between MRI measures of myelin and the histologically measured myelinated axon volume fraction (fA,HIST).
5. DISCUSSION
For the purpose of establishing MRI methods for measuring MVF, this paper presents MRI data and quantitative histology acquired in excised and fixed mouse brains with normal and abnormal myelination. One of the current goals of the authors’ lab is to develop robust MRI assays for routine use in rodent brain studies, and to that end the findings here demonstrate that both MET2 and qMT methods have the potential to provide both specific and accurate whole brain maps of MVF.
Looking beyond excised tissues to in vivo imaging of animals or humans, there are a number of important factors to consider. Previous studies have investigated the effects of chemical fixation on qMT in post-mortem human tissues (Schmierer et al., 2008) and found differences in absolute values but similar predictive value of myelin content. Similarly, previous MET2 studies have shown a close correspondence in the T2 spectrum between fixed and in vivo human white matter (Laule et al., 2006) and between fixed (Dula et al., 2010) and in vivo (Harkins et al., 2013) rat spinal cord. However, there is a noticeable absence of in vivo MET2 data from rat or mouse brain in the literature and even evidence suggesting it cannot be measured (Does and Gore, 2002). This limit may be due to SNR at the resolution necessary for rodent brain imaging, or it may reflect the consequences of more rapid water exchange between myelin and non-myelin compartments in these particular tissues at physiological temperatures in rats and mice. In addition to the tissues being excised and fixed, this study also utilized the addition of Gd-DTPA to boost SNR efficiency (Johnson et al., 2002). To assess the effect of the Gd-DTPA on the MET2 and qMT data, independent scans were run on a control brain that was prepared without the added Gd-DTPA. These scans required a longer TR but were otherwise identical and resulted in similar (< 10% different, not shown) measures of MWF and BPF to those found in the Gd-DTPA loaded brains. Although this was not a thorough investigation, it indicated that loading the tissue with 1 mM Gd-DTPA had no more than small effects on the quantitative myelin measures, and presumably little effect on their relationships to myelin content.
The observations that MWF and BPF correlate strongly with histological measures of myelin content is not novel, having been demonstrated in several previous studies (Laule et al., 2006; Odrobina et al., 2005; Schmierer et al., 2007; Thiessen et al., 2013; Underhill et al., 2011; Webb et al., 2003). However, it is worth noting that with the exception of a recent study of the corpus callosum in cuprizone-fed mice (Thiessen et al., 2013), previous studies that involved brain tissue, as opposed to peripheral nerve, made independent measures of myelin content by optical densitometry of luxol-fast-blue stained histology. Optical densitometry is a practical approach for measuring myelin content over large sections of histology, but it presents challenges with calibration. That is, relating a densitometry measure to MVF itself requires calibration and is sensitive to the extent and spatial uniformity of staining. The Thiessen study included measures akin to MVF and found a strong correlation with BPF, but their MRI measurements did not include MWF. Thus, the present study, which involved direct visualization of myelin and quantitative analysis of approximately 500 TEM images, is unique in providing objective measures of MVF in brain to compare with both MWF and BPF. The TEM analysis also provided measures of myelin thickness, which cannot be extracted from large field optical densitometry studies.
Of course, all histology suffers from changes in tissue during the embedding process, and small-field-of-view TEM presents the potential for sampling bias. In this study, total tissue shrinkage < 10% is expected (Denef et al., 1979; Tang and Nyengaard, 1997), and errors in MVF result only from the difference in shrinkage between myelin and non-myelin regions. If we postulate that the extent of shrinkage in different compartments is proportional to water density, then the effect of tissue shrinkage on MVF may be just a few percent—small compared to the variance in fM,HIST. The area of ultra-thin sections is on the order of our MRI ROIs (see Fig 2a,b) but for high-resolution analysis, we are limited to sampling ~1% of the area. However, by one-way ANOVA across all control sections, we see that variance between brains and ROIs is significantly higher than within a region, suggesting there were minimal effects from sampling bias. Histology also relies on an accurate analysis technique, which has no standard and is quite challenging. The most likely source of inaccuracy in the estimate of fM,HIST comes from the choice of intensity threshold for determining the outer boundary of myelin. Adjusting this threshold higher and lower while still creating qualitatively acceptable myelin segmentation resulted in a variation of fM,HIST by roughly ± 0.03, indicating this as the potential range of systematic error in fM,HIST.
In terms of evaluating and comparing the two MRI methods studied here—MET2 and qMT—the most obvious difference is that the calculation of fM,T2 required a priori information about the volume fractions of water in myelin and non-myelin compartments of white matter, while fM,MT required the same, and a calibration of the value of BPF when MVF = 0, i.e., β in Eqs [9]-[12]. Here, β was estimated from the linear regression of BPF vs fM,HIST (Fig 7b), which makes the evaluation of fM,MT vs fM,HIST somewhat circular. Ideally, the value of β measured here will be applicable to future MRI studies of myelin in excised and fixed mouse brains at 15.2T, but how effectively one can generalize the intercept of BPF and fM,HIST is unclear. There is evidence that BPF changes between fresh and fixed tissue (Schmierer et al., 2008); it may also differ between in vivo and ex vivo tissue states, and perhaps between different white matter tracts and/or species. Moreover, even within a given type/state of tissue, the literature values of BPF vary widely, which is likely due to the wide variety of qMT methods in use. Even using the same IR-based qMT method, the BPF value will depend on assumptions about the bound proton pool—its R1 and the effect of the inversion RF pulse on Mz (van Gelderen et al., 2015)—so it is important to calibrate the qMT method in a consistent manner.
Nonetheless, it may be possible to generalize the offset term, β, by normalizing white matter BPF values to those from cortical gray matter, which contains relatively little myelin. For example, in this study, the relative amplitude of β and BPF in cortical gray matter is 0.72, and similar ratios, 0.76 and 0.77, can be drawn from the data in two previous studies, Janve et al. (Janve et al. 2013) and Thiessen et al. (Thiessen et al., 2013), respectively. The Thiessen et al. study also involved fixed excised mouse brains but a very different qMT protocol, suggesting that a standard β might work for these samples, independently of the qMT protocol. From rat brain in vivo (Underhill et al., 2011), the results were somewhat different with a ratio of 0.62, but this is to be expected given a previous study that showed a greater relative effect of chemical fixation on BPF for normal appearing white matter and multiple sclerosis lesions (Schmierer et al., 2008). Nonetheless, this meta-analysis suggests potential for this approach to estimating β without histology driven calibration.
Unlike fM,MT, fM,T2 does not require an offset term because MWF goes to 0 in the absence of myelin. However, previous studies in rat spinal cord (Dula et al., 2010; Harkins et al., 2012) and rat optic nerve (Dortch et al., 2013) have shown that MWF may be affected by inter-compartmental water exchange. Thus, smaller axons with thinner myelin may result in lower MWF relative to the MVF. This phenomenon may be responsible for the systematic deviation between animal models from the overall linear fit in Fig 7c. That is, all data points from the Pten CKO mice, which exhibited generally thicker myelin, fall above the linear fit line, while 11 of 12 points from the Rictor CKO mice, which exhibited generally thinner myelin, fall below. This additional variance between models may also reflect differences in water densities in myelin and non-myelin regions (ΦW,M and ΦW,NM), but the effect is the same—MWF is not simply a function of MVF. That said, the effect in this study is relatively small. It may be that in vivo and/or at lower magnetic fields (where transverse relaxation rates are slower and, therefore, more sensitive to the rate of water exchange) this effect is more significant and more problematic. While one might speculate that BPF should also be sensitive to the thickness of myelin, since macromolecular protons from deep within myelin may not exchange as effectively with the bulk intra-/extra-axonal water, the effect is not apparent in Fig 7d, which agrees with previous simulation studies (Levesque and Pike, 2009).
Beyond MET2 and qMT, there are a number of other MRI methods that aim to image myelin content, which raises the question of why the present study involved only MET2 and qMT. Similar to MET2, one can image myelin with multi-exponential T2* which permits use of spoiled gradient echo rather than spin echo acquisitions at the cost of introducing a new unknown (the additional dephasing of transverse magnetization). While this approach shows promise (Alonso-Ortiz et al., 2016; Hwang et al., 2010; Lenz et al., 2012) it is not at the point of providing accurate measures of MVF and, in the authors’ experience, is especially challenging at the high magnetic fields used for small animal imaging. Other approaches that use steady state gradient echo are thus far uninterpretable at practical SNR, even for animal imaging (Lankford and Does, 2013).
Exploiting T1 differences between myelin and non-myelin water to selectively excite only myelin water has been used in nerve (Travis and Does, 2005) and white matter (Oh et al., 2013), but again, not for accurate MVF measures because additional unknowns come into play (T1s and water exchange rates). Directly measuring multi-exponential T1 has been proposed (Labadie et al., 2014) but this is complicated not just by water exchange rates but also by magnetization transfer. In fact, the qMT method used here is effectively a bi-exponential measurement of longitudinal relaxation. Because qMT already requires calibration, if the fast relaxing component is due to a combination of MT and multi-exponential T1 (MET1) there is no additional cost in specificity. However, attempting to interpret a bi-exponential recovery of longitudinal relaxation as being specifically a measure of myelin and non-myelin water pools will likely be inaccurate.
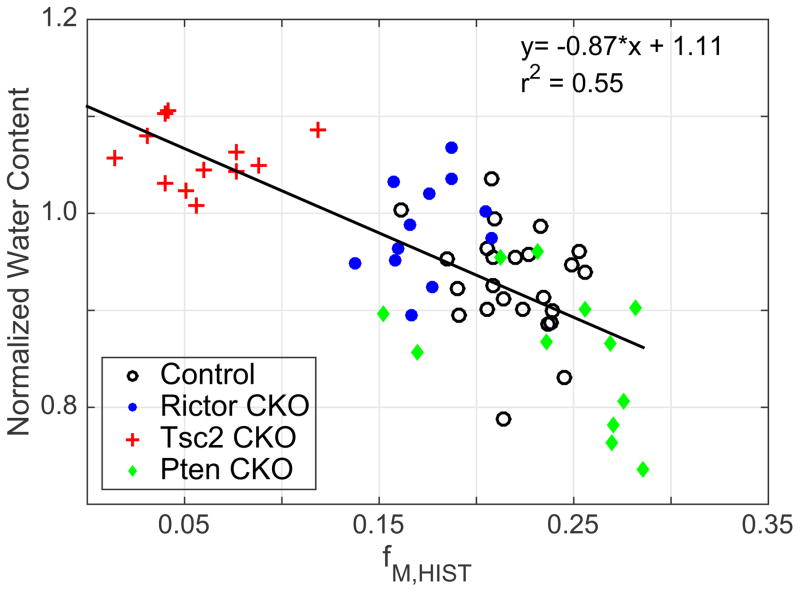
Finally, a few simpler approaches have also been proposed, such as a mono-exponential T1 measurement (Koenig et al., 1990) and measures of the proton density (Mezer et al., 2013). These and similar approaches are essentially a sensitive but not specific measure of myelin, since numerous factors beyond MVF can alter their measurement. That said, from a practical perspective for human imaging, being fast, reproducible, and quantitative measure has obvious advantages. For white matter imaging, the simplest model and, correspondingly, the greatest potential specificity comes from the proton density measurement. For example, in this study, integrating the T2 spectrum and normalizing the amplitude to that of a cortical gray matter voxel results in a measure that correlates strongly with fM,HIST (Fig 8). An interesting feature of this method is that it will be independent of water exchange between myelin and non-myelin compartments. Indeed, variance in fM,T2 between animal models seen above and attributed to water exchange (Fig 7c) is not apparent in Fig 8. In fact, considering a model where MVF is the only determinant of proton density variation in white matter, then a full T2 spectrum with amplitude calibrated to a known water concentration provides enough information to estimate both MVF and water exchange rates, which may in-turn offer an alternative approach to measuring myelin thickness.
Figure 8.
Normalized water content versus fM,HIST with line of best fit and equations shown from control, Rictor CKO, Tsc2 CKO, and Pten CKO mice, (r = −0.74). The linear fit was statistically significant, p ≪ 0.01.
6. CONCLUSION
Here, we assess MRI measures of myelin in control and 3 different models of white matter disease in mouse brain. Both MWF (from MET2) and BPF (from qMT) show strong correlations to quantitative histology. Using a volumetric model of white matter, MWF and BPF were converted to absolute measures of MVF, and displayed strong agreement with histologic myelin volume fraction. Using MET2, MVF measures were derived independently from histology but may be affected by inter-compartmental water exchange depending on myelin microstructure. Using qMT provides a somewhat more accurate estimate of MVF that exhibits less dependence on other microstructure characteristics, but requires calibration, here using histology. This work also provides promise for quantification of g-ratio from MRI where obtaining absolute measures of MVF is important.
Supplementary Material
Supplementary Figure S1. Representative T2 spectra from control, Rictor CKO, Tsc2 CKO, and Pten CKO mice.
Supplementary Figure S2. Regions of interest for the 3 regions in the corpus callosum midbody (orange), genu (green), and splenium (yellow), the anterior commissure (purple), and cortical gray matter (blue).
Supplementary Figure S3. Bound pool fraction (BPF) versus Myelin water fraction (MWF) with line of best fit and equations shown from control, Rictor CKO, Tsc2 CKO, and Pten CKO mice, (r = 0.87). The linear fit was statistically significant, p ≪ 0.01.
Supplementary Figure S4. fM,T2 and fM,MT versus fA,HIST with lines of best fit. r = 0.58 and 0.65, respectively. However, without Tsc2 CKO mice, there is no significant correlation.
Table 1.
Molar concentrations and mass fractions for constituents of myelin and non-myelin macromolecules.
| Constituent | ζi (g/mol 1H) | wi,M | wi,NM |
|---|---|---|---|
|
| |||
| Lipid | 0.80 | 0.43 | |
|
| |||
| Cholesterol | 8.59 | 0.216 | 0.108 |
| Ganglioside | 13.94 | 0.208 | 0 |
| Phosphotidyl ethanolamine | 9.75 | 0.160 | 0.095 |
| Phosphotidyl choline | 9.18 | 0.080 | 0.108 |
| Phosphotidyl serine | 10.51 | 0.068 | 0.043 |
| Sphingomyelin | 9.19 | 0.068 | 0.077 |
|
| |||
| Protein | 0.20 | 0.57 | |
|
| |||
| Myelin basic protein | 20.64 | 0.075 | 0.214 |
| Proteolipid protein | 18.19 | 0.125 | 0.356 |
Acknowledgments
Grant Sponsor: NIH EB001744, NIH EB019980, NSF GRFP DGE-0909667, NIH S10 RR029523, NIH 5K08 NS050484
The authors would like to thank Brittany Parker for assistance with tissue preparation; Vaibhav Janve for supporting data; Julien Cohen-Adad and Tanguy Duval for useful discussions relating to quantitative TEM analysis; and Janice Williams, Mary Dawes, and Maria Vinogradova for help with electron microscopy which was performed through the use of VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and EY08126).
Abbreviations
- MRI
magnetic resonance imaging
- MWF
myelin water fraction
- BPF
bound pool fraction
- fM,T2
myelin volume fraction from MWF
- fM,MT
myelin volume fraction from BPF
- MVF
myelin volume fraction
- AVF
axon volume fraction
- fM,HIST
, myelin volume fraction from histology
- fA,Hist
axon volume fraction from histology
- CKO
conditional knockout
- PBS
phosphate-buffered saline
- MidCC
midbody of corpus callosum
- GCC
genu of corpus callosum
- SCC
splenium of corpus callosum
- AC
anterior commissure
- M0,x
equilibrium magnetization of x
- Vx
volume of x [ml]
- Cx
molar concentration of 1H in x [mol 1H/ml]
- ρx
density of x [g/ml]
- ζx
molar mass of 1H in x [g/mol 1H]
- wx,y
mass fraction of x in y [gx/gy]
- Φx
volume fraction of x in y [mlx/mly]
APPENDIX
The relationship between equilibrium magnetization (M0,x) and volume (Vx) for compartment x, is determined by the molar concentration of protons in each compartment, Cx,
| [A1] |
where k is a constant converting mol 1H to magnetization, Cx = molar concentration of pool x (mol 1H/ml), and Vx = volume of pool x (ml). Further, the concentration, Cx, can be expressed as
| [A2] |
where ρx = mass density of x (g/ml) and ζx = molar mass of 1H in x (g/mol 1H).
For both water compartments, knowing ρW = 1 gH2O/mlH2O, ζW is
| [A3] |
and using ρW and ζW in Eq. A2, CW is
| [A4] |
For the bound proton compartments, CB,M and CB,NM were considered to be the concentrations of carbon-bound protons in myelin and non-myelin compartments, respectively. These values were estimated by first estimating average values of ζB,M, ζB,NM, ρB,M and ρB,NM. The average ζ, for each compartment was computed as the weighted average of molar proton masses of each of the most abundant lipids and proteins as,
| [A5] |
where, x indicates either myelin or non-myelin, and n is the number of different molecules.
For example, cholesterol, [C27H46O], has 45 methylene protons and a molar mass of 386.65 g/mol, making the molar proton (methylene) mass of cholesterol ζcholesterol = 8.59 g/mol 1H. In myelin and non-myelin, the dry mass fraction of cholesterol was estimated as, wcholesterol,M = 0.216 and wcholesterol,NM = 0.108, respectively. Similarly, ζ, wi,M and wi,NM were estimated for all constituent molecules as shown in Table A1 (Gennis, 1989; Horch et al., 2011).
To calculate ρB,M and ρB,NM, first, water mass fractions of myelin and non-myelin tissue (wW,M and wW,NM) were converted to volume fractions (ΦW,M and ΦW,NM) using literature values of ρW, ρM, and ρNM (Laule et al., 2004) as,
| [A6] |
where x indicates either myelin or non-myelin.
Using ρM, ρNM, ΦW,M, and ΦW,NM, the mass densities of bound protons in myelin and non-myelin tissues (ρB,M and ρB,NM, respectively) were determined from,
| [A7] |
where x indicates either myelin or non-myelin. Then, with ζB,M, ζB,NM, ρB,M and ρB,NM, and Eq. A2, CB,M and CB,NM are,
| [A8] |
Thus, the molar concentrations of 1H in the macromolecule compartments of myelin and non-myelin (0.095, and 0.090 mol 1H/ml, respectively) are estimated to be 15–20% lower than that of free water. For simplicity of modeling, we treat all four compartment molar proton concentrations as equal, making magnetization (M0,x) (and, therefore, signal) directly proportional to volume (Vx) (Eq [A1]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso-Ortiz E, Levesque IR, Paquin R, Pike GB. Field inhomogeneity correction for gradient echo myelin water fraction imaging. Magnetic resonance in medicine. 2016 doi: 10.1002/mrm.26334. [DOI] [PubMed] [Google Scholar]
- Carson R, Kelm N, West K, Does M, Fu C, Weaver G, McBrier E, Parker B, Grier M, Ess K. Hypomyelination following deletion of Tsc2 in oligodendrocyte precursors. Annals of Clinical Translational Neurology. 2015;2:1041–54. doi: 10.1002/acn3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RP, Fu C, Winzenburger P, Ess KC. Deletion of Rictor in neural progenitor cells reveals contributions of mTORC2 signaling to tuberous sclerosis complex. Human Molecular Genetics. 2013;22:140–52. doi: 10.1093/hmg/dds414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denef JF, Cordier AC, Mesquita M, Haumont S. The influence of fixation procedure, embedding medium and section thickness on morphometric data in thyroid gland. Histochemistry. 1979;63:163–71. doi: 10.1007/BF00644538. [DOI] [PubMed] [Google Scholar]
- Does MD. Multi-Exponential Relaxation Analysis (MERA) Toolbox, Version 2. 2014 http://www.vuiis.vanderbilt.edu/~doesmd/MERA/MERA_Toolbox.html.
- Does MD, Gore JC. Compartmental study of T1 and T2 in rat brain and trigeminal nerve in vivo. Magnetic Resonance in Medicine. 2002;47:274–83. doi: 10.1002/mrm.10060. [DOI] [PubMed] [Google Scholar]
- Dortch RD, Harkins KD, Juttukonda MR, Gore JC, Does MD. Characterizing inter compartmental water exchange in myelinated tissue using relaxation exchange spectroscopy. Magnetic resonance in medicine. 2013;70:1450–59. doi: 10.1002/mrm.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dula AN, Gochberg DF, Valentine HL, Valentine WM, Does MD. Multiexponential T2, magnetization transfer, and quantitative histology in white matter tracts of rat spinal cord. Magnetic Resonance in Medicine. 2010;63:902–9. doi: 10.1002/mrm.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennis R. Biomembranes: molecular structure and function. Springer-Verlag; New York: 1989. [Google Scholar]
- Gochberg DF, Gore JC. Quantitative magnetization transfer imaging via selective inversion recovery with short repetition times. Magnetic Resonance in Medicine. 2007;57:437–41. doi: 10.1002/mrm.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins K, Dula A, Does M. Effect of intercompartmental water exchange on the apparent myelin water fraction in multiexponential T2 measurements of rat spinal cord. Magnetic Resonance in Medicine. 2012;67:793–800. doi: 10.1002/mrm.23053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins K, Valentine W, Gochberg D, Does M. In-vivo multi-exponential T 2, magnetization transfer and quantitative histology in a rat model of intramyelinic edema. NeuroImage: Clinical. 2013:810–17. doi: 10.1016/j.nicl.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EP, Zhao C, Fancy SP, Kaing S, Franklin RJ, Rowitch DH. Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Annals of Neurology. 2010;68:703–16. doi: 10.1002/ana.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkelman MR, Huang X, Xiang Q, Stanisz G, Swanson SD, Bronskill MJ. Quantitative interpretation of magnetization transfer. Magnetic Resonance in Medicine. 1993;29:759–66. doi: 10.1002/mrm.1910290607. [DOI] [PubMed] [Google Scholar]
- Hennig J. Echoes—how to generate, recognize, use or avoid them in MR-imaging sequences. Part II: Echoes in imaging sequences. Concepts in Magnetic Resonance. 1991;3:179–92. [Google Scholar]
- Horch AR, Gore JC, Does MD. Origins of the ultrashort T21H NMR signals in myelinated nerve: A direct measure of myelin content? Magnetic Resonance in Medicine. 2011;66:24–31. doi: 10.1002/mrm.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D, Kim D, Du Y. In vivo multi-slice mapping of myelin water content using T 2* decay. Neuroimage. 2010;52:198–204. doi: 10.1016/j.neuroimage.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Janve VA, Zu Z, Yao SY, Li K, Zhang FL, Wilson KJ, Ou X, Does MD, Subramaniam S, Gochberg DF. The radial diffusivity and magnetization transfer pool size ratio are sensitive markers for demyelination in a rat model of type III multiple sclerosis (MS) lesions. NeuroImage. 2013;74:298–305. doi: 10.1016/j.neuroimage.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AG, Cofer GP, Gewalt SL, Hedlund LW. Morphologic Phenotyping with MR Microscopy: The Visible Mouse. Radiology. 2002;222:789–93. doi: 10.1148/radiol.2223010531. [DOI] [PubMed] [Google Scholar]
- Kass M, Witkin A, Terzopoulos D. Snakes: Active contour models. International Journal of Computer Vision. 1988;1:321–31. [Google Scholar]
- Koenig S, Brown R, Spiller M, Lundbom N. Relaxometry of brain: Why white matter appears bright in MRI. Magnetic Resonance in Medicine. 1990;14:482–95. doi: 10.1002/mrm.1910140306. [DOI] [PubMed] [Google Scholar]
- Labadie C, Lee J, Rooney W, Jarchow S, Aubert-Frecon M, Springer C, Jr, Moller H. Myelin water mapping by spatially regularized longitudinal relaxographic imaging at high magnetic fields. Magnetic Resonance in Medicine. 2014;71:375–87. doi: 10.1002/mrm.24670. [DOI] [PubMed] [Google Scholar]
- Lankford CL, Does MD. On the inherent precision of mcDESPOT. Magnetic Resonance in Medicine. 2013;69:127–36. doi: 10.1002/mrm.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule C, Leung E, Li D, Traboulsee A. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Multiple Sclerosis. 2006;12:747–53. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- Laule C, Vavasour I, Moore G, Oger J, Li D, Paty D, MacKay A. Water content and myelin water fraction in multiple sclerosis. Journal of Neurology. 2004;251:284–93. doi: 10.1007/s00415-004-0306-6. [DOI] [PubMed] [Google Scholar]
- Lebel MR, Wilman AH. Transverse relaxometry with stimulated echo compensation. Magnetic Resonance in Medicine. 2010;64:1005–14. doi: 10.1002/mrm.22487. [DOI] [PubMed] [Google Scholar]
- Lenz C, Klarhöfer M, Scheffler K. Feasibility of in vivo myelin water imaging using 3D multigradient- echo pulse sequences. Magnetic Resonance in Medicine. 2012;68:523–8. doi: 10.1002/mrm.23241. [DOI] [PubMed] [Google Scholar]
- Levesque I, Pike G. Characterizing healthy and diseased white matter using quantitative magnetization transfer and multicomponent T2 relaxometry: A unified view via a four- pool model. Magnetic Resonance in Medicine. 2009;62:1487–96. doi: 10.1002/mrm.22131. [DOI] [PubMed] [Google Scholar]
- Leys C, Ley C, Klein O, Bernard P, Licata L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Psychology. 2013;49:764–6. [Google Scholar]
- Li K, Zu Z, Xu J, Janve V, Gore J, Does M, Gochberg D. Optimized inversion recovery sequences for quantitative T1 and magnetization transfer imaging. Magnetic Resonance in Medicine. 2010;64:491–500. doi: 10.1002/mrm.22440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay A, Whittall K, Adler J, Li D. In vivo visualization of myelin water in brain by magnetic resonance. Magnetic Resonance in Medicine. 1994;31:673–7. doi: 10.1002/mrm.1910310614. [DOI] [PubMed] [Google Scholar]
- Menon RS, Rusinko MS, Allen PS. Proton relaxation studies of water compartmentalization in a model neurological system. Magnetic Resonance in Medicine. 1992;28:264–74. doi: 10.1002/mrm.1910280208. [DOI] [PubMed] [Google Scholar]
- Mezer A, Yeatman J, Stikov N, Kay K, Cho N, Dougherty R, Perry M, Parvizi J, Hua L, Butts-Pauly K, Wandell B. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Natue Medicine. 2013;19:1667–71. doi: 10.1038/nm.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odrobina E, Lam T, Pun T, Midha R. MR properties of excised neural tissue following experimentally induced demyelination. NMR in Biomedicine. 2005;18:277–84. doi: 10.1002/nbm.951. [DOI] [PubMed] [Google Scholar]
- Oh SH, Bilello M, Schindler M, Markowitz CE, Detre JA, Lee J. Direct visualization of short transverse relaxation time component (ViSTa) Neuroimage. 2013;83:485–492. doi: 10.1016/j.neuroimage.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasloski T, Mädler B, Xiang Q. Applications of stimulated echo correction to multicomponent T2 analysis. Magnetic Resonance in Medicine. 2012;67:1803–14. doi: 10.1002/mrm.23157. [DOI] [PubMed] [Google Scholar]
- Russell-Schulz B, Laule C, Li D, MacKay A. What causes the hyperintense T 2-weighting and increased short T 2 signal in the corticospinal tract? Magnetic Resonance Imaging. 2013;31:329–35. doi: 10.1016/j.mri.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Tozer DJ, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, Miller DH. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. Journal of Magnetic Resonance Imaging. 2007;26:41–51. doi: 10.1002/jmri.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott C, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, Scaravilli F, Barker GJ, Tofts PS, Miller DH. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magnetic Resonance in Medicine. 2008;59:268–77. doi: 10.1002/mrm.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Levesque I, Santos AC, Francis SJ, Narayanan S, Brass SD, Arnold DL, Pike GB. Regional variations in normal brain shown by quantitative magnetization transfer imaging. Magnetic Resonance in Medicine. 2004;51:299–303. doi: 10.1002/mrm.10701. [DOI] [PubMed] [Google Scholar]
- Sled JG, Pike BG. Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magnetic Resonance in Medicine. 2001;46:923–31. doi: 10.1002/mrm.1278. [DOI] [PubMed] [Google Scholar]
- Stikov N, Campbell JS, Stroh T, Lavelée M, Frey S, Novek J, Nuara S, Ho MK, Bedell BJ, Dougherty RF. In vivo histology of the myelin g-ratio with magnetic resonance imaging. NeuroImage. 2015;118:397–405. doi: 10.1016/j.neuroimage.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Stikov N, Perry L, Mezer A, Rykhlevskaia E, Wandell B, Pauly J, Dougherty R. Bound pool fractions complement diffusion measures to describe white matter micro and macrostructure. NeuroImage. 2010;54:1112–21. doi: 10.1016/j.neuroimage.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR. A stereological method for estimating the total length and size of myelin fibers in human brain white matter. Journal of neuroscience methods. 1997;73:193–200. doi: 10.1016/s0165-0270(97)02228-0. [DOI] [PubMed] [Google Scholar]
- Thiessen JD, Zhang Y, Zhang H, Wang L, Buist R, Bigio MR, Kong J, Li X, Martin M. Quantitative MRI and ultrastructural examination of the cuprizone mouse model of demyelination. NMR in Biomedicine. 2013;26:1562–81. doi: 10.1002/nbm.2992. [DOI] [PubMed] [Google Scholar]
- Thirion JP. Image matching as a diffusion process: an analogy with Maxwell’s demons. Medical Image Analysis. 1998;2:243–60. doi: 10.1016/s1361-8415(98)80022-4. [DOI] [PubMed] [Google Scholar]
- Travis AR, Does MD. Selective excitation of myelin water using inversion-recovery-based preparations. Magnetic resonance in medicine. 2005;54:743–7. doi: 10.1002/mrm.20606. [DOI] [PubMed] [Google Scholar]
- Underhill HR, Rostomily RC, Mikheev AM, Yuan C, Yarnykh VL. Fast bound pool fraction imaging of the in vivo rat brain: Association with myelin content and validation in the C6 glioma model. NeuroImage. 2011;54:2052–65. doi: 10.1016/j.neuroimage.2010.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen P, Jiang X, Duyn JH. Effects of magnetization transfer on T1 contrast in human brain white matter. NeuroImage. 2016;128:85–95. doi: 10.1016/j.neuroimage.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S, Munro C, Midha R, Stanisz GJ. Is multicomponent T2 a good measure of myelin content in peripheral nerve? Magnetic Resonance in Medicine. 2003;49:638–45. doi: 10.1002/mrm.10411. [DOI] [PubMed] [Google Scholar]
- West K, Kelm N, Carson R, Does M. A revised model for estimating g-ratio from MRI. NeuroImage. 2016;125:1155–8. doi: 10.1016/j.neuroimage.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittall K, MacKay A. Quantitative interpretation of NMR relaxation data. Journal of Magnetic Resonance. 1989;84:134–52. [Google Scholar]
- Whittall K, Mackay A, Graeb D, Nugent R, Li DK, Paty D. In vivo measurement of T2 distributions and water contents in normal human brain. Magnetic Resonance in Medicine. 1997;37:34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magnetic Resonance in Medicine. 1989;10:135–44. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- Zimmerman J, Brittin W. Nuclear Magnetic Resonance Studies in Multiple Phase Systems: Lifetime of a Water Molecule in an Adsorbing Phase on Silica Gel. The Journal of Physical Chemistry. 1957;61:1328–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Representative T2 spectra from control, Rictor CKO, Tsc2 CKO, and Pten CKO mice.
Supplementary Figure S2. Regions of interest for the 3 regions in the corpus callosum midbody (orange), genu (green), and splenium (yellow), the anterior commissure (purple), and cortical gray matter (blue).
Supplementary Figure S3. Bound pool fraction (BPF) versus Myelin water fraction (MWF) with line of best fit and equations shown from control, Rictor CKO, Tsc2 CKO, and Pten CKO mice, (r = 0.87). The linear fit was statistically significant, p ≪ 0.01.
Supplementary Figure S4. fM,T2 and fM,MT versus fA,HIST with lines of best fit. r = 0.58 and 0.65, respectively. However, without Tsc2 CKO mice, there is no significant correlation.