Summary
Neorickettsia helminthoeca, a type species of the genus Neorickettsia, is an endosymbiont of digenetic trematodes of veterinary importance. Upon ingestion of salmonid fish parasitized with infected trematodes, canids develop salmon poisoning disease (SPD), an acute febrile illness that is particularly severe and often fatal in dogs without adequate treatment. We determined and analysed the complete genome sequence of N. helminthoeca: a single small circular chromosome of 884 232 bp encoding 774 potential proteins. N. helminthoeca is unable to synthesize lipopolysaccharides and most amino acids, but is capable of synthesizing vitamins, cofactors, nucleotides and bacterioferritin. N. helminthoeca is, however, distinct from majority of the family Anaplasmataceae to which it belongs, as it encodes nearly all enzymes required for peptidoglycan biosynthesis, suggesting its structural hardiness and inflammatory potential. Using sera from dogs that were experimentally infected by feeding with parasitized fish or naturally infected in southern California, Western blot analysis revealed that among five predicted N. helminthoeca outer membrane proteins, P51 and strain‐variable surface antigen were uniformly recognized. Our finding will help understanding pathogenesis, prevalence of N. helminthoeca infection among trematodes, canids and potentially other animals in nature to develop effective SPD diagnostic and preventive measures. Recent progresses in large‐scale genome sequencing have been uncovering broad distribution of Neorickettsia spp., the comparative genomics will facilitate understanding of biology and the natural history of these elusive environmental bacteria.
Introduction
Salmon poisoning disease (SPD), an acute and often‐fatal illness in wild and domestic canids, was first discovered in the 1800s when early settlers in Pacific Northwest noted their dogs becoming ill following ingestion of salmon (Philip, 1955). In 1950, a bacterial pathogen was implicated as the causative agent of SPD and named Neorickettsia helminthoeca, due to its biological similarity to the members of the family Rickettsiaceae and the novel invertebrate/helminth vector (Cordy and Gorham, 1950; Philip, 1955). N. helminthoeca exists in all life stages of the fluke Nanophyetus salmincola (Bennington and Pratt, 1960; Schlegel et al., 1968), which has a complicated digenetic life cycle involving both pleurocid freshwater snails (Oxytrema silicula) and salmonid fish as intermediate hosts (Millemann and Knapp, 1970; Headley et al., 2011). Due to the limited geographic range of the vector and intermediate hosts, the distribution of SPD was thought to be limited to the northern Pacific coast. However, SPD cases have been confirmed in southern California (this study; Veterinary Practice News, 2009), Vancouver Island, Canada (Booth et al., 1984), and Maringa, Brazil, using immunohistochemical, histopathological and molecular diagnostic techniques (Table 1; Headley et al., 2004, 2006, 2011), although the vector and life cycle in these regions remain to be identified. The expansion of the geographic distribution of SPD where N. salmincola has not been documented suggests the potential adaptation of this organism to other trematode vectors.
Table 1.
Biological characteristics of Neorickettsia species
| Species | Vertebrate host | Invertebrate vector/hosta | In vivo‐infected mammalian cells | Diseases & symptoms | Geographic distribution |
|---|---|---|---|---|---|
| N. helminthoeca | Canidae | Digenetic trematode Nanophyetus salmincola in snails (Oxytrema silicula) and fish (salmonid) | Monocytes and Macrophages | Salmon Poisoning Disease (pyrexia, anorexia, ocular discharge, weight loss, lethargy and dehydration, > 90% mortality) | California, Washington, Oregon, Idaho, Canada, Brazil |
| N. risticii | Horse, Bat | Digenetic trematode Acanthatrium oregonense in snails (Elimia virginica) and aquatic insects (caddisflies, mayflies) | Monocytes, Macrophages, intestinal epithelial cells and mast cells | Potomac horse fever (fever, depression, anorexia, dehydration, watery diarrhoea, laminitis and/or abortion, ~9% fatality) | USA, Canada, Brazil, Uruguay |
| N. sennetsu | Human | Unknown trematodes in snails and grey mullet fish | Monocytes and Macrophages | Sennetsu neorickettsiosis (fever, fatigue, general malaise and lymphadenopathy) | Japan, South‐East Asia |
a. Transmission mode: all Neorickettsia spp. are transstadially and vertically transmitted through generations of trematodes.
While there is a large range of definitive hosts for the trematode, N. helminthoeca causes severe SPD in members of the Canidae family including dogs, foxes and coyotes (Cordy and Gorham, 1950; Philip et al., 1954a,b; Philip, 1955; Foreyt et al., 1987). Dogs most commonly acquire SPD when they eat raw or undercooked salmonid fish containing encysted trematodes infected with N. helminthoeca. Upon ingestion, the metacercariae stage of the trematode matures in the intestinal lumen for 5–8 days and releases the bacteria to be picked up by monocytes and macrophages in the intestinal wall. The exact mechanism of bacterial entry into these cells is not known, but morphological studies demonstrate the organism existing as clusters termed morulae or singly within a host cell‐derived membrane vacuole in the cytoplasm of the canine host cell (Rikihisa et al., 1991). N. helminthoeca‐infected cells travel throughout the circulation and accumulate in the thoracic and abdominal lymph nodes with the mesenteric and ileocecal lymph nodes being most commonly affected (Philip et al., 1954a; Philip, 1955; Headley et al., 2011). Symptoms begin with pyrexia (39.8–40.9°C) that persists for 6–7 days and anorexia (Rikihisa et al., 1991). Dogs progress to vomiting and diarrhoea that may or may not contain blood 4–6 days following development of a fever. Other symptoms include ocular discharge, weight loss, lethargy and dehydration. If left untreated, death occurs 2–10 days after development of symptoms (Philip, 1955). Current therapies for SPD include fluid therapy, blood transfusions for haemorrhagic diarrhoea, anti‐helminthic praziquantel and oral doxycycline or intravenous oxytetracycline. Affected individuals produce specific immunity to SPD following recovery from the disease (Philip et al., 1954a; Philip, 1955).
Neorickettsia species are obligatory intracellular α‐proteobacteria that belong to the family Anaplasmataceae in the order Rickettsiales (Rikihisa et al., 2005). Neorickettsia spp. are the deepest branching lineage in the family Anaplasmataceae, whereas Anaplasma and Ehrlichia are sister genera that share a common ancestor with Wolbachia spp. (Fig. 1; Pretzman et al., 1995; Wen et al., 1995, 1996). The branching pattern suggests that the speciation of N. helminthoeca occurred earlier than the speciation of N. risticii and N. sennetsu. These findings and many other molecular phylogenetic analyses (Anderson et al., 1992; Wen et al., 1995, 1996; Rikihisa et al., 1997) led to the drastic reclassification of the family Anaplasmataceae (Dumler et al., 2001).
Figure 1.
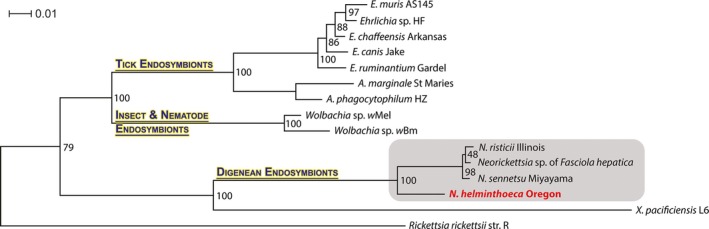
Phylogenetic tree of the family Anaplasmataceae.
16S rRNA sequences of members of the family Anaplasmataceae were aligned using ClustalW, a phylogenetic tree was built using raxml, and the tree was visualized with Dendroscope as described in the ‘Experimental procedures’. Grey box highlights Neorickettsia species.
GenBank Accession numbers and locus tag numbers for the 16S rRNA sequences are N. helminthoeca Oregon, NZ_CP007481/NHE_RS00195; N. risticii Illinois, NC_013009.1/NRI_RS00185; N. sennetsu Miyayama, NC_007798.1/NSE_RS00200; A. phagocytophilum HZ, NC_007797.1/APH_RS03965; A. marginale Florida, NC_012026.1/AMF_RS06130; E. chaffeensis Arkansas, NC_007799.1/ECH_RS03785; E. canis Jake, NC_007354.1/ECAJ_RS00995; E. ruminantium Welgevonden, NC_005295.2/ERUM_RS01035; E. muris AS145, NC_023063.1/MR76_RS00900; Ehrlichia sp. HF, NZ_CP007474.1/EHF_RS03625; Wolbachia pipientis wMel, NC_002978.6/WD_RS05540; Wolbachia endosymbiont of Brugia malayi, NC_006833.1/WBM_RS02885; Rickettsia rickettsii str. R, L36217; Neorickettsia Endobacterium of Fasciola hepatica, LNGI01000001/AS219_00180; Candidatus ‘Xenolissoclinum pacificiensis L6’, AXCJ01000001/P857_926.
Currently, only three pathogenic species of Neorickettsia, namely N. helminthoeca (type species), N. sennetsu (agent of human Sennetsu fever) and N. risticii (agent of Potomac horse fever) have been culture isolated and characterized in sufficient details with documented biological and medical significance (Table 1; Rikihisa et al., 1991, 2005). All of them are known to transmit from trematodes to monocytes/macrophages of mammals (dogs, humans and horses, respectively) and cause severe, sometimes fatal illnesses (Table 1; Rikihisa et al., 2005). In addition, the Stellantochasmus falcatus (SF) agent, which is closely related to N. risticii, was culture isolated from S. falcatus fluke encysting the grey mullet fish in Japan (Wen et al., 1996) and from fish in Oregon (Rikihisa et al., 2004). The initial 16S rRNA gene sequence‐based phylogenetic analysis of N. helminthoeca revealed that the divergence of 16S rRNA sequences is around 5% between N. helminthoeca and N. risticii or N. sennetsu, whereas it is only 0.7% between N. risticii and N. sennetsu.
As endosymbionts of digenetic trematodes (parasitic flatworms or flukes), Neorickettsia species are abundant in nature and have been identified throughout the life cycle of the trematodes and the hosts of trematodes including the essential first intermediate host of snails, the second intermediate hosts such as fish and aquatic insects and the definitive hosts such as mammals and birds wherein the trematodes sexually reproduce fertilized eggs (Cordy and Gorham, 1950; Philip et al., 1954a,b; Philip, 1955; Foreyt et al., 1987; Gibson et al., 2005; Rikihisa et al., 2005; Gibson and Rikihisa, 2008; Greiman et al., 2016). Recent reports revealed more than 10 new genotypes of Neorickettsia in divergent digenean families throughout the world, including Asia, Africa, Australia, Americas and even Antarctica (Ward et al., 2009; Tkach et al., 2012; Greiman et al., 2014, 2017), suggesting a global distribution of Neorickettsia spp. Notably, a Neorickettsia sp. was found in the medically important trematode Fasciola hepatica (the liver fluke, fasciolosis disease agent) isolated from a sheep in Oregon US (McNulty et al., 2017). In addition, a related new species named Candidatus ‘Xenolissoclinum pacificiensis L6’ was identified in the ascidian tunicate Lissoclinum patella, a marine chordate animal at the coast of Papua New Guinea (Kwan and Schmidt, 2013), implicating even boarder distribution of Neorickettsia‐like bacteria among diverse invertebrates. To date, the complete genome sequences have been determined only for N. sennetsu (Dunning Hotopp et al., 2006) and N. risticii (Lin et al., 2009), and almost complete genome sequences were obtained for Neorickettsia endobacterium of F. hepatica (NFh) and Candidatus ‘X. pacificiensis’ (Kwan and Schmidt, 2013; McNulty et al., 2017). The phylogenetic analysis based on 16S rRNA gene sequences suggests that NFh shares > 99% identity with N. risticii and N. sennetsu, while Candidatus ‘X. pacificiensis’ is distantly related to Neorickettsia spp. (Fig. 1). Genomic comparisons indicated that approximately 97% of the predicted proteins (721 of 744) of NFh showed top matches to N. risticii or N. sennetsu, while 22 unique proteins of NFh were hypothetical proteins without functional annotations (McNulty et al., 2017).
Because the mortality rate of SPD is > 90% without rapid antibiotic treatment (Philip, 1955; Rikihisa et al., 1991), the current inefficient diagnostic method (faecal examination for parasite eggs and/or Romanowsky staining of lymph node aspirates), and the expansion of the geographic distribution of SPD, there remains a need for better understanding of N. helminthoeca and development of a simple and rapid serodiagnostic approach. In this study, we sought to (i) determine the complete genome of N. helminthoeca and compare with closely related N. risticii and N. sennetsu genomes, (ii) determine, clone and purify putative immunodominant major outer membrane proteins (OMPs), and (iii) test immunoreactivity of these recombinant OMPs using sera from dogs that were experimentally or naturally infected with N. helminthoeca.
Results and discussion
General features of the genome
The genome of N. helminthoeca Oregon consists of a single double‐stranded circular chromosome spanning 884 232 bp, which is similar to those of N. risticii (Lin et al., 2009) and N. sennetsu (Dunning Hotopp et al., 2006; Table 2), and smaller than those of other members in the family Anaplasmataceae (approximately 1.0–1.5 Mbp; Dunning Hotopp et al., 2006). G+C content of N. helminthoeca genome is 41.7% (Table 2), which is similar to those of other Neorickettsia and Anaplasma spp., but greater than those (approximately 30%) of Ehrlichia spp. and Wolbachia spp. (Dunning Hotopp et al., 2006). The replication origin of N. helminthoeca (Fig. 2) was predicted based on one of the GC‐skew shift points, and the region between hemE (uroporphyrinogen decarboxylase, NHE_RS00005) and an uncharacterized phage protein (NHE_RS04160) as described in N. risticii (Lin et al., 2009), N. sennetsu (Dunning Hotopp et al., 2006) and other members in the family Anaplasmataceae (Ioannidis et al., 2007).
Table 2.
Genome properties of Neorickettsia spp
| Strainsa | NHO | NRI | NSE |
|---|---|---|---|
| RefSeq | NZ_CP007481.1 | NC_013009.1 | NC_007798.1 |
| Size (bp) | 884 232 | 879 977 | 859 006 |
| GC (%) | 41.7 | 41.3 | 41.1 |
| Protein | 774 | 760 | 754 |
| tRNA | 33 | 33 | 33 |
| rRNA | 3 | 3 | 3 |
| Other RNA | 1 | 2 | 3 |
| Pseudogene | 16 | 11 | 2 |
| Total Gene | 827 | 808 | 795 |
| Average gene length | 865 | 842 | 803 |
| Per cent Codingb | 87.1 | 87.0 | 89.3 |
| Assigned functions | 548 | 534 | 540 |
| Unknown functions | 226 (29.2%) | 226 (29.7%) | 214 (28.4%) |
Figure 2.
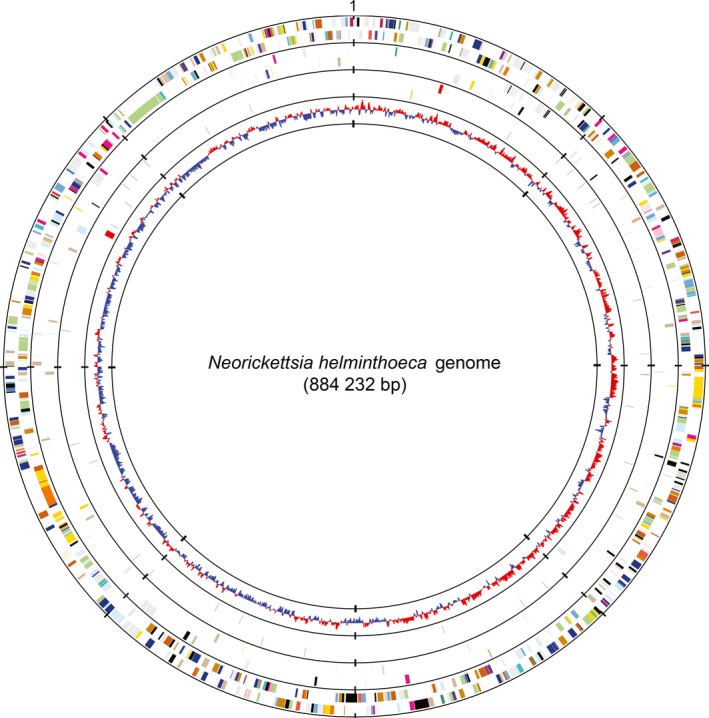
Circular representation of the genome of N. helminthoeca.
From outside to inside, the first circle represents predicted protein‐coding sequences (ORFs) on the plus and minus strands respectively. The second circle represents the unique ORFs of N. helminthoeca in the three‐way comparison with N. risticii and N. sennetsu. Colours indicate the functional role categories of ORFs – dark grey: hypothetical proteins or proteins with unknown functions; gold: amino acid and protein biosynthesis; sky blue: purines, pyrimidines, nucleosides and nucleotides; cyan: fatty acid and phospholipid metabolism; light blue: biosynthesis of cofactors, prosthetic groups and carriers; aquamarine: central intermediary metabolism; royal blue: energy metabolism; pink: transport and binding proteins; dark orange: DNA metabolism and transcription; pale green: protein fate; tomato: regulatory functions and signal transduction; peach puff: cell envelope; pink: cellular processes; maroon: mobile and extrachromosomal element functions. The third circle represent RNA genes, including tRNAs (blue), rRNAs (red) and ncRNAs (orange). The fourth circle represents GC‐skew values [(G‐C)/(G+C)] with a windows size of 500 bp and a step size of 250 bp.
The N. helminthoeca genome encodes one copy each of the 5S, 16S and 23S rRNA genes, which are separated in two loci with the 5S and 23S rRNA genes forming an operon (Fig. 2, red bars in third circle from outside) as in other sequenced members in the family Anaplasmataceae (Massung et al., 2002; Dunning Hotopp et al., 2006). Thirty‐three tRNA genes are identified, which include cognates for all 20 amino acids (Table 2). The numbers of tRNA genes are identical to other Neorickettsia spp., and similar to other members in the family Anaplasmataceae (Dunning Hotopp et al., 2006; Lin et al., 2009), or other bacteria with a single rrn operon (Lee et al., 2009).
With 827 protein‐ and RNA‐coding genes (Fig. 2, Table 2), N. helminthoeca has a smaller number of predicted genes as compared to other members in the family Anaplasmataceae, including Ehrlichia, Anaplasma and Wolbachia endosymbionts of insects or nematodes, each of which have around 1000 or more genes (Crossman, 2006; Dunning Hotopp et al., 2006; Lin et al., 2009). Among the 774 predicted protein‐coding open reading frames (ORFs), 548 genes are assigned with probable functions based on sequence similarity searches. Approximately 29% of the predicted ORFs (226 genes) in the genome are annotated as hypothetical proteins, either with conserved domains or of unknown functions (Table 3).
Table 3.
Role category breakdown of protein‐coding genes in Neorickettsia species
| Role category | NHO | NSE | NRI | Conserveda | Unique in NHOa |
|---|---|---|---|---|---|
| Amino acid biosynthesis | 12 | 9 | 9 | 9 | 3 |
| Biosynthesis of cofactor and vitamin | 62 | 63 | 64 | 60 | 1 |
| Cell envelope | 45 | 31 | 31 | 28 | 17b |
| Cellular processes | 43 | 36 | 36 | 37 | 3 |
| Central intermediary metabolism | 8 | 5 | 5 | 5 | 3 |
| DNA metabolism | 33 | 36 | 37 | 33 | |
| Energy metabolism | 76 | 74 | 76 | 74 | |
| Fatty acid and phospholipid metabolism | 22 | 22 | 22 | 22 | |
| Mobile elements | 4 | 4 | 4 | 4 | |
| Protein fate | 87 | 86 | 88 | 86 | |
| Protein synthesis | 107 | 104 | 105 | 102 | 2 |
| Nucleotide biosynthesis | 37 | 36 | 36 | 36 | |
| Regulatory functions | 10 | 10 | 10 | 10 | |
| Signal transduction | 5 | 5 | 5 | 5 | |
| Transcription | 23 | 23 | 24 | 22 | |
| Transport and binding proteins | 50 | 45 | 45 | 44 | 5 |
| Unknown functions | 226 | 226 | 214 | 160 | 55 |
| Total Proteinsc | 774 | 760 | 754 | 668 | 89 |
| Total Assigned Functions: | 548 | 534 | 540 | 525 |
a. Proteins conserved among three Neorickettsia spp. and specific to N. helminthoeca are based on 3‐way comparison analysis by BlastP (E < e−10).
b. N. helminthoeca encodes nearly complete pathways for peptidoglycan biosynthesis.
c. Certain proteins are assigned to multiple role categories.
Comparison of genomic contents among Neorickettsia species
Previous studies have shown that Anaplasma spp. and Ehrlichia spp. have a single large‐scale symmetrical inversion (X‐alignment) near the replication origin, which is possibly mediated by duplicated rho genes (Dunning Hotopp et al., 2006; Frutos et al., 2007; Nene and Kole, 2009). In addition, Anaplasma and Wolbachia spp. have extensive genomic rearrangement throughout the genome (Wu et al., 2004; Dunning Hotopp et al., 2006). However, the synteny is highly conserved and such genomic rearrangements or a large‐scale inversion is not detected among N. helminthoeca, N. sennetsu and N. risticii (Fig. S1), and rho is not duplicated in three sequenced Neorickettsia spp. In agreement with the 16S rRNA divergence (Fig. 1), N. helminthoeca exhibits multiple synteny divergences from N. risticii and N. sennetsu (Fig. S1).
To compare the genomic contents among Neorickettsia spp., two‐ and three‐way comparisons were performed using reciprocal blastp algorithm with E‐value < 1e−10, and homologous protein clusters were constructed. Three‐way comparison among Neorickettsia spp. showed that > 86% (668 of total 774 protein‐coding ORFs) of N. helminthoeca proteins are conserved with N. risticii and N. sennetsu (Table 3 and Table S1). The vast majority (> 78%, 525/668 ORFs) of these conserved proteins are associated with housekeeping functions and likely essential for Neorickettsia survival (Table 3). Two‐way comparisons revealed that N. risticii and N. sennetsu share an additional 55 conserved proteins, whereas N. helminthoeca shares very limited numbers of orthologues (< 10 proteins) with N. risticii or N. sennetsu (Fig. 3). The result of the two‐way and three‐way comparisons is consistent with the relationship of the species revealed through 16S rRNA‐based phylogeny and whole‐genome synteny analysis.
Figure 3.
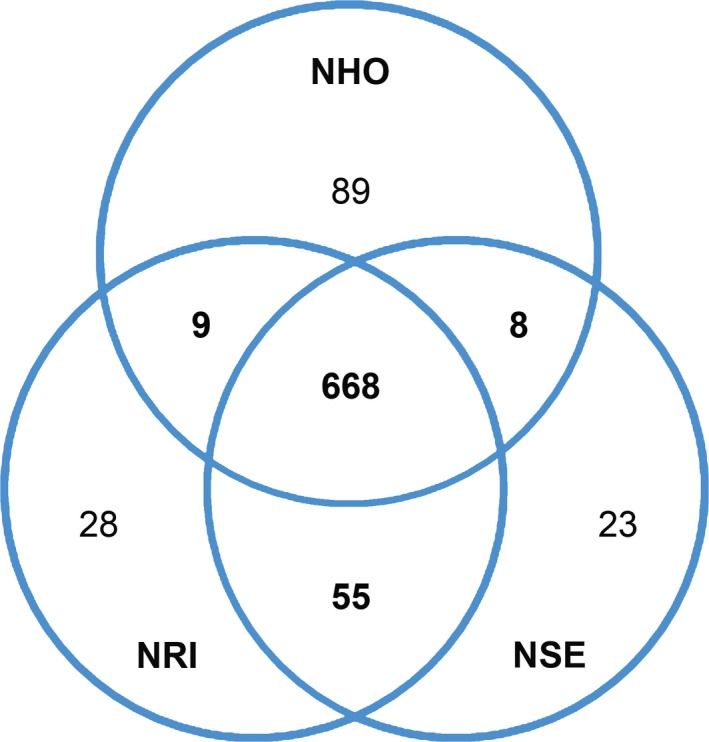
Numbers of protein orthologues shared among Neorickettsia spp.
A Venn diagram was constructed showing the comparison of conserved and unique genes between Neorickettsia spp. as determined by reciprocal blastp algorithm (E < e−10). Numbers within the intersections of different circles indicate orthologue clusters shared by two or three organisms. Species indicated in the diagram are abbreviated as follows: N. helminthoeca (NHO), N. sennetsu (NSE), N. risticii (NRI).
The three Neorickettsia spp. are transmitted by distinct trematodes and cause severe diseases at high mortality in different mammalian hosts (Table 1; Cordes et al., 1986; Dutta et al., 1988; Rikihisa et al., 1991, 2004, 2005; Gibson and Rikihisa, 2008; Lin et al., 2009). We, therefore, analysed the species‐specific genes based on the two‐ and three‐way comparisons. There are 89 species‐specific proteins in N. helminthoeca as compared to 28 and 23 in N. risticii and N. sennetsu respectively (Tables S2–S4). Of the genes unique to N. helminthoeca, more than half of them (50/89 ORFs) are hypothetical proteins without assigned functions (Table S2). Among the N. helminthoeca‐specific proteins with assigned functions, ~38% (15/39 ORFs) are involved in peptidoglycan biosynthesis that are absent in N. risticii and N. sennetsu (Table S2 and Fig. 5), and six proteins are categorized as transporters for iron and other substrates (Table S2). The genomic loci encoding these unique ORFs are distributed throughout N. helminthoeca genome and not clustered in certain islands (Fig. 2, second circle from outside). Blast searches using these N. helminthoeca‐specific proteins against NCBI protein database excluding Neorickettsia spp. showed that only 29 of them match to proteins in other genera, and the majority of them (19, 65.5%) belong to α‐proteobacteria (Table S2). However, whether these proteins are the results of horizontal gene transfer or mutations/deletions from the ancestors of Neorickettsia spp. remains to be determined.
Figure 5.
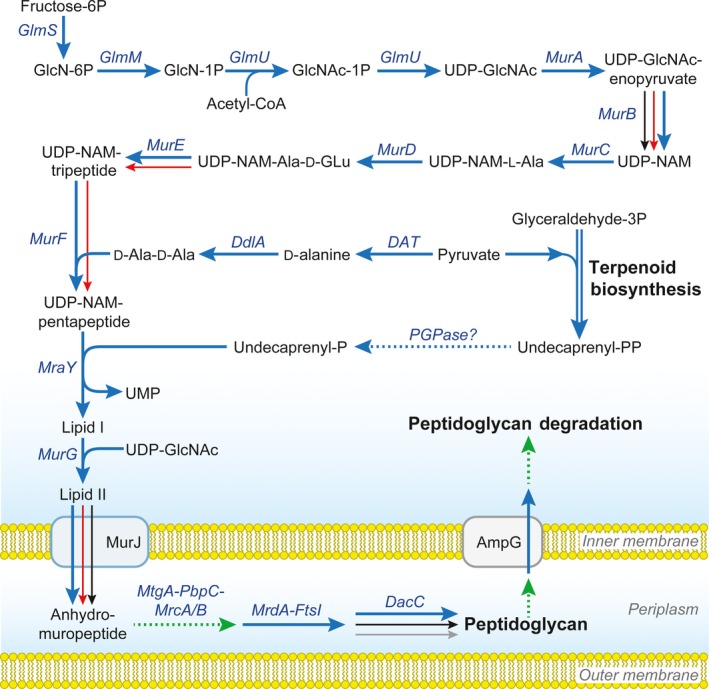
Genes involved in peptidoglycan biosynthesis in selected members of the family Anaplasmataceae.
Biosynthesis pathways of peptidoglycan for N. helminthoeca, N. risticii, N. sennetsu, E. chaffeensis, E. ruminantium, A. phagocytophilum, A. marginale and Wolbachia wMel endosymbiont of Drosophila melanogaster were downloaded from KEGG database (http://www.genome.jp) and analysed. N. helminthoeca, A. marginale and Wolbachia wMel encode nearly all genes for peptidoglycan biosynthesis pathways (blue arrows), except that A. marginale and Wolbachia wMel lacks genes for the biosynthesis of d‐Ala‐d‐Ala. In addition, all members in the family Anaplasmataceae encode terpenoid biosynthesis pathways like isopentenyl‐, farnesyl‐ and geranyl‐diphosphate; however, only Neorickettsia and Wolbachia spp. encode undecaprenyl diphosphate (Und‐PP) synthase (UppS) to produce Und‐PP. N. helminthoeca encodes two PGPases (NHE_RS00895 and NHE_RS01205) that might produce Und‐P from Und‐PP. Genes present in N. risticii and N. sennetsu, red arrows; A. phagocytophilum, black arrows; E. chaffeensis and E. ruminantium, grey arrow. Dashed green lines, genes absent in all bacteria analysed; dashed blue line, potential pathway present. Diagram was modified from KEGG pathways and Gillespie et al. (2010). Abbreviations: GlcN, d‐Glucosamine; GlcNAc, N‐Acetyl‐α‐d‐glucosamine; UDP‐NAM, UDP‐N‐acetylmuramate; Undecaprenyl‐PP (Und‐PP), di‐trans, poly‐cis‐undecaprenyl diphosphate; mDAP, meso‐2,6‐diaminopimelate; UDP‐NAM‐Tripeptide, UDP‐NAM‐l‐Ala‐d‐Glu‐mDAP; UDP‐NAM‐Pentapeptide, UDP‐NAM‐l‐Ala‐d‐Glu‐mDAP‐d‐Ala‐d‐Ala; Lipid I, Und‐PP‐NAM‐l‐Ala‐d‐Glu‐mDAP‐d‐Ala‐d‐Ala; Lipid II, Und‐PP‐NAM‐(GlcNAc)‐l‐Ala‐d‐Glu‐mDAP‐d‐Ala‐d‐Ala; DAT, d‐alanine transaminase; PGPase, phosphatidylglycerophosphatase.
Metabolism
Except for peptidoglycan biosynthesis, most metabolic pathways, transcription, translation and regulatory functions, are highly conserved in N. helminthoeca compared to N. sennetsu and N. risticii (summarized in Fig. 4, Tables 3 and S1; Dunning Hotopp et al., 2006; Lin et al., 2009).
Figure 4.
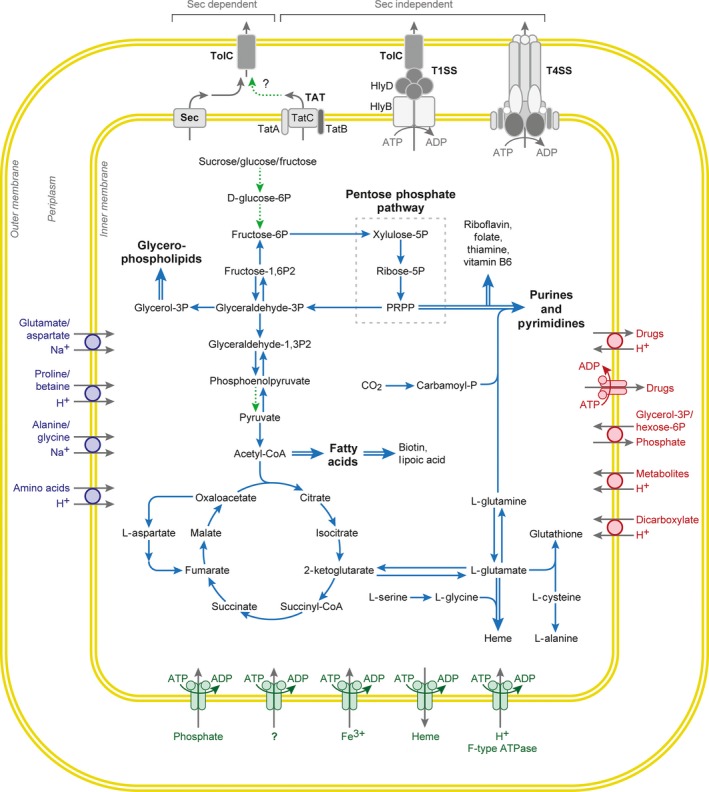
Major metabolic pathways and secretion systems of N. helminthoeca.
N. helminthoeca encodes pathways for aerobic respiration, including the tricarboxylic acid (TCA) cycle and the electron transport chain, but it is unable to use glucose, fructose, or fatty acids directly as a carbon or energy source. N. helminthoeca can synthesize very limited amino acids, but can synthesize most vitamins/cofactors, fatty acids and certain phospholipids, and encodes complete pathways for de novo purine and pyrimidine biosynthesis. Putative transporters were analysed by TransAAP (http://www.membranetransport.org/), and secretion systems were drawn as described in Results. Solid lines, pathways present; dashed lines, pathways absent; double lines, multiple steps involved. Graph was modified from KEGG pathways, Dunning Hotopp et al. (2006) and Gillespie et al. (2015).
Central metabolic pathways
Analysis of the metabolic pathways based on Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.kegg.jp) and BioCyc (http://biocyc.org/) indicates that, similar to other members in the family Anaplasmataceae, N. helminthoeca encodes pathways for aerobic respiration, including the tricarboxylic acid (TCA) cycle and the electron transport chain, but it is unable to use glucose, fructose, or fatty acids directly as a carbon or energy source, as essential enzymes for the utilization of these substrates, such as hexokinases, the first enzyme in the glycolysis pathway that converts glucose to glucose 6‐phosphate, and pyruvate kinase that converts phosphoenolpyruvate to pyruvate, are not identified (Fig. 4). It is likely that N. helminthoeca can synthesize ATP from glutamine as N. risticii, N. sennetsu, or E. chaffeensis does (Weiss et al., 1989; Cheng et al., 2014), as it encodes carbamoyl phosphate synthase (carA/B, NHE_RS00875/NHE_RS02090) and bifunctional glutamate synthase β subunit/2‐polyprenylphenol hydroxylase (GS/PH, NHE_RS02780). These enzymes can convert glutamine to ammonia and glutamate (Fig. 4), and glutamate can be further converted by glutamate dehydrogenase (NHE_RS02165) to 2‐ketoglutarate, which enters the TCA cycle for energy production.
Amino acids, nucleotides, fatty acids and cofactor biosynthesis
Like other Neorickettsia, Ehrlichia and Anaplasma spp. (Dunning Hotopp et al., 2006; Lin et al., 2009), N. helminthoeca synthesizes very limited amino acids including alanine, aspartate, glycine, glutamate and glutamine (Fig. 4 and Table S5). As they are converted from other amino acids or metabolic intermediates, N. helminthoeca must transport most amino acids from its host as discussed further below (Table S7). However, as other members of the family Anaplasmataceae, analysis of KEGG pathways showed that most enzymes are identified for the biosynthesis of fatty acids and certain phospholipids, including phosphatidylglycerol, phosphatidylserine, phosphatidylethanolamine and myo‐inositol‐phosphates (Fig. 4).
Similar to all other sequenced members of Anaplasmataceae (Dunning Hotopp et al., 2006), N. helminthoeca encodes a non‐oxidative pentose‐phosphate pathway that utilizes glyceraldehyde‐3‐phosphate to produce pentose for nucleotide and cofactor biosynthesis. Accordingly, N. helminthoeca encodes complete pathways for de novo purine and pyrimidine biosynthesis and is capable of synthesizing most vitamins or cofactors, such as biotin, folate, FAD, NAD and protoheme (Fig. 4 and Table S6). Overall, N. helminthoeca encodes large number of genes involved in the biosynthesis of cofactors, vitamins and nucleotides (17.2%, 133 of total 774 protein‐coding ORFs), similar to other members of Anaplasmataceae like Ehrlichia (13.4%, 149/1115 ORFs in E. chaffeensis), Anaplasma (10.6%, 145/1370 ORFs in A. phagocytophilum; Dunning Hotopp et al., 2006) and Wolbachia endosymbionts of the insects or nematodes (9.4%, 120/1271 in Wolbachia pipientis wMel; Foster et al., 2005; Brownlie et al., 2009). Unlike tick‐borne members in the family Anaplasmataceae (Ehrlichia and Anaplasma spp.), Neorickettsia spp. are maintained throughout the life cycle of the trematodes (Greiman et al., 2016; Fig. 1). The presence of these biosynthesis pathways suggests that N. helminthoeca do not need to compete with the host for the essential vitamins and nucleotides, which is likely beneficial for their survival especially in invertebrate hosts.
Transporters and porins
To compensate for the incomplete biosynthesis or metabolic pathways, the N. helminthoeca genome encodes several orthologues involved in cytoplasmic membrane transport systems that can supply the necessary amino acids, metabolites and ions, as analysed by TransAAP (Transporter Automatic Annotation Pipeline, http://www.membranetransport.org/; Fig. 4 and Table S7; Ren and Paulsen, 2007; Ren et al., 2007). Transporters for acetyl‐CoA involved in many metabolic pathways and glycerol‐3‐phosphate in phospholipid biosynthesis are identified in N. helminthoeca genome (Table S7). Transport systems for phosphates (pstA/B/C/S), cations, anions, organic ions and multidrug resistance pumps are also present (Table S7). Putative amino acid transporters for alanine, glycine, proline and dicarboxylate amino acids (glutamate or aspartate family) can be found (Table S7). However, as very few amino acids can be synthesized in N. helminthoeca, more transporters are required; it is possible that some ATP‐binding cassette (ABC)‐type transporters with no assigned functions or porins discussed below could act as transporters for amino acids as well as metabolites for protein synthesis and energy production. Orthologues of most identified transporters are conserved in N. risticii and N. sennetsu genomes (Tables S1 and S7), except for few N. helminthoeca‐specific transporters listed in Table S2. Unlike Rickettsia spp. (Winkler, 1976), but similar to all other sequenced members of the Family Anaplasmataceae, N. helminthoeca does not encode translocases for ATP (ATP:ADP antiporters) or NADH, so it likely relies on its own ATP production or encodes unique ATP acquisition mechanisms.
Gram‐negative bacteria also express porins spanning their outer membranes that enable the transport of hydrophilic and large molecules, such as amino acids, sugars and other nutrients (Nikaido, 2003). Similar to other members of the Anaplasmataceae that have limited capabilities of amino acids biosynthesis, intermediary metabolism and glycolysis, nutrient uptake in these bacteria necessitates pores or channels in the bacterial outer membrane (Huang et al., 2007; Kumagai et al., 2008; Gibson et al., 2010). Previous studies have determined that the major outer membrane proteins, including A. phagocytophilum P44s (Huang et al., 2007), E. chaffeensis P28/OMP‐1F (Kumagai et al., 2008) and N. sennetsu P51 (Gibson et al., 2010), possess porin activities as determined by a proteoliposome swelling assay, which allow the diffusion of l‐glutamine, the monosaccharides arabinose and glucose, the disaccharide sucrose and even the tetrasaccharide stachyose. N. helminthoeca encodes a P51 protein (NHE_RS00965) that shares 60% amino acid sequence similarity with N. sennetsu P51 protein (Fig. 6A). Prediction of the two‐dimensional structure of N. helminthoeca P51 using PRED‐TMBB (http://biophysics.biol.uoa.gr/PRED-TMBB/; Bagos et al., 2004) showed that P51 protein contains 18 transmembrane domains with a discrimination value of 2.949 (Fig. S2), suggesting that it is a β‐barrel protein localized to the outer membrane similar to N. sennetsu P51 (Gibson et al., 2010). Therefore, it is likely that N. helminthoeca P51 can function as a porin for nutrient uptake from the host.
DNA, RNA, protein synthesis and DNA repair
Neorickettsia helminthoeca encodes proteins necessary for DNA replication, RNA synthesis and degradation and ribosomal proteins. Although N. helminthoeca encodes proteins required for homologous recombination, including RecA/RecF (but not RecBCD) pathways (Lin et al., 2006) and RuvABC complexes for Holliday junction recombination as other members of the family Anaplasmataceae (Table S8), it has the least amount of enzymes involved in DNA repair compared to other members of the family Anaplasmataceae including N. sennetsu and N. risticii (seven in N. helminthoeca vs. nine in N. sennetsu, 12 in E. chaffeensis and 13 in A. phagocytophilum, Table S8; Dunning Hotopp et al., 2006; Lin et al., 2009). N. helminthoeca lacks most genes required for mismatch repair, nucleotide excision repair (NER, such as uvrABC for UV‐induced DNA damage), various glycosylases for base excision repair (BER) and DNA photolyases, which is an alternative mechanism to repair UV‐damaged DNA identified in E. chaffeensis, A. phagocytophilum and N. risticii (Dunning Hotopp et al., 2006; Lin et al., 2009).
Pathogenesis
Although SPD was recognized more than two centuries ago, the causative agent N. helminthoeca was only stably cultured in a canine cell line in 1990 (Rikihisa et al., 1991), and there is little information available regarding the molecular determinants of N. helminthoeca to invade and cause severe disease in canine hosts. Here, we analysed genes and pathways that are potentially involved in N. helminthoeca pathogenesis, including protein secretion systems, two‐component/one‐component regulatory systems, N. helminthoeca‐specific genes and putative membrane proteins or lipoproteins.
Protein secretion systems
Two major pathways exist to secrete proteins across the cytoplasmic membrane in bacteria. The general Secretion route, termed Sec‐pathway, catalyses the transmembrane translocation of proteins in their unfolded conformation, whereupon they fold into their native structure at the trans‐side of the membrane (Natale et al., 2008). All major components for the Sec‐dependent pathway are identified, including signal recognition particle (SRP) protein, SRP‐docking protein FtsY, the cytosolic protein‐export chaperone SecB, peripheral associated ATP‐dependent motor protein SecA, membrane‐embedded protein conducting channel SecYEG, periplasmic protein YajC that involved in preprotein translocase activity and the membrane complex SecDF that enhances proton motive force (Fig. 4 and summarized under role category ‘Protein fate’ in Table S1). In addition, common chaperones are identified in N. helminthoeca genome, including groEL, groES, dnaK, dnaJ, hscA/B, grpE and htpG (summarized under role category ‘Protein fate’ in Table S1).
Twin‐arginine translocation (Tat)‐pathway, which consists of the TatA, TatB and TatC proteins, can transport folded proteins across the bacterial cytoplasmic membrane by recognizing N‐terminal signal peptides harbouring a distinctive twin‐arginine motif (Lee et al., 2006; Sargent et al., 2006). All genes encoding Tat apparatus are identified in the N. helminthoeca genome (tatA/NHE_RS02000, tatB/NHE_RS02160 and tatC/NHE_RS00490; Fig. 4 and Table S6; Gillespie et al., 2015). However, despite the presence of Tat system, no protein substrate containing a putative Tat signal peptide can be identified in N. helminthoeca using both TAT‐FIND (http://www.cbs.dtu.dk/services/TatP/; Bendtsen et al., 2005) and PRED‐TAT (http://www.compgen.org/tools/PRED-TAT) algorithms (Bagos et al., 2010). Gillespie et al. (2015) reported only a single Tat substrate (PetA) in Rickettsia and suggested that could be due to the substantial differences in signal peptides of Tat substrates in the obligate intracellular bacteria.
Extracellular secretion of various virulence factors across the bacterial cell envelope is one of the major mechanisms by which pathogenic bacteria alter host cell functions, thus enhancing survival of the bacteria and damaging hosts. At least six distinct extracellular protein secretion systems, referred to as type I‐VI secretion systems (T1SS‐T6SS; Papanikou et al., 2007; Costa et al., 2015), have been classified in Gram‐negative bacteria that secrete effector molecules across two lipid bilayers and the periplasm. Except for T2SS, all double‐membrane‐spanning secretion systems (T1SS, T3SS, T4SS and T6SS) use a one‐step mechanism to transport substrates directly from the bacterial cytoplasm into the extracellular space or into a target cell (Costa et al., 2015). Bioinfomatic analysis shows that, similar to all other sequenced members of the family Anaplasmataceae, N. helminthoeca genome encodes both T1SS and T4SS for secretion of proteins across the membranes, but it lacks homologs of T2SS, T3SS, T5SS or T6SS components (Fig. 4; Henderson et al., 2004; Cianciotto, 2005; Bingle et al., 2008). T1SS, a Sec‐independent ATP‐driven ABC transporter system that bypasses the periplasm, is capable of transporting target proteins carrying a C‐terminal uncleaved secretion signal across both inner and outer membranes and into the extracellular medium (Delepelaire, 2004). All of the three components of T1SS, including an inner membrane ATP‐binding cassette (ABC) transporter HlyB (NHE_RS00175), a periplasmic membrane fusion protein (MFP) HlyD (NHE_RS04020) and an outer membrane channel protein TolC (NHE_RS03400) are identified in the N. helminthoeca genome (Fig. 4, Table S1 and S6). A previous study reported that several tandem repeat proteins (TRP120, TRP47 and TRP32/VLPT) are T1SS substrates of E. chaffeensis using an E. coli T1SS surrogate system (Wakeel et al., 2011). Current analysis using the T‐REKS algorism (Jorda and Kajava, 2009) identified several tandem repeat‐containing proteins (not homologous to E. chaffeensis TRPs) like VirB6 and SSAs in all three sequenced Neorickettsia; however, whether these proteins are also secreted by T1SS is unknown (Table S9; Dunning Hotopp et al., 2006; Lin et al., 2009).
T4SS can translocate bacterial effector molecules into host cells, thus often plays a key role in pathogenesis of Gram‐negative host‐associated bacteria (Cascales and Christie, 2003; Backert and Meyer, 2006; Gillespie et al., 2010; Christie et al., 2014). In several intracellular bacteria including the family Anaplasmataceae such as E. chaffeensis and A. phagocytophilum, the T4SS is critical for survival and replication inside host cells, by inducing autophagy for nutrient acquisition and inhibition of host cell apoptosis (Niu et al., 2006; Niu et al., 2010; Liu et al., 2012; Niu et al., 2012; Lin et al., 2007; Lin et al., 2016). In the N. helminthoeca genome, we identified a T4SS encoded by virB/D genes distributed in four separate loci. The organization of virB/D gene clusters is conserved among Neorickettsia spp. as with other Anaplasmataceae, with duplicated genes of virB4, virB8 and virB9, and multiple copies of virB2 and virB6 genes (Tables S1 and S6).
Subcellular fractionation and functional studies have demonstrated that VirB2 is the major pilus component of T4SS extracellular filaments (Cascales and Christie, 2003; Backert and Meyer, 2006). Our previous study has confirmed that N. risticii VirB2 was localized at the opposite poles on the bacterial surface (Lin et al., 2009), suggesting that VirB2 might serve as secretion channels for the T4SS apparatus like that of Agrobacterium (Cascales and Christie, 2003), and play critical roles in mediating the interaction with host cells. Analysis of N. helminthoeca genome reveals three copies of virB2 upstream of virB4, whereas N. risticii and N. sennetsu encode two virB2 genes (Table S6; Lin et al., 2009). Alignment of VirB2 protein sequences indicates that VirB2s of Neorickettsia spp. are closely related to those of other α‐proteobacteria like Rickettsia, Agrobacterium and Caulobacter, but are phylogenetically distinct from VirB2s of E. chaffeensis and A. phagocytophilum that form a separate clade (Fig. S3; Gillespie et al., 2009, 2010). The different numbers of virB2 genes and distinct differences in phylogenetic trees of VirB2 from 16S rRNA gene suggest that virB2 genes might undergo lineage‐specific mutations, duplications, or deletions (Gillespie et al., 2010).
Two‐component regulatory systems
Two‐component regulatory systems (TCRS) are signal transduction systems that allow bacteria to sense and respond rapidly to changing environmental conditions (Mitrophanov and Groisman, 2008; Wuichet et al., 2010). TCRS consists of a sensor histidine protein kinase that responds to specific signals, and a cognate response regulator. Phosphorylation of a response regulator by a cognate histidine kinase changes the biochemical properties of its output domain, which can participate in DNA binding and transcriptional control, perform enzymatic activities, bind RNA, or engage in protein–protein interactions (Gao et al., 2007). TCRS plays a key role in controlling virulence responses in a wide variety of bacterial pathogens (Dorman et al., 2001; Mitrophanov and Groisman, 2008), including E. chaffeensis and A. phagocytophilum in the family Anaplasmataceae, which encode three pairs of TCRS, including CckA/CtrA, PleC/PleD and NtrX/NtrY (Cheng et al., 2006; Cheng et al., 2011; Kumagai et al., 2006; Kumagai et al., 2011).
Computational analysis reveals that the three sequenced Neorickettsia spp. encode two pairs of TCRS: CckA/CtrA and PleC/PleD (Table S6). The histidine kinase CckA/response regulator CtrA pair, identified only in α‐proteobacteria, also have been demonstrated to coordinate multiple cell cycle events at the transcriptional level in E. chaffeensis to regulate bacterial developmental cycle (Cheng et al., 2011). Different from Ehrlichia and Anaplasma, the three Neorickettsia spp. encode two copies of PleC histidine kinase (NHE_RS00035/NHE_RS02255, Tables S1 and S6) and a one‐component signal transduction protein, an EAL domain protein (NHE_RS01830; Fig. S4; Ulrich and Zhulin, 2007; Lin et al., 2009; Romling, 2009; Ulrich and Zhulin, 2010; Lai et al., 2009). The response regulator PleD (NHE_RS02155) can function as diguanyl cyclase that produces cyclic diguanylate (c‐di‐GMP) to regulate cell surface adhesiveness like biofilm or extracellular matrix formation (Tischler and Camilli, 2004), whereas EAL domain protein can function as a diguanylate phosphodiesterase (PDE) that converts c‐di‐GMP to GMP. They likely function synergistically to regulate surface adhesiveness of Neorickettsia, resulting much smaller morulae sizes and more dispersed bacterial colonies compared to Ehrlichia and Anaplasma (Rikihisa, 1991a). In addition, Neorickettsia spp. do not encode genes for NtrY/NtrX, which are thought to be involved in nitrogen metabolism and regulation of nitrogen fixation genes like glnA that encodes a glutamine synthase as in E. chaffeensis (Cheng et al., 2014). Despite this, N. helminthoeca encodes GlnA (NHE_RS01490) and ABC dicarboxylate amino acid transporters (NHE_RS00770) that are predicted to take up glutamine (Table S7) similar to E. chaffeensis (Cheng et al., 2014), suggesting regulation of nitrogen metabolism in Neorickettsia spp. is different from Ehrlichia and Anaplasma spp.
One‐component regulatory systems and transcriptional regulations
One‐component regulatory systems consist of a single protein containing both input and output domains, but lack the phospho‐transfer domains of TCRS, and carry out signalling events in prokaryotes (Ulrich et al., 2005; Ulrich and Zhulin, 2007, 2010). This study found that compared to Ehrlichia and Anaplasma, the three Neorickettsia spp. encode more proteins in one‐component systems (indicated by asterisks in Fig. S4, based on Microbial Signal Transduction Database at http://mistdb.com; Ulrich et al., 2005). Other than an EAL domain protein described above and an HD domain containing deoxyguanosinetriphosphate triphosphohydrolase protein (NHE_RS01895), most one‐component regulatory systems of N. helminthoeca as well as N. risticii and N. sennetsu are predicted to be DNA‐binding transcriptional regulators (Fig. S4, Table S1).
Perhaps due to the relatively homoeostatic intracellular environment of the eukaryotic host cells, members of the order Rickettsiales and Chlamydiaceae have a small number of transcriptional regulators. N. helminthoeca as all other members of the family Anaplasmataceae encodes only two sigma factors: the essential RNA polymerase sigma‐70 factor (RpoD, NHE_RS01300) responsible for most RNA synthesis in exponentially growing cells, and sigma‐32 factor (RpoH, NHE_RS01445) responsible for expression from heat‐shock promoters.
Neorickettsia helminthoeca encodes a putative transcriptional regulator NhxR (N. helminthoeca expression regulator), a 12.5‐kDa DNA‐binding protein (NHE_RS00155) that has 90% amino acid identity with N. risticii NrxR (NRI_RS00145) and N. sennetsu NsxR (NSE_RS00160). NhxR homologues, A. phagocytophilum ApxR and E. chaffeensis EcxR have shown to regulate the expression of P44 outer membrane proteins and the T4SS respectively (Wang et al., 2007b,a; Cheng et al., 2008). The other putative transcriptional regulator Tr1 (NHE_RS00915) is homologous to A. phagocytophilum and E. chaffeensis Tr1, which is regulated by ApxR in A. phagocytophilum and located at the upstream of the tandem genes encoding the major outer membrane proteins (OMPs), like Omp‐1/Msp‐2/P44 expression loci in A. phagocytophilum (Lin et al., 2004) or P28/Omp‐1 gene clusters in E. chaffeensis (Ohashi et al., 2001; Wang et al., 2007a; Rikihisa, 2010). However, Tr1 in N. helminthoeca, N. risticii, or N. sennetsu is not located at upstream of any of genes encoding the major OMPs of N. helminthoeca including P51, SSA or NSPs (Table 4).
Table 4.
Putative outer membrane proteins of Neorickettsia helminthoeca a
| Locus ID | Gene symbol | Protein name | MW (kDa) |
|---|---|---|---|
| Molecular Characterization | |||
| NHE_RS00965 | p51 | P51 Gram‐negative porin family protein | 51.6 |
| NHE_RS03715 | nsp1 | Neorickettsia surface protein 1 | 27.6 |
| NHE_RS03720 | nsp2 | Neorickettsia surface protein 2 | 33.7 |
| NHE_RS03725 | nsp3 | Neorickettsia surface protein 3 | 24.7 |
| NHE_RS03855 | ssa | Strain‐specific surface antigen | 35.5 |
| pSort‐B Prediction | |||
| NHE_RS00040 | Conserved hypothetical protein | 46.3 | |
| NHE_RS01885 | Conserved hypothetical protein | 73.4 | |
| NHE_RS03040 | yaeT | Outer membrane protein assembly complex, YaeT protein | 82.9 |
| NHE_RS03940 | bamD | BamD lipoprotein | 26.5 |
a. Location of outer member proteins is predicted by the pSort‐B algorithm (http://psort.org/psortb). Other putative OMPs (P51, NSP1/2/3 and SSA) are determined by homology searches to N. risticii and N. sennetsu protein database using blastp.
The present study identified several other N. helminthoeca DNA‐binding regulators, which are conserved in N. risticii and N. sennetsu (Fig. S4 and Table S1; Lin et al., 2009). These proteins include (i) a putative transcriptional regulator (NHE_RS02120) containing a helix–turn–helix motif and a peptidase S24 LexA‐like family domain that are likely involved in the SOS response leading to the repair of single‐stranded DNA, (ii) a DNA‐binding protein with a putative transposase domain (NHE_RS04205), (iii) a transcriptional regulator of the MerR (mercuric resistance operon regulator) family (NHE_RS01200), and (iv) an Rrf2 family transcriptional regulator with aminotransferase class V domain (NHE_RS01260; Fig. S4). Functions of any of them remain to be studied.
Ankyrin domain proteins
Ankyrin repeat domains (Ank), found predominantly in eukaryotic proteins, are known to mediate protein‐protein interactions involved in a multitude of host processes, including cytoskeletal motility, tumour suppression and transcriptional regulation (Bennett and Baines, 2001; Mosavi et al., 2004). Compared to free‐living bacteria, Ank proteins are enriched in facultative and obligate intracellular bacteria of eukaryotes (Jernigan and Bordenstein, 2014). Several studies have shown that the ankyrin repeat‐containing protein AnkA of A. phagocytophilum is secreted into host cells by the T4SS and plays an important role in facilitating intracellular infection by activating the Abl‐1 protein tyrosine kinase, interacting with the host tyrosine phosphatase SHP‐1, or regulation of host cell transcription (IJdo et al., 2007; Lin et al., 2007; Garcia‐Garcia et al., 2009). In E. chaffeensis, AnkA homologue Ank200 is translocated into the host cell nucleus through a T1SS‐dependent manner and binds to Alu elements and numerous host proteins (Zhu et al., 2009; Wakeel et al., 2011). Four ankyrin repeat‐containing proteins were identified in the N. helminthoeca genome (four in N. risticii and three in N. sennetsu; Table S6). Phylogenetic analysis indicated that N. helminthoeca encodes one Ank protein (NHE_RS00105) that is clustered with E. chaffeensis T1SS substrate Ank200 (11.6% amino acid similarities; Wakeel et al., 2011) and less related to A. phagocytophilum T4SS substrate AnkA (8.6% amino acid similarities; Lin et al., 2007; Fig. S5). However, whether any of these ankyrin repeat‐containing proteins of Neorickettsia spp. can be secreted into host cytoplasm by the T1SS or T4SS and regulate host cell functions remain to be determined.
Iron uptake and storage
Iron is an essential element for almost all living organisms and serves as a cofactor in key metabolic processes including energy generation, electron transport and DNA synthesis (Skaar, 2010). This study found that the three Neorickettsia spp., E. chaffeensis and A. phagocytophilum encode proteins for iron transport across inner membranes, including periplasmic Fe3+‐binding protein FbpA (NHE_RS00045), cytoplasmic membrane permease component FbpB (NHE_RS01265) and cytoplasmic ABC transporter FbpC (PotC, NHE_RS01995; Table S1). However, homologues to known bacterial siderophore and outer membrane receptors for iron or chelated iron are not identified in these bacteria, suggesting that they might use a unique system to bind and uptake iron from their host. Infection of N. risticii, N. sennetsu and E. chaffeensis, but not A. phagocytophilum, are inhibited by an intracellular labile iron chelator deferoxamine (Park and Rikihisa, 1992; Barnewall and Rikihisa, 1994; Barnewall et al., 1999), suggesting that these bacteria may utilize different iron‐uptake system to obtain iron from the host. Unlike E. chaffeensis and A. phagocytophilum, current analysis found that the three Neorickettsia spp. encode a bacterioferritin (NHE_RS01470; Table S1, under role category ‘Transport and binding proteins’), which can capture soluble but potentially toxic Fe2+ by compartmentalizing it in the form of a bioavailable ferric mineral inside the protein's hollow cavity. In the family Anaplasmataceae, bacterioferritin is also found in the Wolbachia endosymbiont of insects or nematode (Kremer et al., 2009). This could be due to differences in their life cycle and invertebrate host: the entire life cycles of Neorickettsia and Wolbachia spp. are within trematodes, insects, or nematodes with limited labile iron pools, whereas Ehrlichia and Anaplasma live within mammalian blood cells and tick vectors fed on blood rich in iron (Fig. 1).
Cell wall components
Lipopolysaccharide and peptidoglycan
N. helminthoeca lacks all genes encoding lipopolysaccharide (LPS) biosynthesis pathway including lipid A (the core component of LPS) as other sequenced members of the family Anaplasmataceae (Lin and Rikihisa, 2003; Dunning Hotopp et al., 2006; Lin et al., 2009), including the recently sequenced NFh (McNulty et al., 2017). Although few genes involved in LPS biosynthesis were identified in the draft genome of Candidatus ‘X. pacificiensis’, it was not expected to possess a functional LPS biosynthesis pathway (Kwan and Schmidt, 2013).
Interestingly, nearly all genes involved in peptidoglycan biosynthesis are identified in N. helminthoeca, A. marginale and Wolbachia wMel (endosymbiont of insect Drosophila melanogaster) or wBm (endosymbiont of nematode Brugia malayi) in the family Anaplasmataceae. On the contrary, only a very limited numbers of genes in peptidoglycan biosynthesis are present in the genomes of N. risticii, N. sennetsu, E. chaffeensis, E. ruminantium and A. phagocytophilum (Fig. 5). This suggests that the ancestors of the family Anaplasmataceae have undergone independent but parallel loss of the peptidoglycan biosynthetic genes and genome reduction.
Analysis of N. helminthoeca genome suggests that it can perform de novo synthesis of d‐Ala‐d‐Ala from pyruvate, meso‐2,6‐diaminopimelate (mDAP) from l‐Asp and undecaprenyl diphosphate (Und‐PP) through terpenoid biosynthesis pathways (isopentenyl‐ and farnesyl‐diphosphate). Although undecaprenyl diphosphatase like E. coli phosphatidylglycerophosphatase B (PGPase B, PgpB) homologue was not found in N. helminthoeca, N. helminthoeca encodes two putative PgpA superfamily proteins (NHE_RS00895 and NHE_RS01205) that might function as PGPases to produce Und‐P from Und‐PP. A flippase (MurJ, NHE_RS02395) that transports anhydromuropeptide into periplasm was also identified in N. helminthoeca (Fig. 5).
The incorporation of anhydromuropeptide subunits into the murein sacculus requires multiple enzymes like MtgA, MrcA/B, FtsI (PbpB), PbpC, MrdA (Pbp2), MrdB, DacF, Pal, MreB/C (Vollmer and Bertsche, 2008; Gillespie et al., 2010); however, only three genes encoding MrdA, FtsI (PbpB) and DacC were identified in N. helminthoeca (Fig. 5). In addition, except for an AmpG permease (NHE_RS03475) that can transport components of peptidoglycan into the cytoplasm, N. helminthoeca lacks all necessary enzymes required for the degradation and recycling of peptidoglycan, including lytic transglycosylases (LTs), AmpD, AnmK, LdcA, Mpl, YcjI/G, NagA/B/K/Z, PepD and MurQ (Gillespie et al., 2010). Furthermore, the T4SS usually encodes specialized LTs that hydrolyse and facilitate the local disruption of peptidoglycan, allowing for efficient transporter assembly across the entire cell envelope (Mushegian et al., 1996). For example, a specialized LT virB1 homologue (rvhB1) was identified in Rickettsia spp. that encode pathways for biosynthesis and degradation of peptidoglycan; however, virB1 homologue was not identified in N. helminthoeca and other members of the family Anaplasmataceae (Gillespie et al., 2010). Our previous electron microscopy showed that only two layers (outer and inner) of membranes and no thickening of the inner or outer leaflet of the outer membrane were present in N. helminthoeca (Rikihisa et al., 1991), suggesting that N. helminthoeca might not possess a peptidoglycan layer.
However, it is possible that N. helminthoeca can still produce precursors or components of peptidoglycan. As several peptidoglycan components are potent stimulants for innate immunity and antimicrobial responses in host immune defensive cells (Dziarski, 2003; Guan and Mariuzza, 2007; Sukhithasri et al., 2013), the presence of these components in N. helminthoeca could elicit antimicrobial and inflammatory activities in leucocytes and may account for the high acute mortality of SPD (Philip, 1955; Rikihisa et al., 1991) compared to less severe or chronic infections caused by other Neorickettsia, Ehrlichia, or Anaplasma spp. that lack peptidoglycan biosynthesis genes.
Lipoproteins and putative outer membrane proteins
Our previous study indicates that E. chaffeensis expresses mature lipoproteins on the bacterial surface, which induced delayed‐type hypersensitivity reaction in dogs (Huang et al., 2008). This study found N. helminthoeca, like other sequenced members of the family Anaplasmataceae, encodes all three lipoprotein‐processing enzymes (Lgt, LspA and Lnt; Table S10; Gupta and Wu, 1991; Paetzel et al., 2002). Computational analysis with LipoP 1.0 (http://www.cbs.dtu.dk/services/LipoP; Juncker et al., 2003) identified thirteen putative lipoproteins in N. helminthoeca (Table S10), which may also be involved in pathogenesis and immune response in infected canids as in E. chaffeensis (Huang et al., 2008). Homologues of several N. helminthoeca lipoproteins are also identified as lipoproteins in N. risticii, including OmpA, CBS domain protein and VirB6 family proteins (Table S1 and S10; Lin et al., 2009).
Computational analysis using the pSort‐B algorithm predicted only four outer membrane proteins, two of which (BamD lipoprotein and beta‐barrel OMP BamA, also called Omp85/YaeT), are part of the beta‐barrel assembly machinery (BAM) and essential for the folding and insertion of outer membrane proteins of Gram‐negative bacteria (Surana et al., 2004; Table 4). Unlike Ehrlichia and Anaplasma spp. that encode a diverse members of the OMP‐1/P28/MSP2/P44 outer member superfamily proteins (Pfam01617), Neorickettsia spp. encode only one group of putative outer surface proteins that falls into this PFAM family (Dunning Hotopp et al., 2006). This group of proteins consists of three N. helminthoeca surface proteins (NSP1/2/3), which are approximately 30 kDa in mass and likely surface‐exposed based on their similarities to Ehrlichia P28/Omp‐1 (Ohashi et al., 1998a, 2001), A. phagocytophilum P44 (Zhi et al., 1998) and N. risticii/N. sennetsu NSPs (Gibson et al., 2010, 2011; Fig. 6B and Table 4).
Figure 6.
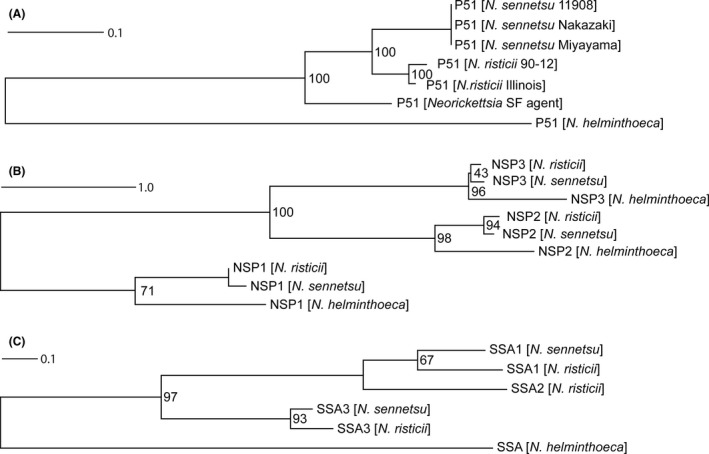
Phylogenetic tree of putative outer membrane proteins in Neorickettsia spp.
The amino acid sequences of putative OMPs (P51, NSPs and SSAs) from N. helminthoeca, N. risticii and N. sennetsu were aligned with clustalw, the phylogenetic tree was built using raxml, and the tree was visualized with Dendroscope as described in the ‘Experimental procedures’. N. helminthoeca encodes P51, NSP1/2/3 and one copy of SSA (closest to SSA3), while ssa2 gene of N. sennetsu is degenerated. For all three putative OMP groups (P51, NSPs, SSAs), N. helminthoeca OMPs forms a separate clade from those of N. risticii and N. sennetsu. GenBank Accession numbers: P51 proteins – N. helminthoeca Oregon, WP_051579521; N. sennetsu Miyayama, WP_011451642; N. sennetsu strain 11908, AAL79561; N. sennetsu Nakazaki, AAR23990; N. risticii Illinois, WP_015816118; N. risticii strain 90‐12, AAB46982; Neorickettsia sp. SF agent, AAR23988.
NSP Proteins: N. helminthoeca Oregon – NSP1, WP_038560103; NSP2, WP_038560106; NSP3, WP_038560109; N. sennetsu Miyayama – NSP1, WP_011452245; NSP2, WP_011452246; NSP3, WP_011452248; N. risticii Illinois – NSP1, WP_015816683; NSP2, WP_015816684; NSP3, WP_015816686.
SSA Proteins: N. helminthoeca Oregon – SSA, WP_038560160; N. sennetsu Miyayama – SSA1, WP_011452276; SSA3, WP_011452279; N. risticii Illinois – SSA1, WP_015816716; SSA2, WP_015816703; SSA3, WP_015816717.
In addition to NSP family OMPs, several studies have identified additional sets of potential surface proteins in other Neorickettsia spp., which include a 51‐kDa protein (P51) and Neorickettsia strain‐specific antigens (SSA; Biswas et al., 1998; Vemulapalli et al., 1998; Rikihisa et al., 2004; Lin et al., 2009; Gibson et al., 2010, 2011). P51 belongs to an orthologue cluster (cluster 409) that exists in all Rickettsiales (Dunning Hotopp et al., 2006), and is highly conserved among all sequenced Neorickettsia spp. including N. helminthoeca (NHE_RS00965) and the SF agent (Rikihisa et al., 2004; Fig. 6A). Previous studies have shown that P51 is the major antigenic protein recognized in horses with Potomac horse fever, and an immunofluorescence assay (IFA) using anti‐P51 antibody on non‐permeabilized N. risticii organisms showed a ring‐like labelling pattern surrounding the bacteria, indicating that P51 is a surface‐exposed antigen (Gibson and Rikihisa, 2008). P51 of N. sennetsu was demonstrated as a porin (Gibson et al., 2010). Phylogeny estimation (Fig. 6A), SignalP prediction (http://www.cbs.dtu.dk/services/SignalP/) and two‐dimensional structures (Fig. S2) suggests that similar to P51 of N. sennetsu and N. risticii, N. helminthoeca P51 is likely a β‐barrel protein localized to the outer membrane.
Strain‐specific antigens (SSAs), proteins of ~50 kDa with extensive intramolecular repeats, have been reported to be a protective antigen of N. risticii against homologous challenge (Biswas et al., 1998; Dutta et al., 1998). Unlike N. risticii or N. sennetsu that encodes two to three tandem genes of non‐identical SSAs, N. helminthoeca only encodes one SSA protein (NHE_RS03855, 35 kDa; Fig. 6C, Table 4 and S9). Phylogenetic analysis reveals that the SSA family proteins in N. sennetsu and N. risticii likely expanded following divergence from N. helminthoeca, but prior to the divergence of N. risticii and N. sennetsu (Fig. 6C). Sequence analysis also identified several intramolecular tandem repeats in N. helminthoeca P51 and SSA proteins (Table S9), suggesting that they might play important roles in pathogenesis and pathogen–host interactions (Citti and Wise, 1995; Smith et al., 1996).
Immunoreactivities of putative outer membrane proteins
Except for Candidatus ‘X. pacificiensis’ that maintains many genes involved in flagella assembly like hook, ring and rod (Kwan and Schmidt, 2013), all members of the family Anaplasmataceae lack LPS, capsule, flagella, or common pili (Dunning Hotopp et al., 2006). In agreement with our previous electron microscope images (Rikihisa et al., 1991), analysis of N. helminthoeca genome indicates that it did not produce a type 4 pili. Therefore, outer membrane proteins play critical roles in bacterium–host cell interactions and induce strong humoral immune responses (Rikihisa et al., 1992, 1994; Zhi et al., 1998; Rikihisa et al., 2004; Ohashi et al., 1998b; Gibson et al., 2011). Analysis of infection‐induced immune reactions to outer membrane proteins provide tools to determine prevalence of N. helminthoeca exposure/infection among various species of animals and provide a groundwork for developing novel rapid immunodiagnostic methods and protective vaccines for SPD.
To elucidate immune reactions of SPD dog sera to P51, NSP1/2/3 and SSA, these proteins were cloned into the pET‐33b(+) expression vector, and recombinant proteins were purified from transformed E. coli (Fig. 7A). The immunoreactivities of these surface proteins were analysed using defined N. helminthoeca IFA‐positive dog sera (Rikihisa et al., 1991). Western blot analysis results showed that P51, NSP1/2/3 and SSA proteins were recognized by antisera from NH1 and NH3 dogs experimentally infected with N. helminthoeca by feeding trematodes‐parasitized fish and seroconverted (IFA titres of 1:640 and 1:1280, respectively, using N. helminthoeca‐infected DH82 cells as the antigen; Rikihisa et al., 1991), with NSP2 and SSA as the strongest sero‐reactive antigens (Fig. 7C,D). In addition, N. helminthoeca‐positive dog sera from naturally infected dogs from southern California recognized P51 and SSA and weakly against NPS3, whereas NSP1 and NSP2 were only detected by ‘M’ sera (Fig. 7E,F). As a control, antisera from the horse experimentally infected with N. risticii did not react with any of these membrane proteins from N. helminthoeca (Fig. 7B). These data indicate that N. helminthoeca OMPs including P51, SSA and NSPs can be recognized by the immune system of N. helminthoeca‐infected dogs.
Figure 7.
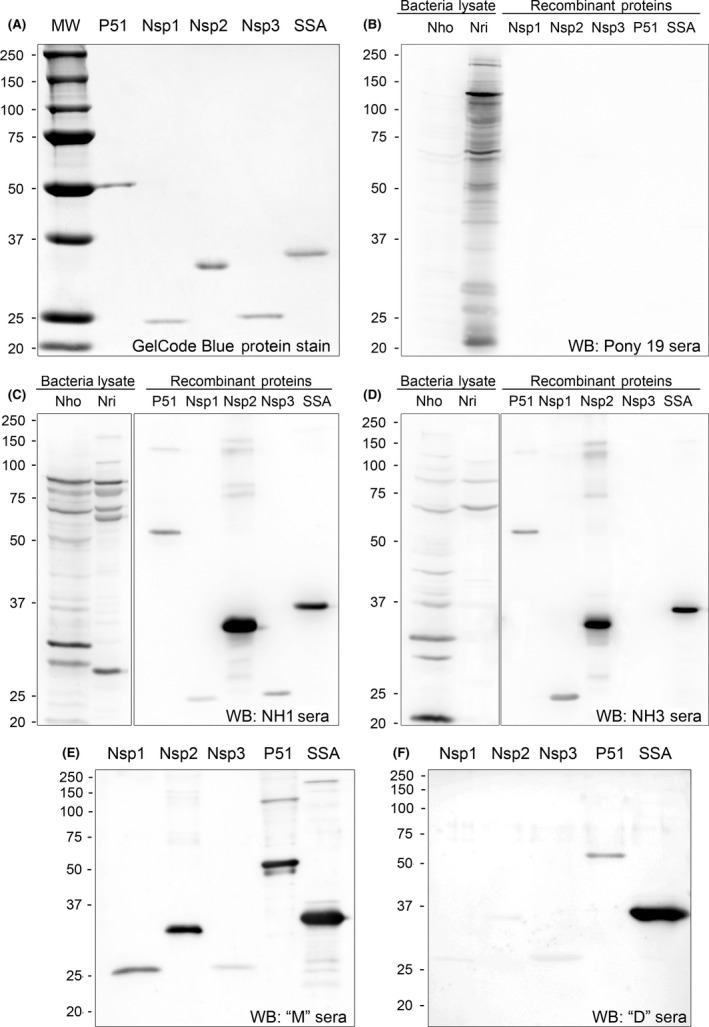
Expression and immunoreactivities of N. helminthoeca putative outer membrane proteins.
P51, NSPs and SSA proteins were cloned into pET33(+) expression vector and recombinant proteins were purified from transformed E. coli BL21(DE3) strain. The size and purity of these recombinant proteins were verified by GelCode blue protein stain (A). N. helminthoeca (70% infected DH82 cells) and N. risticii (90%‐infected P388D1) from 2× T175 flasks were purified by sonication and filtration through 5‐μm filters. ~50 μg each of bacterial lysates from N. risticii (Nri) and N. helminthoeca (Nho) and ~20 μg of purified recombinant outer membrane proteins of N. helminthoeca were subjected to Western blot analysis and probed with (B) Pony 19 sera against N. risticii from experimentally infected pony (1/400 dilution), (C and D) NH1 and NH3 sera against N. helminthoeca from the experimentally infected dogs or (E and F) clinical dog sera from southern California that were positive for N. helminthoeca infection by PCR or IFA. Bands were visualized by ECL. The molecular size of the recombinant proteins are P51, 51.6 kDa; SSA, 33.7 kDa; NSP1, 27.7 kDa; NSP2, 32.2 kDa; NSP3, 23.7 kDa.
Our previous study showed that sera from N. helminthoeca‐infected dogs, N. sennetsu‐infected horse, N. risticii‐infected horses, or E. canis‐infected dogs cross‐reacted with other species but with at least 16‐fold lower than those for homologous antigens by immunofluorescence assay (Rikihisa, 1991b; Rikihisa et al., 1991). This study also showed that approximately 78–80 kDa and 64 kDa proteins were the major antigens shared by N. helminthoeca, N. risticii, N. sennetsu and E. canis (Rikihisa, 1991b; Fig. 7B–D). These cross‐reactive antigens were likely more conserved heat‐shock proteins or molecular chaperones, and their molecular weights were different from predicted outer membrane proteins of N. helminthoeca analysed in the current study (from 23 to 51 kDa). Therefore, in current Western blotting with the dilution of sera at 1:400, horse sera against N. risticii recognized none of N. helminthoeca OMPs (Fig. 7B), whereas dog sera against N. helminthoeca only detected proteins at ~64 and 80‐kD from N. risticii (Fig. 7C,D), suggesting that these recombinant OMPs could be used for specific diagnosis of N. helminthoeca‐infected dogs.
Conclusion and discussion
Despite of expansion of DNA sequences of Neorickettsia spp. in various trematode species worldwide, biology and natural history have been best studied in N. helminthoeca, the type species of the genus Neorickettsia. In this study, we determined and analysed the complete genome sequence of N. helminthoeca, providing a valuable resource necessary for understanding the metabolism of N. helminthoeca and its digenean host associations, the evolution and phylogeny among Neorickettsia spp., potential virulence factors of N. helminthoeca, pathogenic mechanisms of SPD and environmental spreading of N. helminthoeca and trematodes infection in nature. Comparative genomics data of three Neorickettsia spp. of known biological significance is expected to help elucidating biology of other Neorickettsia spp. in the environment.
As SPD progression is rapid, and the case fatality rate is quite high, prevention and early diagnosis of SPD are critical. The serological assay based on defined outer membrane protein antigens is simple, consistent, specific, objective and convenient, thus helps generating epidemiological information on N. helminthoeca exposure among various wild and domestic animals to raise awareness of SPD. Similar to bats that are the definitive hosts of Acanthatrium oregonense trematodes, the vector of N. risticii transmission (Gibson et al., 2005; Gibson and Rikihisa, 2008), the definitive hosts of N. helminthoeca‐infected trematodes in nature are likely asymptomatic, but have antibodies against N. helminthoeca.
Furthermore, these recombinant proteins can be applied to develop a simple and rapid serodiagnostic test for SPD in dogs in the future. The limitation of the assay is, as in any other serologic assays, false negative results at early stages of infection and in immunosuppressed dogs. Future steps necessary for the test to become applicable for clinical diagnosis are to determine sensitivity and specificity of the test using a larger number of well‐defined canine specimens from broader geographic regions. For this and understanding the pathogenesis and canine immune responses in SPD, culture isolation of additional N. helminthoeca strains is desirable. Further characterization of the antigenic surface proteins of N. helminthoeca could provide valuable candidates for the development of rapid, sensitive and specific serodiagnostic approaches or preventive vaccines for SPD.
Experimental procedures
Organisms culture, bacteria purification and DNA preparation
Neorickettsia helminthoeca Oregon strain, which was previously isolated from dog NH1 fed with fluke N. salmincola‐infested salmon kidneys (Rikihisa et al., 1991), was cultured in DH82 cells from the frozen cell stock in Dulbecco's minimal essential medium supplemented with 10% fetal bovine serum and 2 mM l‐glutamine. Cultures were incubated at 37°C under 5% CO2 in a humidified atmosphere. To purify host cell‐free bacteria for genome sequencing, infected cells (> 95% infection) were harvested and Dounce homogenized in SPK buffer (0.2 M sucrose and 0.05 M potassium phosphate, pH 7.4). Lysed cells were centrifuged at 500× g and 700× g to remove unbroken cells and nuclei, filtered through 5.0‐ and 2.7‐μm syringe filters and centrifuged at 10 000× g to pellet host cell‐free bacteria. Genomic DNA was purified using a Genomic‐tip 20/G (QIAGEN, Valencia, CA, USA) according to manufacturer's instructions, and host DNA contamination was verified to be < 0.1% by PCR using specific primers targeting N. helminthoeca 16S rRNA gene and canine G3PDH DNA.
Sequencing and annotation
Indexed Illumina mate pair libraries were prepared following the mate pair library v2 sample preparation guide (Illumina, San Diego, CA, USA), with two modifications. First, the shearing was performed with the Covaris E210 (Covaris, Wobad, MA, USA). The DNA was purified using enzymatic reactions and the size selection of the library was performed with AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA).
Illumina non‐Truseq paired‐end genomic DNA libraries were constructed using the KAPA library preparation kit (Kapa Biosystems, Woburn, MA, USA). DNA was fragmented with the Covaris E210. Then libraries were prepared using a modified version of manufacturer's protocol. The DNA was purified using enzymatic reactions and the size selection of the library was performed with AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA). For indexed samples, the PCR amplification step was performed with primers containing a six nucleotide index sequence.
Concentration and fragment size of libraries were determined using the DNA High Sensitivity Assay on the LabChip GX (Perkin‐Elmer, Waltham, MA, USA) and qPCR using the KAPA Library Quantification Kit (Complete, Universal; Kapa Biosystems, Woburn, MA, USA). The mate pair library was sequenced on an Illumina HiSeq 2500 (Illumina, San Diego, CA, USA) while the paired‐end library was sequenced on an Illumina MiSeq (Illumina, San Diego, CA, USA).
DNA samples for PacBio sequencing were sheared to 8 kbp using the Covaris gTube (Woburn, MA, USA). Sequencing libraries were constructed and prepared for sequencing using the DNA Template Prep Kit 2.0 (3–10 kbp) and the DNA/Polymerase Binding Kit 2.0 (Pacific Biosciences, Menlo Park, CA, USA). Libraries were loaded onto v2 SMRT Cells and sequenced with the DNA Sequencing Kit 2.0 (Pacific Biosciences).
Five assemblies were generated with various combinations of the data and assembly algorithms: (i) celera assembler v7.0 of only PacBio data, (ii) celera assembler v7.0 of PacBio data with correction using Illumina paired‐end data, (iii) HGAP assembly of only PacBio data, (iv) MaSuRCA 1.9.2 assembly of Illumina paired‐end data subsampled to 50× coverage, and (v) MaSuRCA 1.9.2 assembly of Illumina paired‐end data subsampled to 80× coverage. The first assembly was the optimal assembly, namely the one generated with celera assembler v7.0 with only the PacBio data. The data set was subsampled to ~22× coverage of the longest reads using an 8 kbp minimum read length cut‐off, with the remainder of the reads used for the error correction step. The resulting single‐contig assembly totalled ~89.4 Kbp with 41.68% GC‐content. The genome was trimmed to remove overlapping sequences, oriented, circularized and rotated to the predicted origin of replication. Annotation for this finalized genome assembly was generated using the IGS prokaryotic annotation pipeline (Galens et al., 2011) and deposited in GenBank (accession number NZ_CP007481.1).
Bioinformatic analysis
The 16S rRNA, NSP, P51 and SSA proteins were aligned with their Neorickettsia orthologues using clustalw (Thompson et al., 1994) as implemented in bioedit 7.2.5 (Hall, 1999) resulting in 1522 nt, 326 aa, 516 aa and 578 aa alignments respectively. A phylogenetic tree was inferred from the 16S rRNA alignment using raxml v.7.3.0 (Stamatakis et al., 2005) with the GTRGAMMA model, specifically ‘RAxMLHPC ‐f a ‐m GTRGAMMA ‐p 12345 ‐x 12345 ‐N autoMRE ‐n T20’. The MRE‐based bootstopping criterion was not met, resulting in the use of 1000 bootstraps. For the protein alignments, the best‐fit model of amino acid substitution was determined for each alignment separately with prottest 3.2 (Darriba et al., 2011), with all 15 models of protein evolution tested in addition to the +G parameter. WAG+G was determined to be the best model for NSP and SSA while JTT was determined to be the best model for P51. Phylogenetic trees were inferred from the NSP and SSA alignments using raxml v.7.3.0 (Stamatakis et al., 2005) with the best model, specifically ‘RAxMLHPC ‐f a ‐m PROTGAMMAWAG ‐p 12345 ‐x 12345 ‐N autoMRE ‐n T20’. The MRE‐based bootstopping criterion was met at 350 replicates for NSP and SSA. Phylogenetic trees were inferred from the P51 alignment using raxml v.7.3.0 (Stamatakis et al., 2005) with the best model, specifically ‘RAxMLHPC ‐f a ‐m PROTCATJTT ‐p 12345 ‐x 12345 ‐N autoMRE ‐n T20’. The MRE‐based bootstopping criterion was met at 50 replicates for P51. All trees and bootstrap values were visualized in dendroscope v3.5.7 (http://dendroscope.org, Tübingen University, Tübingen, Germany).
The GC skew was calculated as (C‐G)/(C+G) in windows of 500 bp with step size of 250 bp along the chromosome. Synteny plots between Neorickettsia spp. were generated using MUMmer 3 program with default parameters (Delcher et al., 2002). Protein orthologue clusters among Neorickettsia spp., and N. helminthoeca‐specific genes compared to other related organisms were determined using reciprocal blastp with cut‐off scores of E < 10−10.
Metabolic pathways and transporters were compared across genomes using (i) the orthologue clusters generated with reciprocal blastp, (ii) Genome Properties (Haft et al., 2005), (iii) TransportDB (Ren et al., 2007), (iv) Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.kegg.jp), and (v) Biocyc (Krieger et al., 2004). Signal peptides and membrane proteins were predicted using the pSort‐B algorithm (http://psort.org/psortb/;Yu et al., 2010), and lipoproteins were predicted by lipop 1.0 (http://www.cbs.dtu.dk/services/LipoP; Juncker et al., 2003).
Cloning, expression and Western blot analysis of putative N. helminthoeca outer membrane proteins
Full‐length p51, nsp1/2/3 and ssa genes without the signal peptide sequence were PCR amplified from N. helminthoeca genomic DNA, using specific primers (Table S11) and cloned into the pET‐33b(+) vector (Novagen, Billerica, MA, USA). The plasmids were amplified by transformation into Escherichia coli PX5α cells (Protein Express, Cincinnati, OH, USA), and the inserts were confirmed by sequencing. The plasmids were transformed into E. coli BL21 (DE3; Protein Express), and the expression of recombinant proteins was induced with 1 mM isopropyl β‐d‐thiogalactopyranoside. E. coli was sonicated for a total of 5 min (15 s pulse with 45 s interval) on ice, and the pellet containing recombinant protein was washed with 1% Triton X‐100 in sodium phosphate buffer (SPB: 50 mM sodium phosphate, pH 8.0, 0.3 M NaCl). Recombinant proteins were denatured and solubilized with 6 M urea in SPB (for P51, SSA and NSP2/3), or 6M Guanidine HCl in SPB (for NSP1) at 4°C for 1 h. Proteins were purified on a HisPur Cobalt Affinity resin (Pierce, Rockford, IL, USA) and dialysed using Buffer A (50 mM KCl, 100 mM NaCl, 50 mM Tris‐HCl, pH 8.0) containing decreasing concentrations of urea (3 M, 1 M, then 0 M). Protein concentrations were determined by BCA assay (Pierce).
Bacterial lysates of purified N. risticii or N. helminthoeca, and recombinant NSP1/2/3, SSA and P51 were subjected to SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) and Western blot analysis as described previously (Lin et al., 2002). Gels were stained using GelCode Blue (Pierce), and the immunoreactivities of these recombinant proteins were determined by Western blot analysis using SPD dog sera against N. helminthoeca or horse anti‐N. risticii serum as a negative control at 1:400 dilutions. Defined SPD dog sera against N. helminthoeca were obtained from dogs orally fed by fluke N. salmincola‐infested salmon kidneys infected with N. helminthoeca, and sera collected at day 13 and 15 postexposure with IFA titres at 1:640 (NH1) and 1:1280 (NH3) respectively (Rikihisa et al., 1991). Clinical dog sera tested positive for N. helminthoeca infection were received from southern California (‘M’ sera – IFA titre 1:80, from Dana Point, CA in 2012; ‘D’ sera – PCR‐positive for N. helminthoeca 16S rRNA gene, from Aliso Viejo, CA in 2010). Horse anti‐N. risticii serum (Pony 19) was collected from a pony inoculated intravenously with N. risticii‐infected U‐937 cells (IFA titre 1:640; Rikihisa et al., 1988). Reacting bands were detected with Horseradish peroxidase (HRP)‐conjugated goat anti‐dog (KPL Gaithersburg, MD) or anti‐horse (Jackson Immuno Research, West Grove, PA) secondary antibodies and visualized with enhanced chemiluminescence (ECL) by incubating the membranes with LumiGLO™ chemiluminescent reagent (Pierce). Images were captured using an LAS3000 image documentation system (FUJIFILM Medical Systems USA, Stamford, CT, USA).
GenBank Accession numbers and abbreviations of bacteria
N. helminthoeca Oregon (NHO), NZ_CP007481.1 (this study); N. risticii Illinois (NRI), NC_013009.1; N. sennetsu Miyayama (NSE), NC_007798.1; A. phagocytophilum HZ (APH), NC_007797.1; A. marginale Florida (AMA), NC_012026.1; E. chaffeensis Arkansas (ECH), NC_007799.1; E. canis Jake (ECA), NC_007354.1; E. ruminantium Welgevonden (ERU), NC_005295.2; E. muris AS145 (EMU), NC_023063.1; Ehrlichia sp. HF (EHF), NZ_CP007474.1; Wolbachia pipientis (wMel, Wolbachia endosymbiont of Drosophila melanoga), NC_002978.6; Wolbachia endosymbiont of Brugia malayi (wBm), NC_006833.1; Neorickettsia endobacterium of Fasciola hepatica (NFh), NZ_LNGI00000000; Candidatus Xenolissoclinum pacificiensis L6, AXCJ00000000.
Conflict of interest
No potential conflicts of interest were disclosed.
Supporting information
Fig. S1. Synteny plots between Neorickettsia spp.
Fig. S2. Secondary Structure of N. helminthoeca P51 Protein.
Fig. S3. Phylogenetic tree of VirB2 proteins in the family Anaplasmataceae and α‐proteobacteria.
Fig. S4. One‐component regulatory systems of N. helminthoeca.
Fig. S5. Phylogenetic analysis of AnkA or Ank200 homologous proteins in the family Anaplasmataceae.
Table S1. Ortholog clusters conserved among N. helminthoeca, N. risticii and N. sennetsu based on three‐way comparison analysis
Table S2. N. helminthoeca‐specific proteins compared to N. sennetsu and N. risticii
Table S3. N. risticii‐specific proteins compared to N. helminthoeca and N. sennetsu
Table S4. N. sennetsu‐specific proteins compared to N. helminthoeca and N. risticii
Table S5. Amino acid and cofactor biosynthesis in Family Anaplasmataceae
Table S6. Potential pathogenic genes in Neorickettsia species
Table S7. Putative Transporters of N. helminthoeca
Table S8. Genes involved in DNA repair and homologous recombination
Table S9. Proteins with tandem repeats in N. helminthoeca
Table S10. Lipoprotein‐processing enzymes and putative lipoproteins in N. helminthoeca
Table S11. Oligonucleotide primers used for cloning N. helminthoeca outer membrane proteins
Acknowledgements
We thank Tim Vojt at the Ohio State University for assistance in preparing Figs 4 and 5 and Dr. James Munro at University of Maryland School of Medicine for guidance in conducting the phylogenetic analysis.
Microbial Biotechnology (2017) 10(4), 933–957
Funding information Ohio State University College of Veterinary Medicine (Canine Research Grant (2013‐22)), National Institute of Allergy and Infectious Diseases (Contract number ‘HHSN272200900009C’, ‘R01 AI 047885’). KB is partially supported by NIH T35 OD10977 Research Fellowship and American Veterinary Medical Association (AVMA)/American Veterinary Medical Foundation (AVMF) Second Summer Research Opportunity Scholarship.
Contributor Information
Mingqun Lin, Email: lin.427@osu.edu.
Yasuko Rikihisa, Email: rikihisa.1@osu.edu.
References
- Anderson, B.E. , Greene, C.E. , Jones, D.C. , and Dawson, J.E. (1992) Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol 42: 299–302. [DOI] [PubMed] [Google Scholar]
- Backert, S. , and Meyer, T.F. (2006) Type IV secretion systems and their effectors in bacterial pathogenesis. Curr Opin Microbiol 9: 207–217. [DOI] [PubMed] [Google Scholar]
- Bagos, P.G. , Liakopoulos, T.D. , Spyropoulos, I.C. , and Hamodrakas, S.J. (2004) PRED‐TMBB: a web server for predicting the topology of beta‐barrel outer membrane proteins. Nucleic Acids Res 32: W400–W404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagos, P.G. , Nikolaou, E.P. , Liakopoulos, T.D. , and Tsirigos, K.D. (2010) Combined prediction of Tat and Sec signal peptides with hidden Markov models. Bioinformatics 26: 2811–2817. [DOI] [PubMed] [Google Scholar]
- Barnewall, R.E. , and Rikihisa, Y. (1994) Abrogation of gamma interferon‐induced inhibition of Ehrlichia chaffeensis infection in human monocytes with iron‐transferrin. Infect Immun 62: 4804–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnewall, R.E. , Ohashi, N. , and Rikihisa, Y. (1999) Ehrlichia chaffeensis and E. sennetsu, but not the human granulocytic ehrlichiosis agent, colocalize with transferrin receptor and up‐regulate transferrin receptor mRNA by activating iron‐responsive protein 1. Infect Immun 67: 2258–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen, J.D. , Nielsen, H. , Widdick, D. , Palmer, T. , and Brunak, S. (2005) Prediction of twin‐arginine signal peptides. BMC Bioinformatics 6: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, V. , and Baines, A.J. (2001) Spectrin and ankyrin‐based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 81: 1353–1392. [DOI] [PubMed] [Google Scholar]
- Bennington, E. , and Pratt, I. (1960) The life history of the salmonpoisoning fluke, Nanophyetus salmincola (Chapin). J Parasitol 46: 91–100. [PubMed] [Google Scholar]
- Bingle, L.E. , Bailey, C.M. , and Pallen, M.J. (2008) Type VI secretion: a beginner's guide. Curr Opin Microbiol 11: 3–8. [DOI] [PubMed] [Google Scholar]
- Biswas, B. , Vemulapalli, R. , and Dutta, S.K. (1998) Molecular basis for antigenic variation of a protective strain‐specific antigen of Ehrlichia risticii . Infect Immun 66: 3682–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, A.J. , Stogdale, L. , and Grigor, J.A. (1984) Salmon poisoning disease in dogs on southern Vancouver Island. Can Vet J 25: 2–6. [PMC free article] [PubMed] [Google Scholar]
- Brownlie, J.C. , Cass, B.N. , Riegler, M. , Witsenburg, J.J. , Iturbe‐Ormaetxe, I. , McGraw, E.A. , and O'Neill, S.L. (2009) Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5: e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales, E. , and Christie, P.J. (2003) The versatile bacterial type IV secretion systems. Nat Rev Microbiol 1: 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. , Kumagai, Y. , Lin, M. , Zhang, C. , and Rikihisa, Y. (2006) Intra‐leukocyte expression of two‐component systems in Ehrlichia chaffeensis and Anaplasma phagocytophilum and effects of the histidine kinase inhibitor closantel. Cell Microbiol 8: 1241–1252. [DOI] [PubMed] [Google Scholar]
- Cheng, Z. , Wang, X. , and Rikihisa, Y. (2008) Regulation of type IV secretion apparatus genes during Ehrlichia chaffeensis intracellular development by a previously unidentified protein. J Bacteriol 190: 2096–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. , Miura, K. , Popov, V.L. , Kumagai, Y. , and Rikihisa, Y. (2011) Insights into the CtrA regulon in development of stress resistance in obligatory intracellular pathogen Ehrlichia chaffeensis . Mol Microbiol 82: 1217–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. , Lin, M. , and Rikihisa, Y. (2014) Ehrlichia chaffeensis proliferation begins with NtrY/NtrX and PutA/GlnA upregulation and CtrA degradation induced by proline and glutamine uptake. MBio 5: e02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, P.J. , Whitaker, N. , and Gonzalez‐Rivera, C. (2014) Mechanism and structure of the bacterial type IV secretion systems. Biochim Biophys Acta 1843: 1578–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciotto, N.P. (2005) Type II secretion: a protein secretion system for all seasons. Trends Microbiol 13: 581–588. [DOI] [PubMed] [Google Scholar]
- Citti, C. , and Wise, K.S. (1995) Mycoplasma hyorhinis vlp gene transcription: critical role in phase variation and expression of surface lipoproteins. Mol Microbiol 18: 649–660. [DOI] [PubMed] [Google Scholar]
- Cordes, D.O. , Perry, B.D. , Rikihisa, Y. , and Chickering, W.R. (1986) Enterocolitis caused by Ehrlichia sp. in the horse (Potomac horse fever). Vet Pathol 23: 471–477. [DOI] [PubMed] [Google Scholar]
- Cordy, D.R. , and Gorham, J.R. (1950) The pathology and etiology of salmon disease. Am J Pathol 26: 617–637. [PMC free article] [PubMed] [Google Scholar]
- Costa, T.R. , Felisberto‐Rodrigues, C. , Meir, A. , Prevost, M.S. , Redzej, A. , Trokter, M. , and Waksman, G. (2015) Secretion systems in Gram‐negative bacteria: structural and mechanistic insights. Nat Rev Microbiol 13: 343–359. [DOI] [PubMed] [Google Scholar]
- Crossman, L. (2006) Budget genome. Nat Rev Microbiol 4: 326–327. [DOI] [PubMed] [Google Scholar]
- Darriba, D. , Taboada, G.L. , Doallo, R. , and Posada, D. (2011) ProtTest 3: fast selection of best‐fit models of protein evolution. Bioinformatics 27: 1164–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcher, A.L. , Phillippy, A. , Carlton, J. , and Salzberg, S.L. (2002) Fast algorithms for large‐scale genome alignment and comparison. Nucleic Acids Res 30: 2478–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire, P. (2004) Type I secretion in gram‐negative bacteria. Biochim Biophys Acta 1694: 149–161. [DOI] [PubMed] [Google Scholar]
- Dorman, C.J. , McKenna, S. , and Beloin, C. (2001) Regulation of virulence gene expression in Shigella flexneri, a facultative intracellular pathogen. Int J Med Microbiol 291: 89–96. [DOI] [PubMed] [Google Scholar]
- Dumler, J.S. , Barbet, A.F. , Bekker, C.P. , Dasch, G.A. , Palmer, G.H. , Ray, S.C. , et al (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila . Int J Syst Evol Microbiol 51: 2145–2165. [DOI] [PubMed] [Google Scholar]
- Dunning Hotopp, J.C. , Lin, M. , Madupu, R. , Crabtree, J. , Angiuoli, S.V. , Eisen, J.A. , et al (2006) Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet 2: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta, S.K. , Penney, B.E. , Myrup, A.C. , Robl, M.G. , and Rice, R.M. (1988) Disease features in horses with induced equine monocytic ehrlichiosis (Potomac horse fever). Am J Vet Res 49: 1747–1751. [PubMed] [Google Scholar]
- Dutta, S.K. , Vemulapalli, R. , and Biswas, B. (1998) Association of deficiency in antibody response to vaccine and heterogeneity of Ehrlichia risticii strains with Potomac horse fever vaccine failure in horses. J Clin Microbiol 36: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski, R. (2003) Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol Life Sci 60: 1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreyt, W.J. , Gorham, J.R. , Green, J.S. , Leathers, C.W. , and LeaMaster, B.R. (1987) Salmon poisoning disease in juvenile coyotes: clinical evaluation and infectivity of metacercariae and rickettsiae. J Wildl Dis 23: 412–417. [DOI] [PubMed] [Google Scholar]
- Foster, J. , Ganatra, M. , Kamal, I. , Ware, J. , Makarova, K. , Ivanova, N. , et al (2005) The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol 3: e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos, R. , Viari, A. , Vachiery, N. , Boyer, F. , and Martinez, D. (2007) Ehrlichia ruminantium: genomic and evolutionary features. Trends Parasitol 23: 414–419. [DOI] [PubMed] [Google Scholar]
- Galens, K. , Orvis, J. , Daugherty, S. , Creasy, H.H. , Angiuoli, S. , White, O. , et al (2011) The IGS standard operating procedure for automated prokaryotic annotation. Stand Genomic Sci 4: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, R. , Mack, T.R. , and Stock, A.M. (2007) Bacterial response regulators: versatile regulatory strategies from common domains. Trends Biochem Sci 32: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Garcia, J.C. , Rennoll‐Bankert, K.E. , Pelly, S. , Milstone, A.M. , and Dumler, J.S. (2009) Silencing of host cell CYBB gene expression by the nuclear effector AnkA of the intracellular pathogen Anaplasma phagocytophilum . Infect Immun 77: 2385–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, K.E. , and Rikihisa, Y. (2008) Molecular link of different stages of the trematode host of Neorickettsia risticii to Acanthatrium oregonense . Environ Microbiol 10: 2064–2073. [DOI] [PubMed] [Google Scholar]
- Gibson, K.E. , Rikihisa, Y. , Zhang, C. , and Martin, C. (2005) Neorickettsia risticii is vertically transmitted in the trematode Acanthatrium oregonense and horizontally transmitted to bats. Environ Microbiol 7: 203–212. [DOI] [PubMed] [Google Scholar]
- Gibson, K. , Kumagai, Y. , and Rikihisa, Y. (2010) Proteomic analysis of Neorickettsia sennetsu surface‐exposed proteins and porin activity of the major surface protein P51. J Bacteriol 192: 5898–5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, K.E. , Pastenkos, G. , Moesta, S. , and Rikihisa, Y. (2011) Neorickettsia risticii surface‐exposed proteins: proteomics identification, recognition by naturally‐infected horses, and strain variations. Vet Res 42: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, J.J. , Ammerman, N.C. , Dreher‐Lesnick, S.M. , Rahman, M.S. , Worley, M.J. , Setubal, J.C. , et al (2009) An anomalous type IV secretion system in Rickettsia is evolutionarily conserved. PLoS ONE 4: e4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, J.J. , Brayton, K.A. , Williams, K.P. , Diaz, M.A. , Brown, W.C. , Azad, A.F. , and Sobral, B.W. (2010) Phylogenomics reveals a diverse Rickettsiales type IV secretion system. Infect Immun 78: 1809–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, J.J. , Kaur, S.J. , Rahman, M.S. , Rennoll‐Bankert, K. , Sears, K.T. , Beier‐Sexton, M. , and Azad, A.F. (2015) Secretome of obligate intracellular Rickettsia . FEMS Microbiol Rev 39: 47–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiman, S.E. , Tkach, V.V. , Pulis, E. , Fayton, T.J. , and Curran, S.S. (2014) Large scale screening of digeneans for Neorickettsia endosymbionts using real‐time PCR reveals new Neorickettsia genotypes, host associations and geographic records. PLoS ONE 9: e98453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiman, S.E. , Rikihisa, Y. , Cain, J. , Vaughan, J.A. , and Tkach, V.V. (2016) Germs within Worms: localization of Neorickettsia sp. within Life Cycle Stages of the Digenean Plagiorchis elegans . Appl Environ Microbiol 82: 2356–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiman, S.E. , Vaughan, J.A. , Elmahy, R. , Adisakwattana, P. , Van Ha, N. , Fayton, T.J. , et al (2017) Real‐time PCR detection and phylogenetic relationships of Neorickettsia spp. in digeneans from Egypt, Philippines, Thailand, Vietnam and the United States. Parasitol Int 66: 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, R. , and Mariuzza, R.A. (2007) Peptidoglycan recognition proteins of the innate immune system. Trends Microbiol 15: 127–134. [DOI] [PubMed] [Google Scholar]
- Gupta, S.D. , and Wu, H.C. (1991) Identification and subcellular localization of apolipoprotein N‐acyltransferase in Escherichia coli . FEMS Microbiol Lett 62: 37–41. [DOI] [PubMed] [Google Scholar]
- Haft, D.H. , Selengut, J.D. , Brinkac, L.M. , Zafar, N. , and White, O. (2005) Genome Properties: a system for the investigation of prokaryotic genetic content for microbiology, genome annotation and comparative genomics. Bioinformatics 21: 293–306. [DOI] [PubMed] [Google Scholar]
- Hall, T.A. (1999) BioEdit: a user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41: 95–98. [Google Scholar]
- Headley, S.A. , Vidotto, O. , Scorpio, D. , Dumler, J.S. , and Mankowski, J. (2004) Suspected cases of Neorickettsia‐like organisms in Brazilian dogs. Ann N Y Acad Sci 1026: 79–83. [DOI] [PubMed] [Google Scholar]
- Headley, S.A. , Scorpio, D.G. , Barat, N.C. , Vidotto, O. , and Dumler, J.S. (2006) Neorickettsia helminthoeca in dog, Brazil. Emerg Infect Dis 12: 1303–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley, S.A. , Scorpio, D.G. , Vidotto, O. , and Dumler, J.S. (2011) Neorickettsia helminthoeca and salmon poisoning disease: a review. Vet J 187: 165–173. [DOI] [PubMed] [Google Scholar]
- Henderson, I.R. , Navarro‐Garcia, F. , Desvaux, M. , Fernandez, R.C. , and Ala'Aldeen, D. (2004) Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev 68: 692–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Wang, X. , Kikuchi, T. , Kumagai, Y. , and Rikihisa, Y. (2007) Porin activity of Anaplasma phagocytophilum outer membrane fraction and purified P44. J Bacteriol 189: 1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Lin, M. , Wang, X. , Kikuchi, T. , Mottaz, H. , Norbeck, A. , and Rikihisa, Y. (2008) Proteomic analysis of and immune responses to Ehrlichia chaffeensis lipoproteins. Infect Immun 76: 3405–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJdo, J.W. , Carlson, A.C. , and Kennedy, E.L. (2007) Anaplasma phagocytophilum AnkA is tyrosine phosphorylated at EPIYA motifs and recruits SHP‐1 during early infection. Cell Microbiol 9: 1284–1296. [DOI] [PubMed] [Google Scholar]
- Ioannidis, P. , Dunning Hotopp, J.C. , Sapountzis, P. , Siozios, S. , Tsiamis, G. , Bordenstein, S.R. , et al (2007) New criteria for selecting the origin of DNA replication in Wolbachia and closely related bacteria. BMC Genom 8: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan, K.K. , and Bordenstein, S.R. (2014) Ankyrin domains across the Tree of Life. PeerJ 2: e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorda, J. , and Kajava, A.V. (2009) T‐REKS: identification of Tandem REpeats in sequences with a K‐meanS based algorithm. Bioinformatics 25: 2632–2638. [DOI] [PubMed] [Google Scholar]
- Juncker, A.S. , Willenbrock, H. , Von Heijne, G. , Brunak, S. , Nielsen, H. , and Krogh, A. (2003) Prediction of lipoprotein signal peptides in Gram‐negative bacteria. Protein Sci 12: 1652–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, N. , Voronin, D. , Charif, D. , Mavingui, P. , Mollereau, B. , and Vavre, F. (2009) Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog 5: e1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger, C.J. , Zhang, P. , Mueller, L.A. , Wang, A. , Paley, S. , Arnaud, M. , et al (2004) MetaCyc: a multiorganism database of metabolic pathways and enzymes. Nucleic Acids Res 32: D438–D442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, Y. , Cheng, Z. , Lin, M. , and Rikihisa, Y. (2006) Biochemical activities of three pairs of Ehrlichia chaffeensis two‐component regulatory system proteins involved in inhibition of lysosomal fusion. Infect Immun 74: 5014–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, Y. , Huang, H. , and Rikihisa, Y. (2008) Expression and porin activity of P28 and OMP‐1F during intracellular Ehrlichia chaffeensis development. J Bacteriol 190: 3597–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, Y. , Matsuo, J. , Cheng, Z. , Hayakawa, Y. , and Rikihisa, Y. (2011) Cyclic dimeric GMP signaling regulates intracellular aggregation, sessility, and growth of Ehrlichia chaffeensis . Infect Immun 79: 3905–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, J.C. , and Schmidt, E.W. (2013) Bacterial endosymbiosis in a chordate host: long‐term co‐evolution and conservation of secondary metabolism. PLoS ONE 8: e80822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, T.H. , Kumagai, Y. , Hyodo, M. , Hayakawa, Y. , and Rikihisa, Y. (2009) The Anaplasma phagocytophilum PleC histidine kinase and PleD diguanylate cyclase two‐component system and role of cyclic Di‐GMP in host cell infection. J Bacteriol 191: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, P.A. , Tullman‐Ercek, D. , and Georgiou, G. (2006) The bacterial twin‐arginine translocation pathway. Annu Rev Microbiol 60: 373–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Z.M. , Bussema, C. 3rd , and Schmidt, T.M. (2009) rrnDB: documenting the number of rRNA and tRNA genes in bacteria and archaea. Nucleic Acids Res 37: D489–D493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. , and Rikihisa, Y. (2003) Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun 71: 5324–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. , Zhu, M.X. , and Rikihisa, Y. (2002) Rapid activation of protein tyrosine kinase and phospholipase C‐gamma2 and increase in cytosolic free calcium are required by Ehrlichia chaffeensis for internalization and growth in THP‐1 cells. Infect Immun 70: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Q. , Rikihisa, Y. , Felek, S. , Wang, X. , Massung, R.F. , and Woldehiwet, Z. (2004) Anaplasma phagocytophilum has a functional msp2 gene that is distinct from p44 . Infect Immun 72: 3883–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Q. , Zhang, C. , and Rikihisa, Y. (2006) Analysis of involvement of the RecF pathway in p44 recombination in Anaplasma phagocytophilum and in Escherichia coli by using a plasmid carrying the p44 expression and p44 donor loci. Infect Immun 74: 2052–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. , den Dulk‐Ras, A. , Hooykaas, P.J. , and Rikihisa, Y. (2007) Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl‐1 to facilitate infection. Cell Microbiol 9: 2644–2657. [DOI] [PubMed] [Google Scholar]
- Lin, M. , Zhang, C. , Gibson, K. , and Rikihisa, Y. (2009) Analysis of complete genome sequence of Neorickettsia risticii: causative agent of Potomac horse fever. Nucleic Acids Res 37: 6076–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. , Liu, H. , Xiong, Q. , Niu, H. , Cheng, Z. , Yamamoto, A. , and Rikihisa, Y. (2016) Ehrlichia secretes Etf‐1 to induce autophagy and capture nutrients for its growth through RAB5 and class III phosphatidylinositol 3‐kinase. Autophagy 12: 2145–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Bao, W. , Lin, M. , Niu, H. , and Rikihisa, Y. (2012) Ehrlichia type IV secretion effector ECH0825 is translocated to mitochondria and curbs ROS and apoptosis by upregulating host MnSOD. Cell Microbiol 14: 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massung, R.F. , Lee, K. , Mauel, M. , and Gusa, A. (2002) Characterization of the rRNA genes of Ehrlichia chaffeensis and Anaplasma phagocytophila . DNA Cell Biol 21: 587–596. [DOI] [PubMed] [Google Scholar]
- McNulty, S.N. , Tort, J.F. , Rinaldi, G. , Fischer, K. , Rosa, B.A. , Smircich, P. , et al (2017) Genomes of Fasciola hepatica from the Americas reveal colonization with Neorickettsia Endobacteria related to the agents of potomac horse and human sennetsu fevers. PLoS Genet 13: e1006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millemann, R.E. , and Knapp, S.E. (1970) Biology of Nanophyetus salmincola and “salmon poisoning” disease. Adv Parasitol 8: 1–41. [PubMed] [Google Scholar]
- Mitrophanov, A.Y. , and Groisman, E.A. (2008) Signal integration in bacterial two‐component regulatory systems. Genes Dev 22: 2601–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosavi, L.K. , Cammett, T.J. , Desrosiers, D.C. , and Peng, Z.Y. (2004) The ankyrin repeat as molecular architecture for protein recognition. Protein Sci 13: 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushegian, A.R. , Fullner, K.J. , Koonin, E.V. , and Nester, E.W. (1996) A family of lysozyme‐like virulence factors in bacterial pathogens of plants and animals. Proc Natl Acad Sci USA 93: 7321–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale, P. , Bruser, T. , and Driessen, A.J. (2008) Sec‐ and Tat‐mediated protein secretion across the bacterial cytoplasmic membrane–distinct translocases and mechanisms. Biochim Biophys Acta 1778: 1735–1756. [DOI] [PubMed] [Google Scholar]
- Nene, V. , and Kole, C. (2009) Genome Mapping and Genomics in Animal‐Associated Microbes. Berlin: Springer. [Google Scholar]
- Nikaido, H. (2003) Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67: 593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, H. , Rikihisa, Y. , Yamaguchi, M. , and Ohashi, N. (2006) Differential expression of VirB9 and VirB6 during the life cycle of Anaplasma phagocytophilum in human leucocytes is associated with differential binding and avoidance of lysosome pathway. Cell Microbiol 8: 523–534. [DOI] [PubMed] [Google Scholar]
- Niu, H. , Kozjak‐Pavlovic, V. , Rudel, T. , and Rikihisa, Y. (2010) Anaplasma phagocytophilum Ats‐1 is imported into host cell mitochondria and interferes with apoptosis induction. PLoS Pathog 6: e1000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, H. , Xiong, Q. , Yamamoto, A. , Hayashi‐Nishino, M. , and Rikihisa, Y. (2012) Autophagosomes induced by a bacterial Beclin 1 binding protein facilitate obligatory intracellular infection. Proc Natl Acad Sci USA 109: 20800–20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, N. , Unver, A. , Zhi, N. , and Rikihisa, Y. (1998a) Cloning and characterization of multigenes encoding the immunodominant 30‐kilodalton major outer membrane proteins of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J Clin Microbiol 36: 2671–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, N. , Zhi, N. , Zhang, Y. , and Rikihisa, Y. (1998b) Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect Immun 66: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi, N. , Rikihisa, Y. , and Unver, A. (2001) Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis . Infect Immun 69: 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetzel, M. , Karla, A. , Strynadka, N.C. , and Dalbey, R.E. (2002) Signal peptidases. Chem Rev 102: 4549–4580. [DOI] [PubMed] [Google Scholar]
- Papanikou, E. , Karamanou, S. , and Economou, A. (2007) Bacterial protein secretion through the translocase nanomachine. Nat Rev Microbiol 5: 839–851. [DOI] [PubMed] [Google Scholar]
- Park, J. , and Rikihisa, Y. (1992) L‐arginine‐dependent killing of intracellular Ehrlichia risticii by macrophages treated with gamma interferon. Infect Immun 60: 3504–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, C.B. (1955) There's always something new under the parasitological sun (the unique story of helminth‐borne salmon poisoning disease). J Parasitol 41: 125–148. [PubMed] [Google Scholar]
- Philip, C.B. , Hadlow, W.J. , and Hughes, L.E. (1954a) Studies on salmon poisoning disease of canines. I. The rickettsial relationships and pathogenicity of Neorickettsia helmintheca . Exp Parasitol 3: 336–350. [DOI] [PubMed] [Google Scholar]
- Philip, C.B. , Hughes, L.E. , Locker, B. , and Hadlow, W.J. (1954b) Salmon poisoning disease of canines. II. Further observations on etiologic agent. Proc Soc Exp Biol Med 87: 397–400. [DOI] [PubMed] [Google Scholar]
- Pretzman, C. , Ralph, D. , Stothard, D.R. , Fuerst, P.A. , and Rikihisa, Y. (1995) 16S rRNA gene sequence of Neorickettsia helminthoeca and its phylogenetic alignment with members of the genus Ehrlichia . Int J Syst Bacteriol 45: 207–211. [DOI] [PubMed] [Google Scholar]
- Ren, Q. , and Paulsen, I.T. (2007) Large‐scale comparative genomic analyses of cytoplasmic membrane transport systems in prokaryotes. J Mol Microbiol Biotechnol 12: 165–179. [DOI] [PubMed] [Google Scholar]
- Ren, Q. , Chen, K. , and Paulsen, I.T. (2007) TransportDB: a comprehensive database resource for cytoplasmic membrane transport systems and outer membrane channels. Nucleic Acids Res 35: D274–D279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa, Y. (1991a) The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev 4: 286–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa, Y. (1991b) Cross‐reacting antigens between Neorickettsia helminthoeca and Ehrlichia species, shown by immunofluorescence and Western immunoblotting. J Clin Microbiol 29: 2024–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa, Y. (2010) Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol 8: 328–339. [DOI] [PubMed] [Google Scholar]
- Rikihisa, Y. , Pretzman, C.I. , Johnson, G.C. , Reed, S.M. , Yamamoto, S. , and Andrews, F. (1988) Clinical, histopathological, and immunological responses of ponies to Ehrlichia sennetsu and subsequent Ehrlichia risticii challenge. Infect Immun 56: 2960–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa, Y. , Stills, H. , and Zimmerman, G. (1991) Isolation and continuous culture of Neorickettsia helminthoeca in a macrophage cell line. J Clin Microbiol 29: 1928–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa, Y. , Ewing, S.A. , Fox, J.C. , Siregar, A.G. , Pasaribu, F.H. , and Malole, M.B. (1992) Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J Clin Microbiol 30: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa, Y. , Ewing, S.A. , and Fox, J.C. (1994) Western immunoblot analysis of Ehrlichia chaffeensis, E. canis, or E. ewingii infections in dogs and humans. J Clin Microbiol 32: 2107–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa, Y. , Kawahara, M. , Wen, B. , Kociba, G. , Fuerst, P. , Kawamori, F. , et al (1997) Western immunoblot analysis of Haemobartonella muris and comparison of 16S rRNA gene sequences of H. muris, H. felis, and Eperythrozoon suis . J Clin Microbiol 35: 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa, Y. , Zhang, C. , Kanter, M. , Cheng, Z. , Ohashi, N. , and Fukuda, T. (2004) Analysis of p51, groESL, and the major antigen P51 in various species of Neorickettsia, an obligatory intracellular bacterium that infects trematodes and mammals. J Clin Microbiol 42: 3823–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa, Y. , Dumler, J.S. , and Dasch, G.A. (2005) Neorickettsia In The Proteobacteria, Part C, Bergey's Manual of Systematic Bacteriology, 2nd edn Brenner D.J., Krieg N.R., Staley J.T. and Garrity G.M. (eds). New York, NY: Springer, pp. 132–137. [Google Scholar]
- Romling, U. (2009) Cyclic Di‐GMP (c‐Di‐GMP) goes into host cells–c‐Di‐GMP signaling in the obligate intracellular pathogen Anaplasma phagocytophilum . J Bacteriol 191: 683–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent, F. , Berks, B.C. , and Palmer, T. (2006) Pathfinders and trailblazers: a prokaryotic targeting system for transport of folded proteins. FEMS Microbiol Lett 254: 198–207. [DOI] [PubMed] [Google Scholar]
- Schlegel, M.W. , Knapp, S.E. , and Millemann, R.E. (1968) “Salmon poisoning” disease. V. Definitive hosts of the trematode vector, Nanophyetus salmincola . J Parasitol 54: 770–774. [PubMed] [Google Scholar]
- Skaar, E.P. (2010) The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6: e1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G.A. , Theriot, J.A. , and Portnoy, D.A. (1996) The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin‐based motility, the percentage of moving bacteria, and the localization of vasodilator‐stimulated phosphoprotein and profilin. J Cell Biol 135: 647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis, A. , Ludwig, T. , and Meier, H. (2005) RAxML‐III: a fast program for maximum likelihood‐based inference of large phylogenetic trees. Bioinformatics 21: 456–463. [DOI] [PubMed] [Google Scholar]
- Sukhithasri, V. , Nisha, N. , Biswas, L. , Anil Kumar, V. , and Biswas, R. (2013) Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol Res 168: 396–406. [DOI] [PubMed] [Google Scholar]
- Surana, N.K. , Grass, S. , Hardy, G.G. , Li, H. , Thanassi, D.G. , and Geme, J.W. 3rd (2004) Evidence for conservation of architecture and physical properties of Omp85‐like proteins throughout evolution. Proc Natl Acad Sci USA 101: 14497–14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D. , Higgins, D.G. , and Gibson, T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position‐specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler, A.D. , and Camilli, A. (2004) Cyclic diguanylate (c‐di‐GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol 53: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach, V.V. , Schroeder, J.A. , Greiman, S.E. , and Vaughan, J.A. (2012) New genetic lineages, host associations and circulation pathways of Neorickettsia endosymbionts of digeneans. Acta Parasitol 57: 285–292. [DOI] [PubMed] [Google Scholar]
- Ulrich, L.E. , and Zhulin, I.B. (2007) MiST: a microbial signal transduction database. Nucleic Acids Res 35: D386–D390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, L.E. , and Zhulin, I.B. (2010) The MiST2 database: a comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res 38: D401–D407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, L.E. , Koonin, E.V. , and Zhulin, I.B. (2005) One‐component systems dominate signal transduction in prokaryotes. Trends Microbiol 13: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemulapalli, R. , Biswas, B. , and Dutta, S.K. (1998) Cloning and molecular analysis of genes encoding two immunodominant antigens of Ehrlichia risticii . Microb Pathog 24: 361–372. [DOI] [PubMed] [Google Scholar]
- Veterinary Practice News (2009) Salmon poisoning disease found in Southern California [WWW document]. URL http://www.veterinarypracticenews.com/April-2009/Salmon-Poisoning-Disease-Found-In-Southern-California/.
- Vollmer, W. , and Bertsche, U. (2008) Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli . Biochim Biophys Acta 1778: 1714–1734. [DOI] [PubMed] [Google Scholar]
- Wakeel, A. , den Dulk‐Ras, A. , Hooykaas, P.J. , and McBride, J.W. (2011) Ehrlichia chaffeensis tandem repeat proteins and Ank200 are type 1 secretion system substrates related to the repeats‐in‐toxin exoprotein family. Front Cell Infect Microbiol 1: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Kikuchi, T. , and Rikihisa, Y. (2007a) Proteomic identification of a novel Anaplasma phagocytophilum DNA binding protein that regulates a putative transcription factor. J Bacteriol 189: 4880–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Cheng, Z. , Zhang, C. , Kikuchi, T. , and Rikihisa, Y. (2007b) Anaplasma phagocytophilum p44 mRNA expression is differentially regulated in mammalian and tick host cells: involvement of the DNA binding protein ApxR. J Bacteriol 189: 8651–8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, N.L. , Steven, B. , Penn, K. , Methe, B.A. , and Detrich, W.H. 3rd (2009) Characterization of the intestinal microbiota of two Antarctic notothenioid fish species. Extremophiles 13: 679–685. [DOI] [PubMed] [Google Scholar]
- Weiss, E. , Williams, J.C. , Dasch, G.A. , and Kang, Y.H. (1989) Energy metabolism of monocytic Ehrlichia . Proc Natl Acad Sci USA 86: 1674–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, B. , Rikihisa, Y. , Mott, J. , Fuerst, P.A. , Kawahara, M. , and Suto, C. (1995) Ehrlichia muris sp. nov., identified on the basis of 16S rRNA base sequences and serological, morphological, and biological characteristics. Int J Syst Bacteriol 45: 250–254. [DOI] [PubMed] [Google Scholar]
- Wen, B. , Rikihisa, Y. , Yamamoto, S. , Kawabata, N. , and Fuerst, P.A. (1996) Characterization of the SF agent, an Ehrlichia sp. isolated from the fluke Stellantchasmus falcatus, by 16S rRNA base sequence, serological, and morphological analyses. Int J Syst Bacteriol 46: 149–154. [DOI] [PubMed] [Google Scholar]
- Winkler, H.H. (1976) Rickettsial permeability. An ADP‐ATP transport system. J Biol Chem 251: 389–396. [PubMed] [Google Scholar]
- Wu, M. , Sun, L.V. , Vamathevan, J. , Riegler, M. , Deboy, R. , Brownlie, J.C. , et al (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2: E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuichet, K. , Cantwell, B.J. , and Zhulin, I.B. (2010) Evolution and phyletic distribution of two‐component signal transduction systems. Curr Opin Microbiol 13: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, N.Y. , Wagner, J.R. , Laird, M.R. , Melli, G. , Rey, S. , Lo, R. , et al (2010) PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26: 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi, N. , Ohashi, N. , Rikihisa, Y. , Horowitz, H.W. , Wormser, G.P. , and Hechemy, K. (1998) Cloning and expression of the 44‐kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol 36: 1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, B. , Nethery, K.A. , Kuriakose, J.A. , Wakeel, A. , Zhang, X. , and McBride, J.W. (2009) Nuclear Translocated Ehrlichia chaffeensis Ankyrin Protein Interacts with the Mid A‐Stretch of Host Promoter and Intronic Alu Elements. Infect Immun 77: 4243–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Synteny plots between Neorickettsia spp.
Fig. S2. Secondary Structure of N. helminthoeca P51 Protein.
Fig. S3. Phylogenetic tree of VirB2 proteins in the family Anaplasmataceae and α‐proteobacteria.
Fig. S4. One‐component regulatory systems of N. helminthoeca.
Fig. S5. Phylogenetic analysis of AnkA or Ank200 homologous proteins in the family Anaplasmataceae.
Table S1. Ortholog clusters conserved among N. helminthoeca, N. risticii and N. sennetsu based on three‐way comparison analysis
Table S2. N. helminthoeca‐specific proteins compared to N. sennetsu and N. risticii
Table S3. N. risticii‐specific proteins compared to N. helminthoeca and N. sennetsu
Table S4. N. sennetsu‐specific proteins compared to N. helminthoeca and N. risticii
Table S5. Amino acid and cofactor biosynthesis in Family Anaplasmataceae
Table S6. Potential pathogenic genes in Neorickettsia species
Table S7. Putative Transporters of N. helminthoeca
Table S8. Genes involved in DNA repair and homologous recombination
Table S9. Proteins with tandem repeats in N. helminthoeca
Table S10. Lipoprotein‐processing enzymes and putative lipoproteins in N. helminthoeca
Table S11. Oligonucleotide primers used for cloning N. helminthoeca outer membrane proteins


